 Found 6989 hits with Last Name = 'nishimura' and Initial = 'n'
Found 6989 hits with Last Name = 'nishimura' and Initial = 'n' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50344099
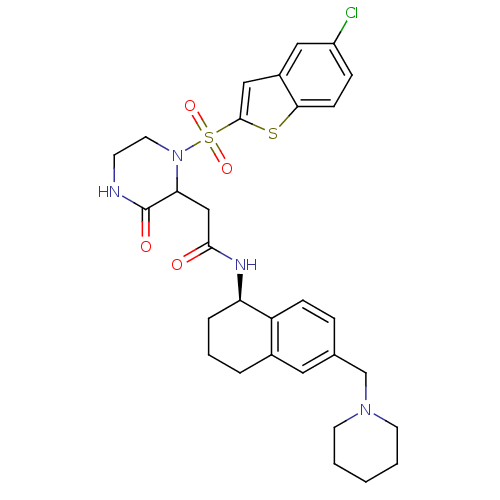
(2-(1-(5-chlorobenzo[b]thiophen-2-ylsulfonyl)-3-oxo...)Show SMILES Clc1ccc2sc(cc2c1)S(=O)(=O)N1CCNC(=O)C1CC(=O)N[C@@H]1CCCc2cc(CN3CCCCC3)ccc12 |r| Show InChI InChI=1S/C30H35ClN4O4S2/c31-23-8-10-27-22(16-23)17-29(40-27)41(38,39)35-14-11-32-30(37)26(35)18-28(36)33-25-6-4-5-21-15-20(7-9-24(21)25)19-34-12-2-1-3-13-34/h7-10,15-17,25-26H,1-6,11-14,18-19H2,(H,32,37)(H,33,36)/t25-,26?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human bradykinin B1 receptor |
Bioorg Med Chem Lett 21: 3384-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.115
BindingDB Entry DOI: 10.7270/Q2FX79R6 |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50344098
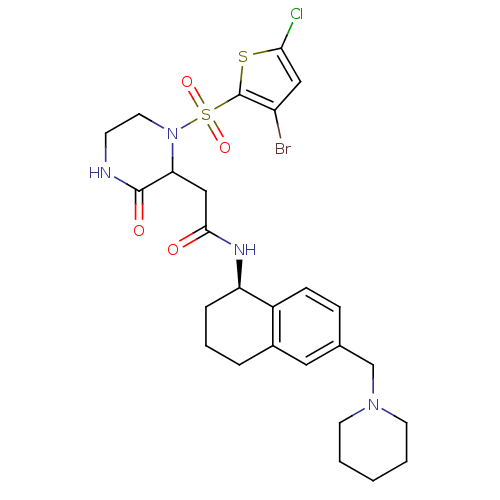
(2-(1-(3-bromo-5-chlorothiophen-2-ylsulfonyl)-3-oxo...)Show SMILES Clc1cc(Br)c(s1)S(=O)(=O)N1CCNC(=O)C1CC(=O)N[C@@H]1CCCc2cc(CN3CCCCC3)ccc12 |r| Show InChI InChI=1S/C26H32BrClN4O4S2/c27-20-14-23(28)37-26(20)38(35,36)32-12-9-29-25(34)22(32)15-24(33)30-21-6-4-5-18-13-17(7-8-19(18)21)16-31-10-2-1-3-11-31/h7-8,13-14,21-22H,1-6,9-12,15-16H2,(H,29,34)(H,30,33)/t21-,22?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human bradykinin B1 receptor |
Bioorg Med Chem Lett 21: 3384-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.115
BindingDB Entry DOI: 10.7270/Q2FX79R6 |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50272453
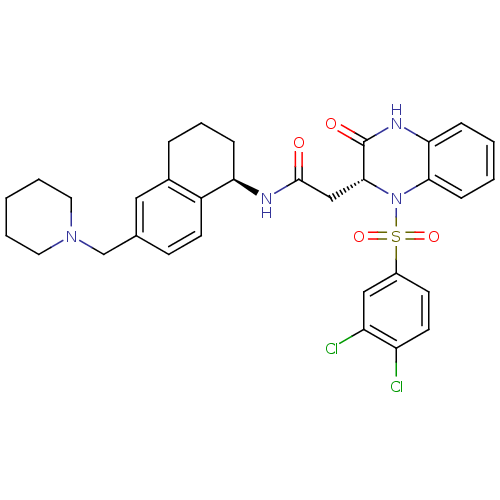
(2-((R)-1-(3,4-dichlorophenylsulfonyl)-3-oxo-1,2,3,...)Show SMILES Clc1ccc(cc1Cl)S(=O)(=O)N1[C@H](CC(=O)N[C@@H]2CCCc3cc(CN4CCCCC4)ccc23)C(=O)Nc2ccccc12 |r| Show InChI InChI=1S/C32H34Cl2N4O4S/c33-25-14-12-23(18-26(25)34)43(41,42)38-29-10-3-2-8-28(29)36-32(40)30(38)19-31(39)35-27-9-6-7-22-17-21(11-13-24(22)27)20-37-15-4-1-5-16-37/h2-3,8,10-14,17-18,27,30H,1,4-7,9,15-16,19-20H2,(H,35,39)(H,36,40)/t27-,30-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human bradykinin B1 receptor |
Bioorg Med Chem Lett 21: 3384-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.115
BindingDB Entry DOI: 10.7270/Q2FX79R6 |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50344111
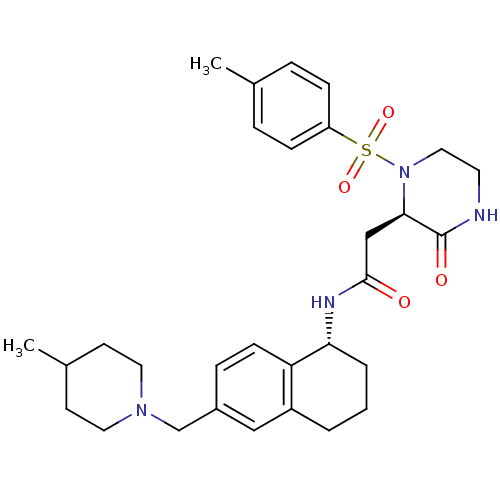
(CHEMBL1777969 | N-((R)-6-((4-methylpiperidin-1-yl)...)Show SMILES CC1CCN(Cc2ccc3[C@@H](CCCc3c2)NC(=O)C[C@H]2N(CCNC2=O)S(=O)(=O)c2ccc(C)cc2)CC1 |r| Show InChI InChI=1S/C30H40N4O4S/c1-21-6-9-25(10-7-21)39(37,38)34-17-14-31-30(36)28(34)19-29(35)32-27-5-3-4-24-18-23(8-11-26(24)27)20-33-15-12-22(2)13-16-33/h6-11,18,22,27-28H,3-5,12-17,19-20H2,1-2H3,(H,31,36)(H,32,35)/t27-,28-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human bradykinin B1 receptor |
Bioorg Med Chem Lett 21: 3384-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.115
BindingDB Entry DOI: 10.7270/Q2FX79R6 |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50344097
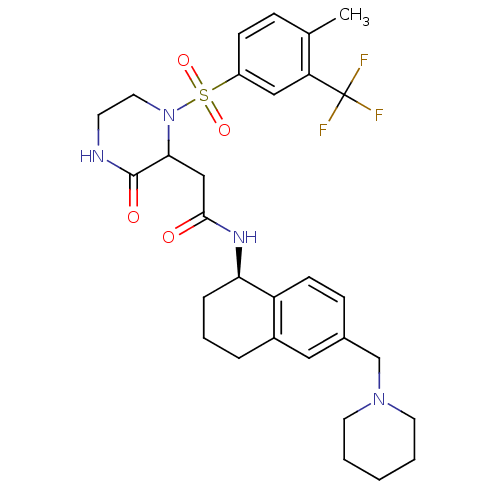
(2-(1-(4-methyl-3-(trifluoromethyl)phenylsulfonyl)-...)Show SMILES Cc1ccc(cc1C(F)(F)F)S(=O)(=O)N1CCNC(=O)C1CC(=O)N[C@@H]1CCCc2cc(CN3CCCCC3)ccc12 |r| Show InChI InChI=1S/C30H37F3N4O4S/c1-20-8-10-23(17-25(20)30(31,32)33)42(40,41)37-15-12-34-29(39)27(37)18-28(38)35-26-7-5-6-22-16-21(9-11-24(22)26)19-36-13-3-2-4-14-36/h8-11,16-17,26-27H,2-7,12-15,18-19H2,1H3,(H,34,39)(H,35,38)/t26-,27?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human bradykinin B1 receptor |
Bioorg Med Chem Lett 21: 3384-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.115
BindingDB Entry DOI: 10.7270/Q2FX79R6 |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50344096
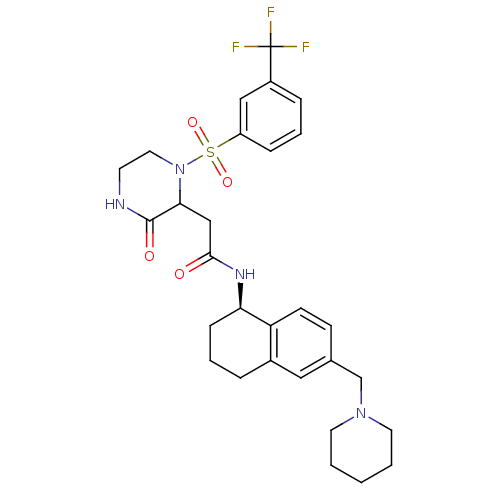
(2-(3-oxo-1-(3-(trifluoromethyl)phenylsulfonyl)pipe...)Show SMILES FC(F)(F)c1cccc(c1)S(=O)(=O)N1CCNC(=O)C1CC(=O)N[C@@H]1CCCc2cc(CN3CCCCC3)ccc12 |r| Show InChI InChI=1S/C29H35F3N4O4S/c30-29(31,32)22-7-5-8-23(17-22)41(39,40)36-15-12-33-28(38)26(36)18-27(37)34-25-9-4-6-21-16-20(10-11-24(21)25)19-35-13-2-1-3-14-35/h5,7-8,10-11,16-17,25-26H,1-4,6,9,12-15,18-19H2,(H,33,38)(H,34,37)/t25-,26?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human bradykinin B1 receptor |
Bioorg Med Chem Lett 21: 3384-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.115
