 Found 108 hits with Last Name = 'bockovich' and Initial = 'n'
Found 108 hits with Last Name = 'bockovich' and Initial = 'n' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1
(Homo sapiens (Human)) | BDBM59229
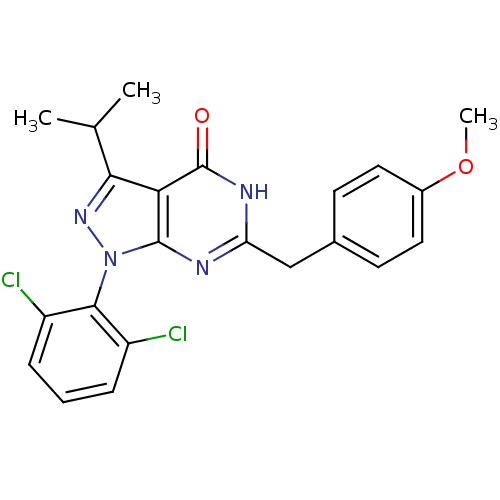
(Pyrazolopyrimidone analog, RGB-286331)Show SMILES COc1ccc(Cc2nc3n(nc(C(C)C)c3c(=O)[nH]2)-c2c(Cl)cccc2Cl)cc1 |(-14.46,2.58,;-13.13,3.35,;-11.8,2.58,;-11.8,1.04,;-10.46,.27,;-9.13,1.04,;-7.8,.27,;-6.46,1.04,;-5.13,.27,;-3.79,1.04,;-2.33,.57,;-1.43,1.81,;-2.33,3.06,;-1.56,4.39,;-2.33,5.73,;-.02,4.39,;-3.79,2.58,;-5.13,3.35,;-5.13,4.89,;-6.46,2.58,;-1.93,-.92,;-.44,-1.32,;.64,-.23,;-.05,-2.8,;-1.13,-3.89,;-2.62,-3.49,;-3.02,-2.01,;-4.51,-1.61,;-9.13,2.58,;-10.46,3.35,)| Show InChI InChI=1S/C22H20Cl2N4O2/c1-12(2)19-18-21(28(27-19)20-15(23)5-4-6-16(20)24)25-17(26-22(18)29)11-13-7-9-14(30-3)10-8-13/h4-10,12H,11H2,1-3H3,(H,25,26,29) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
GPC Biotech, Inc.
| Assay Description
Inhibition of in vitro kinase activity of purified CDKs. |
Chem Biol 12: 1103-15 (2005)
Article DOI: 10.1016/j.chembiol.2005.08.008
BindingDB Entry DOI: 10.7270/Q28W3BR6 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1
(Homo sapiens (Human)) | BDBM59228
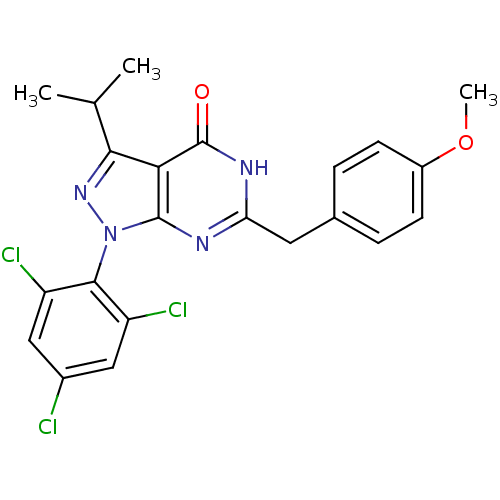
(Pyrazolopyrimidone analog, RGB-285940)Show SMILES COc1ccc(Cc2nc3n(nc(C(C)C)c3c(=O)[nH]2)-c2c(Cl)cc(Cl)cc2Cl)cc1 |(-14.46,2.58,;-13.13,3.35,;-11.8,2.58,;-11.8,1.04,;-10.46,.27,;-9.13,1.04,;-7.8,.27,;-6.46,1.04,;-5.13,.27,;-3.79,1.04,;-2.33,.57,;-1.43,1.81,;-2.33,3.06,;-1.56,4.39,;-2.33,5.73,;-.02,4.39,;-3.79,2.58,;-5.13,3.35,;-5.13,4.89,;-6.46,2.58,;-1.93,-.92,;-.44,-1.32,;.64,-.23,;-.05,-2.8,;-1.13,-3.89,;-.74,-5.38,;-2.62,-3.49,;-3.02,-2.01,;-4.51,-1.61,;-9.13,2.58,;-10.46,3.35,)| Show InChI InChI=1S/C22H19Cl3N4O2/c1-11(2)19-18-21(29(28-19)20-15(24)9-13(23)10-16(20)25)26-17(27-22(18)30)8-12-4-6-14(31-3)7-5-12/h4-7,9-11H,8H2,1-3H3,(H,26,27,30) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
GPC Biotech, Inc.
| Assay Description
Inhibition of in vitro kinase activity of purified CDKs. |
Chem Biol 12: 1103-15 (2005)
Article DOI: 10.1016/j.chembiol.2005.08.008
BindingDB Entry DOI: 10.7270/Q28W3BR6 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1
(Homo sapiens (Human)) | BDBM59230
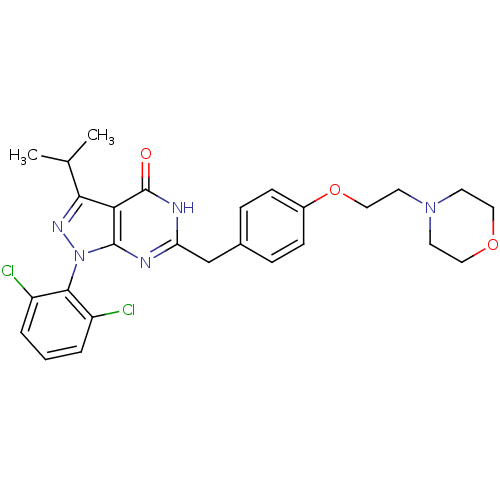
(Pyrazolopyrimidone analog, RGB-285960)Show SMILES CC(C)c1nn(-c2c(Cl)cccc2Cl)c2nc(Cc3ccc(OCCN4CCOCC4)cc3)[nH]c(=O)c12 |(-2.33,5.73,;-1.56,4.39,;-.02,4.39,;-2.33,3.06,;-1.43,1.81,;-2.33,.57,;-1.93,-.92,;-.44,-1.32,;.64,-.23,;-.05,-2.8,;-1.13,-3.89,;-2.62,-3.49,;-3.02,-2.01,;-4.51,-1.61,;-3.79,1.04,;-5.13,.27,;-6.46,1.04,;-7.8,.27,;-9.13,1.04,;-10.46,.27,;-11.8,1.04,;-11.8,2.58,;-13.13,3.35,;-14.46,2.58,;-15.8,3.35,;-17.13,2.58,;-18.47,3.35,;-19.8,2.58,;-19.8,1.04,;-18.47,.27,;-17.13,1.04,;-10.46,3.35,;-9.13,2.58,;-6.46,2.58,;-5.13,3.35,;-5.13,4.89,;-3.79,2.58,)| Show InChI InChI=1S/C27H29Cl2N5O3/c1-17(2)24-23-26(34(32-24)25-20(28)4-3-5-21(25)29)30-22(31-27(23)35)16-18-6-8-19(9-7-18)37-15-12-33-10-13-36-14-11-33/h3-9,17H,10-16H2,1-2H3,(H,30,31,35) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
GPC Biotech, Inc.
| Assay Description
Inhibition of in vitro kinase activity of purified CDKs. |
Chem Biol 12: 1103-15 (2005)
Article DOI: 10.1016/j.chembiol.2005.08.008
BindingDB Entry DOI: 10.7270/Q28W3BR6 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 3/G1/S-specific cyclin-E1
(Homo sapiens (Human)) | BDBM59227
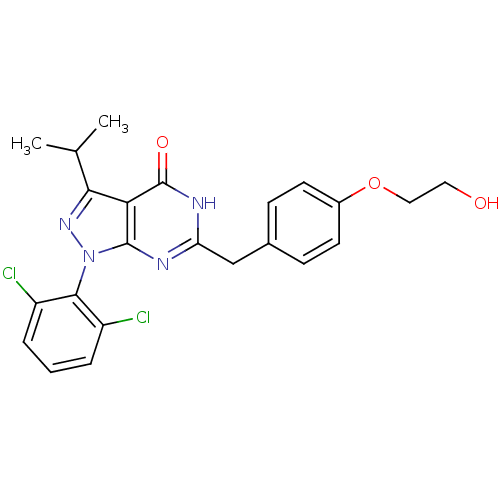
(Pyrazolopyrimidone analog, RGB-286147)Show SMILES CC(C)c1nn(-c2c(Cl)cccc2Cl)c2nc(Cc3ccc(OCCO)cc3)[nH]c(=O)c12 |(-2.33,5.73,;-1.56,4.39,;-.02,4.39,;-2.33,3.06,;-1.43,1.81,;-2.33,.57,;-1.93,-.92,;-.44,-1.32,;.64,-.23,;-.05,-2.8,;-1.13,-3.89,;-2.62,-3.49,;-3.02,-2.01,;-4.51,-1.61,;-3.79,1.04,;-5.13,.27,;-6.46,1.04,;-7.8,.27,;-9.13,1.04,;-10.46,.27,;-11.8,1.04,;-11.8,2.58,;-13.13,3.35,;-14.46,2.58,;-15.8,3.35,;-17.13,2.58,;-10.46,3.35,;-9.13,2.58,;-6.46,2.58,;-5.13,3.35,;-5.13,4.89,;-3.79,2.58,)| Show InChI InChI=1S/C23H22Cl2N4O3/c1-13(2)20-19-22(29(28-20)21-16(24)4-3-5-17(21)25)26-18(27-23(19)31)12-14-6-8-15(9-7-14)32-11-10-30/h3-9,13,30H,10-12H2,1-2H3,(H,26,27,31) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
GPC Biotech, Inc.
| Assay Description
Inhibition of in vitro kinase activity of purified CDKs. |
Chem Biol 12: 1103-15 (2005)
Article DOI: 10.1016/j.chembiol.2005.08.008
BindingDB Entry DOI: 10.7270/Q28W3BR6 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5
(Homo sapiens (Human)) | BDBM59227

