 Found 323 hits with Last Name = 'ali' and Initial = 'mm'
Found 323 hits with Last Name = 'ali' and Initial = 'mm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50005397
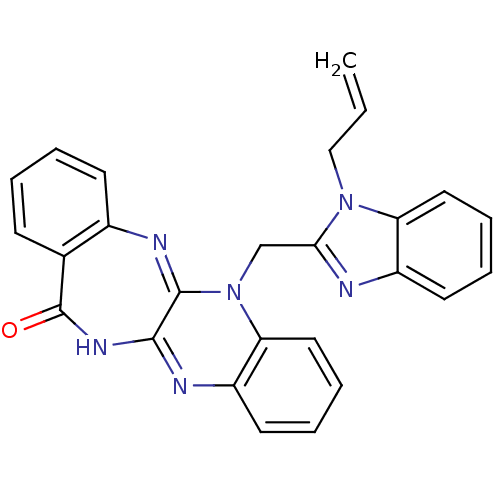
(CHEMBL2206684)Show SMILES C=CCn1c(CN2C3=Nc4ccccc4C(=O)NC3=Nc3ccccc23)nc2ccccc12 |c:20,t:7| Show InChI InChI=1S/C26H20N6O/c1-2-15-31-21-13-7-5-11-19(21)27-23(31)16-32-22-14-8-6-12-20(22)28-24-25(32)29-18-10-4-3-9-17(18)26(33)30-24/h2-14H,1,15-16H2,(H,28,30,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402366

(CHEMBL2206696)Show SMILES Nc1c(cc(-c2ccccc2)n1-c1ccncc1)-c1nc2ccccc2[nH]1 Show InChI InChI=1S/C22H17N5/c23-21-17(22-25-18-8-4-5-9-19(18)26-22)14-20(15-6-2-1-3-7-15)27(21)16-10-12-24-13-11-16/h1-14H,23H2,(H,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50005398
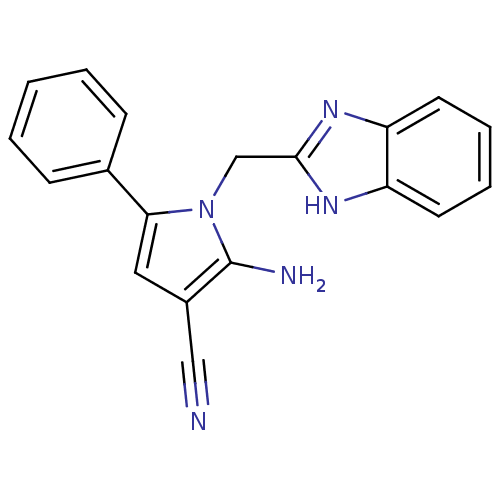
(CHEMBL2206694)Show InChI InChI=1S/C19H15N5/c20-11-14-10-17(13-6-2-1-3-7-13)24(19(14)21)12-18-22-15-8-4-5-9-16(15)23-18/h1-10H,12,21H2,(H,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402361
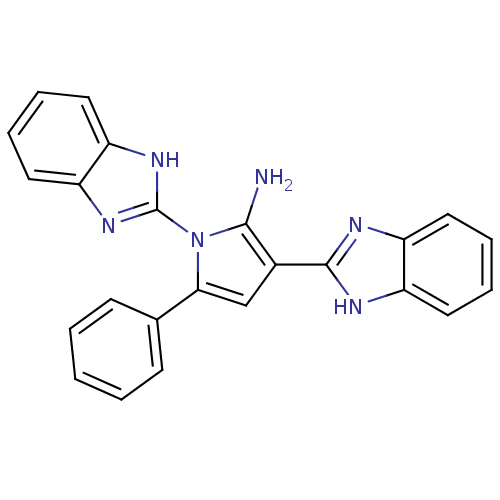
(CHEMBL2206680)Show SMILES Nc1c(cc(-c2ccccc2)n1-c1nc2ccccc2[nH]1)-c1nc2ccccc2[nH]1 Show InChI InChI=1S/C24H18N6/c25-22-16(23-26-17-10-4-5-11-18(17)27-23)14-21(15-8-2-1-3-9-15)30(22)24-28-19-12-6-7-13-20(19)29-24/h1-14H,25H2,(H,26,27)(H,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402378
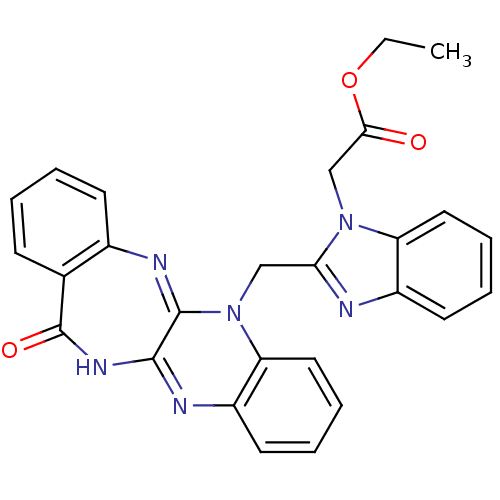
(CHEMBL2206685)Show SMILES CCOC(=O)Cn1c(CN2C3=Nc4ccccc4C(=O)NC3=Nc3ccccc23)nc2ccccc12 |c:23,t:10| Show InChI InChI=1S/C27H22N6O3/c1-2-36-24(34)16-32-21-13-7-5-11-19(21)28-23(32)15-33-22-14-8-6-12-20(22)29-25-26(33)30-18-10-4-3-9-17(18)27(35)31-25/h3-14H,2,15-16H2,1H3,(H,29,31,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402373
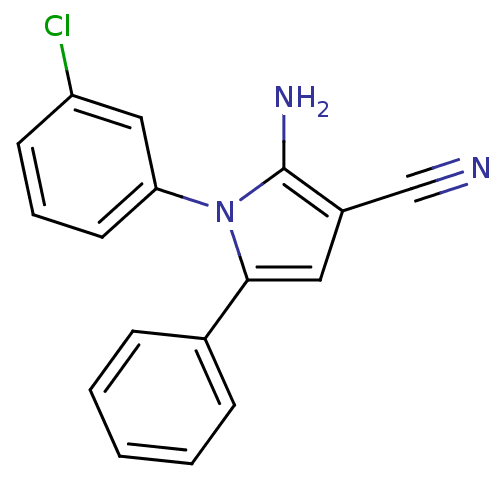
(CHEMBL2206691)Show InChI InChI=1S/C17H12ClN3/c18-14-7-4-8-15(10-14)21-16(9-13(11-19)17(21)20)12-5-2-1-3-6-12/h1-10H,20H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402360

