 Found 1054 hits with Last Name = 'ballard' and Initial = 'p'
Found 1054 hits with Last Name = 'ballard' and Initial = 'p' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50236369
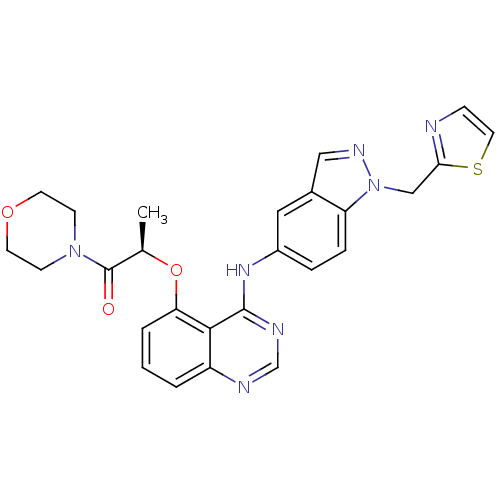
((R)-1-morpholino-2-(4-(1-(thiazol-2-ylmethyl)-1H-i...)Show SMILES C[C@@H](Oc1cccc2ncnc(Nc3ccc4n(Cc5nccs5)ncc4c3)c12)C(=O)N1CCOCC1 Show InChI InChI=1S/C26H25N7O3S/c1-17(26(34)32-8-10-35-11-9-32)36-22-4-2-3-20-24(22)25(29-16-28-20)31-19-5-6-21-18(13-19)14-30-33(21)15-23-27-7-12-37-23/h2-7,12-14,16-17H,8-11,15H2,1H3,(H,28,29,31)/t17-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 18: 1799-803 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.035
BindingDB Entry DOI: 10.7270/Q23T9H0F |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50236369

((R)-1-morpholino-2-(4-(1-(thiazol-2-ylmethyl)-1H-i...)Show SMILES C[C@@H](Oc1cccc2ncnc(Nc3ccc4n(Cc5nccs5)ncc4c3)c12)C(=O)N1CCOCC1 Show InChI InChI=1S/C26H25N7O3S/c1-17(26(34)32-8-10-35-11-9-32)36-22-4-2-3-20-24(22)25(29-16-28-20)31-19-5-6-21-18(13-19)14-30-33(21)15-23-27-7-12-37-23/h2-7,12-14,16-17H,8-11,15H2,1H3,(H,28,29,31)/t17-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibition of erbB2 |
Bioorg Med Chem Lett 18: 1799-803 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.035
BindingDB Entry DOI: 10.7270/Q23T9H0F |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50169743

((13S,17S)-13-Methyl-7-[9-(4,4,5,5,5-pentafluoro-pe...)Show SMILES C[C@]12CC[C@H]3[C@H]([C@@H]1CC[C@@H]2O)[C@H](CCCCCCCCCS(=O)CCCC(F)(F)C(F)(F)F)Cc1cc(O)ccc31 |r| Show InChI InChI=1S/C32H47F5O3S/c1-30-17-15-26-25-12-11-24(38)21-23(25)20-22(29(26)27(30)13-14-28(30)39)10-7-5-3-2-4-6-8-18-41(40)19-9-16-31(33,34)32(35,36)37/h11-12,21-22,26-29,38-39H,2-10,13-20H2,1H3/t22-,26-,27+,28+,29-,30+,41?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at ERalpha receptor in human MCF7 cells |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50125052
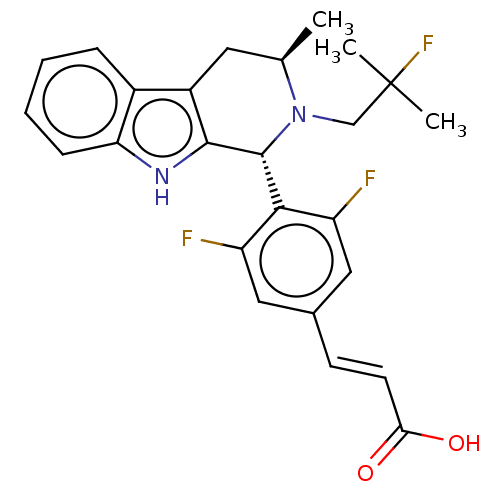
(CHEMBL3623004 | US10130617, Example 1 | US20240043...)Show SMILES C[C@@H]1Cc2c([nH]c3ccccc23)[C@H](N1CC(C)(C)F)c1c(F)cc(\C=C\C(O)=O)cc1F |r| Show InChI InChI=1S/C25H25F3N2O2/c1-14-10-17-16-6-4-5-7-20(16)29-23(17)24(30(14)13-25(2,3)28)22-18(26)11-15(12-19(22)27)8-9-21(31)32/h4-9,11-12,14,24,29H,10,13H2,1-3H3,(H,31,32)/b9-8+/t14-,24-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.138 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at ERalpha receptor in human MCF7 cells |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50084948
(CHEMBL195515 | GW7604)Show SMILES CC\C(=C(/c1ccc(O)cc1)c1ccc(\C=C\C(O)=O)cc1)c1ccccc1 Show InChI InChI=1S/C25H22O3/c1-2-23(19-6-4-3-5-7-19)25(21-13-15-22(26)16-14-21)20-11-8-18(9-12-20)10-17-24(27)28/h3-17,26H,2H2,1H3,(H,27,28)/b17-10+,25-23+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.145 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to ERalpha receptor (unknown origin) |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50169743