BindingDB Entry DOI: 10.7270/Q2FX79R6 |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50344100
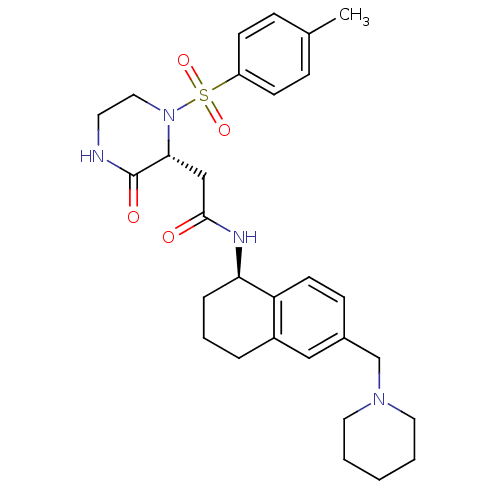
(2-((2R)-1-((4-methylphenyl)sulfonyl)-3-oxo-2-piper...)Show SMILES Cc1ccc(cc1)S(=O)(=O)N1CCNC(=O)[C@H]1CC(=O)N[C@@H]1CCCc2cc(CN3CCCCC3)ccc12 |r| Show InChI InChI=1S/C29H38N4O4S/c1-21-8-11-24(12-9-21)38(36,37)33-17-14-30-29(35)27(33)19-28(34)31-26-7-5-6-23-18-22(10-13-25(23)26)20-32-15-3-2-4-16-32/h8-13,18,26-27H,2-7,14-17,19-20H2,1H3,(H,30,35)(H,31,34)/t26-,27-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human bradykinin B1 receptor |
Bioorg Med Chem Lett 21: 3384-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.115
BindingDB Entry DOI: 10.7270/Q2FX79R6 |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50344087
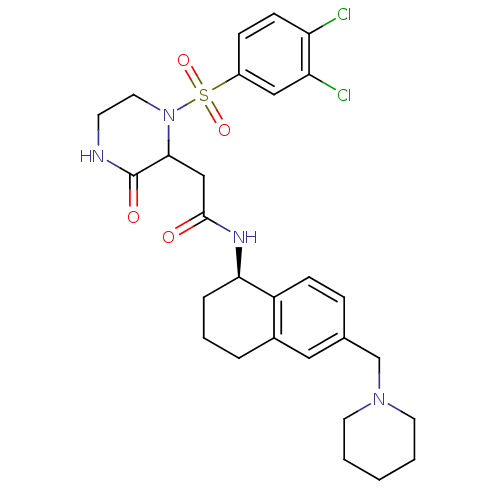
(2-(1-(3,4-dichlorophenylsulfonyl)-3-oxopiperazin-2...)Show SMILES Clc1ccc(cc1Cl)S(=O)(=O)N1CCNC(=O)C1CC(=O)N[C@@H]1CCCc2cc(CN3CCCCC3)ccc12 |r| Show InChI InChI=1S/C28H34Cl2N4O4S/c29-23-10-8-21(16-24(23)30)39(37,38)34-14-11-31-28(36)26(34)17-27(35)32-25-6-4-5-20-15-19(7-9-22(20)25)18-33-12-2-1-3-13-33/h7-10,15-16,25-26H,1-6,11-14,17-18H2,(H,31,36)(H,32,35)/t25-,26?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human bradykinin B1 receptor |
Bioorg Med Chem Lett 21: 3384-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.115
BindingDB Entry DOI: 10.7270/Q2FX79R6 |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50344110

(2-((R)-3-oxo-1-tosylpiperazin-2-yl)-N-((R)-7-(pipe...)Show SMILES Cc1ccc(cc1)S(=O)(=O)N1CCNC(=O)[C@H]1CC(=O)N[C@@H]1CCOc2cc(CN3CCCCC3)ccc12 |r| Show InChI InChI=1S/C28H36N4O5S/c1-20-5-8-22(9-6-20)38(35,36)32-15-12-29-28(34)25(32)18-27(33)30-24-11-16-37-26-17-21(7-10-23(24)26)19-31-13-3-2-4-14-31/h5-10,17,24-25H,2-4,11-16,18-19H2,1H3,(H,29,34)(H,30,33)/t24-,25-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human bradykinin B1 receptor |
Bioorg Med Chem Lett 21: 3384-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.115
BindingDB Entry DOI: 10.7270/Q2FX79R6 |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50344092
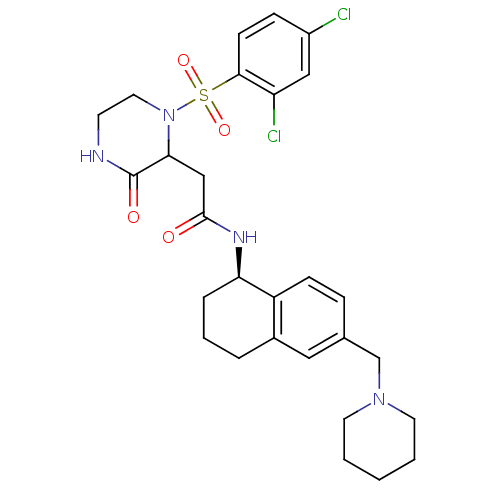
(2-(1-(2,4-dichlorophenylsulfonyl)-3-oxopiperazin-2...)Show SMILES Clc1ccc(c(Cl)c1)S(=O)(=O)N1CCNC(=O)C1CC(=O)N[C@@H]1CCCc2cc(CN3CCCCC3)ccc12 |r| Show InChI InChI=1S/C28H34Cl2N4O4S/c29-21-8-10-26(23(30)16-21)39(37,38)34-14-11-31-28(36)25(34)17-27(35)32-24-6-4-5-20-15-19(7-9-22(20)24)18-33-12-2-1-3-13-33/h7-10,15-16,24-25H,1-6,11-14,17-18H2,(H,31,36)(H,32,35)/t24-,25?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human bradykinin B1 receptor |
Bioorg Med Chem Lett 21: 3384-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.115
BindingDB Entry DOI: 10.7270/Q2FX79R6 |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50344119
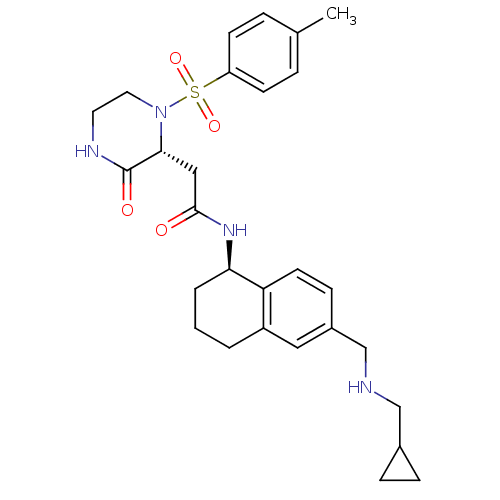
(CHEMBL1777977 | N-((R)-6-((cyclopropylmethylamino)...)Show SMILES Cc1ccc(cc1)S(=O)(=O)N1CCNC(=O)[C@H]1CC(=O)N[C@@H]1CCCc2cc(CNCC3CC3)ccc12 |r| Show InChI InChI=1S/C28H36N4O4S/c1-19-5-10-23(11-6-19)37(35,36)32-14-13-30-28(34)26(32)16-27(33)31-25-4-2-3-22-15-21(9-12-24(22)25)18-29-17-20-7-8-20/h5-6,9-12,15,20,25-26,29H,2-4,7-8,13-14,16-18H2,1H3,(H,30,34)(H,31,33)/t25-,26-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human bradykinin B1 receptor |
Bioorg Med Chem Lett 21: 3384-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.115
BindingDB Entry DOI: 10.7270/Q2FX79R6 |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50344120
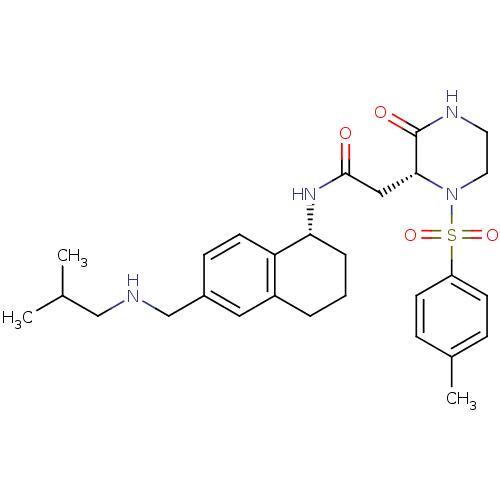
(CHEMBL1777978 | N-((R)-6-((isobutylamino)methyl)-1...)Show SMILES CC(C)CNCc1ccc2[C@@H](CCCc2c1)NC(=O)C[C@H]1N(CCNC1=O)S(=O)(=O)c1ccc(C)cc1 |r| Show InChI InChI=1S/C28H38N4O4S/c1-19(2)17-29-18-21-9-12-24-22(15-21)5-4-6-25(24)31-27(33)16-26-28(34)30-13-14-32(26)37(35,36)23-10-7-20(3)8-11-23/h7-12,15,19,25-26,29H,4-6,13-14,16-18H2,1-3H3,(H,30,34)(H,31,33)/t25-,26-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human bradykinin B1 receptor |
Bioorg Med Chem Lett 21: 3384-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.115
BindingDB Entry DOI: 10.7270/Q2FX79R6 |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50344093
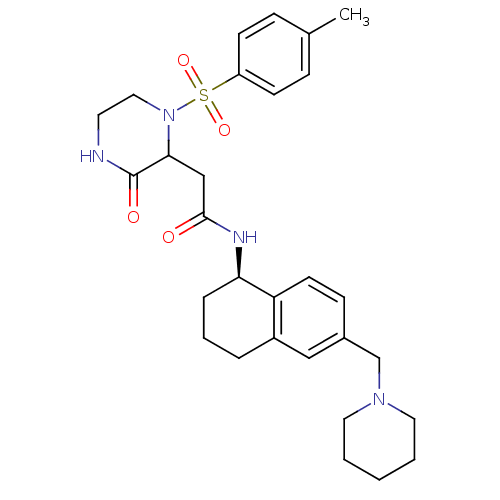
