

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 21 | -43.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of Notre Dame | Assay Description To assess the effect of test compounds on histone deacetylase enzyme function in Vitro, a fluorometric assay was performed using HDAC, which incubate... | J Med Chem 51: 2898-906 (2008) Article DOI: 10.1021/jm7015254 BindingDB Entry DOI: 10.7270/Q2NC5ZG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM22450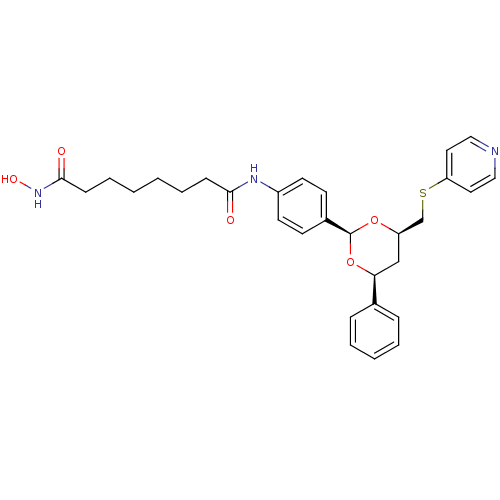 (N-hydroxy-N -(4-{(2R,4S,6R)-4-phenyl-6-[(pyridin-4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 28 | -42.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of Notre Dame | Assay Description To assess the effect of test compounds on histone deacetylase enzyme function in Vitro, a fluorometric assay was performed using HDAC, which incubate... | J Med Chem 51: 2898-906 (2008) Article DOI: 10.1021/jm7015254 BindingDB Entry DOI: 10.7270/Q2NC5ZG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 48 | -41.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of Notre Dame | Assay Description To assess the effect of test compounds on histone deacetylase enzyme function in Vitro, a fluorometric assay was performed using HDAC, which incubate... | J Med Chem 51: 2898-906 (2008) Article DOI: 10.1021/jm7015254 BindingDB Entry DOI: 10.7270/Q2NC5ZG2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM22450 (N-hydroxy-N -(4-{(2R,4S,6R)-4-phenyl-6-[(pyridin-4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 88 | -39.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of Notre Dame | Assay Description To assess the effect of test compounds on histone deacetylase enzyme function in Vitro, a fluorometric assay was performed using HDAC, which incubate... | J Med Chem 51: 2898-906 (2008) Article DOI: 10.1021/jm7015254 BindingDB Entry DOI: 10.7270/Q2NC5ZG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM22449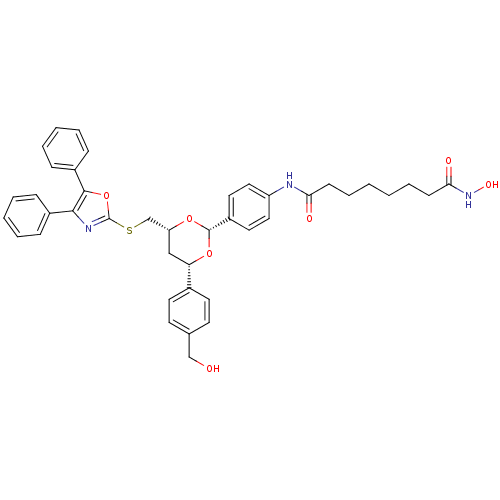 (CHEMBL356769 | N-(4-{(2R,4R,6S)-4-{[(4,5-diphenyl-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 142 | -38.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of Notre Dame | Assay Description To assess the effect of test compounds on histone deacetylase enzyme function in Vitro, a fluorometric assay was performed using HDAC, which incubate... | J Med Chem 51: 2898-906 (2008) Article DOI: 10.1021/jm7015254 BindingDB Entry DOI: 10.7270/Q2NC5ZG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM22449 (CHEMBL356769 | N-(4-{(2R,4R,6S)-4-{[(4,5-diphenyl-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 995 | -33.