(Pyrazolopyrimidone analog, RGB-286147)Show SMILES CC(C)c1nn(-c2c(Cl)cccc2Cl)c2nc(Cc3ccc(OCCO)cc3)[nH]c(=O)c12 |(-2.33,5.73,;-1.56,4.39,;-.02,4.39,;-2.33,3.06,;-1.43,1.81,;-2.33,.57,;-1.93,-.92,;-.44,-1.32,;.64,-.23,;-.05,-2.8,;-1.13,-3.89,;-2.62,-3.49,;-3.02,-2.01,;-4.51,-1.61,;-3.79,1.04,;-5.13,.27,;-6.46,1.04,;-7.8,.27,;-9.13,1.04,;-10.46,.27,;-11.8,1.04,;-11.8,2.58,;-13.13,3.35,;-14.46,2.58,;-15.8,3.35,;-17.13,2.58,;-10.46,3.35,;-9.13,2.58,;-6.46,2.58,;-5.13,3.35,;-5.13,4.89,;-3.79,2.58,)| Show InChI InChI=1S/C23H22Cl2N4O3/c1-13(2)20-19-22(29(28-20)21-16(24)4-3-5-17(21)25)26-18(27-23(19)31)12-14-6-8-15(9-7-14)32-11-10-30/h3-9,13,30H,10-12H2,1-2H3,(H,26,27,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GPC Biotech, Inc.
| Assay Description
Inhibition of in vitro kinase activity of purified CDKs. |
Chem Biol 12: 1103-15 (2005)
Article DOI: 10.1016/j.chembiol.2005.08.008
BindingDB Entry DOI: 10.7270/Q28W3BR6 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1
(Homo sapiens (Human)) | BDBM59227

(Pyrazolopyrimidone analog, RGB-286147)Show SMILES CC(C)c1nn(-c2c(Cl)cccc2Cl)c2nc(Cc3ccc(OCCO)cc3)[nH]c(=O)c12 |(-2.33,5.73,;-1.56,4.39,;-.02,4.39,;-2.33,3.06,;-1.43,1.81,;-2.33,.57,;-1.93,-.92,;-.44,-1.32,;.64,-.23,;-.05,-2.8,;-1.13,-3.89,;-2.62,-3.49,;-3.02,-2.01,;-4.51,-1.61,;-3.79,1.04,;-5.13,.27,;-6.46,1.04,;-7.8,.27,;-9.13,1.04,;-10.46,.27,;-11.8,1.04,;-11.8,2.58,;-13.13,3.35,;-14.46,2.58,;-15.8,3.35,;-17.13,2.58,;-10.46,3.35,;-9.13,2.58,;-6.46,2.58,;-5.13,3.35,;-5.13,4.89,;-3.79,2.58,)| Show InChI InChI=1S/C23H22Cl2N4O3/c1-13(2)20-19-22(29(28-20)21-16(24)4-3-5-17(21)25)26-18(27-23(19)31)12-14-6-8-15(9-7-14)32-11-10-30/h3-9,13,30H,10-12H2,1-2H3,(H,26,27,31) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
GPC Biotech, Inc.
| Assay Description
Inhibition of in vitro kinase activity of purified CDKs. |
Chem Biol 12: 1103-15 (2005)
Article DOI: 10.1016/j.chembiol.2005.08.008
BindingDB Entry DOI: 10.7270/Q28W3BR6 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1
(Homo sapiens (Human)) | BDBM59235
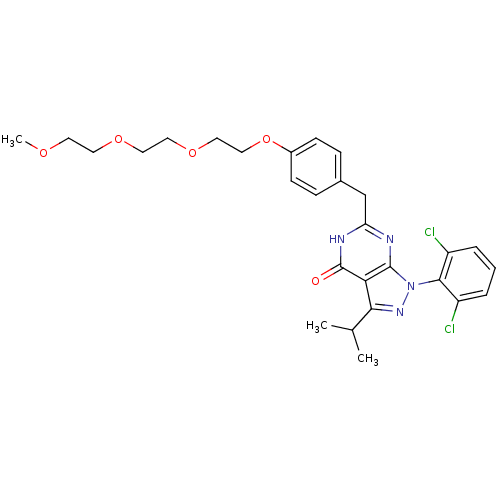
(PEGylated derivative, RGB-286276)Show SMILES COCCOCCOCCOc1ccc(Cc2nc3n(nc(C(C)C)c3c(=O)[nH]2)-c2c(Cl)cccc2Cl)cc1 |(-26.47,3.35,;-25.13,2.58,;-23.8,3.35,;-22.47,2.58,;-21.13,3.35,;-19.8,2.58,;-18.47,3.35,;-17.13,2.58,;-15.8,3.35,;-14.46,2.58,;-13.13,3.35,;-11.8,2.58,;-11.8,1.04,;-10.46,.27,;-9.13,1.04,;-7.8,.27,;-6.46,1.04,;-5.13,.27,;-3.79,1.04,;-2.33,.57,;-1.43,1.81,;-2.33,3.06,;-1.93,4.55,;-.44,4.95,;-3.02,5.64,;-3.79,2.58,;-5.13,3.35,;-5.13,4.89,;-6.46,2.58,;-1.93,-.92,;-.44,-1.32,;.33,.02,;-.05,-2.8,;-1.13,-3.89,;-2.62,-3.49,;-3.02,-2.01,;-4.51,-1.61,;-9.13,2.58,;-10.46,3.35,)| Show InChI InChI=1S/C28H32Cl2N4O5/c1-18(2)25-24-27(34(33-25)26-21(29)5-4-6-22(26)30)31-23(32-28(24)35)17-19-7-9-20(10-8-19)39-16-15-38-14-13-37-12-11-36-3/h4-10,18H,11-17H2,1-3H3,(H,31,32,35) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
GPC Biotech, Inc.
| Assay Description
In vitro kinase activity of PEGylated pyrazolopyrimidone CDK inhibitor or a 5 PEG-amine was coupled to ReactiGel agarose beads (Pierce, No. 20259). |
Chem Biol 12: 1103-15 (2005)
Article DOI: 10.1016/j.chembiol.2005.08.008
BindingDB Entry DOI: 10.7270/Q28W3BR6 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase/G2/mitotic-specific cyclin- 1
(Homo sapiens (Human)) | BDBM59235

(PEGylated derivative, RGB-286276)Show SMILES COCCOCCOCCOc1ccc(Cc2nc3n(nc(C(C)C)c3c(=O)[nH]2)-c2c(Cl)cccc2Cl)cc1 |(-26.47,3.35,;-25.13,2.58,;-23.8,3.35,;-22.47,2.58,;-21.13,3.35,;-19.8,2.58,;-18.47,3.35,;-17.13,2.58,;-15.8,3.35,;-14.46,2.58,;-13.13,3.35,;-11.8,2.58,;-11.8,1.04,;-10.46,.27,;-9.13,1.04,;-7.8,.27,;-6.46,1.04,;-5.13,.27,;-3.79,1.04,;-2.33,.57,;-1.43,1.81,;-2.33,3.06,;-1.93,4.55,;-.44,4.95,;-3.02,5.64,;-3.79,2.58,;-5.13,3.35,;-5.13,4.89,;-6.46,2.58,;-1.93,-.92,;-.44,-1.32,;.33,.02,;-.05,-2.8,;-1.13,-3.89,;-2.62,-3.49,;-3.02,-2.01,;-4.51,-1.61,;-9.13,2.58,;-10.46,3.35,)| Show InChI InChI=1S/C28H32Cl2N4O5/c1-18(2)25-24-27(34(33-25)26-21(29)5-4-6-22(26)30)31-23(32-28(24)35)17-19-7-9-20(10-8-19)39-16-15-38-14-13-37-12-11-36-3/h4-10,18H,11-17H2,1-3H3,(H,31,32,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
GPC Biotech, Inc.
| Assay Description
Inhibition of HCT116 cell proliferation with compounds using calcein AM Assay. |
Chem Biol 12: 1103-15 (2005)
Article DOI: 10.1016/j.chembiol.2005.08.008
BindingDB Entry DOI: 10.7270/Q28W3BR6 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase/G2/mitotic-specific cyclin- 1
(Homo sapiens (Human)) | BDBM59229

(Pyrazolopyrimidone analog, RGB-286331)Show SMILES COc1ccc(Cc2nc3n(nc(C(C)C)c3c(=O)[nH]2)-c2c(Cl)cccc2Cl)cc1 |(-14.46,2.58,;-13.13,3.35,;-11.8,2.58,;-11.8,1.04,;-10.46,.27,;-9.13,1.04,;-7.8,.27,;-6.46,1.04,;-5.13,.27,;-3.79,1.04,;-2.33,.57,;-1.43,1.81,;-2.33,3.06,;-1.56,4.39,;-2.33,5.73,;-.02,4.39,;-3.79,2.58,;-5.13,3.35,;-5.13,4.89,;-6.46,2.58,;-1.93,-.92,;-.44,-1.32,;.64,-.23,;-.05,-2.8,;-1.13,-3.89,;-2.62,-3.49,;-3.02,-2.01,;-4.51,-1.61,;-9.13,2.58,;-10.46,3.35,)| Show InChI InChI=1S/C22H20Cl2N4O2/c1-12(2)19-18-21(28(27-19)20-15(23)5-4-6-16(20)24)25-17(26-22(18)29)11-13-7-9-14(30-3)10-8-13/h4-10,12H,11H2,1-3H3,(H,25,26,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
GPC Biotech, Inc.
| Assay Description
Inhibition of in vitro kinase activity of purified CDKs. |
Chem Biol 12: 1103-15 (2005)
Article DOI: 10.1016/j.chembiol.2005.08.008
BindingDB Entry DOI: 10.7270/Q28W3BR6 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase/G2/mitotic-specific cyclin- 1
(Homo sapiens (Human)) | BDBM59227

