

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coagulation factor X (Oryctolagus cuniculus) | BDBM35743 (CHEMBL479863 | cis-1,2-diaminocyclohexane derivati...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1.74 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of rabbit factor 10a by Lineweaver-Burk plot | Bioorg Med Chem 17: 1193-206 (2009) Article DOI: 10.1016/j.bmc.2008.12.037 BindingDB Entry DOI: 10.7270/Q2V69JGR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM35743 (CHEMBL479863 | cis-1,2-diaminocyclohexane derivati...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 2.85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of human factor 10a by Lineweaver-Burk plot | Bioorg Med Chem 17: 1193-206 (2009) Article DOI: 10.1016/j.bmc.2008.12.037 BindingDB Entry DOI: 10.7270/Q2V69JGR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Rattus norvegicus (rat)) | BDBM35743 (CHEMBL479863 | cis-1,2-diaminocyclohexane derivati...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 8.77 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of rat factor 10a by Lineweaver-Burk plot | Bioorg Med Chem 17: 1193-206 (2009) Article DOI: 10.1016/j.bmc.2008.12.037 BindingDB Entry DOI: 10.7270/Q2V69JGR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17283 ((2S)-3-(7-carbamimidoylnaphthalen-2-yl)-2-(4-{[(3S...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of human factor 10a by Lineweaver-Burk plot | Bioorg Med Chem 17: 1193-206 (2009) Article DOI: 10.1016/j.bmc.2008.12.037 BindingDB Entry DOI: 10.7270/Q2V69JGR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM35743 (CHEMBL479863 | cis-1,2-diaminocyclohexane derivati...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of human thrombin by Lineweaver-Burk plot | Bioorg Med Chem 17: 1193-206 (2009) Article DOI: 10.1016/j.bmc.2008.12.037 BindingDB Entry DOI: 10.7270/Q2V69JGR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM17283 ((2S)-3-(7-carbamimidoylnaphthalen-2-yl)-2-(4-{[(3S...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of human recombinant tissue plasminogen activator by Lineweaver-Burk plot | Bioorg Med Chem 17: 1193-206 (2009) Article DOI: 10.1016/j.bmc.2008.12.037 BindingDB Entry DOI: 10.7270/Q2V69JGR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM17283 ((2S)-3-(7-carbamimidoylnaphthalen-2-yl)-2-(4-{[(3S...