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of Notre Dame | Assay Description To assess the effect of test compounds on histone deacetylase enzyme function in Vitro, a fluorometric assay was performed using HDAC, which incubate... | J Med Chem 51: 2898-906 (2008) Article DOI: 10.1021/jm7015254 BindingDB Entry DOI: 10.7270/Q2NC5ZG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | 2.00E+3 | -32.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of Notre Dame | Assay Description To assess the effect of test compounds on histone deacetylase enzyme function in Vitro, a fluorometric assay was performed using HDAC, which incubate... | J Med Chem 51: 2898-906 (2008) Article DOI: 10.1021/jm7015254 BindingDB Entry DOI: 10.7270/Q2NC5ZG2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM22450 (N-hydroxy-N -(4-{(2R,4S,6R)-4-phenyl-6-[(pyridin-4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.10E+3 | -29.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of Notre Dame | Assay Description To assess the effect of test compounds on histone deacetylase enzyme function in Vitro, a fluorometric assay was performed using HDAC, which incubate... | J Med Chem 51: 2898-906 (2008) Article DOI: 10.1021/jm7015254 BindingDB Entry DOI: 10.7270/Q2NC5ZG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM22449 (CHEMBL356769 | N-(4-{(2R,4R,6S)-4-{[(4,5-diphenyl-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 6.30E+3 | -29.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of Notre Dame | Assay Description To assess the effect of test compounds on histone deacetylase enzyme function in Vitro, a fluorometric assay was performed using HDAC, which incubate... | J Med Chem 51: 2898-906 (2008) Article DOI: 10.1021/jm7015254 BindingDB Entry DOI: 10.7270/Q2NC5ZG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-T1/Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM50539902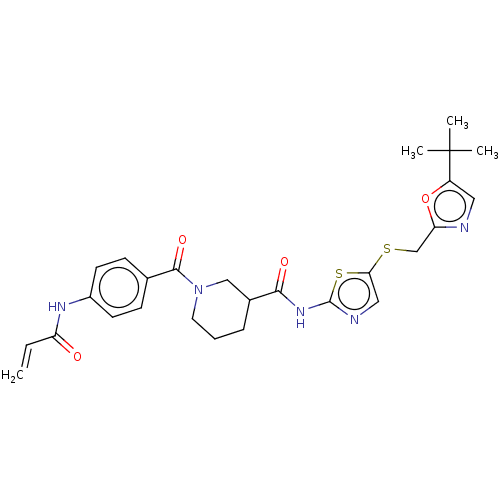 (CHEMBL4644893) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of recombinant human full-length His-tagged CDK9/Cyclin T1 expressed in baculovirus infection system using Cdk7/9tide as substrate measure... | J Med Chem 63: 6708-6726 (2020) Article DOI: 10.1021/acs.jmedchem.9b01929 BindingDB Entry DOI: 10.7270/Q25M698Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-T1/Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM50539903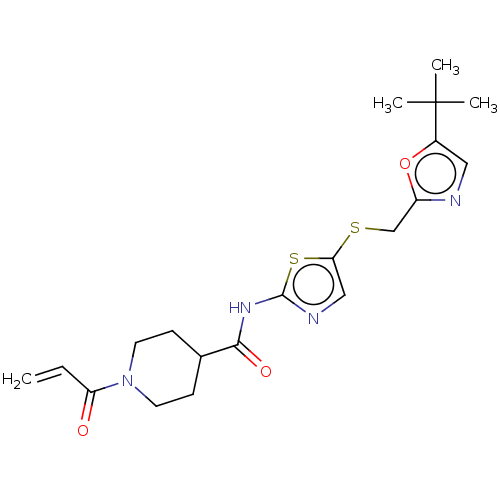 (CHEMBL4641226) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of recombinant human full-length His-tagged CDK9/Cyclin T1 expressed in baculovirus infection system using Cdk7/9tide as substrate measure... | J Med Chem 63: 6708-6726 (2020) Article DOI: 10.1021/acs.jmedchem.9b01929 BindingDB Entry DOI: 