(Pyrazolopyrimidone analog, RGB-286147)Show SMILES CC(C)c1nn(-c2c(Cl)cccc2Cl)c2nc(Cc3ccc(OCCO)cc3)[nH]c(=O)c12 |(-2.33,5.73,;-1.56,4.39,;-.02,4.39,;-2.33,3.06,;-1.43,1.81,;-2.33,.57,;-1.93,-.92,;-.44,-1.32,;.64,-.23,;-.05,-2.8,;-1.13,-3.89,;-2.62,-3.49,;-3.02,-2.01,;-4.51,-1.61,;-3.79,1.04,;-5.13,.27,;-6.46,1.04,;-7.8,.27,;-9.13,1.04,;-10.46,.27,;-11.8,1.04,;-11.8,2.58,;-13.13,3.35,;-14.46,2.58,;-15.8,3.35,;-17.13,2.58,;-10.46,3.35,;-9.13,2.58,;-6.46,2.58,;-5.13,3.35,;-5.13,4.89,;-3.79,2.58,)| Show InChI InChI=1S/C23H22Cl2N4O3/c1-13(2)20-19-22(29(28-20)21-16(24)4-3-5-17(21)25)26-18(27-23(19)31)12-14-6-8-15(9-7-14)32-11-10-30/h3-9,13,30H,10-12H2,1-2H3,(H,26,27,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
GPC Biotech, Inc.
| Assay Description
Inhibition of in vitro kinase activity of purified CDKs. |
Chem Biol 12: 1103-15 (2005)
Article DOI: 10.1016/j.chembiol.2005.08.008
BindingDB Entry DOI: 10.7270/Q28W3BR6 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase/G2/mitotic-specific cyclin- 1
(Homo sapiens (Human)) | BDBM59227

(Pyrazolopyrimidone analog, RGB-286147)Show SMILES CC(C)c1nn(-c2c(Cl)cccc2Cl)c2nc(Cc3ccc(OCCO)cc3)[nH]c(=O)c12 |(-2.33,5.73,;-1.56,4.39,;-.02,4.39,;-2.33,3.06,;-1.43,1.81,;-2.33,.57,;-1.93,-.92,;-.44,-1.32,;.64,-.23,;-.05,-2.8,;-1.13,-3.89,;-2.62,-3.49,;-3.02,-2.01,;-4.51,-1.61,;-3.79,1.04,;-5.13,.27,;-6.46,1.04,;-7.8,.27,;-9.13,1.04,;-10.46,.27,;-11.8,1.04,;-11.8,2.58,;-13.13,3.35,;-14.46,2.58,;-15.8,3.35,;-17.13,2.58,;-10.46,3.35,;-9.13,2.58,;-6.46,2.58,;-5.13,3.35,;-5.13,4.89,;-3.79,2.58,)| Show InChI InChI=1S/C23H22Cl2N4O3/c1-13(2)20-19-22(29(28-20)21-16(24)4-3-5-17(21)25)26-18(27-23(19)31)12-14-6-8-15(9-7-14)32-11-10-30/h3-9,13,30H,10-12H2,1-2H3,(H,26,27,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
GPC Biotech, Inc.
| Assay Description
Inhibition of HCT116 cell proliferation after 72hr of incubation with compounds was determined by SRB Assay. |
Chem Biol 12: 1103-15 (2005)
Article DOI: 10.1016/j.chembiol.2005.08.008
BindingDB Entry DOI: 10.7270/Q28W3BR6 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase/G2/mitotic-specific cyclin- 1
(Homo sapiens (Human)) | BDBM59230

(Pyrazolopyrimidone analog, RGB-285960)Show SMILES CC(C)c1nn(-c2c(Cl)cccc2Cl)c2nc(Cc3ccc(OCCN4CCOCC4)cc3)[nH]c(=O)c12 |(-2.33,5.73,;-1.56,4.39,;-.02,4.39,;-2.33,3.06,;-1.43,1.81,;-2.33,.57,;-1.93,-.92,;-.44,-1.32,;.64,-.23,;-.05,-2.8,;-1.13,-3.89,;-2.62,-3.49,;-3.02,-2.01,;-4.51,-1.61,;-3.79,1.04,;-5.13,.27,;-6.46,1.04,;-7.8,.27,;-9.13,1.04,;-10.46,.27,;-11.8,1.04,;-11.8,2.58,;-13.13,3.35,;-14.46,2.58,;-15.8,3.35,;-17.13,2.58,;-18.47,3.35,;-19.8,2.58,;-19.8,1.04,;-18.47,.27,;-17.13,1.04,;-10.46,3.35,;-9.13,2.58,;-6.46,2.58,;-5.13,3.35,;-5.13,4.89,;-3.79,2.58,)| Show InChI InChI=1S/C27H29Cl2N5O3/c1-17(2)24-23-26(34(32-24)25-20(28)4-3-5-21(25)29)30-22(31-27(23)35)16-18-6-8-19(9-7-18)37-15-12-33-10-13-36-14-11-33/h3-9,17H,10-16H2,1-2H3,(H,30,31,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
GPC Biotech, Inc.
| Assay Description
Inhibition of HCT116 cell proliferation after 72hr of incubation with compounds was determined by SRB Assay. |
Chem Biol 12: 1103-15 (2005)
Article DOI: 10.1016/j.chembiol.2005.08.008
BindingDB Entry DOI: 10.7270/Q28W3BR6 |
More data for this
Ligand-Target Pair | |
CDK-activating kinase assembly factor MAT1
(Homo sapiens (Human)) | BDBM59227

(Pyrazolopyrimidone analog, RGB-286147)Show SMILES CC(C)c1nn(-c2c(Cl)cccc2Cl)c2nc(Cc3ccc(OCCO)cc3)[nH]c(=O)c12 |(-2.33,5.73,;-1.56,4.39,;-.02,4.39,;-2.33,3.06,;-1.43,1.81,;-2.33,.57,;-1.93,-.92,;-.44,-1.32,;.64,-.23,;-.05,-2.8,;-1.13,-3.89,;-2.62,-3.49,;-3.02,-2.01,;-4.51,-1.61,;-3.79,1.04,;-5.13,.27,;-6.46,1.04,;-7.8,.27,;-9.13,1.04,;-10.46,.27,;-11.8,1.04,;-11.8,2.58,;-13.13,3.35,;-14.46,2.58,;-15.8,3.35,;-17.13,2.58,;-10.46,3.35,;-9.13,2.58,;-6.46,2.58,;-5.13,3.35,;-5.13,4.89,;-3.79,2.58,)| Show InChI InChI=1S/C23H22Cl2N4O3/c1-13(2)20-19-22(29(28-20)21-16(24)4-3-5-17(21)25)26-18(27-23(19)31)12-14-6-8-15(9-7-14)32-11-10-30/h3-9,13,30H,10-12H2,1-2H3,(H,26,27,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
GPC Biotech, Inc.
| Assay Description
Inhibition of in vitro kinase activity of purified CDKs. |
Chem Biol 12: 1103-15 (2005)
Article DOI: 10.1016/j.chembiol.2005.08.008
BindingDB Entry DOI: 10.7270/Q28W3BR6 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase/G2/mitotic-specific cyclin- 1
(Homo sapiens (Human)) | BDBM59235

(PEGylated derivative, RGB-286276)Show SMILES COCCOCCOCCOc1ccc(Cc2nc3n(nc(C(C)C)c3c(=O)[nH]2)-c2c(Cl)cccc2Cl)cc1 |(-26.47,3.35,;-25.13,2.58,;-23.8,3.35,;-22.47,2.58,;-21.13,3.35,;-19.8,2.58,;-18.47,3.35,;-17.13,2.58,;-15.8,3.35,;-14.46,2.58,;-13.13,3.35,;-11.8,2.58,;-11.8,1.04,;-10.46,.27,;-9.13,1.04,;-7.8,.27,;-6.46,1.04,;-5.13,.27,;-3.79,1.04,;-2.33,.57,;-1.43,1.81,;-2.33,3.06,;-1.93,4.55,;-.44,4.95,;-3.02,5.64,;-3.79,2.58,;-5.13,3.35,;-5.13,4.89,;-6.46,2.58,;-1.93,-.92,;-.44,-1.32,;.33,.02,;-.05,-2.8,;-1.13,-3.89,;-2.62,-3.49,;-3.02,-2.01,;-4.51,-1.61,;-9.13,2.58,;-10.46,3.35,)| Show InChI InChI=1S/C28H32Cl2N4O5/c1-18(2)25-24-27(34(33-25)26-21(29)5-4-6-22(26)30)31-23(32-28(24)35)17-19-7-9-20(10-8-19)39-16-15-38-14-13-37-12-11-36-3/h4-10,18H,11-17H2,1-3H3,(H,31,32,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
GPC Biotech, Inc.
| Assay Description
In vitro kinase activity of PEGylated pyrazolopyrimidone CDK inhibitor or a 5 PEG-amine was coupled to ReactiGel agarose beads (Pierce, No. 20259). |
Chem Biol 12: 1103-15 (2005)
Article DOI: 10.1016/j.chembiol.2005.08.008
BindingDB Entry DOI: 10.7270/Q28W3BR6 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM50079331
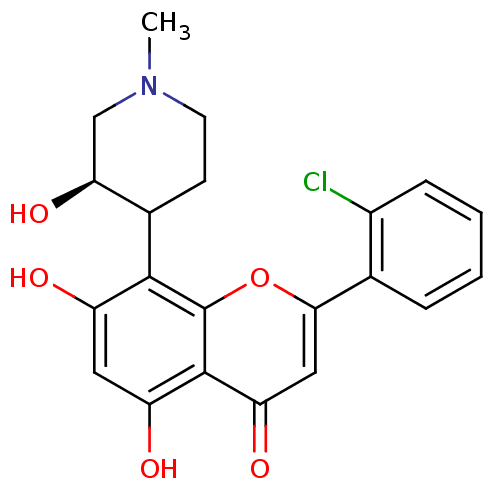
(2-(2-Chloro-phenyl)-5,7-dihydroxy-8-(3-hydroxy-1-m...)Show SMILES CN1CCC([C@@H](O)C1)c1c(O)cc(O)c2c1oc(cc2=O)-c1ccccc1Cl Show InChI InChI=1S/C21H20ClNO5/c1-23-7-6-12(17(27)10-23)19-14(24)8-15(25)20-16(26)9-18(28-21(19)20)11-4-2-3-5-13(11)22/h2-5,8-9,12,17,24-25,27H,6-7,10H2,1H3/t12?,17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 155 | n/a | n/a | n/a | n/a | n/a | n/a |
GPC Biotech, Inc.
| Assay Description
Inhibition of in vitro kinase activity of purified CDKs. |
Chem Biol 12: 1103-15 (2005)
Article DOI: 10.1016/j.chembiol.2005.08.008
BindingDB Entry DOI: 10.7270/Q28W3BR6 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase/G2/mitotic-specific cyclin- 1
(Homo sapiens (Human)) | BDBM59229