(CHEMBL2206681)Show SMILES Nc1c(cc(-c2ccccc2)n1Cc1nc2ccccc2[nH]1)-c1nc2ccccc2[nH]1 Show InChI InChI=1S/C25H20N6/c26-24-17(25-29-20-12-6-7-13-21(20)30-25)14-22(16-8-2-1-3-9-16)31(24)15-23-27-18-10-4-5-11-19(18)28-23/h1-14H,15,26H2,(H,27,28)(H,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402372
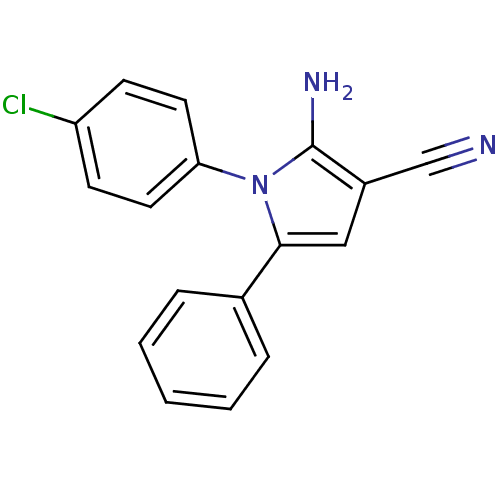
(CHEMBL2206692)Show InChI InChI=1S/C17H12ClN3/c18-14-6-8-15(9-7-14)21-16(10-13(11-19)17(21)20)12-4-2-1-3-5-12/h1-10H,20H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402374

(CHEMBL2206690)Show InChI InChI=1S/C17H12BrN3/c18-14-6-8-15(9-7-14)21-16(10-13(11-19)17(21)20)12-4-2-1-3-5-12/h1-10H,20H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402371
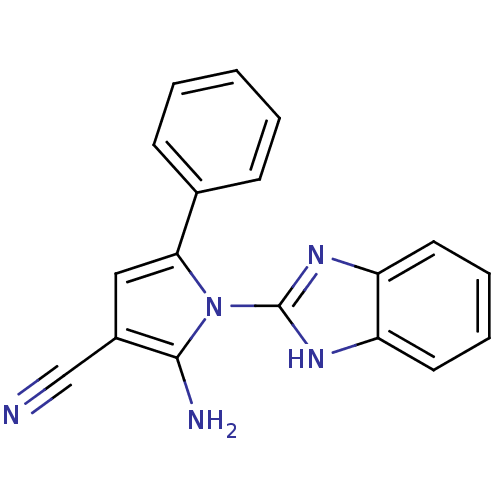
(CHEMBL2206693)Show InChI InChI=1S/C18H13N5/c19-11-13-10-16(12-6-2-1-3-7-12)23(17(13)20)18-21-14-8-4-5-9-15(14)22-18/h1-10H,20H2,(H,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402375
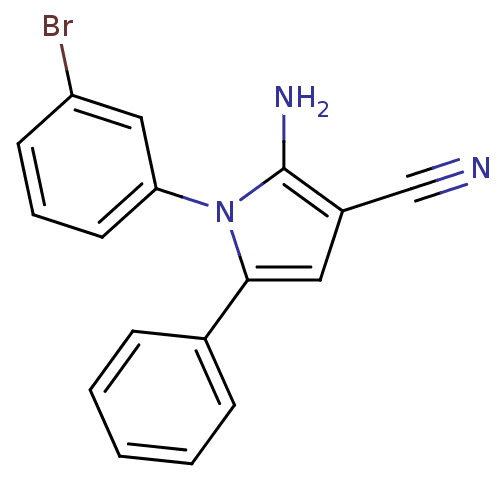
(CHEMBL2206689)Show InChI InChI=1S/C17H12BrN3/c18-14-7-4-8-15(10-14)21-16(9-13(11-19)17(21)20)12-5-2-1-3-6-12/h1-10H,20H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402363
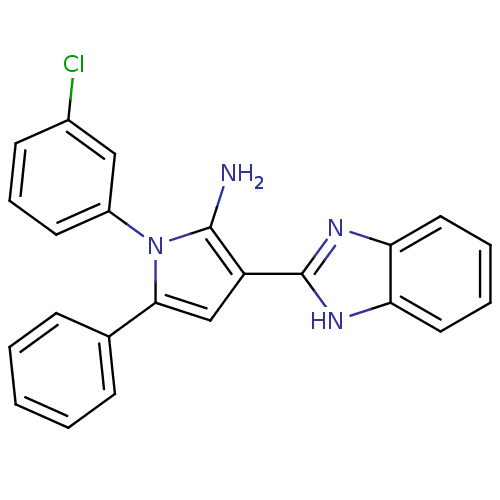
(CHEMBL2206699)Show SMILES Nc1c(cc(-c2ccccc2)n1-c1cccc(Cl)c1)-c1nc2ccccc2[nH]1 Show InChI InChI=1S/C23H17ClN4/c24-16-9-6-10-17(13-16)28-21(15-7-2-1-3-8-15)14-18(22(28)25)23-26-19-11-4-5-12-20(19)27-23/h1-14H,25H2,(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402362
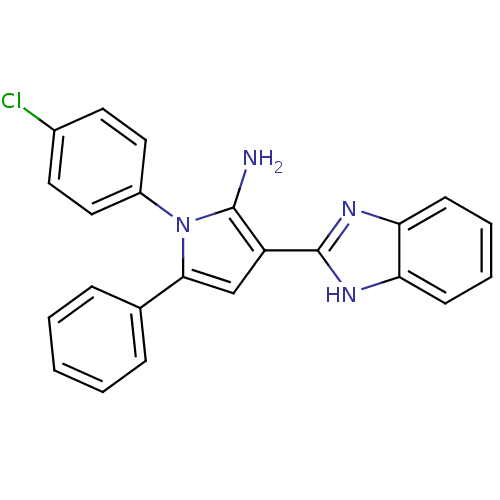
(CHEMBL2206700)Show SMILES Nc1c(cc(-c2ccccc2)n1-c1ccc(Cl)cc1)-c1nc2ccccc2[nH]1 Show InChI InChI=1S/C23H17ClN4/c24-16-10-12-17(13-11-16)28-21(15-6-2-1-3-7-15)14-18(22(28)25)23-26-19-8-4-5-9-20(19)27-23/h1-14H,25H2,(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402370
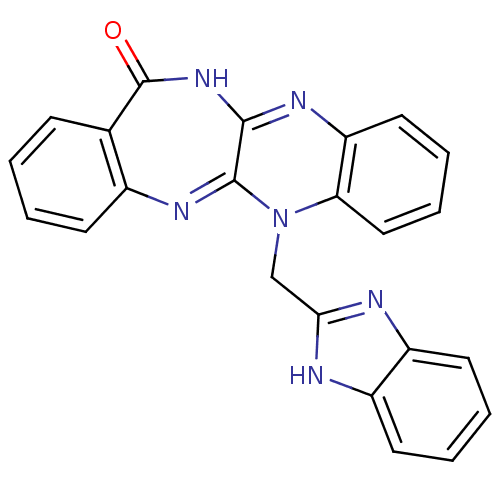
(CHEMBL2206683)Show SMILES O=C1NC2=Nc3ccccc3N(Cc3nc4ccccc4[nH]3)C2=Nc2ccccc12 |c:26,t:3| Show InChI InChI=1S/C23H16N6O/c30-23-14-7-1-2-8-15(14)27-22-21(28-23)26-18-11-5-6-12-19(18)29(22)13-20-24-16-9-3-4-10-17(16)25-20/h1-12H,13H2,(H,24,25)(H,26,28,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402367