10.7270/Q25M698Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-T1/Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM5931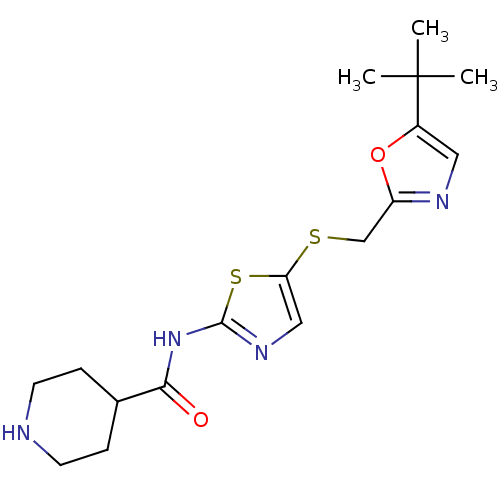 (BMS-387072 | CHEMBL296468 | N-(5-{[(5-tert-butyl-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of recombinant human full-length His-tagged CDK9/Cyclin T1 expressed in baculovirus infection system using Cdk7/9tide as substrate measure... | J Med Chem 63: 6708-6726 (2020) Article DOI: 10.1021/acs.jmedchem.9b01929 BindingDB Entry DOI: 10.7270/Q25M698Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM519553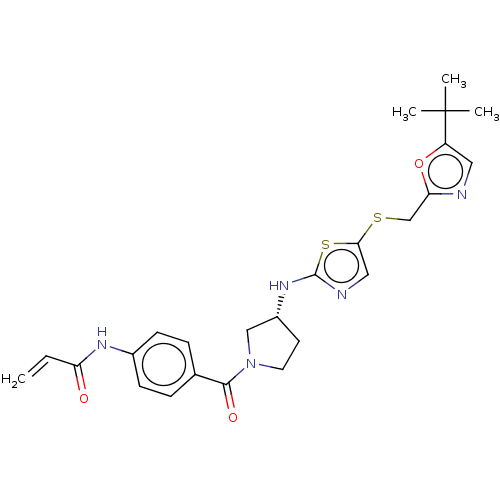 (US11142507, Compound MFH-4-70-1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9.86 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Jurkat cells were treated with DMSO or concentration of compound indicated. 6 hours after treatment, cells were washed and harvested by resuspending ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DN486D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A1/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50539903 (CHEMBL4641226) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of recombinant human full-length N-terminal GST-tagged CDK2/cyclinA1 expressed in baculovirus infected Sf9 insect cells using FRET-labeled... | J Med Chem 63: 6708-6726 (2020) Article DOI: 10.1021/acs.jmedchem.9b01929 BindingDB Entry DOI: 10.7270/Q25M698Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A1/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50539902 (CHEMBL4644893) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of recombinant human full-length N-terminal GST-tagged CDK2/cyclinA1 expressed in baculovirus infected Sf9 insect cells using FRET-labeled... | J Med Chem 63: 6708-6726 (2020) Article DOI: 10.1021/acs.jmedchem.9b01929 BindingDB Entry DOI: 10.7270/Q25M698Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-H/Cyclin-dependent kinase 7 (Homo sapiens (Human)) | BDBM50110178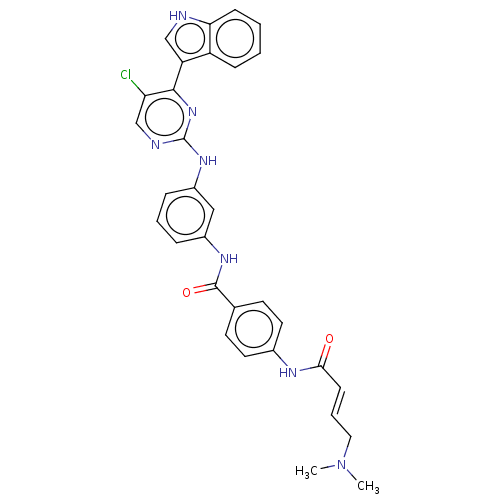 (CHEMBL3603847 | US10787436, Compound I-23) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 11.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute, Inc. US Patent | Assay Description In vitro kinase assays with immunoprecipitated proteins (or recombinant CAK complexes) were performed as follows. Kinase reactions were performed in ... | US Patent US10787436 (2020) BindingDB Entry DOI: 10.7270/Q20G3P6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM519554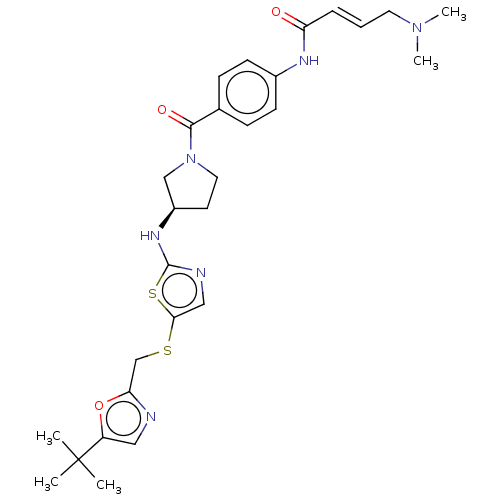 (US11142507, Compound MFH-4-73-1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11.9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Jurkat cells were treated with DMSO or concentration of compound indicated. 