(Pyrazolopyrimidone analog, RGB-286331)Show SMILES COc1ccc(Cc2nc3n(nc(C(C)C)c3c(=O)[nH]2)-c2c(Cl)cccc2Cl)cc1 |(-14.46,2.58,;-13.13,3.35,;-11.8,2.58,;-11.8,1.04,;-10.46,.27,;-9.13,1.04,;-7.8,.27,;-6.46,1.04,;-5.13,.27,;-3.79,1.04,;-2.33,.57,;-1.43,1.81,;-2.33,3.06,;-1.56,4.39,;-2.33,5.73,;-.02,4.39,;-3.79,2.58,;-5.13,3.35,;-5.13,4.89,;-6.46,2.58,;-1.93,-.92,;-.44,-1.32,;.64,-.23,;-.05,-2.8,;-1.13,-3.89,;-2.62,-3.49,;-3.02,-2.01,;-4.51,-1.61,;-9.13,2.58,;-10.46,3.35,)| Show InChI InChI=1S/C22H20Cl2N4O2/c1-12(2)19-18-21(28(27-19)20-15(23)5-4-6-16(20)24)25-17(26-22(18)29)11-13-7-9-14(30-3)10-8-13/h4-10,12H,11H2,1-3H3,(H,25,26,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
GPC Biotech, Inc.
| Assay Description
Inhibition of HCT116 cell proliferation after 72hr of incubation with compounds was determined by SRB Assay. |
Chem Biol 12: 1103-15 (2005)
Article DOI: 10.1016/j.chembiol.2005.08.008
BindingDB Entry DOI: 10.7270/Q28W3BR6 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase/G2/mitotic-specific cyclin- 1
(Homo sapiens (Human)) | BDBM50079331

(2-(2-Chloro-phenyl)-5,7-dihydroxy-8-(3-hydroxy-1-m...)Show SMILES CN1CCC([C@@H](O)C1)c1c(O)cc(O)c2c1oc(cc2=O)-c1ccccc1Cl Show InChI InChI=1S/C21H20ClNO5/c1-23-7-6-12(17(27)10-23)19-14(24)8-15(25)20-16(26)9-18(28-21(19)20)11-4-2-3-5-13(11)22/h2-5,8-9,12,17,24-25,27H,6-7,10H2,1H3/t12?,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 221 | n/a | n/a | n/a | n/a | n/a | n/a |
GPC Biotech, Inc.
| Assay Description
Inhibition of HCT116 cell proliferation after 72hr of incubation with compounds was determined by SRB Assay. |
Chem Biol 12: 1103-15 (2005)
Article DOI: 10.1016/j.chembiol.2005.08.008
BindingDB Entry DOI: 10.7270/Q28W3BR6 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase/G2/mitotic-specific cyclin- 1
(Homo sapiens (Human)) | BDBM59228

(Pyrazolopyrimidone analog, RGB-285940)Show SMILES COc1ccc(Cc2nc3n(nc(C(C)C)c3c(=O)[nH]2)-c2c(Cl)cc(Cl)cc2Cl)cc1 |(-14.46,2.58,;-13.13,3.35,;-11.8,2.58,;-11.8,1.04,;-10.46,.27,;-9.13,1.04,;-7.8,.27,;-6.46,1.04,;-5.13,.27,;-3.79,1.04,;-2.33,.57,;-1.43,1.81,;-2.33,3.06,;-1.56,4.39,;-2.33,5.73,;-.02,4.39,;-3.79,2.58,;-5.13,3.35,;-5.13,4.89,;-6.46,2.58,;-1.93,-.92,;-.44,-1.32,;.64,-.23,;-.05,-2.8,;-1.13,-3.89,;-.74,-5.38,;-2.62,-3.49,;-3.02,-2.01,;-4.51,-1.61,;-9.13,2.58,;-10.46,3.35,)| Show InChI InChI=1S/C22H19Cl3N4O2/c1-11(2)19-18-21(29(28-19)20-15(24)9-13(23)10-16(20)25)26-17(27-22(18)30)8-12-4-6-14(31-3)7-5-12/h4-7,9-11H,8H2,1-3H3,(H,26,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
GPC Biotech, Inc.
| Assay Description
Inhibition of in vitro kinase activity of purified CDKs. |
Chem Biol 12: 1103-15 (2005)
Article DOI: 10.1016/j.chembiol.2005.08.008
BindingDB Entry DOI: 10.7270/Q28W3BR6 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 6/G1/S-specific cyclin-D3
(Homo sapiens (Human)) | BDBM59227

(Pyrazolopyrimidone analog, RGB-286147)Show SMILES CC(C)c1nn(-c2c(Cl)cccc2Cl)c2nc(Cc3ccc(OCCO)cc3)[nH]c(=O)c12 |(-2.33,5.73,;-1.56,4.39,;-.02,4.39,;-2.33,3.06,;-1.43,1.81,;-2.33,.57,;-1.93,-.92,;-.44,-1.32,;.64,-.23,;-.05,-2.8,;-1.13,-3.89,;-2.62,-3.49,;-3.02,-2.01,;-4.51,-1.61,;-3.79,1.04,;-5.13,.27,;-6.46,1.04,;-7.8,.27,;-9.13,1.04,;-10.46,.27,;-11.8,1.04,;-11.8,2.58,;-13.13,3.35,;-14.46,2.58,;-15.8,3.35,;-17.13,2.58,;-10.46,3.35,;-9.13,2.58,;-6.46,2.58,;-5.13,3.35,;-5.13,4.89,;-3.79,2.58,)| Show InChI InChI=1S/C23H22Cl2N4O3/c1-13(2)20-19-22(29(28-20)21-16(24)4-3-5-17(21)25)26-18(27-23(19)31)12-14-6-8-15(9-7-14)32-11-10-30/h3-9,13,30H,10-12H2,1-2H3,(H,26,27,31) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 282 | n/a | n/a | n/a | n/a | n/a | n/a |
GPC Biotech, Inc.
| Assay Description
Inhibition of in vitro kinase activity of purified CDKs. |
Chem Biol 12: 1103-15 (2005)
Article DOI: 10.1016/j.chembiol.2005.08.008
BindingDB Entry DOI: 10.7270/Q28W3BR6 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase/G2/mitotic-specific cyclin- 1
(Homo sapiens (Human)) | BDBM59230

(Pyrazolopyrimidone analog, RGB-285960)Show SMILES CC(C)c1nn(-c2c(Cl)cccc2Cl)c2nc(Cc3ccc(OCCN4CCOCC4)cc3)[nH]c(=O)c12 |(-2.33,5.73,;-1.56,4.39,;-.02,4.39,;-2.33,3.06,;-1.43,1.81,;-2.33,.57,;-1.93,-.92,;-.44,-1.32,;.64,-.23,;-.05,-2.8,;-1.13,-3.89,;-2.62,-3.49,;-3.02,-2.01,;-4.51,-1.61,;-3.79,1.04,;-5.13,.27,;-6.46,1.04,;-7.8,.27,;-9.13,1.04,;-10.46,.27,;-11.8,1.04,;-11.8,2.58,;-13.13,3.35,;-14.46,2.58,;-15.8,3.35,;-17.13,2.58,;-18.47,3.35,;-19.8,2.58,;-19.8,1.04,;-18.47,.27,;-17.13,1.04,;-10.46,3.35,;-9.13,2.58,;-6.46,2.58,;-5.13,3.35,;-5.13,4.89,;-3.79,2.58,)| Show InChI InChI=1S/C27H29Cl2N5O3/c1-17(2)24-23-26(34(32-24)25-20(28)4-3-5-21(25)29)30-22(31-27(23)35)16-18-6-8-19(9-7-18)37-15-12-33-10-13-36-14-11-33/h3-9,17H,10-16H2,1-2H3,(H,30,31,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
GPC Biotech, Inc.
| Assay Description
Inhibition of in vitro kinase activity of purified CDKs. |
Chem Biol 12: 1103-15 (2005)
Article DOI: 10.1016/j.chembiol.2005.08.008
BindingDB Entry DOI: 10.7270/Q28W3BR6 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase/G2/mitotic-specific cyclin- 1
(Homo sapiens (Human)) | BDBM59228