(CHEMBL2206695)Show SMILES Nc1c(cc(-c2ccccc2)n1-c1ccccn1)-c1nc2ccccc2[nH]1 Show InChI InChI=1S/C22H17N5/c23-21-16(22-25-17-10-4-5-11-18(17)26-22)14-19(15-8-2-1-3-9-15)27(21)20-12-6-7-13-24-20/h1-14H,23H2,(H,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402376
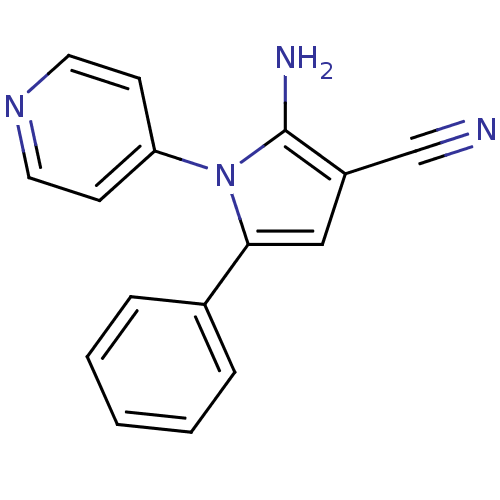
(CHEMBL2206688)Show InChI InChI=1S/C16H12N4/c17-11-13-10-15(12-4-2-1-3-5-12)20(16(13)18)14-6-8-19-9-7-14/h1-10H,18H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402364
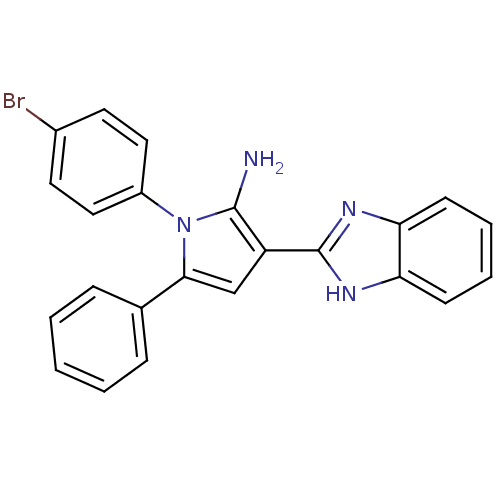
(CHEMBL2206698)Show SMILES Nc1c(cc(-c2ccccc2)n1-c1ccc(Br)cc1)-c1nc2ccccc2[nH]1 Show InChI InChI=1S/C23H17BrN4/c24-16-10-12-17(13-11-16)28-21(15-6-2-1-3-7-15)14-18(22(28)25)23-26-19-8-4-5-9-20(19)27-23/h1-14H,25H2,(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402365
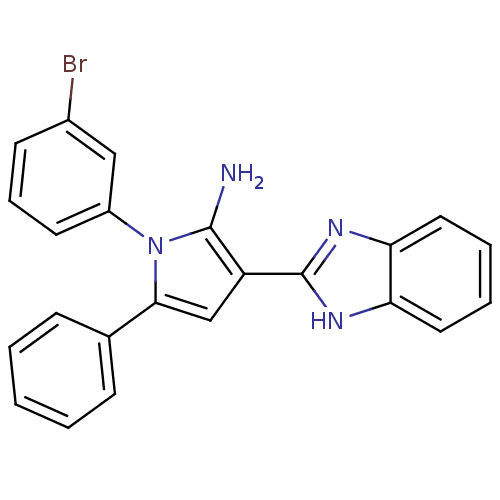
(CHEMBL2206697)Show SMILES Nc1c(cc(-c2ccccc2)n1-c1cccc(Br)c1)-c1nc2ccccc2[nH]1 Show InChI InChI=1S/C23H17BrN4/c24-16-9-6-10-17(13-16)28-21(15-7-2-1-3-8-15)14-18(22(28)25)23-26-19-11-4-5-12-20(19)27-23/h1-14H,25H2,(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402377

(CHEMBL2206687)Show InChI InChI=1S/C16H12N4/c17-11-13-10-14(12-6-2-1-3-7-12)20(16(13)18)15-8-4-5-9-19-15/h1-10H,18H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402379
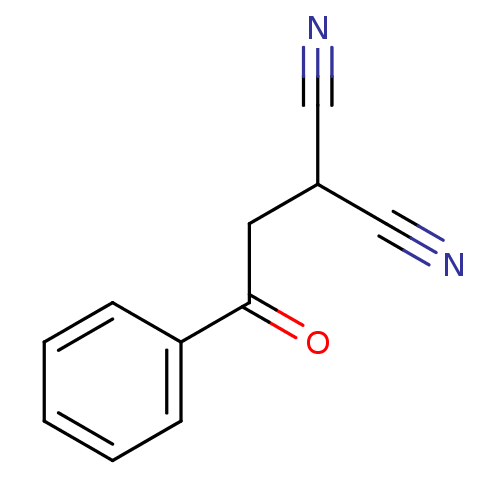
(CHEMBL2206686)Show InChI InChI=1S/C11H8N2O/c12-7-9(8-13)6-11(14)10-4-2-1-3-5-10/h1-5,9H,6H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.296 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402368
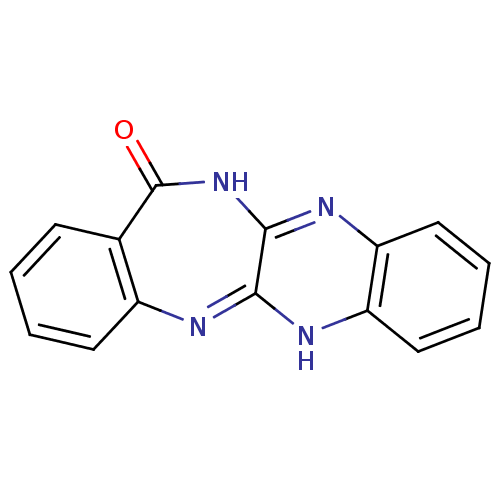
(CHEMBL2206682)Show InChI InChI=1S/C15H10N4O/c20-15-9-5-1-2-6-10(9)16-13-14(19-15)18-12-8-4-3-7-11(12)17-13/h1-8H,(H,16,17)(H,18,19,20) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402369