(2-(3-oxo-1-tosylpiperazin-2-yl)-N-((R)-6-(piperidi...)Show SMILES Cc1ccc(cc1)S(=O)(=O)N1CCNC(=O)C1CC(=O)N[C@@H]1CCCc2cc(CN3CCCCC3)ccc12 |r| Show InChI InChI=1S/C29H38N4O4S/c1-21-8-11-24(12-9-21)38(36,37)33-17-14-30-29(35)27(33)19-28(34)31-26-7-5-6-23-18-22(10-13-25(23)26)20-32-15-3-2-4-16-32/h8-13,18,26-27H,2-7,14-17,19-20H2,1H3,(H,30,35)(H,31,34)/t26-,27?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human bradykinin B1 receptor |
Bioorg Med Chem Lett 21: 3384-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.115
BindingDB Entry DOI: 10.7270/Q2FX79R6 |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50344109
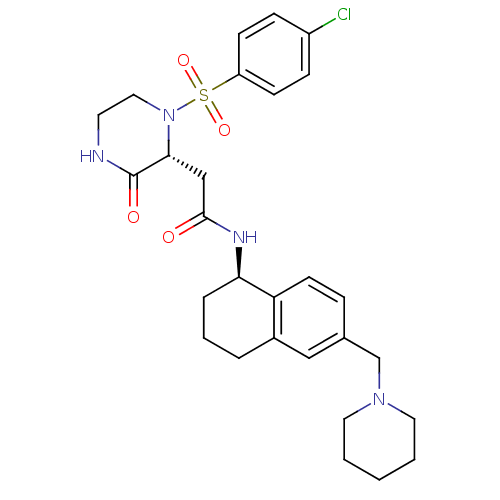
(2-((R)-1-(4-chlorophenylsulfonyl)-3-oxopiperazin-2...)Show SMILES Clc1ccc(cc1)S(=O)(=O)N1CCNC(=O)[C@H]1CC(=O)N[C@@H]1CCCc2cc(CN3CCCCC3)ccc12 |r| Show InChI InChI=1S/C28H35ClN4O4S/c29-22-8-10-23(11-9-22)38(36,37)33-16-13-30-28(35)26(33)18-27(34)31-25-6-4-5-21-17-20(7-12-24(21)25)19-32-14-2-1-3-15-32/h7-12,17,25-26H,1-6,13-16,18-19H2,(H,30,35)(H,31,34)/t25-,26-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human bradykinin B1 receptor |
Bioorg Med Chem Lett 21: 3384-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.115
BindingDB Entry DOI: 10.7270/Q2FX79R6 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50401261
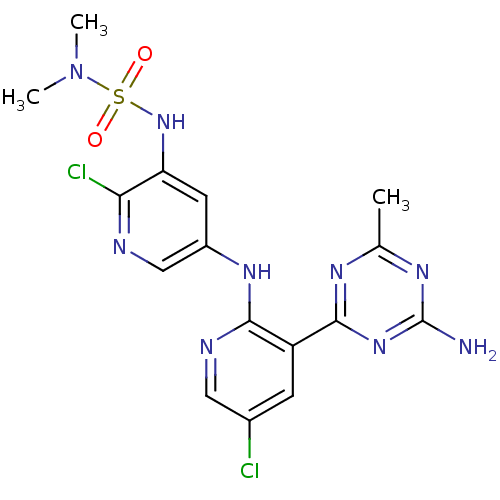
(CHEMBL2206921 | US8772480, 384)Show SMILES CN(C)S(=O)(=O)Nc1cc(Nc2ncc(Cl)cc2-c2nc(C)nc(N)n2)cnc1Cl Show InChI InChI=1S/C16H17Cl2N9O2S/c1-8-22-15(25-16(19)23-8)11-4-9(17)6-21-14(11)24-10-5-12(13(18)20-7-10)26-30(28,29)27(2)3/h4-7,26H,1-3H3,(H,21,24)(H2,19,22,23,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal polyHis-tagged PI3K p110delta expressed in baculovirus infected Hi5 cells using Phosphatidylinositol-4,5-bisphosphate ... |
Bioorg Med Chem Lett 22: 5714-20 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.078
BindingDB Entry DOI: 10.7270/Q2MG7QNW |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50209744
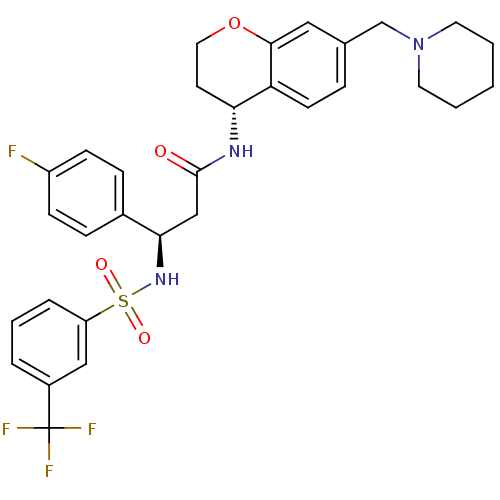
((R)-3-(4-fluorophenyl)-N-((R)-7-(piperidin-1-ylmet...)Show SMILES Fc1ccc(cc1)[C@@H](CC(=O)N[C@@H]1CCOc2cc(CN3CCCCC3)ccc12)NS(=O)(=O)c1cccc(c1)C(F)(F)F |r| Show InChI InChI=1S/C31H33F4N3O4S/c32-24-10-8-22(9-11-24)28(37-43(40,41)25-6-4-5-23(18-25)31(33,34)35)19-30(39)36-27-13-16-42-29-17-21(7-12-26(27)29)20-38-14-2-1-3-15-38/h4-12,17-18,27-28,37H,1-3,13-16,19-20H2,(H,36,39)/t27-,28-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human bradykinin B1 receptor |
Bioorg Med Chem Lett 21: 3384-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.115
BindingDB Entry DOI: 10.7270/Q2FX79R6 |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50344090
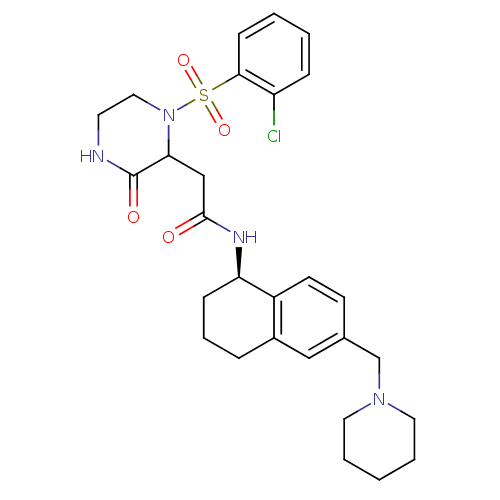
(2-(1-(2-chlorophenylsulfonyl)-3-oxopiperazin-2-yl)...)Show SMILES Clc1ccccc1S(=O)(=O)N1CCNC(=O)C1CC(=O)N[C@@H]1CCCc2cc(CN3CCCCC3)ccc12 |r| Show InChI InChI=1S/C28H35ClN4O4S/c29-23-8-2-3-10-26(23)38(36,37)33-16-13-30-28(35)25(33)18-27(34)31-24-9-6-7-21-17-20(11-12-22(21)24)19-32-14-4-1-5-15-32/h2-3,8,10-12,17,24-25H,1,4-7,9,13-16,18-19H2,(H,30,35)(H,31,34)/t24-,25?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human bradykinin B1 receptor |
Bioorg Med Chem Lett 21: 3384-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.115
BindingDB Entry DOI: 10.7270/Q2FX79R6 |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50344095
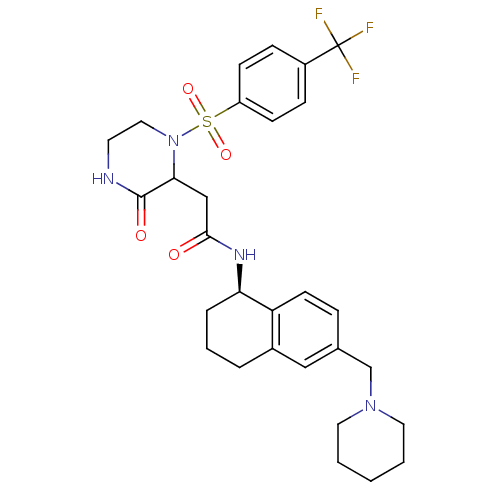
(2-(3-oxo-1-(4-(trifluoromethyl)phenylsulfonyl)pipe...)Show SMILES FC(F)(F)c1ccc(cc1)S(=O)(=O)N1CCNC(=O)C1CC(=O)N[C@@H]1CCCc2cc(CN3CCCCC3)ccc12 |r| Show InChI InChI=1S/C29H35F3N4O4S/c30-29(31,32)22-8-10-23(11-9-22)41(39,40)36-16-13-33-28(38)26(36)18-27(37)34-25-6-4-5-21-17-20(7-12-24(21)25)19-35-14-2-1-3-15-35/h7-12,17,25-26H,1-6,13-16,18-19H2,(H,33,38)(H,34,37)/t25-,26?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human bradykinin B1 receptor |
Bioorg Med Chem Lett 21: 3384-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.115
BindingDB Entry DOI: 10.7270/Q2FX79R6 |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50344108
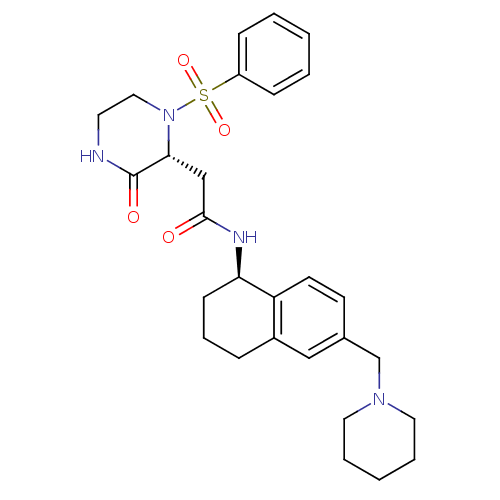
(2-((R)-3-oxo-1-(phenylsulfonyl)piperazin-2-yl)-N-(...)Show SMILES O=C(C[C@H]1N(CCNC1=O)S(=O)(=O)c1ccccc1)N[C@@H]1CCCc2cc(CN3CCCCC3)ccc12 |r| Show InChI InChI=1S/C28H36N4O4S/c33-27(19-26-28(34)29-14-17-32(26)37(35,36)23-9-3-1-4-10-23)30-25-11-7-8-22-18-21(12-13-24(22)25)20-31-15-5-2-6-16-31/h1,3-4,9-10,12-13,18,25-26H,2,5-8,11,14-17,19-20H2,(H,29,34)(H,30,33)/t25-,26-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human bradykinin B1 receptor |