6 hours after treatment, cells were washed and harvested by resuspending ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DN486D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-H/Cyclin-dependent kinase 7 (Homo sapiens (Human)) | BDBM50526794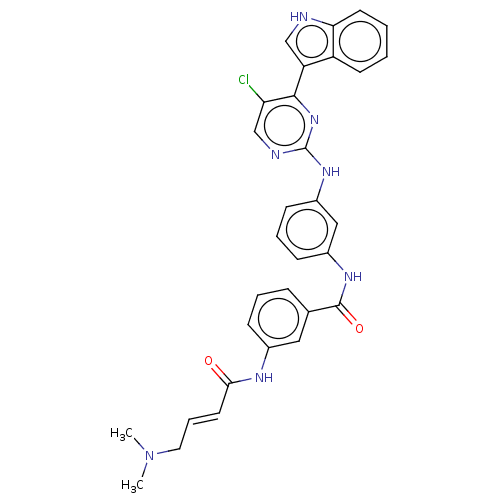 (CHEMBL4303287 | US10787436, Compound I-18) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 13.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute, Inc. US Patent | Assay Description In vitro kinase assays with immunoprecipitated proteins (or recombinant CAK complexes) were performed as follows. Kinase reactions were performed in ... | US Patent US10787436 (2020) BindingDB Entry DOI: 10.7270/Q20G3P6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-T1/Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM50573506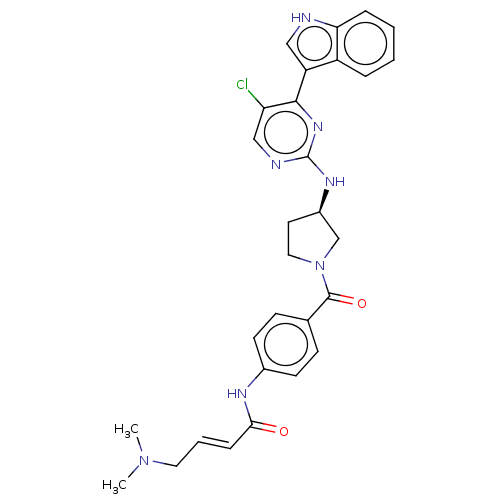 (CHEMBL4861833) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CDK9/Cyclin T1 (unknown origin) | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113481 BindingDB Entry DOI: 10.7270/Q22B92TP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-T1/Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM50539909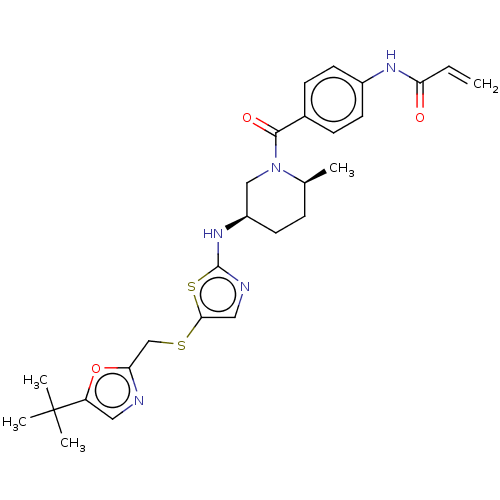 (CHEMBL4644643) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of recombinant human full-length His-tagged CDK9/Cyclin T1 expressed in baculovirus infection system using Cdk7/9tide as substrate measure... | J Med Chem 63: 6708-6726 (2020) Article DOI: 10.1021/acs.jmedchem.9b01929 BindingDB Entry DOI: 10.7270/Q25M698Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM519536 (US11142507, Compound MFH-3-110-1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15.1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2DN486D | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-H/Cyclin-dependent kinase 7 (Homo sapiens (Human)) | BDBM464083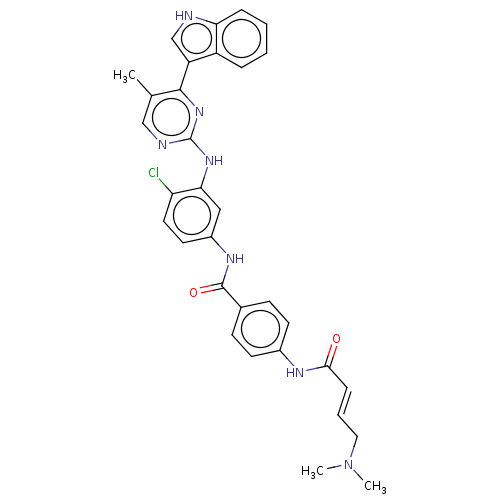 (US10787436, Compound I-19) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute, Inc. US Patent | Assay Description In vitro kinase assays with immunoprecipitated proteins (or recombinant CAK complexes) were performed as follows. Kinase reactions were performed in ... | US Patent US10787436 (2020) BindingDB Entry DOI: 10.7270/Q20G3P6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50539906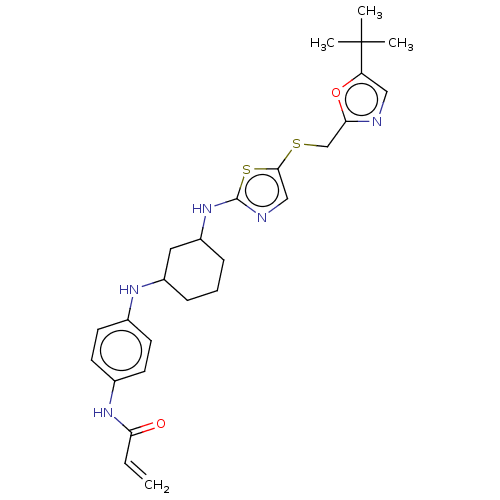 (CHEMBL4648005 | US11142507, Compound MFH-3-35-1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15.7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Jurkat cells were treated with DMSO or concentration of compound indicated. 