(CHEMBL1652555)Show InChI InChI=1S/C8H7N3O/c9-7-8(12)11-6-4-2-1-3-5(6)10-7/h1-4H,(H2,9,10)(H,11,12) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 20.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM50322535

(3-(imidazo[1,2-b]pyridazin-3-ylethynyl)-4-methyl-N...)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3cnc4cccnn34)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C29H27F3N6O/c1-20-5-6-22(16-21(20)8-10-25-18-33-27-4-3-11-34-38(25)27)28(39)35-24-9-7-23(26(17-24)29(30,31)32)19-37-14-12-36(2)13-15-37/h3-7,9,11,16-18H,12-15,19H2,1-2H3,(H,35,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Kafrelsheikh University
Curated by ChEMBL
| Assay Description
Inhibition of FGFR-1 (unknown origin) |
Eur J Med Chem 163: 37-53 (2019)
Article DOI: 10.1016/j.ejmech.2018.11.061
BindingDB Entry DOI: 10.7270/Q25B05S0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50363397

(CHEMBL1946170 | REGORAFENIB | US10183928, Regorafe...)Show SMILES CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3ccc(Cl)c(c3)C(F)(F)F)c(F)c2)ccn1 Show InChI InChI=1S/C21H15ClF4N4O3/c1-27-19(31)18-10-13(6-7-28-18)33-12-3-5-17(16(23)9-12)30-20(32)29-11-2-4-15(22)14(8-11)21(24,25)26/h2-10H,1H3,(H,27,31)(H2,29,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of BRAF (unknown origin) |
Eur J Med Chem 179: 707-722 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.063
BindingDB Entry DOI: 10.7270/Q2PC35S4 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 3
(Mus musculus (mouse)) | BDBM50363397

(CHEMBL1946170 | REGORAFENIB | US10183928, Regorafe...)Show SMILES CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3ccc(Cl)c(c3)C(F)(F)F)c(F)c2)ccn1 Show InChI InChI=1S/C21H15ClF4N4O3/c1-27-19(31)18-10-13(6-7-28-18)33-12-3-5-17(16(23)9-12)30-20(32)29-11-2-4-15(22)14(8-11)21(24,25)26/h2-10H,1H3,(H,27,31)(H2,29,30,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of mouse VEGFR-3 (818 to 1363 residues) |
Eur J Med Chem 179: 707-722 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.063
BindingDB Entry DOI: 10.7270/Q2PC35S4 |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM50363397

(CHEMBL1946170 | REGORAFENIB | US10183928, Regorafe...)Show SMILES CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3ccc(Cl)c(c3)C(F)(F)F)c(F)c2)ccn1 Show InChI InChI=1S/C21H15ClF4N4O3/c1-27-19(31)18-10-13(6-7-28-18)33-12-3-5-17(16(23)9-12)30-20(32)29-11-2-4-15(22)14(8-11)21(24,25)26/h2-10H,1H3,(H,27,31)(H2,29,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of FGFR1 (unknown origin) |
Eur J Med Chem 179: 707-722 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.063
BindingDB Entry DOI: 10.7270/Q2PC35S4 |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50363397

(CHEMBL1946170 | REGORAFENIB | US10183928, Regorafe...)Show SMILES CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3ccc(Cl)c(c3)C(F)(F)F)c(F)c2)ccn1 Show InChI InChI=1S/C21H15ClF4N4O3/c1-27-19(31)18-10-13(6-7-28-18)33-12-3-5-17(16(23)9-12)30-20(32)29-11-2-4-15(22)14(8-11)21(24,25)26/h2-10H,1H3,(H,27,31)(H2,29,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of PDGFR beta (561 to 1106 residues) (unknown origin) |
Eur J Med Chem 179: 707-722 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.063
BindingDB Entry DOI: 10.7270/Q2PC35S4 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Mus musculus) | BDBM50363397

(CHEMBL1946170 | REGORAFENIB | US10183928, Regorafe...)Show SMILES CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3ccc(Cl)c(c3)C(F)(F)F)c(F)c2)ccn1 Show InChI InChI=1S/C21H15ClF4N4O3/c1-27-19(31)18-10-13(6-7-28-18)33-12-3-5-17(16(23)9-12)30-20(32)29-11-2-4-15(22)14(8-11)21(24,25)26/h2-10H,1H3,(H,27,31)(H2,29,30,32) | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of mouse VEGFR-2 (785 to 1376 residues) |
Eur J Med Chem 179: 707-722 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.063
BindingDB Entry DOI: 10.7270/Q2PC35S4 |
More data for this
Ligand-Target Pair | |
Angiopoietin-1 receptor
(Homo sapiens (Human)) | BDBM50363397

(CHEMBL1946170 | REGORAFENIB | US10183928, Regorafe...)Show SMILES CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3ccc(Cl)c(c3)C(F)(F)F)c(F)c2)ccn1 Show InChI InChI=1S/C21H15ClF4N4O3/c1-27-19(31)18-10-13(6-7-28-18)33-12-3-5-17(16(23)9-12)30-20(32)29-11-2-4-15(22)14(8-11)21(24,25)26/h2-10H,1H3,(H,27,31)(H2,29,30,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged TIE2 (unknown origin) using biotin-Ahx-EPKDDAYPLYSDFG peptide as substrate by HTRF method |
Eur J Med Chem 179: 707-722 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.063
BindingDB Entry DOI: 10.7270/Q2PC35S4 |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50363397

(CHEMBL1946170 | REGORAFENIB | US10183928, Regorafe...)Show SMILES CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3ccc(Cl)c(c3)C(F)(F)F)c(F)c2)ccn1 Show InChI InChI=1S/C21H15ClF4N4O3/c1-27-19(31)18-10-13(6-7-28-18)33-12-3-5-17(16(23)9-12)30-20(32)29-11-2-4-15(22)14(8-11)21(24,25)26/h2-10H,1H3,(H,27,31)(H2,29,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of RAF1 (unknown origin) |
Eur J Med Chem 179: 707-722 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.063
BindingDB Entry DOI: 10.7270/Q2PC35S4 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 1
(Homo sapiens (Human)) | BDBM50363397