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of human plasmin by Lineweaver-Burk plot | Bioorg Med Chem 17: 1193-206 (2009) Article DOI: 10.1016/j.bmc.2008.12.037 BindingDB Entry DOI: 10.7270/Q2V69JGR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM35743 (CHEMBL479863 | cis-1,2-diaminocyclohexane derivati...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 6.82E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of human recombinant tissue plasminogen activator by Lineweaver-Burk plot | Bioorg Med Chem 17: 1193-206 (2009) Article DOI: 10.1016/j.bmc.2008.12.037 BindingDB Entry DOI: 10.7270/Q2V69JGR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM35743 (CHEMBL479863 | cis-1,2-diaminocyclohexane derivati...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of human recombinant factor 7a/soluble tissue factor by Lineweaver-Burk plot | Bioorg Med Chem 17: 1193-206 (2009) Article DOI: 10.1016/j.bmc.2008.12.037 BindingDB Entry DOI: 10.7270/Q2V69JGR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM35743 (CHEMBL479863 | cis-1,2-diaminocyclohexane derivati...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of human plasmin by Lineweaver-Burk plot | Bioorg Med Chem 17: 1193-206 (2009) Article DOI: 10.1016/j.bmc.2008.12.037 BindingDB Entry DOI: 10.7270/Q2V69JGR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM17283 ((2S)-3-(7-carbamimidoylnaphthalen-2-yl)-2-(4-{[(3S...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | >2.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of human thrombin by Lineweaver-Burk plot | Bioorg Med Chem 17: 1193-206 (2009) Article DOI: 10.1016/j.bmc.2008.12.037 BindingDB Entry DOI: 10.7270/Q2V69JGR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM532034 (US11208403, Example 111) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A kinase dilution solution containing 170 ng/ml AXL (the 464th to 885th amino acids of the intracellular domain of human AXL expressed as a fusion pr... | Citation and Details BindingDB Entry DOI: 10.7270/Q29026Z6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM532033 (N-[6-(2-Amino-5-{3-methoxy-4-[2-(morpholin-4-yl)et...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A kinase dilution solution containing 170 ng/ml AXL (the 464th to 885th amino acids of the intracellular domain of human AXL expressed as a fusion pr... | Citation and Details BindingDB Entry DOI: 10.7270/Q29026Z6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM532023 (N-(6-[2-Amino-5-(3,4-dimethoxyphenyl)pyridin-3-yl]...