6 hours after treatment, cells were washed and harvested by resuspending ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DN486D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A1/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50539906 (CHEMBL4648005 | US11142507, Compound MFH-3-35-1) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of recombinant human full-length N-terminal GST-tagged CDK2/cyclinA1 expressed in baculovirus infected Sf9 insect cells using FRET-labeled... | J Med Chem 63: 6708-6726 (2020) Article DOI: 10.1021/acs.jmedchem.9b01929 BindingDB Entry DOI: 10.7270/Q25M698Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM519528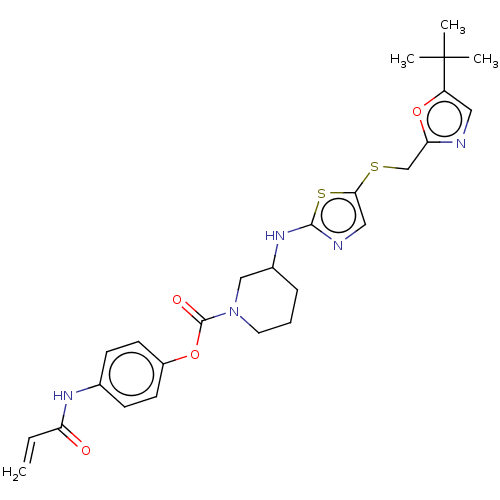 (US11142507, Compound MFH-2-102-1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 20.4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2DN486D | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-H/Cyclin-dependent kinase 7 (Homo sapiens (Human)) | BDBM464084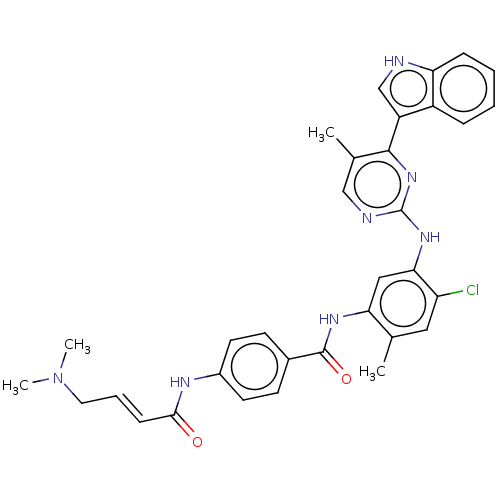 (US10787436, Compound I-20) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 20.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute, Inc. US Patent | Assay Description In vitro kinase assays with immunoprecipitated proteins (or recombinant CAK complexes) were performed as follows. Kinase reactions were performed in ... | US Patent US10787436 (2020) BindingDB Entry DOI: 10.7270/Q20G3P6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A1/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM5931 (BMS-387072 | CHEMBL296468 | N-(5-{[(5-tert-butyl-1...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of recombinant human full-length N-terminal GST-tagged CDK2/cyclinA1 expressed in baculovirus infected Sf9 insect cells using FRET-labeled... | J Med Chem 63: 6708-6726 (2020) Article DOI: 10.1021/acs.jmedchem.9b01929 BindingDB Entry DOI: 10.7270/Q25M698Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM519493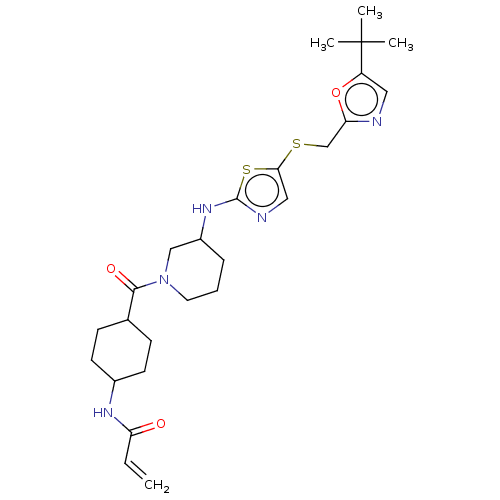 (US11142507, Compound MFH-2-95-1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2DN486D | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-H/Cyclin-dependent kinase 7 (Homo sapiens (Human)) | BDBM464085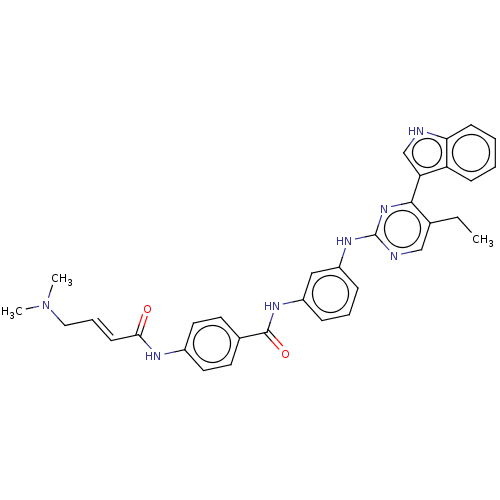 (US10787436, Compound I-21) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 22.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute, Inc. US Patent | Assay Description In vitro kinase assays with immunoprecipitated proteins (or recombinant CAK complexes) were performed as follows. Kinase reactions were performed in ... | US Patent US10787436 (2020) BindingDB Entry DOI: 10.7270/Q20G3P6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM519536 (US11142507, Compound MFH-3-110-1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 22.9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Jurkat cells were treated with DMSO or concentration of compound indicated. 