(Pyrazolopyrimidone analog, RGB-285940)Show SMILES COc1ccc(Cc2nc3n(nc(C(C)C)c3c(=O)[nH]2)-c2c(Cl)cc(Cl)cc2Cl)cc1 |(-14.46,2.58,;-13.13,3.35,;-11.8,2.58,;-11.8,1.04,;-10.46,.27,;-9.13,1.04,;-7.8,.27,;-6.46,1.04,;-5.13,.27,;-3.79,1.04,;-2.33,.57,;-1.43,1.81,;-2.33,3.06,;-1.56,4.39,;-2.33,5.73,;-.02,4.39,;-3.79,2.58,;-5.13,3.35,;-5.13,4.89,;-6.46,2.58,;-1.93,-.92,;-.44,-1.32,;.64,-.23,;-.05,-2.8,;-1.13,-3.89,;-.74,-5.38,;-2.62,-3.49,;-3.02,-2.01,;-4.51,-1.61,;-9.13,2.58,;-10.46,3.35,)| Show InChI InChI=1S/C22H19Cl3N4O2/c1-11(2)19-18-21(29(28-19)20-15(24)9-13(23)10-16(20)25)26-17(27-22(18)30)8-12-4-6-14(31-3)7-5-12/h4-7,9-11H,8H2,1-3H3,(H,26,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
GPC Biotech, Inc.
| Assay Description
Inhibition of HCT116 cell proliferation after 72hr of incubation with compounds was determined by SRB Assay. |
Chem Biol 12: 1103-15 (2005)
Article DOI: 10.1016/j.chembiol.2005.08.008
BindingDB Entry DOI: 10.7270/Q28W3BR6 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase/G2/mitotic-specific cyclin- 1
(Homo sapiens (Human)) | BDBM50079331

(2-(2-Chloro-phenyl)-5,7-dihydroxy-8-(3-hydroxy-1-m...)Show SMILES CN1CCC([C@@H](O)C1)c1c(O)cc(O)c2c1oc(cc2=O)-c1ccccc1Cl Show InChI InChI=1S/C21H20ClNO5/c1-23-7-6-12(17(27)10-23)19-14(24)8-15(25)20-16(26)9-18(28-21(19)20)11-4-2-3-5-13(11)22/h2-5,8-9,12,17,24-25,27H,6-7,10H2,1H3/t12?,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 485 | n/a | n/a | n/a | n/a | n/a | n/a |
GPC Biotech, Inc.
| Assay Description
Inhibition of in vitro kinase activity of purified CDKs. |
Chem Biol 12: 1103-15 (2005)
Article DOI: 10.1016/j.chembiol.2005.08.008
BindingDB Entry DOI: 10.7270/Q28W3BR6 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 6/G1/S-specific cyclin-D3
(Homo sapiens (Human)) | BDBM50079331

(2-(2-Chloro-phenyl)-5,7-dihydroxy-8-(3-hydroxy-1-m...)Show SMILES CN1CCC([C@@H](O)C1)c1c(O)cc(O)c2c1oc(cc2=O)-c1ccccc1Cl Show InChI InChI=1S/C21H20ClNO5/c1-23-7-6-12(17(27)10-23)19-14(24)8-15(25)20-16(26)9-18(28-21(19)20)11-4-2-3-5-13(11)22/h2-5,8-9,12,17,24-25,27H,6-7,10H2,1H3/t12?,17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 663 | n/a | n/a | n/a | n/a | n/a | n/a |
GPC Biotech, Inc.
| Assay Description
Inhibition of in vitro kinase activity of purified CDKs. |
Chem Biol 12: 1103-15 (2005)
Article DOI: 10.1016/j.chembiol.2005.08.008
BindingDB Entry DOI: 10.7270/Q28W3BR6 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM59227

(Pyrazolopyrimidone analog, RGB-286147)Show SMILES CC(C)c1nn(-c2c(Cl)cccc2Cl)c2nc(Cc3ccc(OCCO)cc3)[nH]c(=O)c12 |(-2.33,5.73,;-1.56,4.39,;-.02,4.39,;-2.33,3.06,;-1.43,1.81,;-2.33,.57,;-1.93,-.92,;-.44,-1.32,;.64,-.23,;-.05,-2.8,;-1.13,-3.89,;-2.62,-3.49,;-3.02,-2.01,;-4.51,-1.61,;-3.79,1.04,;-5.13,.27,;-6.46,1.04,;-7.8,.27,;-9.13,1.04,;-10.46,.27,;-11.8,1.04,;-11.8,2.58,;-13.13,3.35,;-14.46,2.58,;-15.8,3.35,;-17.13,2.58,;-10.46,3.35,;-9.13,2.58,;-6.46,2.58,;-5.13,3.35,;-5.13,4.89,;-3.79,2.58,)| Show InChI InChI=1S/C23H22Cl2N4O3/c1-13(2)20-19-22(29(28-20)21-16(24)4-3-5-17(21)25)26-18(27-23(19)31)12-14-6-8-15(9-7-14)32-11-10-30/h3-9,13,30H,10-12H2,1-2H3,(H,26,27,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 754 | n/a | n/a | n/a | n/a | n/a | n/a |
GPC Biotech, Inc.
| Assay Description
Inhibition of in vitro kinase activity of purified CDKs. |
Chem Biol 12: 1103-15 (2005)
Article DOI: 10.1016/j.chembiol.2005.08.008
BindingDB Entry DOI: 10.7270/Q28W3BR6 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM59227

(Pyrazolopyrimidone analog, RGB-286147)Show SMILES CC(C)c1nn(-c2c(Cl)cccc2Cl)c2nc(Cc3ccc(OCCO)cc3)[nH]c(=O)c12 |(-2.33,5.73,;-1.56,4.39,;-.02,4.39,;-2.33,3.06,;-1.43,1.81,;-2.33,.57,;-1.93,-.92,;-.44,-1.32,;.64,-.23,;-.05,-2.8,;-1.13,-3.89,;-2.62,-3.49,;-3.02,-2.01,;-4.51,-1.61,;-3.79,1.04,;-5.13,.27,;-6.46,1.04,;-7.8,.27,;-9.13,1.04,;-10.46,.27,;-11.8,1.04,;-11.8,2.58,;-13.13,3.35,;-14.46,2.58,;-15.8,3.35,;-17.13,2.58,;-10.46,3.35,;-9.13,2.58,;-6.46,2.58,;-5.13,3.35,;-5.13,4.89,;-3.79,2.58,)| Show InChI InChI=1S/C23H22Cl2N4O3/c1-13(2)20-19-22(29(28-20)21-16(24)4-3-5-17(21)25)26-18(27-23(19)31)12-14-6-8-15(9-7-14)32-11-10-30/h3-9,13,30H,10-12H2,1-2H3,(H,26,27,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 839 | n/a | n/a | n/a | n/a | n/a | n/a |
GPC Biotech, Inc.
| Assay Description
Inhibition of in vitro kinase activity of purified CDKs. |
Chem Biol 12: 1103-15 (2005)
Article DOI: 10.1016/j.chembiol.2005.08.008
BindingDB Entry DOI: 10.7270/Q28W3BR6 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM59228

(Pyrazolopyrimidone analog, RGB-285940)Show SMILES COc1ccc(Cc2nc3n(nc(C(C)C)c3c(=O)[nH]2)-c2c(Cl)cc(Cl)cc2Cl)cc1 |(-14.46,2.58,;-13.13,3.35,;-11.8,2.58,;-11.8,1.04,;-10.46,.27,;-9.13,1.04,;-7.8,.27,;-6.46,1.04,;-5.13,.27,;-3.79,1.04,;-2.33,.57,;-1.43,1.81,;-2.33,3.06,;-1.56,4.39,;-2.33,5.73,;-.02,4.39,;-3.79,2.58,;-5.13,3.35,;-5.13,4.89,;-6.46,2.58,;-1.93,-.92,;-.44,-1.32,;.64,-.23,;-.05,-2.8,;-1.13,-3.89,;-.74,-5.38,;-2.62,-3.49,;-3.02,-2.01,;-4.51,-1.61,;-9.13,2.58,;-10.46,3.35,)| Show InChI InChI=1S/C22H19Cl3N4O2/c1-11(2)19-18-21(29(28-19)20-15(24)9-13(23)10-16(20)25)26-17(27-22(18)30)8-12-4-6-14(31-3)7-5-12/h4-7,9-11H,8H2,1-3H3,(H,26,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 858 | n/a | n/a | n/a | n/a | n/a | n/a |
GPC Biotech, Inc.
| Assay Description
Inhibition of in vitro kinase activity of purified CDKs. |
Chem Biol 12: 1103-15 (2005)
Article DOI: 10.1016/j.chembiol.2005.08.008
BindingDB Entry DOI: 10.7270/Q28W3BR6 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM59235

(PEGylated derivative, RGB-286276)Show SMILES COCCOCCOCCOc1ccc(Cc2nc3n(nc(C(C)C)c3c(=O)[nH]2)-c2c(Cl)cccc2Cl)cc1 |(-26.47,3.35,;-25.13,2.58,;-23.8,3.35,;-22.47,2.58,;-21.13,3.35,;-19.8,2.58,;-18.47,3.35,;-17.13,2.58,;-15.8,3.35,;-14.46,2.58,;-13.13,3.35,;-11.8,2.58,;-11.8,1.04,;-10.46,.27,;-9.13,1.04,;-7.8,.27,;-6.46,1.04,;-5.13,.27,;-3.79,1.04,;-2.33,.57,;-1.43,1.81,;-2.33,3.06,;-1.93,4.55,;-.44,4.95,;-3.02,5.64,;-3.79,2.58,;-5.13,3.35,;-5.13,4.89,;-6.46,2.58,;-1.93,-.92,;-.44,-1.32,;.33,.02,;-.05,-2.8,;-1.13,-3.89,;-2.62,-3.49,;-3.02,-2.01,;-4.51,-1.61,;-9.13,2.58,;-10.46,3.35,)| Show InChI InChI=1S/C28H32Cl2N4O5/c1-18(2)25-24-27(34(33-25)26-21(29)5-4-6-22(26)30)31-23(32-28(24)35)17-19-7-9-20(10-8-19)39-16-15-38-14-13-37-12-11-36-3/h4-10,18H,11-17H2,1-3H3,(H,31,32,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 890 | n/a | n/a | n/a | n/a | n/a | n/a |
GPC Biotech, Inc.
| Assay Description
In vitro kinase activity of PEGylated pyrazolopyrimidone CDK inhibitor or a 5 PEG-amine was coupled to ReactiGel agarose beads (Pierce, No. 20259). |
Chem Biol 12: 1103-15 (2005)
Article DOI: 10.1016/j.chembiol.2005.08.008
BindingDB Entry DOI: 10.7270/Q28W3BR6 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1
(Homo sapiens (Human)) | BDBM50079331