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A kinase dilution solution containing 170 ng/ml AXL (the 464th to 885th amino acids of the intracellular domain of human AXL expressed as a fusion pr... | Citation and Details BindingDB Entry DOI: 10.7270/Q29026Z6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM532007 (US11208403, Example 84) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A kinase dilution solution containing 170 ng/ml AXL (the 464th to 885th amino acids of the intracellular domain of human AXL expressed as a fusion pr... | Citation and Details BindingDB Entry DOI: 10.7270/Q29026Z6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50442114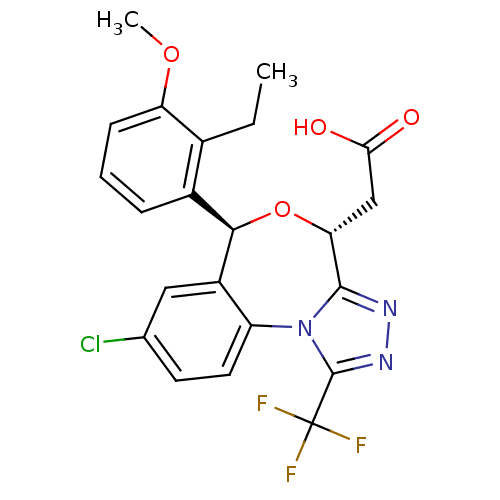 (CHEMBL2441090) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of rat hepatic microsomal squalene synthase using [3H]FPP as substrate after by scintillation spectrophotometry | ACS Med Chem Lett 4: 932-6 (2013) Article DOI: 10.1021/ml400151c BindingDB Entry DOI: 10.7270/Q2J67JCV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM532009 (US11208403, Example 86) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A kinase dilution solution containing 170 ng/ml AXL (the 464th to 885th amino acids of the intracellular domain of human AXL expressed as a fusion pr... | Citation and Details BindingDB Entry DOI: 10.7270/Q29026Z6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM532008 (N-(4-{2-Amino-5-[1-(tetrahydro-2H-pyran-4-yl)-1H-p...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A kinase dilution solution containing 170 ng/ml AXL (the 464th to 885th amino acids of the intracellular domain of human AXL expressed as a fusion pr... | Citation and Details BindingDB Entry DOI: 10.7270/Q29026Z6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM531957 (US11208403, Example 34) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A kinase dilution solution containing 170 ng/ml AXL (the 464th to 885th amino acids of the intracellular domain of human AXL expressed as a fusion pr... | Citation and Details BindingDB Entry DOI: 10.7270/Q29026Z6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM35767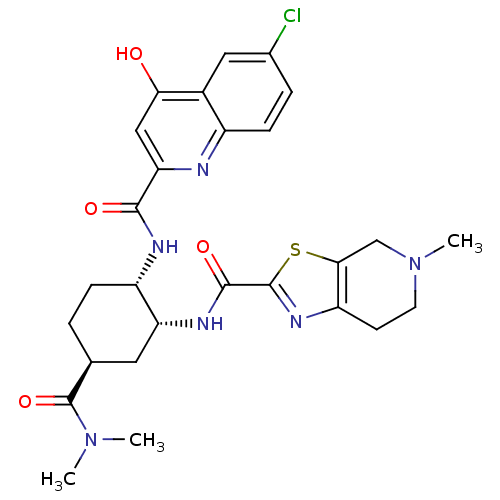 (cis-1,2-diaminocyclohexane derivative, 5h) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | 7.4 | 23 |