((13S,17S)-13-Methyl-7-[9-(4,4,5,5,5-pentafluoro-pe...)Show SMILES C[C@]12CC[C@H]3[C@H]([C@@H]1CC[C@@H]2O)[C@H](CCCCCCCCCS(=O)CCCC(F)(F)C(F)(F)F)Cc1cc(O)ccc31 |r| Show InChI InChI=1S/C32H47F5O3S/c1-30-17-15-26-25-12-11-24(38)21-23(25)20-22(29(26)27(30)13-14-28(30)39)10-7-5-3-2-4-6-8-18-41(40)19-9-16-31(33,34)32(35,36)37/h11-12,21-22,26-29,38-39H,2-10,13-20H2,1H3/t22-,26-,27+,28+,29-,30+,41?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.209 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at progesterone receptor in human MCF cells assessed as estradiol-induced receptor response |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50125052

(CHEMBL3623004 | US10130617, Example 1 | US20240043...)Show SMILES C[C@@H]1Cc2c([nH]c3ccccc23)[C@H](N1CC(C)(C)F)c1c(F)cc(\C=C\C(O)=O)cc1F |r| Show InChI InChI=1S/C25H25F3N2O2/c1-14-10-17-16-6-4-5-7-20(16)29-23(17)24(30(14)13-25(2,3)28)22-18(26)11-15(12-19(22)27)8-9-21(31)32/h4-9,11-12,14,24,29H,10,13H2,1-3H3,(H,31,32)/b9-8+/t14-,24-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.209 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at ERalpha receptor in human MCF7 cells in presence of 0.25 uM tamoxifen |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50125052

(CHEMBL3623004 | US10130617, Example 1 | US20240043...)Show SMILES C[C@@H]1Cc2c([nH]c3ccccc23)[C@H](N1CC(C)(C)F)c1c(F)cc(\C=C\C(O)=O)cc1F |r| Show InChI InChI=1S/C25H25F3N2O2/c1-14-10-17-16-6-4-5-7-20(16)29-23(17)24(30(14)13-25(2,3)28)22-18(26)11-15(12-19(22)27)8-9-21(31)32/h4-9,11-12,14,24,29H,10,13H2,1-3H3,(H,31,32)/b9-8+/t14-,24-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.282 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at progesterone receptor in human MCF cells assessed as estradiol-induced receptor response |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM20608
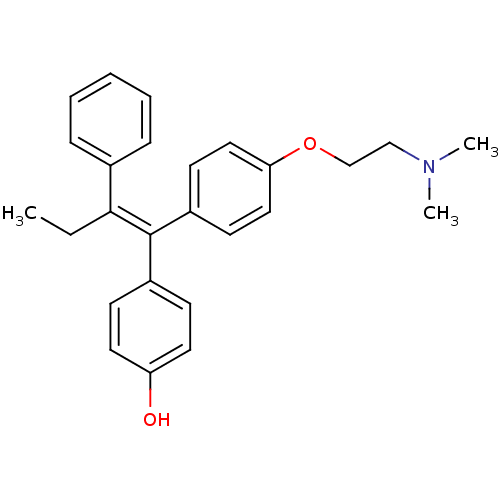
(4-Hydroxytamoxifen | 4-Hydroxytamoxifen (9) | 4-[(...)Show SMILES CC\C(=C(/c1ccc(O)cc1)c1ccc(OCCN(C)C)cc1)c1ccccc1 Show InChI InChI=1S/C26H29NO2/c1-4-25(20-8-6-5-7-9-20)26(21-10-14-23(28)15-11-21)22-12-16-24(17-13-22)29-19-18-27(2)3/h5-17,28H,4,18-19H2,1-3H3/b26-25- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.309 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to ERalpha receptor (unknown origin) |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50125055
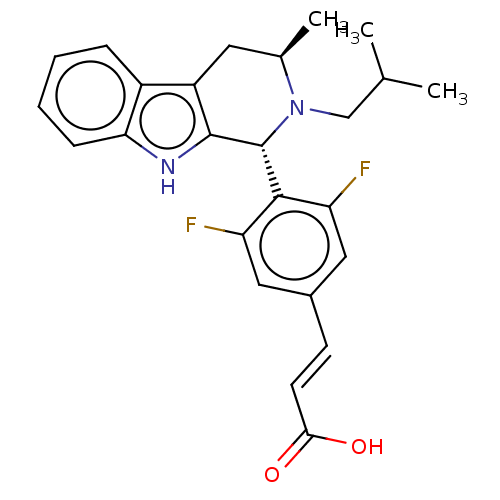
(CHEMBL3623002)Show SMILES CC(C)CN1[C@H](C)Cc2c([nH]c3ccccc23)[C@H]1c1c(F)cc(\C=C\C(O)=O)cc1F |r| Show InChI InChI=1S/C25H26F2N2O2/c1-14(2)13-29-15(3)10-18-17-6-4-5-7-21(17)28-24(18)25(29)23-19(26)11-16(12-20(23)27)8-9-22(30)31/h4-9,11-12,14-15,25,28H,10,13H2,1-3H3,(H,30,31)/b9-8+/t15-,25-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.417 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at ERalpha receptor in human MCF7 cells |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50125055