6 hours after treatment, cells were washed and harvested by resuspending ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DN486D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM519528 (US11142507, Compound MFH-2-102-1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 24.6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Jurkat cells were treated with DMSO or concentration of compound indicated. 6 hours after treatment, cells were washed and harvested by resuspending ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DN486D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 12 (Homo sapiens) | BDBM50539904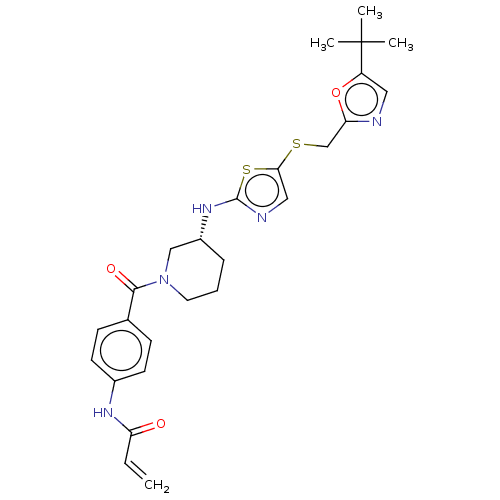 (CHEMBL4647764) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of human CDK12/cyclin K using Pol2-CTD as substrate by [gamma-33P]ATP-based radioisotope filter binding assay | J Med Chem 63: 6708-6726 (2020) Article DOI: 10.1021/acs.jmedchem.9b01929 BindingDB Entry DOI: 10.7270/Q25M698Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-T1/Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM50539901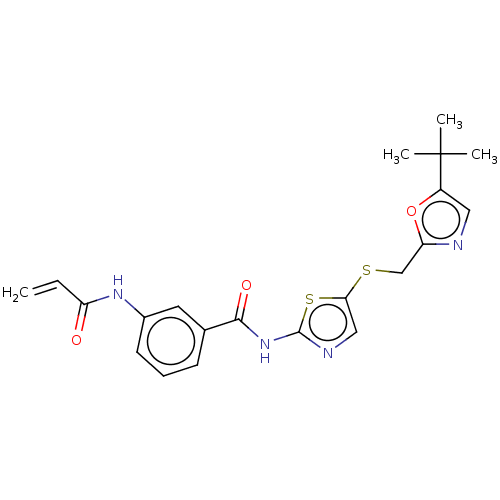 (CHEMBL4643057) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of recombinant human full-length His-tagged CDK9/Cyclin T1 expressed in baculovirus infection system using Cdk7/9tide as substrate measure... | J Med Chem 63: 6708-6726 (2020) Article DOI: 10.1021/acs.jmedchem.9b01929 BindingDB Entry DOI: 10.7270/Q25M698Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM50539906 (CHEMBL4648005 | US11142507, Compound MFH-3-35-1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 30.9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2DN486D | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-T1/Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM50539906 (CHEMBL4648005 | US11142507, Compound MFH-3-35-1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of recombinant human full-length His-tagged CDK9/Cyclin T1 expressed in baculovirus infection system using Cdk7/9tide as substrate measure... | J Med Chem 63: 6708-6726 (2020) Article DOI: 10.1021/acs.jmedchem.9b01929 BindingDB Entry DOI: 10.7270/Q25M698Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-H/Cyclin-dependent kinase 7 (Homo sapiens (Human)) | BDBM464086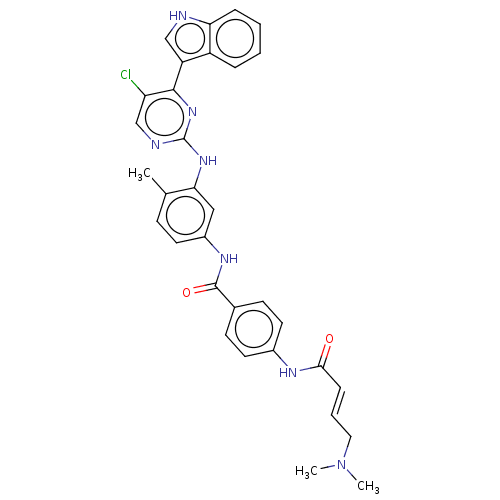 (US10787436, Compound I-22) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 32.