

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Enoyl-acyl-carrier protein reductase (Plasmodium falciparum) | BDBM50220547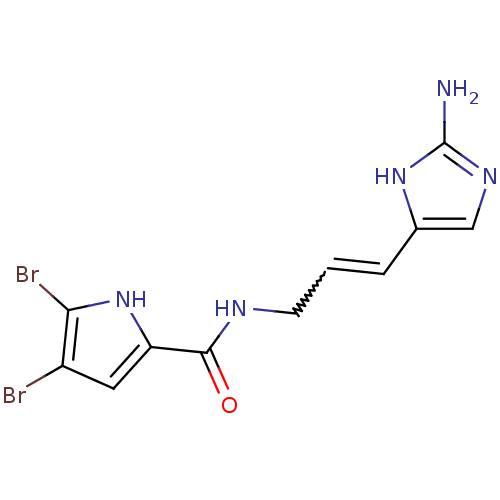 (4,5-Dibromo-1H-pyrrole-2-carboxylic acid [3-(2-ami...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of London Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum FabI in presence of variable crotonyl-coA level | Bioorg Med Chem 15: 6834-45 (2007) Article DOI: 10.1016/j.bmc.2007.07.032 BindingDB Entry DOI: 10.7270/Q2VQ32DZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-acyl-carrier protein reductase (Plasmodium falciparum) | BDBM50220547 (4,5-Dibromo-1H-pyrrole-2-carboxylic acid [3-(2-ami...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of London Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum FabI in presence of variable NADH levels | Bioorg Med Chem 15: 6834-45 (2007) Article DOI: 10.1016/j.bmc.2007.07.032 BindingDB Entry DOI: 10.7270/Q2VQ32DZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADH] (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50329257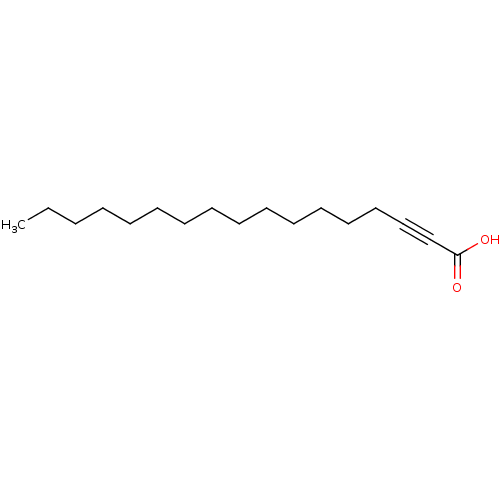 (2-Hexadecynoic acid | CHEMBL1269411) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of London Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis inhA | Bioorg Med Chem 18: 7475-85 (2010) Article DOI: 10.1016/j.bmc.2010.08.055 BindingDB Entry DOI: 10.7270/Q25Q4WB5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-ACP reductase (Plasmodium falciparum) | BDBM50329257 (2-Hexadecynoic acid | CHEMBL1269411) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of London Curated by ChEMBL | Assay Description Non-competitive inhibition of Plasmodium falciparum FabI using NADH as cofactor by Michaelis-Menten steady state analysis | Bioorg Med Chem 18: 7475-85 (2010) Article DOI: 10.1016/j.bmc.2010.08.055 BindingDB Entry DOI: 10.7270/Q25Q4WB5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-ACP reductase (Plasmodium falciparum) | BDBM50329257 (2-Hexadecynoic acid | CHEMBL1269411) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of London Curated by ChEMBL | Assay Description Non-competitive inhibition of Plasmodium falciparum FabI using crotonyl-CoA as substrate by Michaelis-Menten steady state analysis | Bioorg Med Chem 18: 7475-85 (2010) Article DOI: 10.1016/j.bmc.2010.08.055 BindingDB Entry DOI: 10.7270/Q25Q4WB5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxyacyl-[acyl-carrier-protein] dehydratase (Plasmodium falciparum) | BDBM50329257 (2-Hexadecynoic acid | CHEMBL1269411) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of London Curated by ChEMBL | Assay Description Competitive inhibition of Plasmodium falciparum FabZ using crotonyl-CoA as substrate by Michaelis-Menten steady state analysis | Bioorg Med Chem 18: 7475-85 (2010) Article DOI: 10.1016/j.bmc.2010.08.055 BindingDB Entry DOI: 10.7270/Q25Q4WB5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxoacyl-acyl-carrier protein reductase (Plasmodium falciparum) | BDBM50329257 (2-Hexadecynoic acid | CHEMBL1269411) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of London Curated by ChEMBL | Assay Description Non-competitive inhibition of Plasmodium falciparum FabG using NADH as cofactor by Michaelis-Menten steady state analysis | Bioorg Med Chem 18: 7475-85 (2010) Article DOI: 10.1016/j.bmc.2010.08.055 BindingDB Entry DOI: 10.7270/Q25Q4WB5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxoacyl-acyl-carrier protein reductase (Plasmodium falciparum) | BDBM50329257 (2-Hexadecynoic acid | CHEMBL1269411) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.43E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of London Curated by ChEMBL | Assay Description Competitive inhibition of Plasmodium falciparum FabG using acetoacetyl-CoA as substrate by Michaelis-Menten steady state analysis | Bioorg Med Chem 18: 7475-85 (2010) Article DOI: 10.1016/j.bmc.2010.08.055 BindingDB Entry DOI: 10.7270/Q25Q4WB5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50250903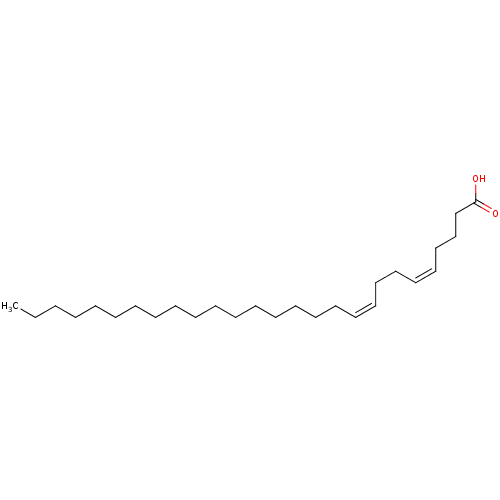 ((5Z,9Z)-5,9-heptacosadienoic acid | CHEMBL463437) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Puerto Rico Curated by ChEMBL | Assay Description Inhibition of human DNA topoisomerase 1 | J Nat Prod 65: 1715-8 (2002) BindingDB Entry DOI: 10.7270/Q2WW7HFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50150484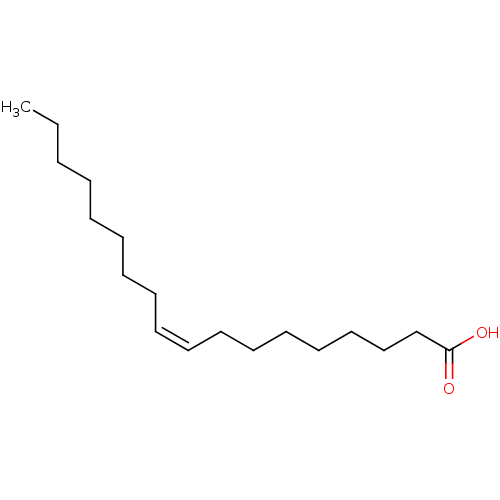 ((Z)-9-octadecenoic acid | (Z)-Octadec-9-enoic acid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 3.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Puerto Rico Curated by ChEMBL | Assay Description Inhibition of DNA topoisomerase 1 (unknown origin) | J Nat Prod 65: 1715-8 (2002) BindingDB Entry DOI: 10.7270/Q2WW7HFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesterol side-chain cleavage enzyme, mitochondrial (Rattus norvegicus) | BDBM50478451 (CHEMBL442810) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <3.96E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Puerto Rico Curated by ChEMBL | Assay Description Antibacterial activity against Streptococcus faecalis group D ATCC 29212 after overnight incubation | J Nat Prod 60: 502-4 (1997) Article DOI: 10.1021/np970034t BindingDB Entry DOI: 10.7270/Q2445Q8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Staphylococcus aureus) | BDBM50329257 (2-Hexadecynoic acid | CHEMBL1269411) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Inter American University of Puerto Rico Curated by ChEMBL | Assay Description Inhibition of supercoiling activity of Staphylococcus aureus DNA gyrase using relaxed pHOT1 plasmid DNA after 60 mins using ethidium bromide staining... | Bioorg Med Chem Lett 25: 5067-71 (2015) Article DOI: 10.1016/j.bmcl.2015.10.022 BindingDB Entry DOI: 10.7270/Q2GF0WBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Staphylococcus aureus) | BDBM50133156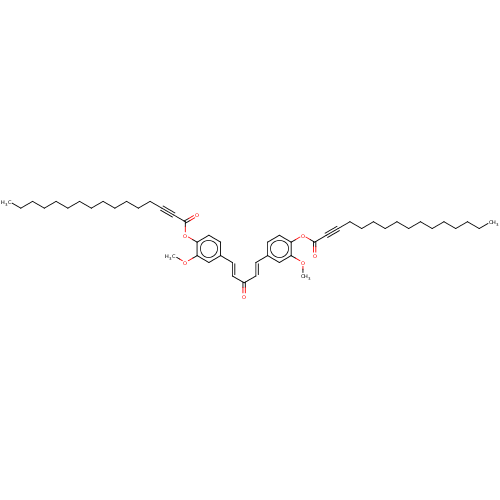 (CHEMBL3634703) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.26E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Inter American University of Puerto Rico