(2-(2-Chloro-phenyl)-5,7-dihydroxy-8-(3-hydroxy-1-m...)Show SMILES CN1CCC([C@@H](O)C1)c1c(O)cc(O)c2c1oc(cc2=O)-c1ccccc1Cl Show InChI InChI=1S/C21H20ClNO5/c1-23-7-6-12(17(27)10-23)19-14(24)8-15(25)20-16(26)9-18(28-21(19)20)11-4-2-3-5-13(11)22/h2-5,8-9,12,17,24-25,27H,6-7,10H2,1H3/t12?,17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GPC Biotech, Inc.
| Assay Description
Inhibition of in vitro kinase activity of purified CDKs. |
Chem Biol 12: 1103-15 (2005)
Article DOI: 10.1016/j.chembiol.2005.08.008
BindingDB Entry DOI: 10.7270/Q28W3BR6 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM59230

(Pyrazolopyrimidone analog, RGB-285960)Show SMILES CC(C)c1nn(-c2c(Cl)cccc2Cl)c2nc(Cc3ccc(OCCN4CCOCC4)cc3)[nH]c(=O)c12 |(-2.33,5.73,;-1.56,4.39,;-.02,4.39,;-2.33,3.06,;-1.43,1.81,;-2.33,.57,;-1.93,-.92,;-.44,-1.32,;.64,-.23,;-.05,-2.8,;-1.13,-3.89,;-2.62,-3.49,;-3.02,-2.01,;-4.51,-1.61,;-3.79,1.04,;-5.13,.27,;-6.46,1.04,;-7.8,.27,;-9.13,1.04,;-10.46,.27,;-11.8,1.04,;-11.8,2.58,;-13.13,3.35,;-14.46,2.58,;-15.8,3.35,;-17.13,2.58,;-18.47,3.35,;-19.8,2.58,;-19.8,1.04,;-18.47,.27,;-17.13,1.04,;-10.46,3.35,;-9.13,2.58,;-6.46,2.58,;-5.13,3.35,;-5.13,4.89,;-3.79,2.58,)| Show InChI InChI=1S/C27H29Cl2N5O3/c1-17(2)24-23-26(34(32-24)25-20(28)4-3-5-21(25)29)30-22(31-27(23)35)16-18-6-8-19(9-7-18)37-15-12-33-10-13-36-14-11-33/h3-9,17H,10-16H2,1-2H3,(H,30,31,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GPC Biotech, Inc.
| Assay Description
Inhibition of in vitro kinase activity of purified CDKs. |
Chem Biol 12: 1103-15 (2005)
Article DOI: 10.1016/j.chembiol.2005.08.008
BindingDB Entry DOI: 10.7270/Q28W3BR6 |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 1
(Homo sapiens (Human)) | BDBM50289899
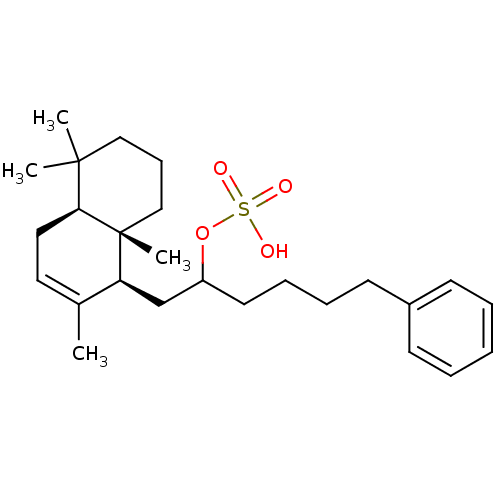
(CHEMBL292556 | Sulfuric acid mono-[5-phenyl-1-((1S...)Show SMILES CC1=CC[C@H]2C(C)(C)CCC[C@]2(C)[C@H]1CC(CCCCc1ccccc1)OS(O)(=O)=O |t:1| Show InChI InChI=1S/C26H40O4S/c1-20-15-16-24-25(2,3)17-10-18-26(24,4)23(20)19-22(30-31(27,28)29)14-9-8-13-21-11-6-5-7-12-21/h5-7,11-12,15,22-24H,8-10,13-14,16-19H2,1-4H3,(H,27,28,29)/t22?,23-,24-,26+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required for its inhibitory activity against Cdc25A phosphatase |
Bioorg Med Chem Lett 7: 2015-2020 (1997)
Article DOI: 10.1016/S0960-894X(97)00357-0
BindingDB Entry DOI: 10.7270/Q28S4PXX |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 1
(Homo sapiens (Human)) | BDBM50289900
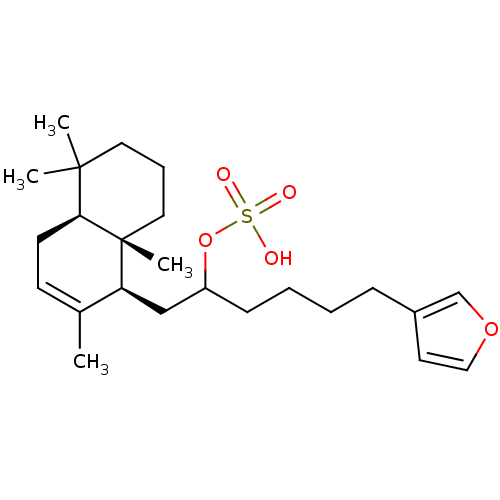
(CHEMBL303581 | Sulfuric acid mono-[5-furan-3-yl-1-...)Show SMILES CC1=CC[C@H]2C(C)(C)CCC[C@]2(C)[C@H]1CC(CCCCc1ccoc1)OS(O)(=O)=O |t:1| Show InChI InChI=1S/C24H38O5S/c1-18-10-11-22-23(2,3)13-7-14-24(22,4)21(18)16-20(29-30(25,26)27)9-6-5-8-19-12-15-28-17-19/h10,12,15,17,20-22H,5-9,11,13-14,16H2,1-4H3,(H,25,26,27)/t20?,21-,22-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required for its inhibitory activity against Cdc25A phosphatase |
Bioorg Med Chem Lett 7: 2015-2020 (1997)
Article DOI: 10.1016/S0960-894X(97)00357-0
BindingDB Entry DOI: 10.7270/Q28S4PXX |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 1
(Homo sapiens (Human)) | BDBM50289900

(CHEMBL303581 | Sulfuric acid mono-[5-furan-3-yl-1-...)Show SMILES CC1=CC[C@H]2C(C)(C)CCC[C@]2(C)[C@H]1CC(CCCCc1ccoc1)OS(O)(=O)=O |t:1| Show InChI InChI=1S/C24H38O5S/c1-18-10-11-22-23(2,3)13-7-14-24(22,4)21(18)16-20(29-30(25,26)27)9-6-5-8-19-12-15-28-17-19/h10,12,15,17,20-22H,5-9,11,13-14,16H2,1-4H3,(H,25,26,27)/t20?,21-,22-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required for its inhibitory activity against Cdc25A phosphatase |
Bioorg Med Chem Lett 7: 2015-2020 (1997)
Article DOI: 10.1016/S0960-894X(97)00357-0
BindingDB Entry DOI: 10.7270/Q28S4PXX |
More data for this
Ligand-Target Pair | |
Dual specificity protein phosphatase 3
(Homo sapiens (Human)) | BDBM50289899

(CHEMBL292556 | Sulfuric acid mono-[5-phenyl-1-((1S...)Show SMILES CC1=CC[C@H]2C(C)(C)CCC[C@]2(C)[C@H]1CC(CCCCc1ccccc1)OS(O)(=O)=O |t:1| Show InChI InChI=1S/C26H40O4S/c1-20-15-16-24-25(2,3)17-10-18-26(24,4)23(20)19-22(30-31(27,28)29)14-9-8-13-21-11-6-5-7-12-21/h5-7,11-12,15,22-24H,8-10,13-14,16-19H2,1-4H3,(H,27,28,29)/t22?,23-,24-,26+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required for its inhibitory activity against VHR phosphatase |
Bioorg Med Chem Lett 7: 2015-2020 (1997)
Article DOI: 10.1016/S0960-894X(97)00357-0
BindingDB Entry DOI: 10.7270/Q28S4PXX |
More data for this
Ligand-Target Pair | |
Dual specificity protein phosphatase 3
(Homo sapiens (Human)) | BDBM50286608

(CHEMBL60072 | Sulfircin analogue | Sulfuric acid m...)Show SMILES C[C@H](CCCc1ccoc1)[C@H](C[C@H]1C(C)=CC[C@H]2C(C)(C)CCC[C@]12C)OS([O-])(=O)=O |c:15| Show InChI InChI=1S/C25H40O5S/c1-18-10-11-23-24(3,4)13-7-14-25(23,5)21(18)16-22(30-31(26,27)28)19(2)8-6-9-20-12-15-29-17-20/h10,12,15,17,19,21-23H,6-9,11,13-14,16H2,1-5H3,(H,26,27,28)/p-1/t19-,21+,22+,23+,25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required for its inhibitory activity against VHR phosphatase |
Bioorg Med Chem Lett 7: 2015-2020 (1997)
Article DOI: 10.1016/S0960-894X(97)00357-0
BindingDB Entry DOI: 10.7270/Q28S4PXX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50289899