Bioorg Med Chem Lett 21: 3384-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.115
BindingDB Entry DOI: 10.7270/Q2FX79R6 |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50344117
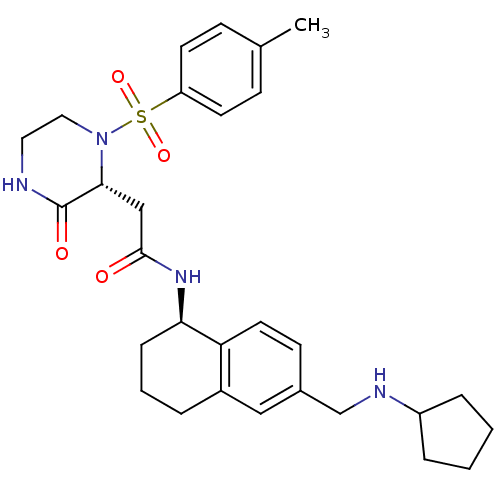
(CHEMBL1777975 | N-((R)-6-((cyclopentylamino)methyl...)Show SMILES Cc1ccc(cc1)S(=O)(=O)N1CCNC(=O)[C@H]1CC(=O)N[C@@H]1CCCc2cc(CNC3CCCC3)ccc12 |r| Show InChI InChI=1S/C29H38N4O4S/c1-20-9-12-24(13-10-20)38(36,37)33-16-15-30-29(35)27(33)18-28(34)32-26-8-4-5-22-17-21(11-14-25(22)26)19-31-23-6-2-3-7-23/h9-14,17,23,26-27,31H,2-8,15-16,18-19H2,1H3,(H,30,35)(H,32,34)/t26-,27-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human bradykinin B1 receptor |
Bioorg Med Chem Lett 21: 3384-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.115
BindingDB Entry DOI: 10.7270/Q2FX79R6 |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50344094
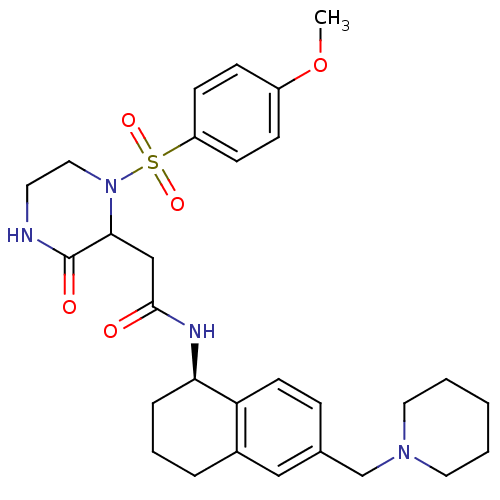
(2-(1-(4-methoxyphenylsulfonyl)-3-oxopiperazin-2-yl...)Show SMILES COc1ccc(cc1)S(=O)(=O)N1CCNC(=O)C1CC(=O)N[C@@H]1CCCc2cc(CN3CCCCC3)ccc12 |r| Show InChI InChI=1S/C29H38N4O5S/c1-38-23-9-11-24(12-10-23)39(36,37)33-17-14-30-29(35)27(33)19-28(34)31-26-7-5-6-22-18-21(8-13-25(22)26)20-32-15-3-2-4-16-32/h8-13,18,26-27H,2-7,14-17,19-20H2,1H3,(H,30,35)(H,31,34)/t26-,27?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human bradykinin B1 receptor |
Bioorg Med Chem Lett 21: 3384-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.115
BindingDB Entry DOI: 10.7270/Q2FX79R6 |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50344091
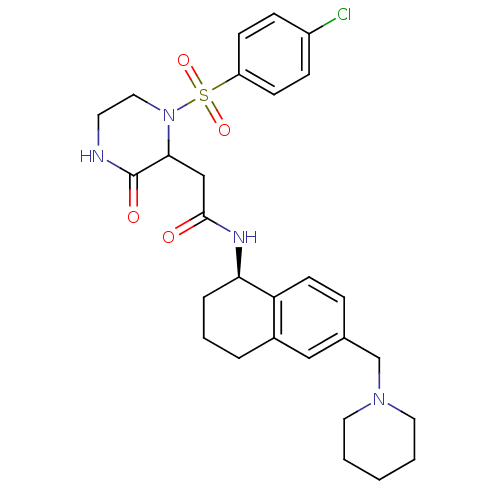
(2-(1-(4-chlorophenylsulfonyl)-3-oxopiperazin-2-yl)...)Show SMILES Clc1ccc(cc1)S(=O)(=O)N1CCNC(=O)C1CC(=O)N[C@@H]1CCCc2cc(CN3CCCCC3)ccc12 |r| Show InChI InChI=1S/C28H35ClN4O4S/c29-22-8-10-23(11-9-22)38(36,37)33-16-13-30-28(35)26(33)18-27(34)31-25-6-4-5-21-17-20(7-12-24(21)25)19-32-14-2-1-3-15-32/h7-12,17,25-26H,1-6,13-16,18-19H2,(H,30,35)(H,31,34)/t25-,26?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human bradykinin B1 receptor |
Bioorg Med Chem Lett 21: 3384-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.115
BindingDB Entry DOI: 10.7270/Q2FX79R6 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50401261

(CHEMBL2206921 | US8772480, 384)Show SMILES CN(C)S(=O)(=O)Nc1cc(Nc2ncc(Cl)cc2-c2nc(C)nc(N)n2)cnc1Cl Show InChI InChI=1S/C16H17Cl2N9O2S/c1-8-22-15(25-16(19)23-8)11-4-9(17)6-21-14(11)24-10-5-12(13(18)20-7-10)26-30(28,29)27(2)3/h4-7,26H,1-3H3,(H,21,24)(H2,19,22,23,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal polyHis-tagged PI3K p110beta expressed in baculovirus infected Hi5 cells using Phosphatidylinositol-4,5-bisphosphate a... |
Bioorg Med Chem Lett 22: 5714-20 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.078
BindingDB Entry DOI: 10.7270/Q2MG7QNW |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50357645
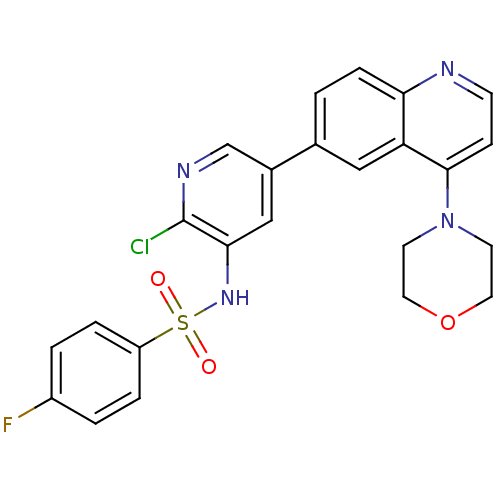
(CHEMBL1738719)Show SMILES Fc1ccc(cc1)S(=O)(=O)Nc1cc(cnc1Cl)-c1ccc2nccc(N3CCOCC3)c2c1 Show InChI InChI=1S/C24H20ClFN4O3S/c25-24-22(29-34(31,32)19-4-2-18(26)3-5-19)14-17(15-28-24)16-1-6-21-20(13-16)23(7-8-27-21)30-9-11-33-12-10-30/h1-8,13-15,29H,9-12H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminus polyHis-tagged human recombinant PI3Kalpha expressed in baculovirus infected insect Sf9 cells using phosphoinositol-4,5-bisp... |
J Med Chem 54: 4735-51 (2011)
Article DOI: 10.1021/jm200386s
BindingDB Entry DOI: 10.7270/Q2FB53C5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50357674
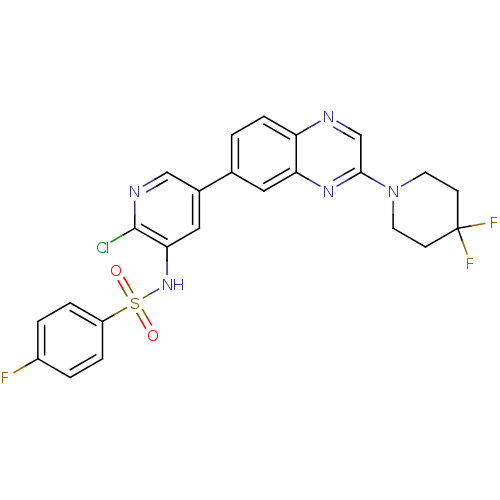
(CHEMBL1914739)Show SMILES Fc1ccc(cc1)S(=O)(=O)Nc1cc(cnc1Cl)-c1ccc2ncc(nc2c1)N1CCC(F)(F)CC1 Show InChI InChI=1S/C24H19ClF3N5O2S/c25-23-21(32-36(34,35)18-4-2-17(26)3-5-18)12-16(13-30-23)15-1-6-19-20(11-15)31-22(14-29-19)33-9-7-24(27,28)8-10-33/h1-6,11-14,32H,7-10H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminus polyHis-tagged human recombinant PI3Kalpha expressed in baculovirus infected insect Sf9 cells using phosphoinositol-4,5-bisp... |
J Med Chem 54: 4735-51 (2011)
Article DOI: 10.1021/jm200386s