(CHEMBL1946170 | REGORAFENIB | US10183928, Regorafe...)Show SMILES CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3ccc(Cl)c(c3)C(F)(F)F)c(F)c2)ccn1 Show InChI InChI=1S/C21H15ClF4N4O3/c1-27-19(31)18-10-13(6-7-28-18)33-12-3-5-17(16(23)9-12)30-20(32)29-11-2-4-15(22)14(8-11)21(24,25)26/h2-10H,1H3,(H,27,31)(H2,29,30,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR1 (unknown origin) |
Eur J Med Chem 179: 707-722 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.063
BindingDB Entry DOI: 10.7270/Q2PC35S4 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50369571
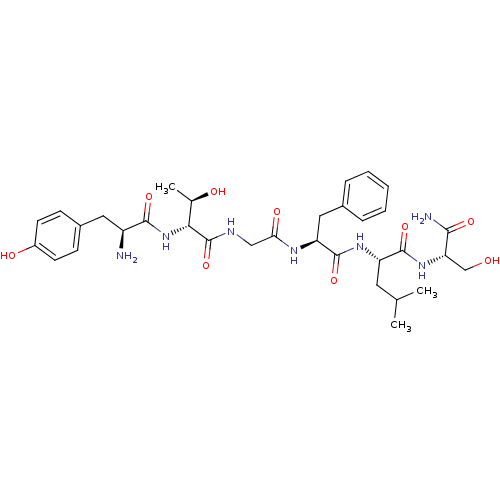
(CHEMBL1790710)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)[C@@H](C)O)C(=O)N[C@@H](CO)C(N)=O Show InChI InChI=1S/C33H47N7O9/c1-18(2)13-24(31(47)39-26(17-41)29(35)45)38-32(48)25(15-20-7-5-4-6-8-20)37-27(44)16-36-33(49)28(19(3)42)40-30(46)23(34)14-21-9-11-22(43)12-10-21/h4-12,18-19,23-26,28,41-43H,13-17,34H2,1-3H3,(H2,35,45)(H,36,49)(H,37,44)(H,38,48)(H,39,47)(H,40,46)/t19-,23+,24+,25+,26+,28-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Northern Colorado
Curated by ChEMBL
| Assay Description
Binding affinity towards Opioid receptor delta 1 was evaluated |
J Med Chem 43: 2586-90 (2000)
BindingDB Entry DOI: 10.7270/Q2JQ11Q1 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1
(Mus musculus (Mouse)-MOUSE) | BDBM50369571

(CHEMBL1790710)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)[C@@H](C)O)C(=O)N[C@@H](CO)C(N)=O Show InChI InChI=1S/C33H47N7O9/c1-18(2)13-24(31(47)39-26(17-41)29(35)45)38-32(48)25(15-20-7-5-4-6-8-20)37-27(44)16-36-33(49)28(19(3)42)40-30(46)23(34)14-21-9-11-22(43)12-10-21/h4-12,18-19,23-26,28,41-43H,13-17,34H2,1-3H3,(H2,35,45)(H,36,49)(H,37,44)(H,38,48)(H,39,47)(H,40,46)/t19-,23+,24+,25+,26+,28-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Northern Colorado
Curated by ChEMBL
| Assay Description
Opioid receptor activity was evaluated using mouse vas deferens (MVD) assay |
J Med Chem 43: 2586-90 (2000)
BindingDB Entry DOI: 10.7270/Q2JQ11Q1 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50369572
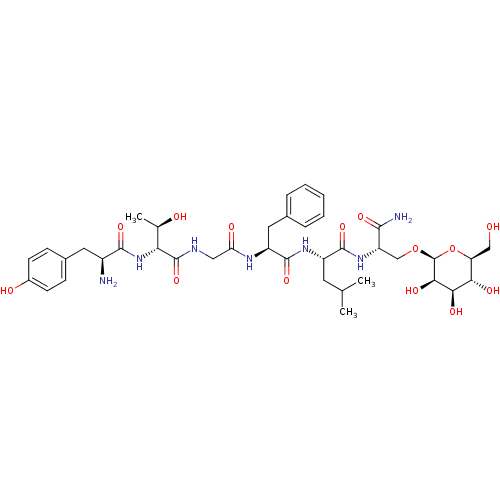
(CHEMBL1790709)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)[C@@H](C)O)C(=O)N[C@@H](CO[C@H]1O[C@@H](CO)[C@H](O)[C@@H](O)[C@H]1O)C(N)=O Show InChI InChI=1S/C39H57N7O14/c1-19(2)13-25(36(56)45-27(34(41)54)18-59-39-33(53)32(52)31(51)28(17-47)60-39)44-37(57)26(15-21-7-5-4-6-8-21)43-29(50)16-42-38(58)30(20(3)48)46-35(55)24(40)14-22-9-11-23(49)12-10-22/h4-12,19-20,24-28,30-33,39,47-49,51-53H,13-18,40H2,1-3H3,(H2,41,54)(H,42,58)(H,43,50)(H,44,57)(H,45,56)(H,46,55)/t20-,24+,25+,26+,27+,28+,30-,31+,32-,33-,39+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Northern Colorado
Curated by ChEMBL
| Assay Description
Binding affinity towards Opioid receptor delta 1 was evaluated |
J Med Chem 43: 2586-90 (2000)
BindingDB Entry DOI: 10.7270/Q2JQ11Q1 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50369571

(CHEMBL1790710)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)[C@@H](C)O)C(=O)N[C@@H](CO)C(N)=O Show InChI InChI=1S/C33H47N7O9/c1-18(2)13-24(31(47)39-26(17-41)29(35)45)38-32(48)25(15-20-7-5-4-6-8-20)37-27(44)16-36-33(49)28(19(3)42)40-30(46)23(34)14-21-9-11-22(43)12-10-21/h4-12,18-19,23-26,28,41-43H,13-17,34H2,1-3H3,(H2,35,45)(H,36,49)(H,37,44)(H,38,48)(H,39,47)(H,40,46)/t19-,23+,24+,25+,26+,28-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Northern Colorado
Curated by ChEMBL
| Assay Description
Binding affinity towards Opioid receptor mu 1 was evaluated |
J Med Chem 43: 2586-90 (2000)
BindingDB Entry DOI: 10.7270/Q2JQ11Q1 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50363397