Curated by ChEMBL | Assay Description Inhibition of supercoiling activity of Staphylococcus aureus DNA gyrase using relaxed pHOT1 plasmid DNA after 60 mins using ethidium bromide staining... | Bioorg Med Chem Lett 25: 5067-71 (2015) Article DOI: 10.1016/j.bmcl.2015.10.022 BindingDB Entry DOI: 10.7270/Q2GF0WBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50250904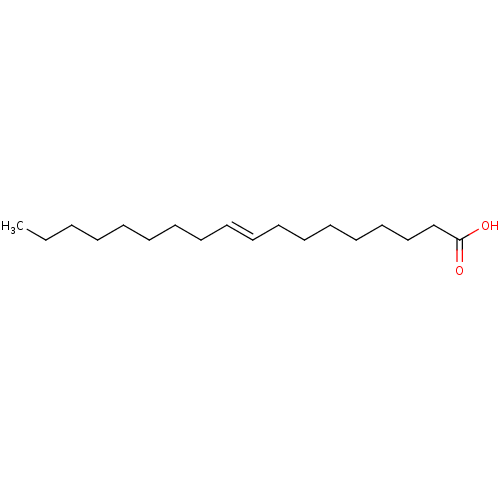 (CHEMBL460657 | Elaidinsaeure | elaidic acid | tran...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Puerto Rico Curated by ChEMBL | Assay Description Inhibition of DNA topoisomerase 1 (unknown origin) | J Nat Prod 65: 1715-8 (2002) BindingDB Entry DOI: 10.7270/Q2WW7HFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Topoisomerase I subunit B (Leishmania major) | BDBM50392268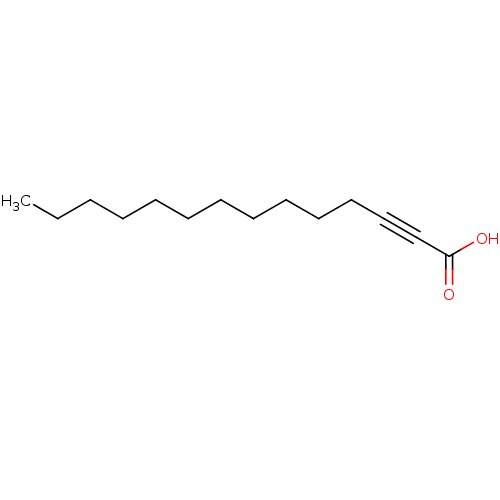 (CHEMBL2153501) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 6.78E+4 | n/a | n/a | n/a | n/a |
University of Puerto Rico Curated by ChEMBL | Assay Description Inhibition of Leishmania donovani topoisomerase 1B-mediated relaxation of supercoiled pSK DNA after 30 mins by ethidium bromide staining based agaros... | Bioorg Med Chem Lett 22: 6185-9 (2012) Article DOI: 10.1016/j.bmcl.2012.08.019 BindingDB Entry DOI: 10.7270/Q2R49RW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Topoisomerase I subunit B (Leishmania major) | BDBM50329257 (2-Hexadecynoic acid | CHEMBL1269411) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.87E+4 | n/a | n/a | n/a | n/a |
University of Puerto Rico Curated by ChEMBL | Assay Description Inhibition of Leishmania donovani topoisomerase 1B-mediated relaxation of supercoiled pSK DNA after 30 mins by ethidium bromide staining based agaros... | Bioorg Med Chem Lett 22: 6185-9 (2012) Article DOI: 10.1016/j.bmcl.2012.08.019 BindingDB Entry DOI: 10.7270/Q2R49RW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Topoisomerase I subunit B (Leishmania major) | BDBM50392269 (CHEMBL2153502) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 5.30E+3 | n/a | n/a | n/a | n/a |
University of Puerto Rico Curated by ChEMBL | Assay Description Inhibition of Leishmania donovani topoisomerase 1B-mediated relaxation of supercoiled pSK DNA after 30 mins by ethidium bromide staining based agaros... | Bioorg Med Chem Lett 22: 6185-9 (2012) Article DOI: 10.1016/j.bmcl.2012.08.019 BindingDB Entry DOI: 10.7270/Q2R49RW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Topoisomerase I subunit B (Leishmania major) | BDBM50008923 ((S)-4-ethyl-4-hydroxy-1,12-dihydro-4H-2-oxa-6,12a-...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 670 | n/a | n/a | n/a | n/a |
University of Puerto Rico Curated by ChEMBL | Assay Description Inhibition of Leishmania donovani topoisomerase 1B-mediated relaxation of supercoiled pSK DNA after 30 mins by ethidium bromide staining based agaros... | Bioorg Med Chem Lett 22: 6185-9 (2012) Article DOI: 10.1016/j.bmcl.2012.08.019 BindingDB Entry DOI: 10.7270/Q2R49RW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||