BindingDB Entry DOI: 10.7270/Q2FB53C5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50357665

(CHEMBL1914728)Show SMILES CN(C)c1ccc(cc1)-c1ccnc2ccc(cc12)-c1cnc(Cl)c(NS(=O)(=O)c2ccc(F)cc2)c1 Show InChI InChI=1S/C28H22ClFN4O2S/c1-34(2)22-8-3-18(4-9-22)24-13-14-31-26-12-5-19(15-25(24)26)20-16-27(28(29)32-17-20)33-37(35,36)23-10-6-21(30)7-11-23/h3-17,33H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminus polyHis-tagged human recombinant PI3Kalpha expressed in baculovirus infected insect Sf9 cells using phosphoinositol-4,5-bisp... |
J Med Chem 54: 4735-51 (2011)
Article DOI: 10.1021/jm200386s
BindingDB Entry DOI: 10.7270/Q2FB53C5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50357652
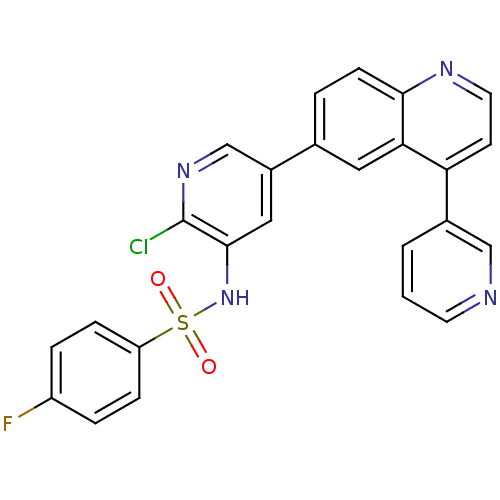
(CHEMBL1914726)Show SMILES Fc1ccc(cc1)S(=O)(=O)Nc1cc(cnc1Cl)-c1ccc2nccc(-c3cccnc3)c2c1 Show InChI InChI=1S/C25H16ClFN4O2S/c26-25-24(31-34(32,33)20-6-4-19(27)5-7-20)13-18(15-30-25)16-3-8-23-22(12-16)21(9-11-29-23)17-2-1-10-28-14-17/h1-15,31H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminus polyHis-tagged human recombinant PI3Kalpha expressed in baculovirus infected insect Sf9 cells using phosphoinositol-4,5-bisp... |
J Med Chem 54: 4735-51 (2011)
Article DOI: 10.1021/jm200386s
BindingDB Entry DOI: 10.7270/Q2FB53C5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50357650
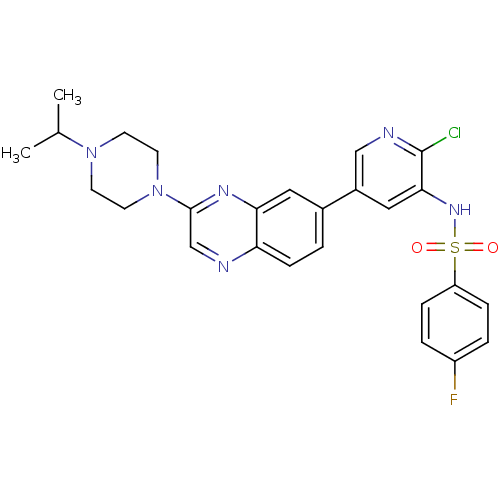
(CHEMBL1914742)Show SMILES CC(C)N1CCN(CC1)c1cnc2ccc(cc2n1)-c1cnc(Cl)c(NS(=O)(=O)c2ccc(F)cc2)c1 Show InChI InChI=1S/C26H26ClFN6O2S/c1-17(2)33-9-11-34(12-10-33)25-16-29-22-8-3-18(13-23(22)31-25)19-14-24(26(27)30-15-19)32-37(35,36)21-6-4-20(28)5-7-21/h3-8,13-17,32H,9-12H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminus polyHis-tagged human recombinant PI3Kalpha expressed in baculovirus infected insect Sf9 cells using phosphoinositol-4,5-bisp... |
J Med Chem 54: 4735-51 (2011)
Article DOI: 10.1021/jm200386s
BindingDB Entry DOI: 10.7270/Q2FB53C5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50357653
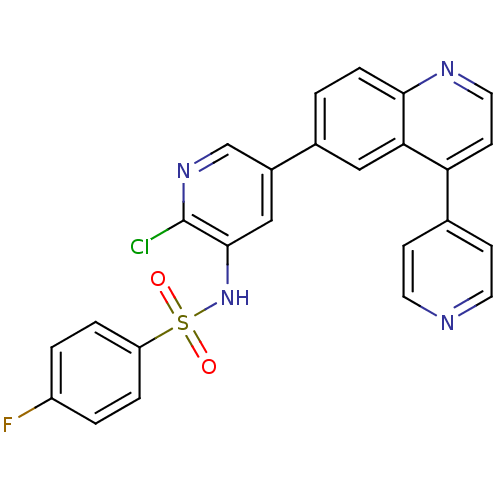
(CHEMBL1914727)Show SMILES Fc1ccc(cc1)S(=O)(=O)Nc1cc(cnc1Cl)-c1ccc2nccc(-c3ccncc3)c2c1 Show InChI InChI=1S/C25H16ClFN4O2S/c26-25-24(31-34(32,33)20-4-2-19(27)3-5-20)14-18(15-30-25)17-1-6-23-22(13-17)21(9-12-29-23)16-7-10-28-11-8-16/h1-15,31H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminus polyHis-tagged human recombinant PI3Kalpha expressed in baculovirus infected insect Sf9 cells using phosphoinositol-4,5-bisp... |
J Med Chem 54: 4735-51 (2011)
Article DOI: 10.1021/jm200386s
BindingDB Entry DOI: 10.7270/Q2FB53C5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50357668
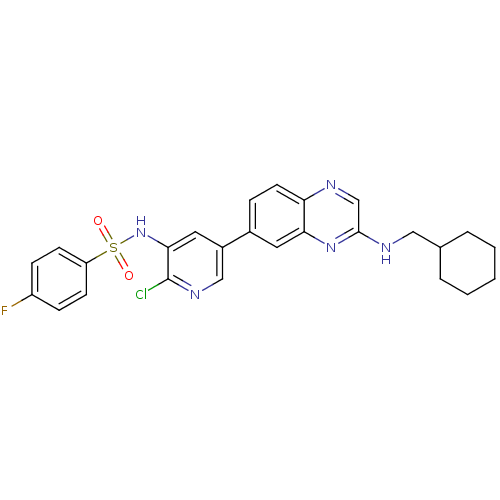
(CHEMBL1914731)Show SMILES Fc1ccc(cc1)S(=O)(=O)Nc1cc(cnc1Cl)-c1ccc2ncc(NCC3CCCCC3)nc2c1 Show InChI InChI=1S/C26H25ClFN5O2S/c27-26-24(33-36(34,35)21-9-7-20(28)8-10-21)13-19(15-31-26)18-6-11-22-23(12-18)32-25(16-29-22)30-14-17-4-2-1-3-5-17/h6-13,15-17,33H,1-5,14H2,(H,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminus polyHis-tagged human recombinant PI3Kalpha expressed in baculovirus infected insect Sf9 cells using phosphoinositol-4,5-bisp... |
J Med Chem 54: 4735-51 (2011)
Article DOI: 10.1021/jm200386s
BindingDB Entry DOI: 10.7270/Q2FB53C5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50357670
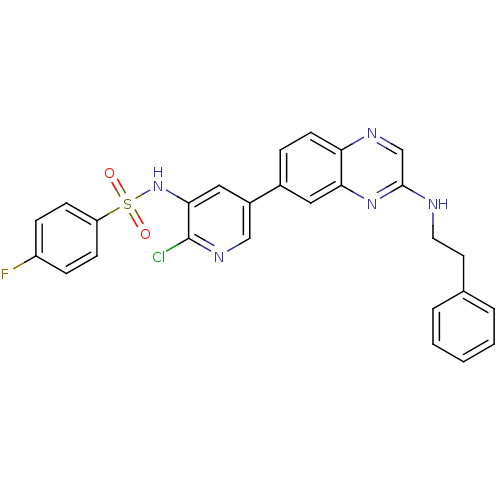
(CHEMBL1914734)Show SMILES Fc1ccc(cc1)S(=O)(=O)Nc1cc(cnc1Cl)-c1ccc2ncc(NCCc3ccccc3)nc2c1 Show InChI InChI=1S/C27H21ClFN5O2S/c28-27-25(34-37(35,36)22-9-7-21(29)8-10-22)15-20(16-32-27)19-6-11-23-24(14-19)33-26(17-31-23)30-13-12-18-4-2-1-3-5-18/h1-11,14-17,34H,12-13H2,(H,30,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminus polyHis-tagged human recombinant PI3Kalpha expressed in baculovirus infected insect Sf9 cells using phosphoinositol-4,5-bisp... |
J Med Chem 54: 4735-51 (2011)
Article DOI: 10.1021/jm200386s
BindingDB Entry DOI: 10.7270/Q2FB53C5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50357666
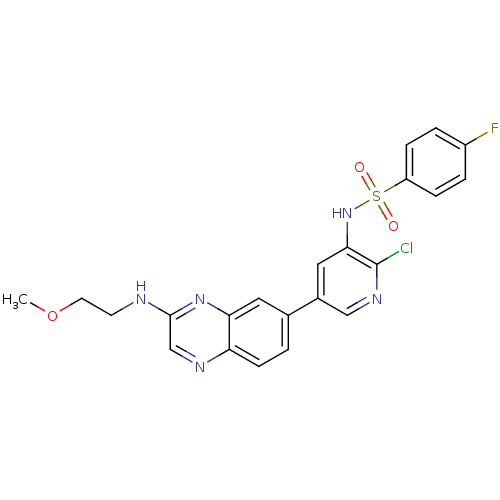
(CHEMBL1914729)Show SMILES COCCNc1cnc2ccc(cc2n1)-c1cnc(Cl)c(NS(=O)(=O)c2ccc(F)cc2)c1 Show InChI InChI=1S/C22H19ClFN5O3S/c1-32-9-8-25-21-13-26-18-7-2-14(10-19(18)28-21)15-11-20(22(23)27-12-15)29-33(30,31)17-5-3-16(24)4-6-17/h2-7,10-13,29H,8-9H2,1H3,(H,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminus polyHis-tagged human recombinant PI3Kalpha expressed in baculovirus infected insect Sf9 cells using phosphoinositol-4,5-bisp... |