(CHEMBL292556 | Sulfuric acid mono-[5-phenyl-1-((1S...)Show SMILES CC1=CC[C@H]2C(C)(C)CCC[C@]2(C)[C@H]1CC(CCCCc1ccccc1)OS(O)(=O)=O |t:1| Show InChI InChI=1S/C26H40O4S/c1-20-15-16-24-25(2,3)17-10-18-26(24,4)23(20)19-22(30-31(27,28)29)14-9-8-13-21-11-6-5-7-12-21/h5-7,11-12,15,22-24H,8-10,13-14,16-19H2,1-4H3,(H,27,28,29)/t22?,23-,24-,26+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required for its inhibitory activity against PTB1B phosphatase |
Bioorg Med Chem Lett 7: 2015-2020 (1997)
Article DOI: 10.1016/S0960-894X(97)00357-0
BindingDB Entry DOI: 10.7270/Q28S4PXX |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase/G2/mitotic-specific cyclin- 1
(Homo sapiens (Human)) | BDBM59233

(Pyrazolopyrimidone analog, RGB-310590)Show SMILES COc1ccc(Cc2nc3n(ncc3c(=O)[nH]2)-c2c(Cl)cc(Cl)cc2Cl)cc1 |(-14.46,2.58,;-13.13,3.35,;-11.8,2.58,;-11.8,1.04,;-10.46,.27,;-9.13,1.04,;-7.8,.27,;-6.46,1.04,;-5.13,.27,;-3.79,1.04,;-2.33,.57,;-1.43,1.81,;-2.33,3.06,;-3.79,2.58,;-5.13,3.35,;-5.13,4.89,;-6.46,2.58,;-1.93,-.92,;-.44,-1.32,;.33,.02,;-.05,-2.8,;-1.13,-3.89,;-.74,-5.38,;-2.62,-3.49,;-3.02,-2.01,;-4.51,-1.61,;-9.13,2.58,;-10.46,3.35,)| Show InChI InChI=1S/C19H13Cl3N4O2/c1-28-12-4-2-10(3-5-12)6-16-24-18-13(19(27)25-16)9-23-26(18)17-14(21)7-11(20)8-15(17)22/h2-5,7-9H,6H2,1H3,(H,24,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GPC Biotech, Inc.
| Assay Description
Inhibition of HCT116 cell proliferation after 72hr of incubation with compounds was determined by SRB Assay. |
Chem Biol 12: 1103-15 (2005)
Article DOI: 10.1016/j.chembiol.2005.08.008
BindingDB Entry DOI: 10.7270/Q28W3BR6 |
More data for this
Ligand-Target Pair | |
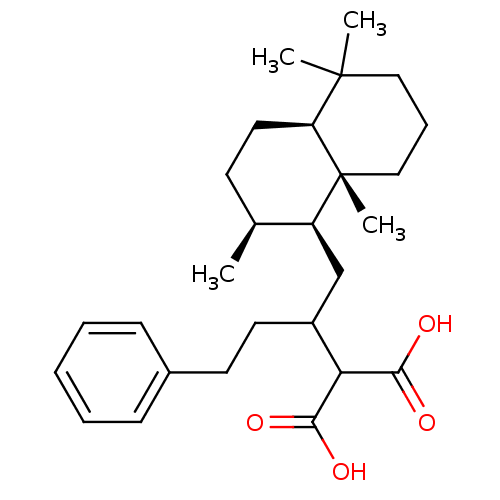
Dual specificity protein phosphatase 3
(Homo sapiens (Human)) | BDBM50289906
(2-[3-Phenyl-1-((1S,2S,4aS,8aR)-2,5,5,8a-tetramethy...)Show SMILES C[C@H]1CC[C@H]2C(C)(C)CCC[C@]2(C)[C@H]1CC(CCc1ccccc1)C(C(O)=O)C(O)=O Show InChI InChI=1S/C27H40O4/c1-18-11-14-22-26(2,3)15-8-16-27(22,4)21(18)17-20(23(24(28)29)25(30)31)13-12-19-9-6-5-7-10-19/h5-7,9-10,18,20-23H,8,11-17H2,1-4H3,(H,28,29)(H,30,31)/t18-,20?,21-,22-,27+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required for its inhibitory activity against VHR phosphatase |
Bioorg Med Chem Lett 7: 2015-2020 (1997)
Article DOI: 10.1016/S0960-894X(97)00357-0
BindingDB Entry DOI: 10.7270/Q28S4PXX |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 1
(Homo sapiens (Human)) | BDBM50289906

(2-[3-Phenyl-1-((1S,2S,4aS,8aR)-2,5,5,8a-tetramethy...)Show SMILES C[C@H]1CC[C@H]2C(C)(C)CCC[C@]2(C)[C@H]1CC(CCc1ccccc1)C(C(O)=O)C(O)=O Show InChI InChI=1S/C27H40O4/c1-18-11-14-22-26(2,3)15-8-16-27(22,4)21(18)17-20(23(24(28)29)25(30)31)13-12-19-9-6-5-7-10-19/h5-7,9-10,18,20-23H,8,11-17H2,1-4H3,(H,28,29)(H,30,31)/t18-,20?,21-,22-,27+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required for its inhibitory activity against Cdc25A phosphatase |
Bioorg Med Chem Lett 7: 2015-2020 (1997)
Article DOI: 10.1016/S0960-894X(97)00357-0
BindingDB Entry DOI: 10.7270/Q28S4PXX |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 1
(Homo sapiens (Human)) | BDBM50286608

(CHEMBL60072 | Sulfircin analogue | Sulfuric acid m...)Show SMILES C[C@H](CCCc1ccoc1)[C@H](C[C@H]1C(C)=CC[C@H]2C(C)(C)CCC[C@]12C)OS([O-])(=O)=O |c:15| Show InChI InChI=1S/C25H40O5S/c1-18-10-11-23-24(3,4)13-7-14-25(23,5)21(18)16-22(30-31(26,27)28)19(2)8-6-9-20-12-15-29-17-20/h10,12,15,17,19,21-23H,6-9,11,13-14,16H2,1-5H3,(H,26,27,28)/p-1/t19-,21+,22+,23+,25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required for its inhibitory activity against Cdc25A phosphatase |
Bioorg Med Chem Lett 7: 2015-2020 (1997)
Article DOI: 10.1016/S0960-894X(97)00357-0
BindingDB Entry DOI: 10.7270/Q28S4PXX |
More data for this
Ligand-Target Pair | |
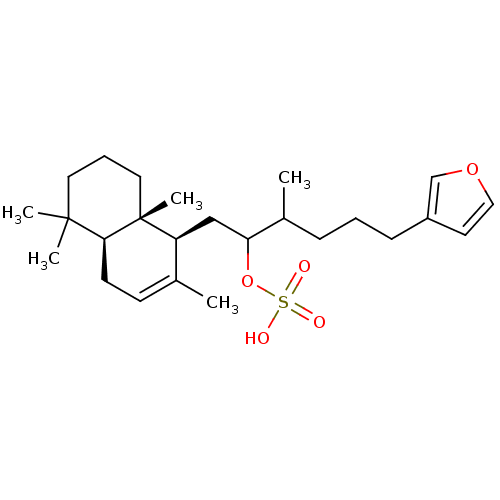
Dual specificity protein phosphatase 3
(Homo sapiens (Human)) | BDBM50289897
(CHEMBL60297 | Sulfuric acid mono-[5-furan-3-yl-2-m...)Show SMILES CC(CCCc1ccoc1)C(C[C@H]1C(C)=CC[C@H]2C(C)(C)CCC[C@]12C)OS(O)(=O)=O |c:15| Show InChI InChI=1S/C25H40O5S/c1-18-10-11-23-24(3,4)13-7-14-25(23,5)21(18)16-22(30-31(26,27)28)19(2)8-6-9-20-12-15-29-17-20/h10,12,15,17,19,21-23H,6-9,11,13-14,16H2,1-5H3,(H,26,27,28)/t19?,21-,22?,23-,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 7.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required for its inhibitory activity against VHR phosphatase |
Bioorg Med Chem Lett 7: 2015-2020 (1997)
Article DOI: 10.1016/S0960-894X(97)00357-0
BindingDB Entry DOI: 10.7270/Q28S4PXX |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 1
(Homo sapiens (Human)) | BDBM50289897