(CHEMBL1946170 | REGORAFENIB | US10183928, Regorafe...)Show SMILES CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3ccc(Cl)c(c3)C(F)(F)F)c(F)c2)ccn1 Show InChI InChI=1S/C21H15ClF4N4O3/c1-27-19(31)18-10-13(6-7-28-18)33-12-3-5-17(16(23)9-12)30-20(32)29-11-2-4-15(22)14(8-11)21(24,25)26/h2-10H,1H3,(H,27,31)(H2,29,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal GST tagged VEGFR-2 expressed in baculovirus infected Sf9 cells using poly (Glu,Tyr) 4:1 as substrate in presence of AT... |
Eur J Med Chem 179: 707-722 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.063
BindingDB Entry DOI: 10.7270/Q2PC35S4 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk2
(Homo sapiens (Human)) | BDBM50252231
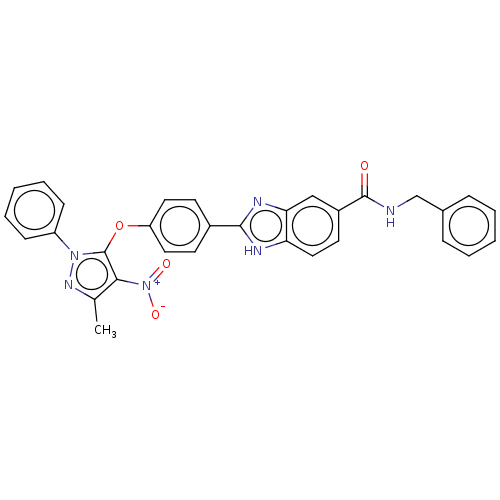
(CHEMBL4072515)Show SMILES Cc1nn(c(Oc2ccc(cc2)-c2nc3cc(ccc3[nH]2)C(=O)NCc2ccccc2)c1[N+]([O-])=O)-c1ccccc1 Show InChI InChI=1S/C31H24N6O4/c1-20-28(37(39)40)31(36(35-20)24-10-6-3-7-11-24)41-25-15-12-22(13-16-25)29-33-26-17-14-23(18-27(26)34-29)30(38)32-19-21-8-4-2-5-9-21/h2-18H,19H2,1H3,(H,32,38)(H,33,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of Chk2 (unknown origin) by ELISA based spectrophotometric analysis |
Eur J Med Chem 134: 392-405 (2017)
Article DOI: 10.1016/j.ejmech.2017.03.090
BindingDB Entry DOI: 10.7270/Q27P91T8 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk2
(Homo sapiens (Human)) | BDBM50252253
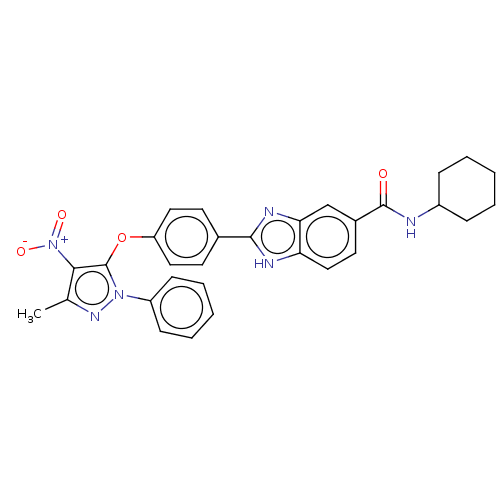
(CHEMBL4093249)Show SMILES Cc1nn(c(Oc2ccc(cc2)-c2nc3cc(ccc3[nH]2)C(=O)NC2CCCCC2)c1[N+]([O-])=O)-c1ccccc1 Show InChI InChI=1S/C30H28N6O4/c1-19-27(36(38)39)30(35(34-19)23-10-6-3-7-11-23)40-24-15-12-20(13-16-24)28-32-25-17-14-21(18-26(25)33-28)29(37)31-22-8-4-2-5-9-22/h3,6-7,10-18,22H,2,4-5,8-9H2,1H3,(H,31,37)(H,32,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of Chk2 (unknown origin) by ELISA based spectrophotometric analysis |
Eur J Med Chem 134: 392-405 (2017)
Article DOI: 10.1016/j.ejmech.2017.03.090
BindingDB Entry DOI: 10.7270/Q27P91T8 |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM16673

(4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamo...)Show SMILES CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3ccc(Cl)c(c3)C(F)(F)F)cc2)ccn1 Show InChI InChI=1S/C21H16ClF3N4O3/c1-26-19(30)18-11-15(8-9-27-18)32-14-5-2-12(3-6-14)28-20(31)29-13-4-7-17(22)16(10-13)21(23,24)25/h2-11H,1H3,(H,26,30)(H2,28,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of RAF1 (unknown origin) |
Eur J Med Chem 179: 707-722 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.063
BindingDB Entry DOI: 10.7270/Q2PC35S4 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50369572

(CHEMBL1790709)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)[C@@H](C)O)C(=O)N[C@@H](CO[C@H]1O[C@@H](CO)[C@H](O)[C@@H](O)[C@H]1O)C(N)=O Show InChI InChI=1S/C39H57N7O14/c1-19(2)13-25(36(56)45-27(34(41)54)18-59-39-33(53)32(52)31(51)28(17-47)60-39)44-37(57)26(15-21-7-5-4-6-8-21)43-29(50)16-42-38(58)30(20(3)48)46-35(55)24(40)14-22-9-11-23(49)12-10-22/h4-12,19-20,24-28,30-33,39,47-49,51-53H,13-18,40H2,1-3H3,(H2,41,54)(H,42,58)(H,43,50)(H,44,57)(H,45,56)(H,46,55)/t20-,24+,25+,26+,27+,28+,30-,31+,32-,33-,39+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Northern Colorado
Curated by ChEMBL
| Assay Description
Binding affinity towards Opioid receptor mu 1 was evaluated |
J Med Chem 43: 2586-90 (2000)
BindingDB Entry DOI: 10.7270/Q2JQ11Q1 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk2
(Homo sapiens (Human)) | BDBM50291408
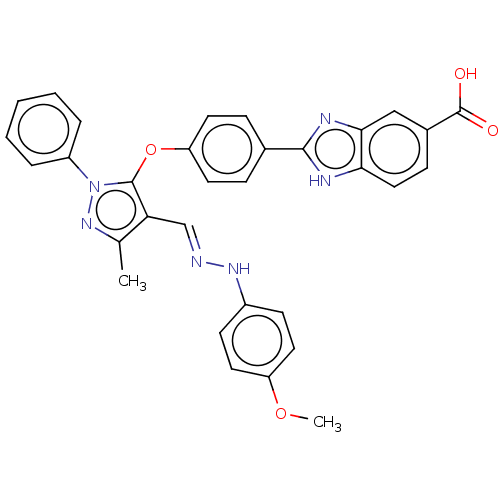
(CHEMBL4165600)Show SMILES COc1ccc(N\N=C\c2c(C)nn(c2Oc2ccc(cc2)-c2nc3cc(ccc3[nH]2)C(O)=O)-c2ccccc2)cc1 Show InChI InChI=1S/C32H26N6O4/c1-20-27(19-33-36-23-11-15-25(41-2)16-12-23)31(38(37-20)24-6-4-3-5-7-24)42-26-13-8-21(9-14-26)30-34-28-17-10-22(32(39)40)18-29(28)35-30/h3-19,36H,1-2H3,(H,34,35)(H,39,40)/b33-19+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of Chk2 (unknown origin) by spectrophotometric analysis |
Eur J Med Chem 144: 859-873 (2018)
Article DOI: 10.1016/j.ejmech.2017.12.023
BindingDB Entry DOI: 10.7270/Q2KD21F1 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk2
(Homo sapiens (Human)) | BDBM50291372