J Med Chem 54: 4735-51 (2011)
Article DOI: 10.1021/jm200386s
BindingDB Entry DOI: 10.7270/Q2FB53C5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50357662
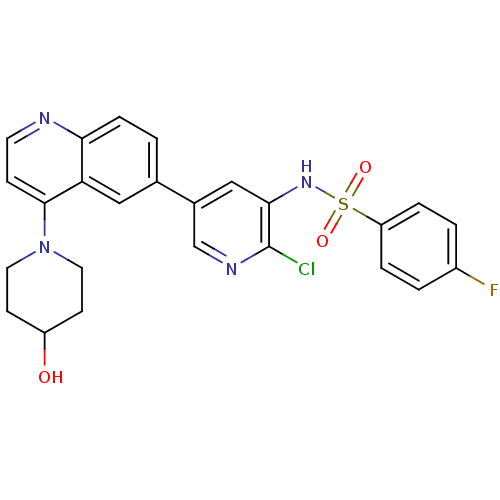
(CHEMBL1914721)Show SMILES OC1CCN(CC1)c1ccnc2ccc(cc12)-c1cnc(Cl)c(NS(=O)(=O)c2ccc(F)cc2)c1 Show InChI InChI=1S/C25H22ClFN4O3S/c26-25-23(30-35(33,34)20-4-2-18(27)3-5-20)14-17(15-29-25)16-1-6-22-21(13-16)24(7-10-28-22)31-11-8-19(32)9-12-31/h1-7,10,13-15,19,30,32H,8-9,11-12H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha-mediated Akt phosphorylation at Ser 473 in human U87MG cells |
J Med Chem 54: 4735-51 (2011)
Article DOI: 10.1021/jm200386s
BindingDB Entry DOI: 10.7270/Q2FB53C5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50357651
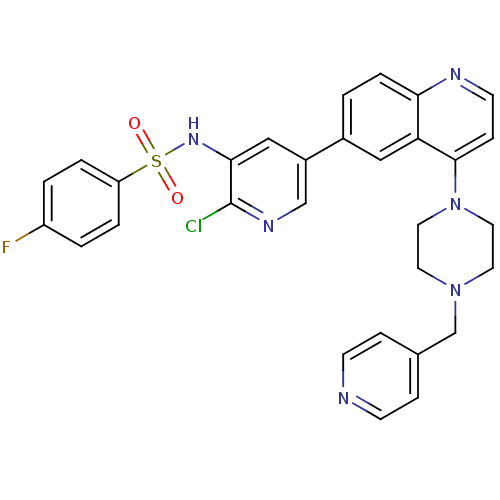
(CHEMBL1914724)Show SMILES Fc1ccc(cc1)S(=O)(=O)Nc1cc(cnc1Cl)-c1ccc2nccc(N3CCN(Cc4ccncc4)CC3)c2c1 Show InChI InChI=1S/C30H26ClFN6O2S/c31-30-28(36-41(39,40)25-4-2-24(32)3-5-25)18-23(19-35-30)22-1-6-27-26(17-22)29(9-12-34-27)38-15-13-37(14-16-38)20-21-7-10-33-11-8-21/h1-12,17-19,36H,13-16,20H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminus polyHis-tagged human recombinant PI3Kalpha expressed in baculovirus infected insect Sf9 cells using phosphoinositol-4,5-bisp... |
J Med Chem 54: 4735-51 (2011)
Article DOI: 10.1021/jm200386s
BindingDB Entry DOI: 10.7270/Q2FB53C5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50357673
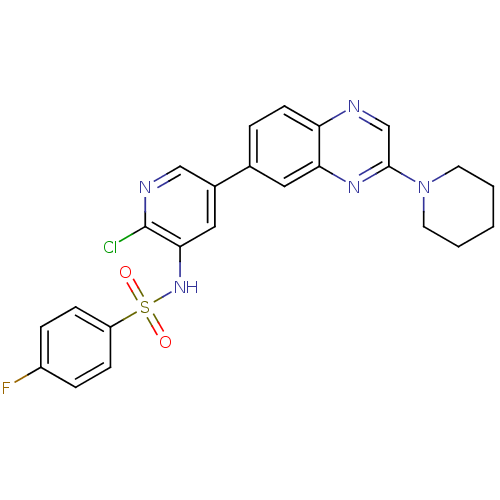
(CHEMBL1914738)Show SMILES Fc1ccc(cc1)S(=O)(=O)Nc1cc(cnc1Cl)-c1ccc2ncc(nc2c1)N1CCCCC1 Show InChI InChI=1S/C24H21ClFN5O2S/c25-24-22(30-34(32,33)19-7-5-18(26)6-8-19)13-17(14-28-24)16-4-9-20-21(12-16)29-23(15-27-20)31-10-2-1-3-11-31/h4-9,12-15,30H,1-3,10-11H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminus polyHis-tagged human recombinant PI3Kalpha expressed in baculovirus infected insect Sf9 cells using phosphoinositol-4,5-bisp... |
J Med Chem 54: 4735-51 (2011)
Article DOI: 10.1021/jm200386s
BindingDB Entry DOI: 10.7270/Q2FB53C5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM122451
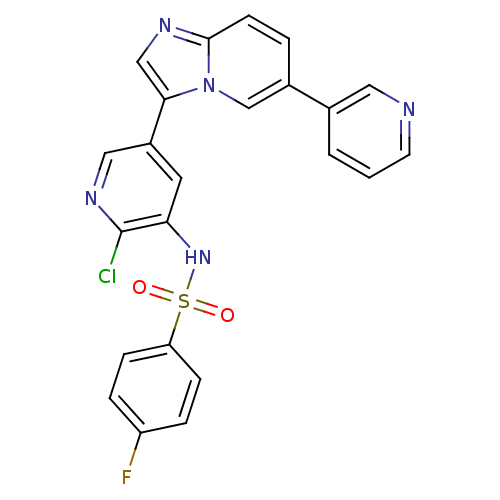
(US8729074, 8)Show SMILES Fc1ccc(cc1)S(=O)(=O)Nc1cc(cnc1Cl)-c1cnc2ccc(cn12)-c1cccnc1 Show InChI InChI=1S/C23H15ClFN5O2S/c24-23-20(29-33(31,32)19-6-4-18(25)5-7-19)10-17(12-28-23)21-13-27-22-8-3-16(14-30(21)22)15-2-1-9-26-11-15/h1-14,29H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1 | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
US Patent
| Assay Description
The PI3K AlphaScreen assay (PerkinElmer, Waltham, Mass.) measures the activity of a panel of four phosphoinositide 3-kinases: PI3Kα, PI3Kβ,... |
US Patent US8729074 (2014)
BindingDB Entry DOI: 10.7270/Q2RJ4H4G |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50357675
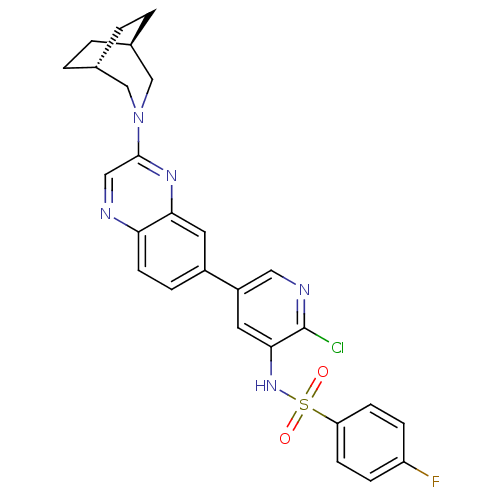
(CHEMBL1914740)Show SMILES Fc1ccc(cc1)S(=O)(=O)Nc1cc(cnc1Cl)-c1ccc2ncc(nc2c1)N1C[C@H]2CC[C@H](CC2)C1 |r,wD:30.39,33.37,(27.04,-36.48,;27.04,-38.02,;28.38,-38.79,;28.39,-40.33,;27.05,-41.09,;25.72,-40.34,;25.71,-38.8,;27.05,-42.63,;25.96,-43.72,;25.56,-42.23,;28.39,-43.4,;28.4,-44.94,;29.73,-45.71,;29.74,-47.26,;28.4,-48.03,;27.06,-47.26,;27.07,-45.71,;25.73,-44.94,;31.07,-48.03,;31.07,-49.57,;32.4,-50.34,;33.74,-49.57,;35.08,-50.34,;36.43,-49.56,;36.42,-47.99,;35.07,-47.23,;33.73,-48.01,;32.4,-47.25,;37.75,-47.21,;37.63,-45.67,;38.78,-44.64,;38.36,-46.12,;39.13,-47.45,;40.5,-47.6,;41.08,-46.19,;40.36,-44.9,;39.08,-48.09,)| Show InChI InChI=1S/C27H25ClFN5O2S/c28-27-25(33-37(35,36)22-8-6-21(29)7-9-22)12-20(13-31-27)19-5-10-23-24(11-19)32-26(14-30-23)34-15-17-1-2-18(16-34)4-3-17/h5-14,17-18,33H,1-4,15-16H2/t17-,18+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminus polyHis-tagged human recombinant PI3Kalpha expressed in baculovirus infected insect Sf9 cells using phosphoinositol-4,5-bisp... |
J Med Chem 54: 4735-51 (2011)
Article DOI: 10.1021/jm200386s
BindingDB Entry DOI: 10.7270/Q2FB53C5 |
More data for this
Ligand-Target Pair | |
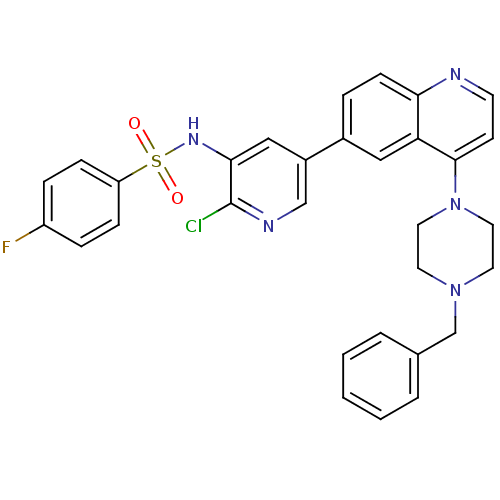
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50357664