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute, Inc. US Patent | Assay Description In vitro kinase assays with immunoprecipitated proteins (or recombinant CAK complexes) were performed as follows. Kinase reactions were performed in ... | US Patent US10787436 (2020) BindingDB Entry DOI: 10.7270/Q20G3P6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM519553 (US11142507, Compound MFH-4-70-1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2DN486D | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-H/Cyclin-dependent kinase 7 (Homo sapiens (Human)) | BDBM50110178 (CHEMBL3603847 | US10787436, Compound I-23) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 33.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute, Inc. US Patent | Assay Description In vitro kinase assays with immunoprecipitated proteins (or recombinant CAK complexes) were performed as follows. Kinase reactions were performed in ... | US Patent US10787436 (2020) BindingDB Entry DOI: 10.7270/Q20G3P6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 12 (Homo sapiens) | BDBM50573512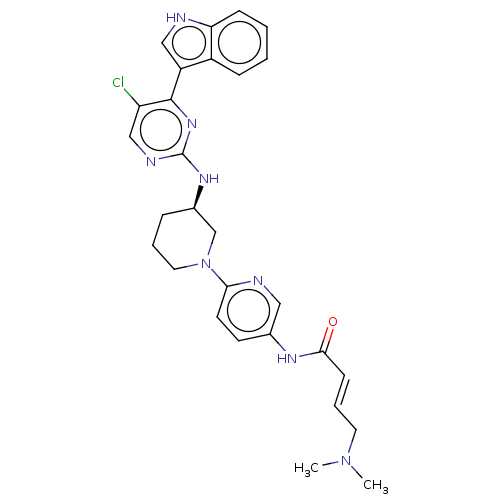 (CHEMBL4748005) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant CDK12/CyclinK assessed as reduction in substrate phosphorylation using His-c-Myc as substrate preincubated with enzym... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113481 BindingDB Entry DOI: 10.7270/Q22B92TP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-H/Cyclin-dependent kinase 7 (Homo sapiens (Human)) | BDBM464104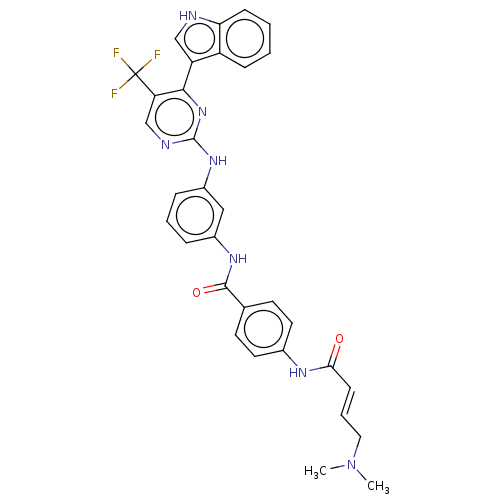 (US10787436, Compound I-42) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 35.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute, Inc. US Patent | Assay Description In vitro kinase assays with immunoprecipitated proteins (or recombinant CAK complexes) were performed as follows. Kinase reactions were performed in ... | US Patent US10787436 (2020) BindingDB Entry DOI: 10.7270/Q20G3P6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-H/Cyclin-dependent kinase 7 (Homo sapiens (Human)) | BDBM464105 (US10787436, Compound I-43) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 36.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute, Inc. US Patent | Assay Description In vitro kinase assays with immunoprecipitated proteins (or recombinant CAK complexes) were performed as follows. Kinase reactions were performed in ... | US Patent US10787436 (2020) BindingDB Entry DOI: 10.7270/Q20G3P6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-H/Cyclin-dependent kinase 7 (Homo sapiens (Human)) | BDBM464088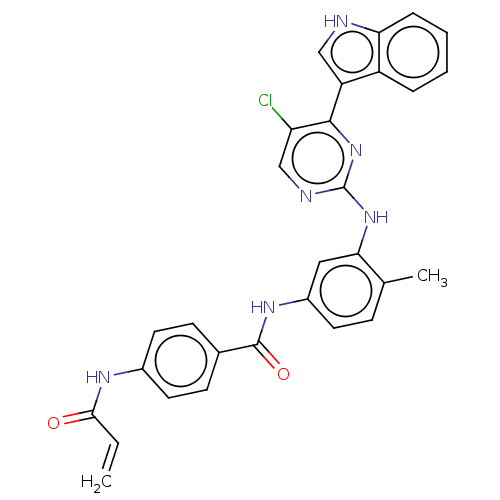 (US10787436, Compound I-24) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute, Inc. US Patent | Assay Description In vitro kinase assays with immunoprecipitated proteins (or recombinant CAK complexes) were performed as follows. Kinase reactions