(CHEMBL4177291)Show SMILES Cc1nn(c(Oc2ccc(cc2)-c2nc3cc(ccc3[nH]2)[N+]([O-])=O)c1\C=N\NC(N)=S)-c1ccccc1 Show InChI InChI=1S/C25H20N8O3S/c1-15-20(14-27-30-25(26)37)24(32(31-15)17-5-3-2-4-6-17)36-19-10-7-16(8-11-19)23-28-21-12-9-18(33(34)35)13-22(21)29-23/h2-14H,1H3,(H,28,29)(H3,26,30,37)/b27-14+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of Chk2 (unknown origin) by spectrophotometric analysis |
Eur J Med Chem 144: 859-873 (2018)
Article DOI: 10.1016/j.ejmech.2017.12.023
BindingDB Entry DOI: 10.7270/Q2KD21F1 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk2
(Homo sapiens (Human)) | BDBM50291380
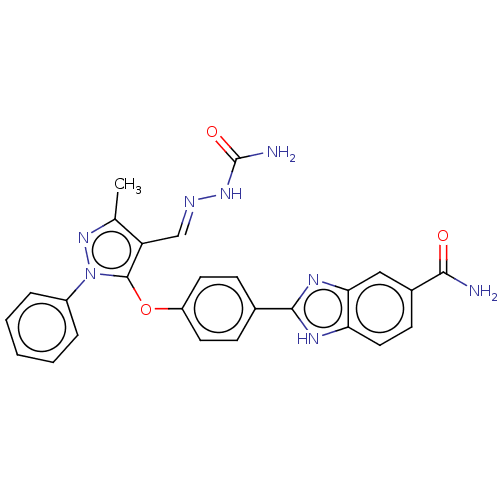
(CHEMBL4170221)Show SMILES Cc1nn(c(Oc2ccc(cc2)-c2nc3cc(ccc3[nH]2)C(N)=O)c1\C=N\NC(N)=O)-c1ccccc1 Show InChI InChI=1S/C26H22N8O3/c1-15-20(14-29-32-26(28)36)25(34(33-15)18-5-3-2-4-6-18)37-19-10-7-16(8-11-19)24-30-21-12-9-17(23(27)35)13-22(21)31-24/h2-14H,1H3,(H2,27,35)(H,30,31)(H3,28,32,36)/b29-14+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of Chk2 (unknown origin) by spectrophotometric analysis |
Eur J Med Chem 144: 859-873 (2018)
Article DOI: 10.1016/j.ejmech.2017.12.023
BindingDB Entry DOI: 10.7270/Q2KD21F1 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk2
(Homo sapiens (Human)) | BDBM50291376
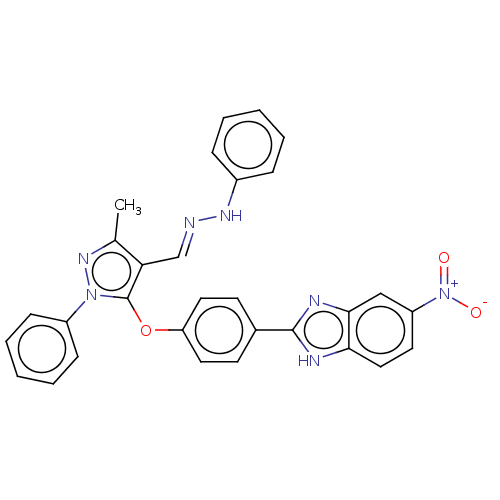
(CHEMBL4170505)Show SMILES Cc1nn(c(Oc2ccc(cc2)-c2nc3cc(ccc3[nH]2)[N+]([O-])=O)c1\C=N\Nc1ccccc1)-c1ccccc1 Show InChI InChI=1S/C30H23N7O3/c1-20-26(19-31-34-22-8-4-2-5-9-22)30(36(35-20)23-10-6-3-7-11-23)40-25-15-12-21(13-16-25)29-32-27-17-14-24(37(38)39)18-28(27)33-29/h2-19,34H,1H3,(H,32,33)/b31-19+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of Chk2 (unknown origin) by spectrophotometric analysis |
Eur J Med Chem 144: 859-873 (2018)
Article DOI: 10.1016/j.ejmech.2017.12.023
BindingDB Entry DOI: 10.7270/Q2KD21F1 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk2
(Homo sapiens (Human)) | BDBM50291405
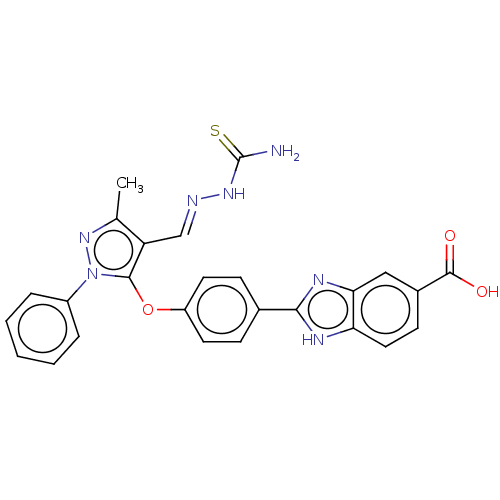
(CHEMBL4173655)Show SMILES Cc1nn(c(Oc2ccc(cc2)-c2nc3cc(ccc3[nH]2)C(O)=O)c1\C=N\NC(N)=S)-c1ccccc1 Show InChI InChI=1S/C26H21N7O3S/c1-15-20(14-28-31-26(27)37)24(33(32-15)18-5-3-2-4-6-18)36-19-10-7-16(8-11-19)23-29-21-12-9-17(25(34)35)13-22(21)30-23/h2-14H,1H3,(H,29,30)(H,34,35)(H3,27,31,37)/b28-14+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of Chk2 (unknown origin) by spectrophotometric analysis |
Eur J Med Chem 144: 859-873 (2018)
Article DOI: 10.1016/j.ejmech.2017.12.023
BindingDB Entry DOI: 10.7270/Q2KD21F1 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk2
(Homo sapiens (Human)) | BDBM50252229
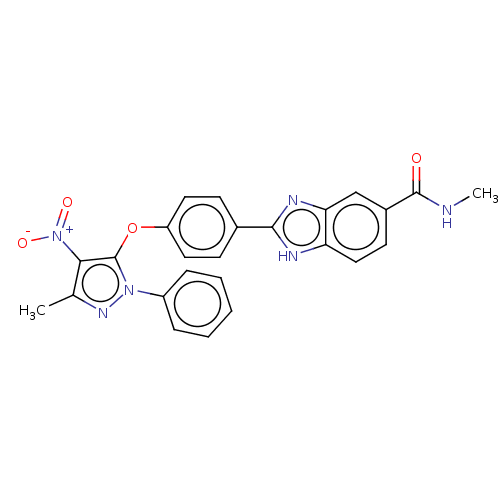
(CHEMBL4063921)Show SMILES CNC(=O)c1ccc2[nH]c(nc2c1)-c1ccc(Oc2c(c(C)nn2-c2ccccc2)[N+]([O-])=O)cc1 Show InChI InChI=1S/C25H20N6O4/c1-15-22(31(33)34)25(30(29-15)18-6-4-3-5-7-18)35-19-11-8-16(9-12-19)23-27-20-13-10-17(24(32)26-2)14-21(20)28-23/h3-14H,1-2H3,(H,26,32)(H,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of Chk2 (unknown origin) by ELISA based spectrophotometric analysis |
Eur J Med Chem 134: 392-405 (2017)
Article DOI: 10.1016/j.ejmech.2017.03.090
BindingDB Entry DOI: 10.7270/Q27P91T8 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk2
(Homo sapiens (Human)) | BDBM50252230
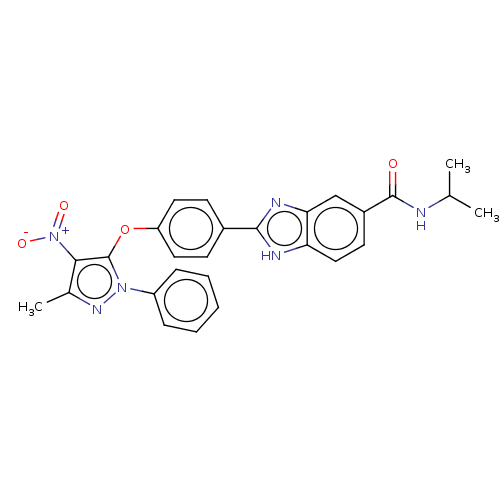
(CHEMBL4085496)Show SMILES CC(C)NC(=O)c1ccc2[nH]c(nc2c1)-c1ccc(Oc2c(c(C)nn2-c2ccccc2)[N+]([O-])=O)cc1 Show InChI InChI=1S/C27H24N6O4/c1-16(2)28-26(34)19-11-14-22-23(15-19)30-25(29-22)18-9-12-21(13-10-18)37-27-24(33(35)36)17(3)31-32(27)20-7-5-4-6-8-20/h4-16H,1-3H3,(H,28,34)(H,29,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of Chk2 (unknown origin) by ELISA based spectrophotometric analysis |
Eur J Med Chem 134: 392-405 (2017)
Article DOI: 10.1016/j.ejmech.2017.03.090
BindingDB Entry DOI: 10.7270/Q27P91T8 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk2
(Homo sapiens (Human)) | BDBM50291406