(CHEMBL1914723)Show SMILES Fc1ccc(cc1)S(=O)(=O)Nc1cc(cnc1Cl)-c1ccc2nccc(N3CCN(Cc4ccccc4)CC3)c2c1 Show InChI InChI=1S/C31H27ClFN5O2S/c32-31-29(36-41(39,40)26-9-7-25(33)8-10-26)19-24(20-35-31)23-6-11-28-27(18-23)30(12-13-34-28)38-16-14-37(15-17-38)21-22-4-2-1-3-5-22/h1-13,18-20,36H,14-17,21H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha-mediated Akt phosphorylation at Ser 473 in human U87MG cells |
J Med Chem 54: 4735-51 (2011)
Article DOI: 10.1021/jm200386s
BindingDB Entry DOI: 10.7270/Q2FB53C5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM122459
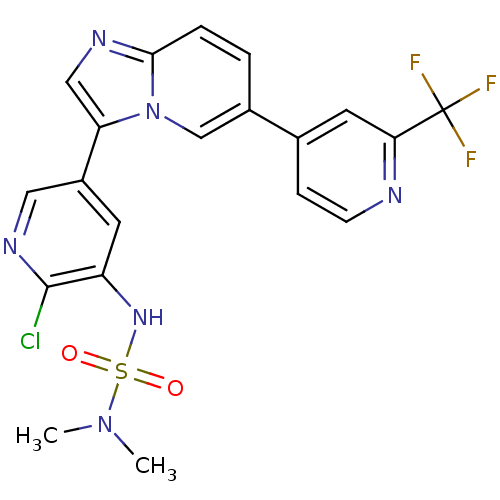
(US8729074, 19)Show SMILES CN(C)S(=O)(=O)Nc1cc(cnc1Cl)-c1cnc2ccc(cn12)-c1ccnc(c1)C(F)(F)F Show InChI InChI=1S/C20H16ClF3N6O2S/c1-29(2)33(31,32)28-15-7-14(9-27-19(15)21)16-10-26-18-4-3-13(11-30(16)18)12-5-6-25-17(8-12)20(22,23)24/h3-11,28H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
US Patent
| Assay Description
The PI3K AlphaScreen assay (PerkinElmer, Waltham, Mass.) measures the activity of a panel of four phosphoinositide 3-kinases: PI3Kα, PI3Kβ,... |
US Patent US8729074 (2014)
BindingDB Entry DOI: 10.7270/Q2RJ4H4G |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50401261

(CHEMBL2206921 | US8772480, 384)Show SMILES CN(C)S(=O)(=O)Nc1cc(Nc2ncc(Cl)cc2-c2nc(C)nc(N)n2)cnc1Cl Show InChI InChI=1S/C16H17Cl2N9O2S/c1-8-22-15(25-16(19)23-8)11-4-9(17)6-21-14(11)24-10-5-12(13(18)20-7-10)26-30(28,29)27(2)3/h4-7,26H,1-3H3,(H,21,24)(H2,19,22,23,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal polyHis-tagged PI3K p110gamma expressed in baculovirus infected Hi5 cells using Phosphatidylinositol-4,5-bisphosphate ... |
Bioorg Med Chem Lett 22: 5714-20 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.078
BindingDB Entry DOI: 10.7270/Q2MG7QNW |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM122468
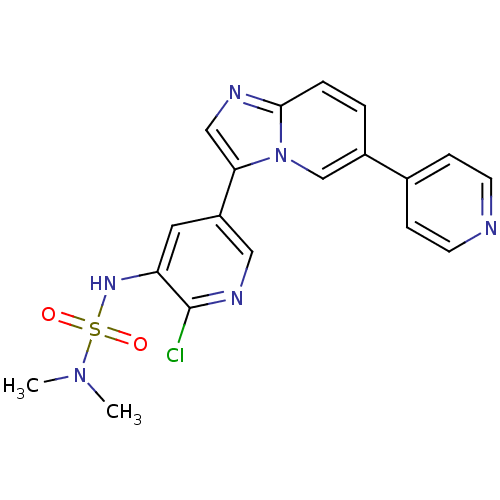
(US8729074, 18)Show SMILES CN(C)S(=O)(=O)Nc1cc(cnc1Cl)-c1cnc2ccc(cn12)-c1ccncc1 Show InChI InChI=1S/C19H17ClN6O2S/c1-25(2)29(27,28)24-16-9-15(10-23-19(16)20)17-11-22-18-4-3-14(12-26(17)18)13-5-7-21-8-6-13/h3-12,24H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
US Patent
| Assay Description
The PI3K AlphaScreen assay (PerkinElmer, Waltham, Mass.) measures the activity of a panel of four phosphoinositide 3-kinases: PI3Kα, PI3Kβ,... |
US Patent US8729074 (2014)
BindingDB Entry DOI: 10.7270/Q2RJ4H4G |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50394838
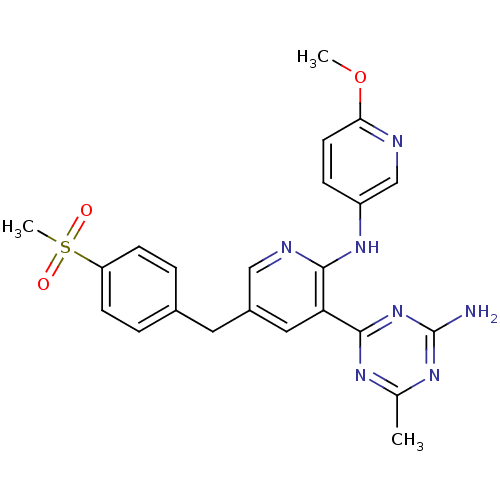
(CHEMBL2165007 | US8772480, 141)Show SMILES COc1ccc(Nc2ncc(Cc3ccc(cc3)S(C)(=O)=O)cc2-c2nc(C)nc(N)n2)cn1 Show InChI InChI=1S/C23H23N7O3S/c1-14-27-22(30-23(24)28-14)19-11-16(10-15-4-7-18(8-5-15)34(3,31)32)12-26-21(19)29-17-6-9-20(33-2)25-13-17/h4-9,11-13H,10H2,1-3H3,(H,26,29)(H2,24,27,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal poly-His tagged-PI3Kdelta expressed in Sf9 baculovirus system co-expressing p85alpha after 20 mins by AlphaScreen assa... |
J Med Chem 55: 5188-219 (2012)
Article DOI: 10.1021/jm300184s
BindingDB Entry DOI: 10.7270/Q23F4QSJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50396809
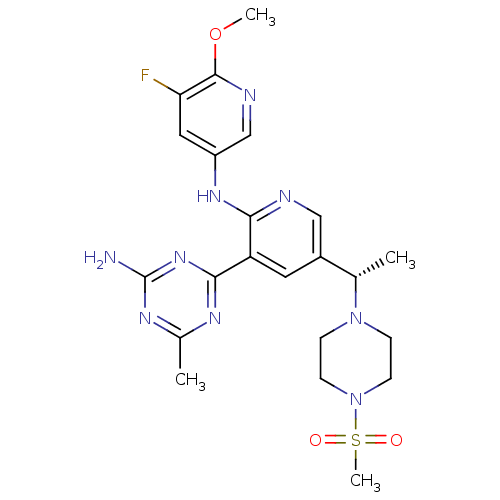
(CHEMBL2170099 | US8772480, 147)Show SMILES COc1ncc(Nc2ncc(cc2-c2nc(C)nc(N)n2)[C@H](C)N2CCN(CC2)S(C)(=O)=O)cc1F |r| Show InChI InChI=1S/C22H28FN9O3S/c1-13(31-5-7-32(8-6-31)36(4,33)34)15-9-17(20-27-14(2)28-22(24)30-20)19(25-11-15)29-16-10-18(23)21(35-3)26-12-16/h9-13H,5-8H2,1-4H3,(H,25,29)(H2,24,27,28,30)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal polyHis-tagged PI3K p110delta/p85alpha expressed in baculovirus infected Sf9 cells using phosphatidylinositol-4,5-bisp... |
J Med Chem 55: 7796-816 (2012)
Article DOI: 10.1021/jm300846z
BindingDB Entry DOI: 10.7270/Q2028SPT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50396807
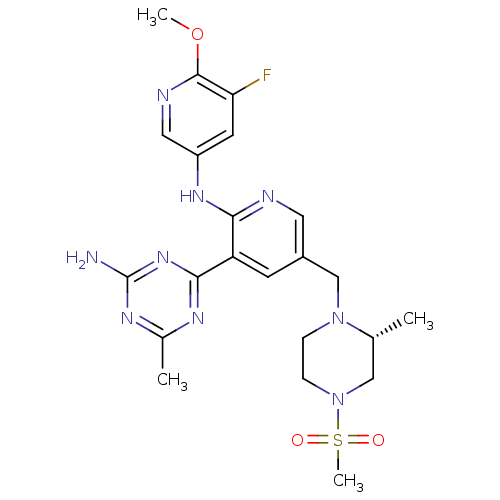
(CHEMBL2170082 | US8772480, 316)Show SMILES COc1ncc(Nc2ncc(CN3CCN(C[C@H]3C)S(C)(=O)=O)cc2-c2nc(C)nc(N)n2)cc1F |r| Show InChI InChI=1S/C22H28FN9O3S/c1-13-11-32(36(4,33)34)6-5-31(13)12-15-7-17(20-27-14(2)28-22(24)30-20)19(25-9-15)29-16-8-18(23)21(35-3)26-10-16/h7-10,13H,5-6,11-12H2,1-4H3,(H,25,29)(H2,24,27,28,30)/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal polyHis-tagged PI3K p110delta/p85alpha expressed in baculovirus infected Sf9 cells using phosphatidylinositol-4,5-bisp... |