were performed in ... | US Patent US10787436 (2020) BindingDB Entry DOI: 10.7270/Q20G3P6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50573506 (CHEMBL4861833) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CDK2/Cyclin A (unknown origin) | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113481 BindingDB Entry DOI: 10.7270/Q22B92TP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 13/Cyclin-K (Homo sapiens (Human)) | BDBM50539904 (CHEMBL4647764) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of human CDK13/cyclin K using Pol2-CTD as substrate by [gamma-33P]ATP-based radioisotope filter binding assay | J Med Chem 63: 6708-6726 (2020) Article DOI: 10.1021/acs.jmedchem.9b01929 BindingDB Entry DOI: 10.7270/Q25M698Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 13/Cyclin-K (Homo sapiens (Human)) | BDBM50573506 (CHEMBL4861833) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant CDK13/CyclinK assessed as reduction in substrate phosphorylation using His-c-Myc as substrate preincubated with enzym... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113481 BindingDB Entry DOI: 10.7270/Q22B92TP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM519554 (US11142507, Compound MFH-4-73-1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 54.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2DN486D | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A1/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50539901 (CHEMBL4643057) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of recombinant human full-length N-terminal GST-tagged CDK2/cyclinA1 expressed in baculovirus infected Sf9 insect cells using FRET-labeled... | J Med Chem 63: 6708-6726 (2020) Article DOI: 10.1021/acs.jmedchem.9b01929 BindingDB Entry DOI: 10.7270/Q25M698Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM519551 (US11142507, Compound MFH-4-13-1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2DN486D | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM519534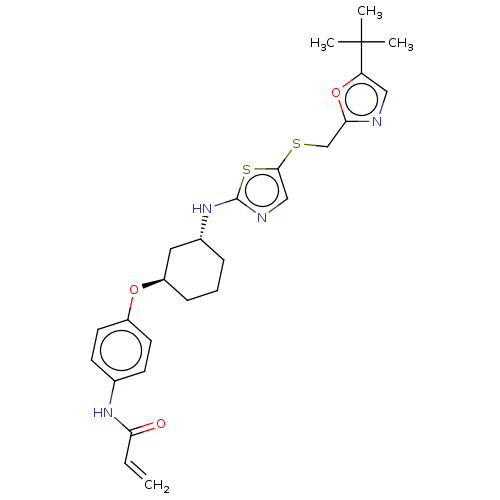 (US11142507, Compound MFH-3-103-1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 66.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2DN486D | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 12 (Homo sapiens) | BDBM50573513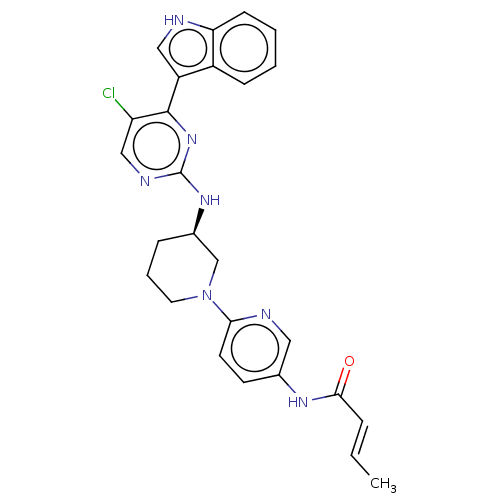 (CHEMBL4866757) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant CDK12/CyclinK assessed as reduction in substrate phosphorylation using His-c-Myc as substrate preincubated with enzym... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113481 BindingDB Entry DOI: 10.7270/Q22B92TP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 465 total ) | Next | Last >> |