(CHEMBL4165737)Show SMILES Cc1nn(c(Oc2ccc(cc2)-c2nc3cc(ccc3[nH]2)C(O)=O)c1\C=N\NC(N)=O)-c1ccccc1 Show InChI InChI=1S/C26H21N7O4/c1-15-20(14-28-31-26(27)36)24(33(32-15)18-5-3-2-4-6-18)37-19-10-7-16(8-11-19)23-29-21-12-9-17(25(34)35)13-22(21)30-23/h2-14H,1H3,(H,29,30)(H,34,35)(H3,27,31,36)/b28-14+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of Chk2 (unknown origin) by spectrophotometric analysis |
Eur J Med Chem 144: 859-873 (2018)
Article DOI: 10.1016/j.ejmech.2017.12.023
BindingDB Entry DOI: 10.7270/Q2KD21F1 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk2
(Homo sapiens (Human)) | BDBM50291383

(CHEMBL4177437)Show SMILES Cc1nn(c(Oc2ccc(cc2)-c2nc3cc(ccc3[nH]2)C(N)=O)c1\C=N\Nc1ccccc1)-c1ccccc1 Show InChI InChI=1S/C31H25N7O2/c1-20-26(19-33-36-23-8-4-2-5-9-23)31(38(37-20)24-10-6-3-7-11-24)40-25-15-12-21(13-16-25)30-34-27-17-14-22(29(32)39)18-28(27)35-30/h2-19,36H,1H3,(H2,32,39)(H,34,35)/b33-19+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of Chk2 (unknown origin) by spectrophotometric analysis |
Eur J Med Chem 144: 859-873 (2018)
Article DOI: 10.1016/j.ejmech.2017.12.023
BindingDB Entry DOI: 10.7270/Q2KD21F1 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk2
(Homo sapiens (Human)) | BDBM50291384

(CHEMBL4177025)Show SMILES Cc1nn(c(Sc2ccc(cc2)-c2nc3cc(ccc3[nH]2)C(O)=O)c1\C=N\NC(N)=O)-c1ccccc1 Show InChI InChI=1S/C26H21N7O3S/c1-15-20(14-28-31-26(27)36)24(33(32-15)18-5-3-2-4-6-18)37-19-10-7-16(8-11-19)23-29-21-12-9-17(25(34)35)13-22(21)30-23/h2-14H,1H3,(H,29,30)(H,34,35)(H3,27,31,36)/b28-14+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of Chk2 (unknown origin) by spectrophotometric analysis |
Eur J Med Chem 144: 859-873 (2018)
Article DOI: 10.1016/j.ejmech.2017.12.023
BindingDB Entry DOI: 10.7270/Q2KD21F1 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data