J Med Chem 55: 7796-816 (2012)
Article DOI: 10.1021/jm300846z
BindingDB Entry DOI: 10.7270/Q2028SPT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50396806
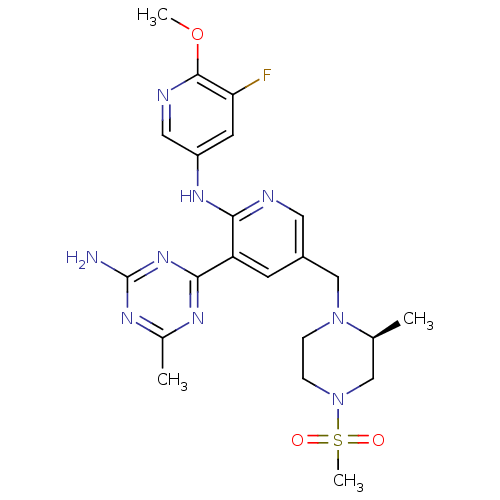
(CHEMBL2170083 | US8772480, 270)Show SMILES COc1ncc(Nc2ncc(CN3CCN(C[C@@H]3C)S(C)(=O)=O)cc2-c2nc(C)nc(N)n2)cc1F |r| Show InChI InChI=1S/C22H28FN9O3S/c1-13-11-32(36(4,33)34)6-5-31(13)12-15-7-17(20-27-14(2)28-22(24)30-20)19(25-9-15)29-16-8-18(23)21(35-3)26-10-16/h7-10,13H,5-6,11-12H2,1-4H3,(H,25,29)(H2,24,27,28,30)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal polyHis-tagged PI3K p110delta/p85alpha expressed in baculovirus infected Sf9 cells using phosphatidylinositol-4,5-bisp... |
J Med Chem 55: 7796-816 (2012)
Article DOI: 10.1021/jm300846z
BindingDB Entry DOI: 10.7270/Q2028SPT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50396820
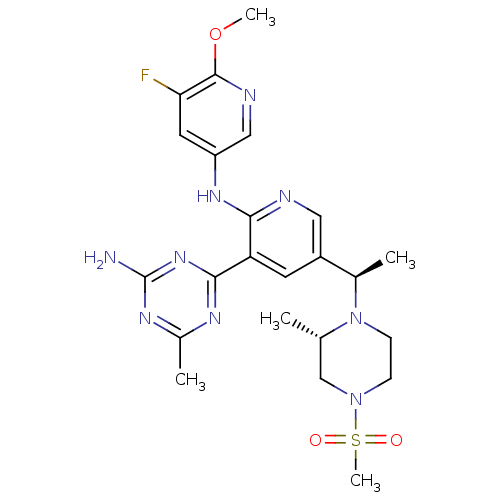
(CHEMBL2170088 | US8772480, 272)Show SMILES COc1ncc(Nc2ncc(cc2-c2nc(C)nc(N)n2)[C@@H](C)N2CCN(C[C@@H]2C)S(C)(=O)=O)cc1F |r| Show InChI InChI=1S/C23H30FN9O3S/c1-13-12-32(37(5,34)35)6-7-33(13)14(2)16-8-18(21-28-15(3)29-23(25)31-21)20(26-10-16)30-17-9-19(24)22(36-4)27-11-17/h8-11,13-14H,6-7,12H2,1-5H3,(H,26,30)(H2,25,28,29,31)/t13-,14+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal polyHis-tagged PI3K p110alpha/p85alpha expressed in baculovirus infected Sf9 cells using phosphatidylinositol-4,5-bisp... |
J Med Chem 55: 7796-816 (2012)
Article DOI: 10.1021/jm300846z
BindingDB Entry DOI: 10.7270/Q2028SPT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50396808
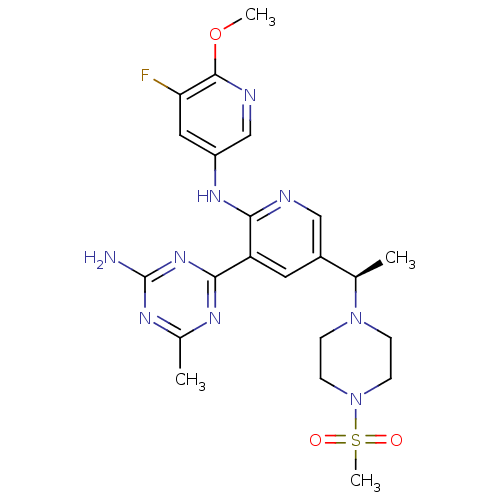
(CHEMBL2170081 | US8772480, 148)Show SMILES COc1ncc(Nc2ncc(cc2-c2nc(C)nc(N)n2)[C@@H](C)N2CCN(CC2)S(C)(=O)=O)cc1F |r| Show InChI InChI=1S/C22H28FN9O3S/c1-13(31-5-7-32(8-6-31)36(4,33)34)15-9-17(20-27-14(2)28-22(24)30-20)19(25-11-15)29-16-10-18(23)21(35-3)26-12-16/h9-13H,5-8H2,1-4H3,(H,25,29)(H2,24,27,28,30)/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal polyHis-tagged PI3K p110gamma expressed in baculovirus infected Hi5 cells using ATP as substrate after 20 mins by spec... |
J Med Chem 55: 7796-816 (2012)
Article DOI: 10.1021/jm300846z
BindingDB Entry DOI: 10.7270/Q2028SPT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50357667
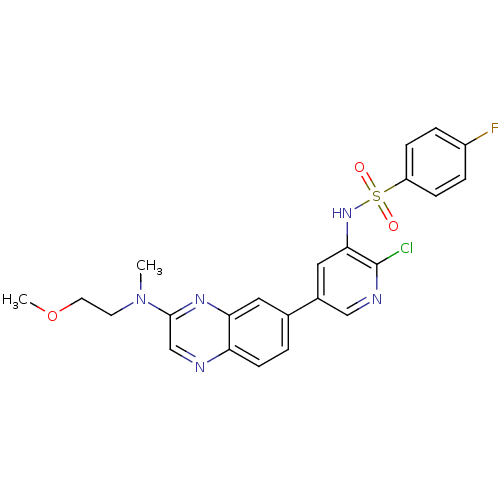
(CHEMBL1914730)Show SMILES COCCN(C)c1cnc2ccc(cc2n1)-c1cnc(Cl)c(NS(=O)(=O)c2ccc(F)cc2)c1 Show InChI InChI=1S/C23H21ClFN5O3S/c1-30(9-10-33-2)22-14-26-19-8-3-15(11-20(19)28-22)16-12-21(23(24)27-13-16)29-34(31,32)18-6-4-17(25)5-7-18/h3-8,11-14,29H,9-10H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha-mediated Akt phosphorylation at Ser 473 in human U87MG cells |
J Med Chem 54: 4735-51 (2011)
Article DOI: 10.1021/jm200386s
BindingDB Entry DOI: 10.7270/Q2FB53C5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM125327
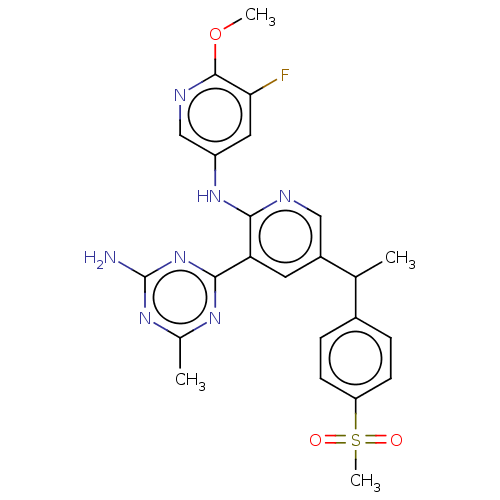
(US8772480, 150)Show SMILES COc1ncc(Nc2ncc(cc2-c2nc(C)nc(N)n2)C(C)c2ccc(cc2)S(C)(=O)=O)cc1F Show InChI InChI=1S/C24H24FN7O3S/c1-13(15-5-7-18(8-6-15)36(4,33)34)16-9-19(22-29-14(2)30-24(26)32-22)21(27-11-16)31-17-10-20(25)23(35-3)28-12-17/h5-13H,1-4H3,(H,27,31)(H2,26,29,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
US Patent
| Assay Description
The PI3K AlphaScreen assay (PerkinElmer, Waltham, Mass.) measures the activity of a panel of four phosphoinositide 3-kinases: PI3Kalpha, PI3Kbeta, PI... |
US Patent US8772480 (2014)
BindingDB Entry DOI: 10.7270/Q2V123FJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM125451
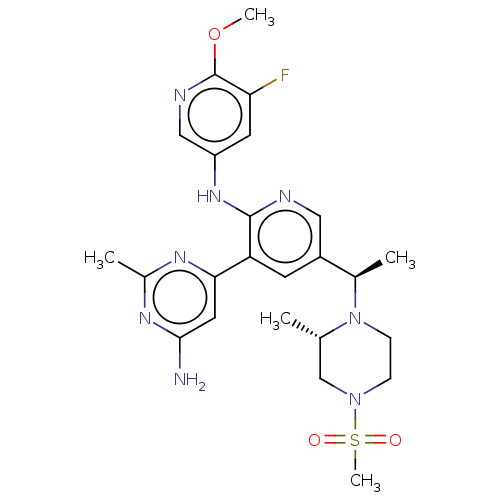
(US8772480, 306)Show SMILES COc1ncc(Nc2ncc(cc2-c2cc(N)nc(C)n2)[C@@H](C)N2CCN(C[C@@H]2C)S(C)(=O)=O)cc1F |r| Show InChI InChI=1S/C24H31FN8O3S/c1-14-13-32(37(5,34)35)6-7-33(14)15(2)17-8-19(21-10-22(26)30-16(3)29-21)23(27-11-17)31-18-9-20(25)24(36-4)28-12-18/h8-12,14-15H,6-7,13H2,1-5H3,(H,27,31)(H2,26,29,30)/t14-,15+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
US Patent
| Assay Description
The PI3K AlphaScreen assay (PerkinElmer, Waltham, Mass.) measures the activity of a panel of four phosphoinositide 3-kinases: PI3Kalpha, PI3Kbeta, PI... |
US Patent US8772480 (2014)
BindingDB Entry DOI: 10.7270/Q2V123FJ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data