Daiichi Sankyo Co, Ltd | Assay Description The in vitro anti-fXa activity was measured by using a chromogenic substrate S-2222 and human fXa. Aqueous DMSO or test compounds in aqueous DMSO and... | Bioorg Med Chem 17: 8221-33 (2009) Article DOI: 10.1016/j.bmc.2009.10.024 BindingDB Entry DOI: 10.7270/Q2CC0Z1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM531976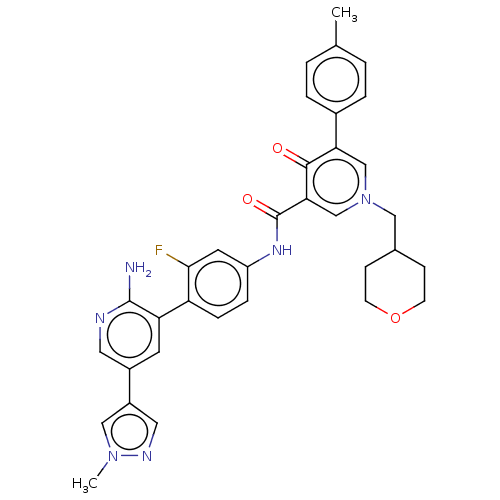 (US11208403, Example 53) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A kinase dilution solution containing 170 ng/ml AXL (the 464th to 885th amino acids of the intracellular domain of human AXL expressed as a fusion pr... | Citation and Details BindingDB Entry DOI: 10.7270/Q29026Z6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM532029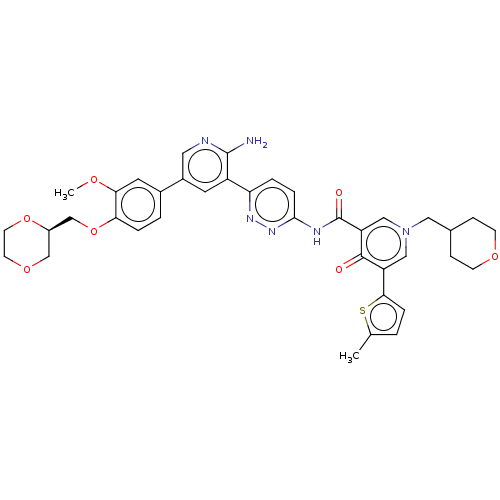 (US11208403, Example 106) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A kinase dilution solution containing 170 ng/ml AXL (the 464th to 885th amino acids of the intracellular domain of human AXL expressed as a fusion pr... | Citation and Details BindingDB Entry DOI: 10.7270/Q29026Z6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM532021 (US11208403, Example 98) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A kinase dilution solution containing 170 ng/ml AXL (the 464th to 885th amino acids of the intracellular domain of human AXL expressed as a fusion pr... | Citation and Details BindingDB Entry DOI: 10.7270/Q29026Z6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM532027 (US11208403, Example 104) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A kinase dilution solution containing 170 ng/ml AXL (the 464th to 885th amino acids of the intracellular domain of human AXL expressed as a fusion pr... | Citation and Details BindingDB Entry DOI: 10.7270/Q29026Z6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM531958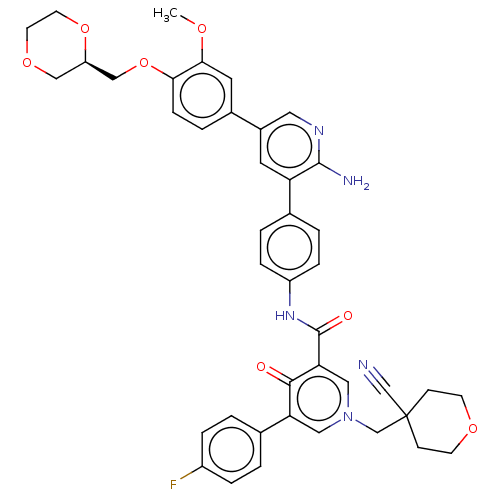 (US11208403, Example 35) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A kinase dilution solution containing 170 ng/ml AXL (the 464th to 885th amino acids of the intracellular domain of human AXL expressed as a fusion pr... | Citation and Details BindingDB Entry DOI: 