(CHEMBL60297 | Sulfuric acid mono-[5-furan-3-yl-2-m...)Show SMILES CC(CCCc1ccoc1)C(C[C@H]1C(C)=CC[C@H]2C(C)(C)CCC[C@]12C)OS(O)(=O)=O |c:15| Show InChI InChI=1S/C25H40O5S/c1-18-10-11-23-24(3,4)13-7-14-25(23,5)21(18)16-22(30-31(26,27)28)19(2)8-6-9-20-12-15-29-17-20/h10,12,15,17,19,21-23H,6-9,11,13-14,16H2,1-5H3,(H,26,27,28)/t19?,21-,22?,23-,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 8.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required for its inhibitory activity against Cdc25A phosphatase |
Bioorg Med Chem Lett 7: 2015-2020 (1997)
Article DOI: 10.1016/S0960-894X(97)00357-0
BindingDB Entry DOI: 10.7270/Q28S4PXX |
More data for this
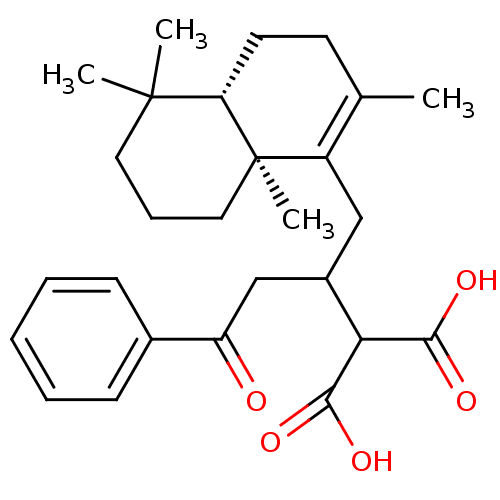
Ligand-Target Pair | |
Dual specificity protein phosphatase 3
(Homo sapiens (Human)) | BDBM50289902
(2-[3-Oxo-3-phenyl-1-((4aS,8aS)-2,5,5,8a-tetramethy...)Show SMILES CC1=C(CC(CC(=O)c2ccccc2)C(C(O)=O)C(O)=O)[C@@]2(C)CCCC(C)(C)[C@@H]2CC1 |c:1| Show InChI InChI=1S/C27H36O5/c1-17-11-12-22-26(2,3)13-8-14-27(22,4)20(17)15-19(23(24(29)30)25(31)32)16-21(28)18-9-6-5-7-10-18/h5-7,9-10,19,22-23H,8,11-16H2,1-4H3,(H,29,30)(H,31,32)/t19?,22-,27+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| n/a | n/a | 8.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required for its inhibitory activity against VHR phosphatase |
Bioorg Med Chem Lett 7: 2015-2020 (1997)
Article DOI: 10.1016/S0960-894X(97)00357-0
BindingDB Entry DOI: 10.7270/Q28S4PXX |
More data for this
Ligand-Target Pair | |
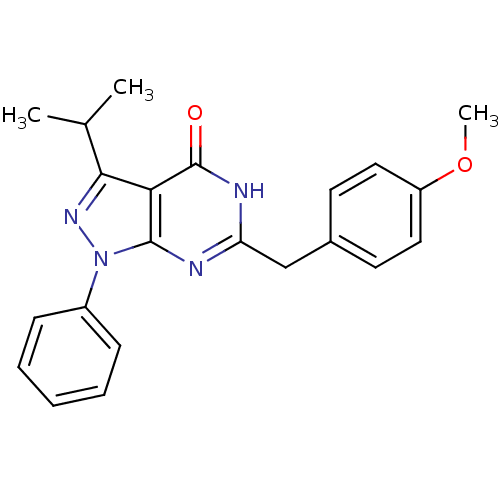
Cyclin-dependent kinase/G2/mitotic-specific cyclin- 1
(Homo sapiens (Human)) | BDBM59231
(Pyrazolopyrimidone analog, RGB-310596)Show SMILES COc1ccc(Cc2nc3n(nc(C(C)C)c3c(=O)[nH]2)-c2ccccc2)cc1 Show InChI InChI=1S/C22H22N4O2/c1-14(2)20-19-21(26(25-20)16-7-5-4-6-8-16)23-18(24-22(19)27)13-15-9-11-17(28-3)12-10-15/h4-12,14H,13H2,1-3H3,(H,23,24,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GPC Biotech, Inc.
| Assay Description
Inhibition of HCT116 cell proliferation after 72hr of incubation with compounds was determined by SRB Assay. |
Chem Biol 12: 1103-15 (2005)
Article DOI: 10.1016/j.chembiol.2005.08.008
BindingDB Entry DOI: 10.7270/Q28W3BR6 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase/G2/mitotic-specific cyclin- 1
(Homo sapiens (Human)) | BDBM59232

(Pyrazolopyrimidone analog, RGB-310595)Show SMILES COc1ccc(Cc2nc3n[nH]c(C(C)C)c3c(=O)[nH]2)cc1 Show InChI InChI=1S/C16H18N4O2/c1-9(2)14-13-15(20-19-14)17-12(18-16(13)21)8-10-4-6-11(22-3)7-5-10/h4-7,9H,8H2,1-3H3,(H2,17,18,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GPC Biotech, Inc.
| Assay Description
Inhibition of HCT116 cell proliferation after 72hr of incubation with compounds was determined by SRB Assay. |
Chem Biol 12: 1103-15 (2005)
Article DOI: 10.1016/j.chembiol.2005.08.008
BindingDB Entry DOI: 10.7270/Q28W3BR6 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase/G2/mitotic-specific cyclin- 1
(Homo sapiens (Human)) | BDBM59234
(Pyrazolopyrimidone analog, RGB-310591)Show SMILES COc1ccc(Cc2nc3n(nc(Cc4ccccc4)c3c(=O)[nH]2)-c2c(Cl)cc(Cl)cc2Cl)cc1 |(-14.46,2.58,;-13.13,3.35,;-11.8,2.58,;-11.8,1.04,;-10.46,.27,;-9.13,1.04,;-7.8,.27,;-6.46,1.04,;-5.13,.27,;-3.79,1.04,;-2.33,.57,;-1.43,1.81,;-2.33,3.06,;-1.85,4.53,;-.37,4.92,;.03,6.41,;1.52,6.81,;2.61,5.72,;2.21,4.23,;.72,3.84,;-3.79,2.58,;-5.13,3.35,;-5.13,4.89,;-6.46,2.58,;-1.93,-.92,;-.44,-1.32,;.33,.02,;-.05,-2.8,;-1.13,-3.89,;-.74,-5.38,;-2.62,-3.49,;-3.02,-2.01,;-4.51,-1.61,;-9.13,2.58,;-10.46,3.35,)| Show InChI InChI=1S/C26H19Cl3N4O2/c1-35-18-9-7-16(8-10-18)12-22-30-25-23(26(34)31-22)21(11-15-5-3-2-4-6-15)32-33(25)24-19(28)13-17(27)14-20(24)29/h2-10,13-14H,11-12H2,1H3,(H,30,31,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GPC Biotech, Inc.
| Assay Description
Inhibition of HCT116 cell proliferation after 72hr of incubation with compounds was determined by SRB Assay. |
Chem Biol 12: 1103-15 (2005)
Article DOI: 10.1016/j.chembiol.2005.08.008
BindingDB Entry DOI: 10.7270/Q28W3BR6 |
More data for this
Ligand-Target Pair | |
Dual specificity protein phosphatase 3
(Homo sapiens (Human)) | BDBM50289900

(CHEMBL303581 | Sulfuric acid mono-[5-furan-3-yl-1-...)Show SMILES CC1=CC[C@H]2C(C)(C)CCC[C@]2(C)[C@H]1CC(CCCCc1ccoc1)OS(O)(=O)=O |t:1| Show InChI InChI=1S/C24H38O5S/c1-18-10-11-22-23(2,3)13-7-14-24(22,4)21(18)16-20(29-30(25,26)27)9-6-5-8-19-12-15-28-17-19/h10,12,15,17,20-22H,5-9,11,13-14,16H2,1-4H3,(H,25,26,27)/t20?,21-,22-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required for its inhibitory activity against VHR phosphatase |
Bioorg Med Chem Lett 7: 2015-2020 (1997)
Article DOI: 10.1016/S0960-894X(97)00357-0
BindingDB Entry DOI: 10.7270/Q28S4PXX |
More data for this
Ligand-Target Pair | |
Dual specificity protein phosphatase 3
(Homo sapiens (Human)) | BDBM50289900

(CHEMBL303581 | Sulfuric acid mono-[5-furan-3-yl-1-...)Show SMILES CC1=CC[C@H]2C(C)(C)CCC[C@]2(C)[C@H]1CC(CCCCc1ccoc1)OS(O)(=O)=O |t:1| Show InChI InChI=1S/C24H38O5S/c1-18-10-11-22-23(2,3)13-7-14-24(22,4)21(18)16-20(29-30(25,26)27)9-6-5-8-19-12-15-28-17-19/h10,12,15,17,20-22H,5-9,11,13-14,16H2,1-4H3,(H,25,26,27)/t20?,21-,22-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required for its inhibitory activity against Cdc25A phosphatase |
Bioorg Med Chem Lett 7: 2015-2020 (1997)
Article DOI: 10.1016/S0960-894X(97)00357-0
BindingDB Entry DOI: 10.7270/Q28S4PXX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50289900

(CHEMBL303581 | Sulfuric acid mono-[5-furan-3-yl-1-...)Show SMILES CC1=CC[C@H]2C(C)(C)CCC[C@]2(C)[C@H]1CC(CCCCc1ccoc1)OS(O)(=O)=O |t:1| Show InChI InChI=1S/C24H38O5S/c1-18-10-11-22-23(2,3)13-7-14-24(22,4)21(18)16-20(29-30(25,26)27)9-6-5-8-19-12-15-28-17-19/h10,12,15,17,20-22H,5-9,11,13-14,16H2,1-4H3,(H,25,26,27)/t20?,21-,22-,24+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required for its inhibitory activity against PTB1B phosphatase |
Bioorg Med Chem Lett 7: 2015-2020 (1997)
Article DOI: 10.1016/S0960-894X(97)00357-0
BindingDB Entry DOI: 10.7270/Q28S4PXX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50289900

(CHEMBL303581 | Sulfuric acid mono-[5-furan-3-yl-1-...)Show SMILES CC1=CC[C@H]2C(C)(C)CCC[C@]2(C)[C@H]1CC(CCCCc1ccoc1)OS(O)(=O)=O |t:1| Show InChI InChI=1S/C24H38O5S/c1-18-10-11-22-23(2,3)13-7-14-24(22,4)21(18)16-20(29-30(25,26)27)9-6-5-8-19-12-15-28-17-19/h10,12,15,17,20-22H,5-9,11,13-14,16H2,1-4H3,(H,25,26,27)/t20?,21-,22-,24+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required for its inhibitory activity against PTB1B phosphatase |
Bioorg Med Chem Lett 7: 2015-2020 (1997)
Article DOI: 10.1016/S0960-894X(97)00357-0
BindingDB Entry DOI: 10.7270/Q28S4PXX |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 1
(Homo sapiens (Human)) | BDBM50289903
(CHEMBL64690 | Sulfuric acid mono-[3-phenyl-1-((1S,...)Show SMILES CC1=CC[C@H]2C(C)(C)CCC[C@]2(C)[C@H]1CC(CCc1ccccc1)OS(O)(=O)=O |t:1| Show InChI InChI=1S/C24H36O4S/c1-18-11-14-22-23(2,3)15-8-16-24(22,4)21(18)17-20(28-29(25,26)27)13-12-19-9-6-5-7-10-19/h5-7,9-11,20-22H,8,12-17H2,1-4H3,(H,25,26,27)/t20?,21-,22-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.65E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required for its inhibitory activity against Cdc25A phosphatase |
Bioorg Med Chem Lett 7: 2015-2020 (1997)
Article DOI: 10.1016/S0960-894X(97)00357-0
BindingDB Entry DOI: 10.7270/Q28S4PXX |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data