10.7270/Q29026Z6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50388376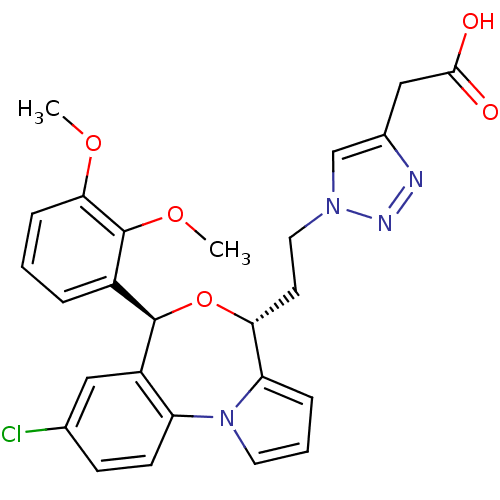 (CHEMBL2057637) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co. Ltd Curated by ChEMBL | Assay Description Inhibition of squalene synthase in rat hepatic cells | Bioorg Med Chem 20: 3072-93 (2012) Article DOI: 10.1016/j.bmc.2012.02.054 BindingDB Entry DOI: 10.7270/Q2B8595B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50388375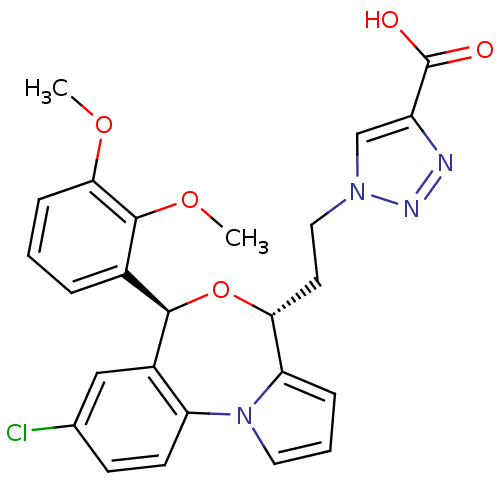 (CHEMBL2057636) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co. Ltd Curated by ChEMBL | Assay Description Inhibition of squalene synthase in rat hepatic cells | Bioorg Med Chem 20: 3072-93 (2012) Article DOI: 10.1016/j.bmc.2012.02.054 BindingDB Entry DOI: 10.7270/Q2B8595B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM532013 (US11208403, Example 90) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A kinase dilution solution containing 170 ng/ml AXL (the 464th to 885th amino acids of the intracellular domain of human AXL expressed as a fusion pr... | Citation and Details BindingDB Entry DOI: 10.7270/Q29026Z6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM531981 (US11208403, Example 58) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A kinase dilution solution containing 170 ng/ml AXL (the 464th to 885th amino acids of the intracellular domain of human AXL expressed as a fusion pr... | Citation and Details BindingDB Entry DOI: 10.7270/Q29026Z6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM531955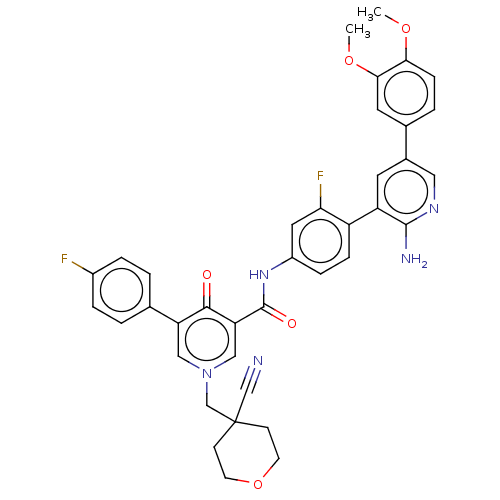 (US11208403, Example 32) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A kinase dilution solution containing 170 ng/ml AXL (the 464th to 885th amino acids of the intracellular domain of human AXL expressed as a fusion pr... | Citation and Details BindingDB Entry DOI: 10.7270/Q29026Z6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM531991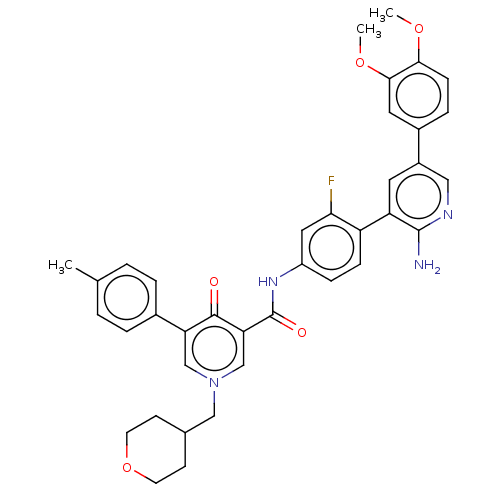 (N-{4-[2-Amino-5-(3,4-dimethoxyphenyl)pyridin-3-yl]...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A kinase dilution solution containing 170 ng/ml AXL (the 464th to 885th amino acids of the intracellular domain of human AXL expressed as a fusion pr... | Citation and Details BindingDB Entry DOI: 10.7270/Q29026Z6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM531994 (N-[4-(2-Amino-5-{4-[(4-methylpiperazin-1-yl)methyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A kinase dilution solution containing 170 ng/ml AXL (the 464th to 885th amino acids of the intracellular domain of human AXL expressed as a fusion pr... | Citation and Details BindingDB Entry DOI: 10.7270/Q29026Z6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM531999 (US11208403, Example 76) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A kinase dilution solution containing 170 ng/ml AXL (the 464th to 885th amino acids of the intracellular domain of human AXL expressed as a fusion pr... | Citation and Details BindingDB Entry DOI: 10.7270/Q29026Z6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50388378 (CHEMBL2057639) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co. Ltd Curated by ChEMBL | Assay Description Inhibition of squalene synthase in rat hepatic cells | Bioorg Med Chem 20: 3072-93 (2012) Article DOI: 10.1016/j.bmc.2012.02.054 BindingDB Entry DOI: 10.7270/Q2B8595B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50388348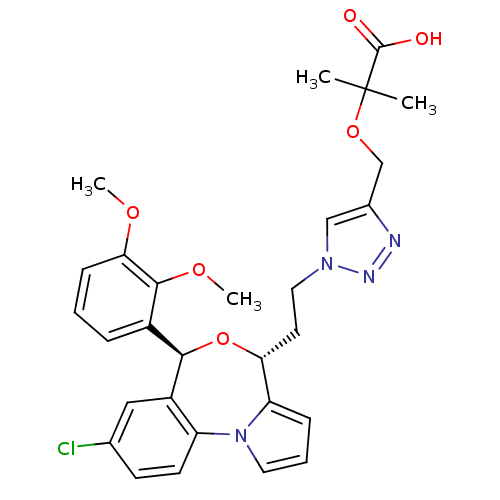 (CHEMBL2057952) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co. Ltd Curated by ChEMBL | Assay Description Inhibition of squalene synthase in rat hepatic cells | Bioorg Med Chem 20: 3072-93 (2012) Article DOI: 10.1016/j.bmc.2012.02.054 BindingDB Entry DOI: 10.7270/Q2B8595B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM532024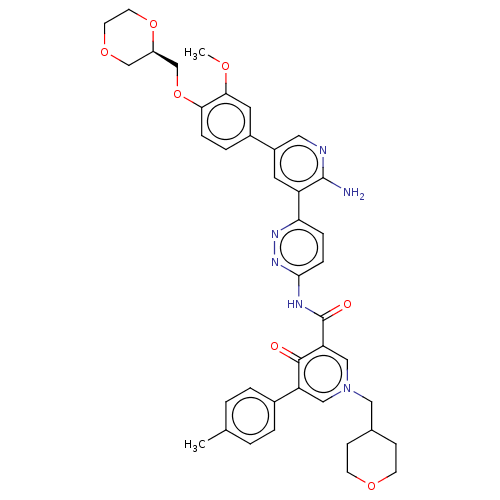 (US11208403, Example 101) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A kinase dilution solution containing 170 ng/ml AXL (the 464th to 885th amino acids of the intracellular domain of human AXL expressed as a fusion pr... | Citation and Details BindingDB Entry DOI: 10.7270/Q29026Z6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM531986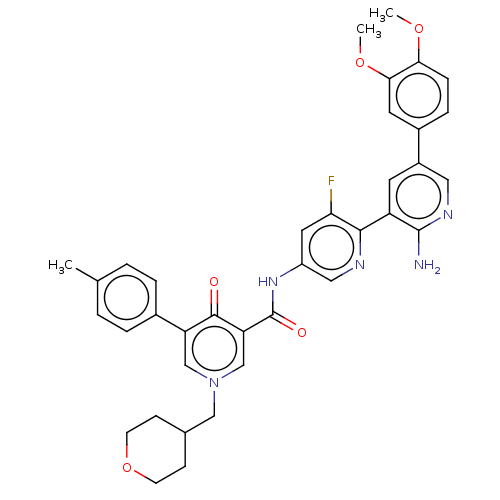 (US11208403, Example 63) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A kinase dilution solution containing 170 ng/ml AXL (the 464th to 885th amino acids of the intracellular domain of human AXL expressed as a fusion pr... | Citation and Details BindingDB Entry DOI: 10.7270/Q29026Z6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM531964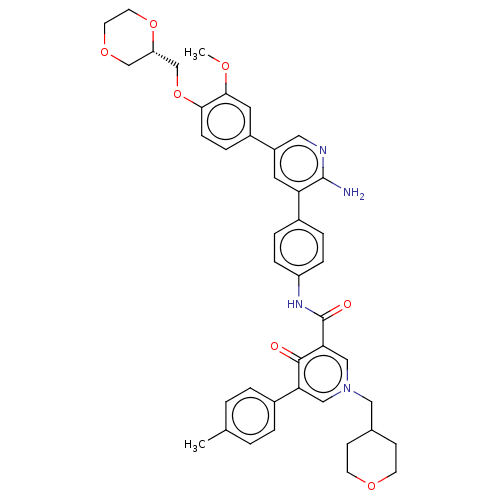 (US11208403, Example 41) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A kinase dilution solution containing 170 ng/ml AXL (the 464th to 885th amino acids of the intracellular domain of human AXL expressed as a fusion pr... | Citation and Details BindingDB Entry DOI: 10.7270/Q29026Z6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM531956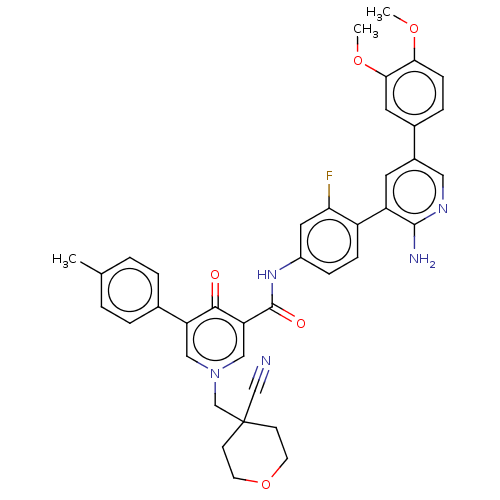 (US11208403, Example 33) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A kinase dilution solution containing 170 ng/ml AXL (the 464th to 885th amino acids of the intracellular domain of human AXL expressed as a fusion pr... | Citation and Details BindingDB Entry DOI: 10.7270/Q29026Z6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM531952 (US11208403, Example 29) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A kinase dilution solution containing 170 ng/ml AXL (the 464th to 885th amino acids of the intracellular domain of human AXL expressed as a fusion pr... | Citation and Details BindingDB Entry DOI: 10.7270/Q29026Z6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM532010 (US11208403, Example 87) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A kinase dilution solution containing 170 ng/ml AXL (the 464th to 885th amino acids of the intracellular domain of human AXL expressed as a fusion pr... | Citation and Details BindingDB Entry DOI: 10.7270/Q29026Z6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM531928 (US11208403, Example 5) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A kinase dilution solution containing 170 ng/ml AXL (the 464th to 885th amino acids of the intracellular domain of human AXL expressed as a fusion pr... | Citation and Details BindingDB Entry DOI: 10.7270/Q29026Z6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50442115 (CHEMBL2440128) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of rat hepatic microsomal squalene synthase using [3H]FPP as substrate after by scintillation spectrophotometry | ACS Med Chem Lett 4: 932-6 (2013) Article DOI: 10.1021/ml400151c BindingDB Entry DOI: 10.7270/Q2J67JCV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50388370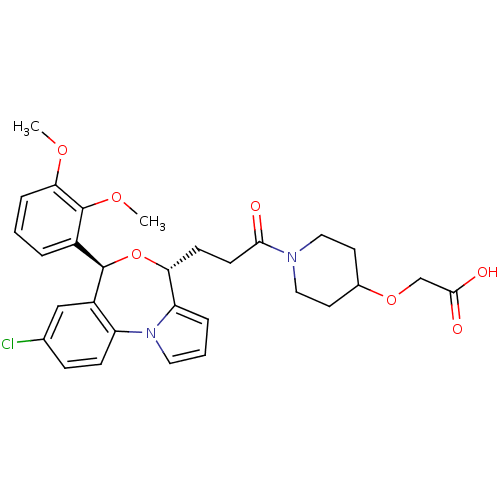 (CHEMBL2057631) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co. Ltd Curated by ChEMBL | Assay Description Inhibition of squalene synthase in rat hepatic cells | Bioorg Med Chem 20: 3072-93 (2012) Article DOI: 10.1016/j.bmc.2012.02.054 BindingDB Entry DOI: 10.7270/Q2B8595B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM532012 (US11208403, Example 89) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A kinase dilution solution containing 170 ng/ml AXL (the 464th to 885th amino acids of the intracellular domain of human AXL expressed as a fusion pr... | Citation and Details BindingDB Entry DOI: 10.7270/Q29026Z6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM531953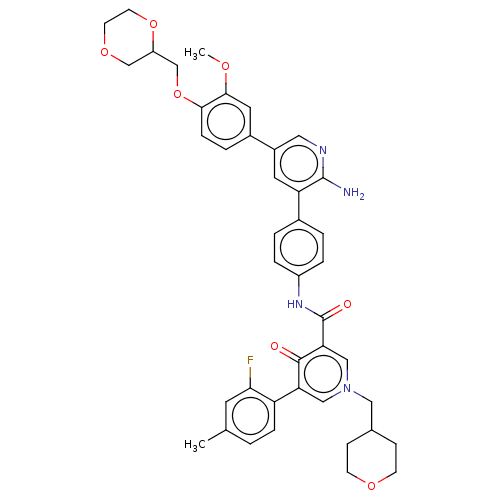 (US11208403, Example 30) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A kinase dilution solution containing 170 ng/ml AXL (the 464th to 885th amino acids of the intracellular domain of human AXL expressed as a fusion pr... | Citation and Details BindingDB Entry DOI: 10.7270/Q29026Z6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM531937 (US11208403, Example 14) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A kinase dilution solution containing 170 ng/ml AXL (the 464th to 885th amino acids of the intracellular domain of human AXL expressed as a fusion pr... | Citation and Details BindingDB Entry DOI: 10.7270/Q29026Z6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM532044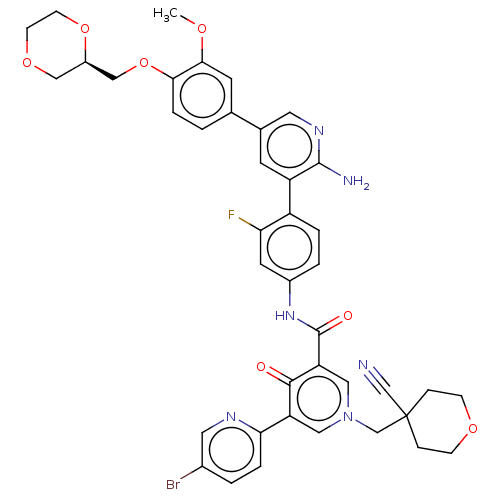 (N-[4-(2-Amino-5-{4-[(2R)-1,4-dioxan-2-ylmethoxy]-3...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A kinase dilution solution containing 170 ng/ml AXL (the 464th to 885th amino acids of the intracellular domain of human AXL expressed as a fusion pr... | Citation and Details BindingDB Entry DOI: 10.7270/Q29026Z6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50388377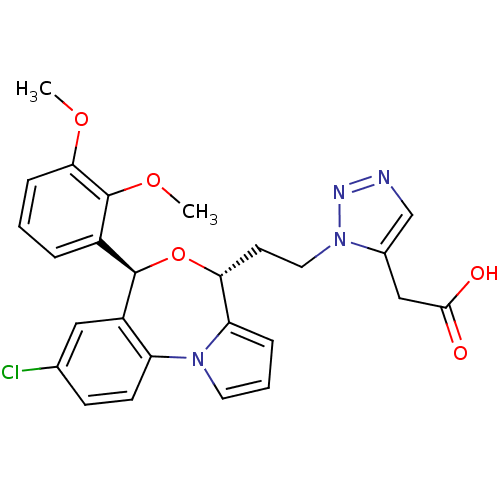 (CHEMBL2057638) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co. Ltd Curated by ChEMBL | Assay Description Inhibition of squalene synthase in rat hepatic cells | Bioorg Med Chem 20: 3072-93 (2012) Article DOI: 10.1016/j.bmc.2012.02.054 BindingDB Entry DOI: 10.7270/Q2B8595B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50442113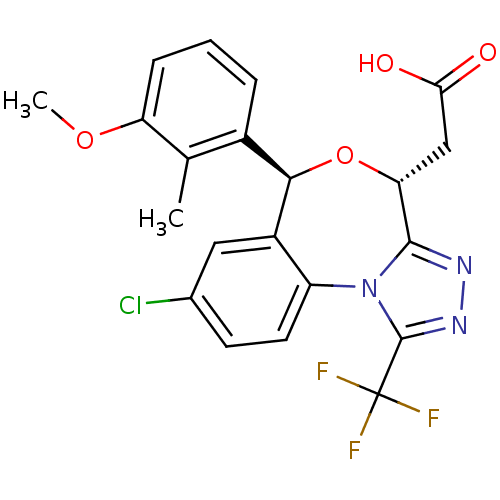 (CHEMBL2441083) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of rat hepatic microsomal squalene synthase using [3H]FPP as substrate after by scintillation spectrophotometry | ACS Med Chem Lett 4: 932-6 (2013) Article DOI: 10.1021/ml400151c BindingDB Entry DOI: 10.7270/Q2J67JCV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 475 total ) | Next | Last >> |