(CHEMBL3623002)Show SMILES CC(C)CN1[C@H](C)Cc2c([nH]c3ccccc23)[C@H]1c1c(F)cc(\C=C\C(O)=O)cc1F |r| Show InChI InChI=1S/C25H26F2N2O2/c1-14(2)13-29-15(3)10-18-17-6-4-5-7-21(17)28-24(18)25(29)23-19(26)11-16(12-20(23)27)8-9-22(30)31/h4-9,11-12,14-15,25,28H,10,13H2,1-3H3,(H,30,31)/b9-8+/t15-,25-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.708 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to ERalpha receptor (unknown origin) |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM20608

(4-Hydroxytamoxifen | 4-Hydroxytamoxifen (9) | 4-[(...)Show SMILES CC\C(=C(/c1ccc(O)cc1)c1ccc(OCCN(C)C)cc1)c1ccccc1 Show InChI InChI=1S/C26H29NO2/c1-4-25(20-8-6-5-7-9-20)26(21-10-14-23(28)15-11-21)22-12-16-24(17-13-22)29-19-18-27(2)3/h5-17,28H,4,18-19H2,1-3H3/b26-25- | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at progesterone receptor in human MCF cells assessed as estradiol-induced receptor response |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50125052

(CHEMBL3623004 | US10130617, Example 1 | US20240043...)Show SMILES C[C@@H]1Cc2c([nH]c3ccccc23)[C@H](N1CC(C)(C)F)c1c(F)cc(\C=C\C(O)=O)cc1F |r| Show InChI InChI=1S/C25H25F3N2O2/c1-14-10-17-16-6-4-5-7-20(16)29-23(17)24(30(14)13-25(2,3)28)22-18(26)11-15(12-19(22)27)8-9-21(31)32/h4-9,11-12,14,24,29H,10,13H2,1-3H3,(H,31,32)/b9-8+/t14-,24-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to ERalpha receptor (unknown origin) |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50125052

(CHEMBL3623004 | US10130617, Example 1 | US20240043...)Show SMILES C[C@@H]1Cc2c([nH]c3ccccc23)[C@H](N1CC(C)(C)F)c1c(F)cc(\C=C\C(O)=O)cc1F |r| Show InChI InChI=1S/C25H25F3N2O2/c1-14-10-17-16-6-4-5-7-20(16)29-23(17)24(30(14)13-25(2,3)28)22-18(26)11-15(12-19(22)27)8-9-21(31)32/h4-9,11-12,14,24,29H,10,13H2,1-3H3,(H,31,32)/b9-8+/t14-,24-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to ERalpha receptor (unknown origin) |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50169743

((13S,17S)-13-Methyl-7-[9-(4,4,5,5,5-pentafluoro-pe...)Show SMILES C[C@]12CC[C@H]3[C@H]([C@@H]1CC[C@@H]2O)[C@H](CCCCCCCCCS(=O)CCCC(F)(F)C(F)(F)F)Cc1cc(O)ccc31 |r| Show InChI InChI=1S/C32H47F5O3S/c1-30-17-15-26-25-12-11-24(38)21-23(25)20-22(29(26)27(30)13-14-28(30)39)10-7-5-3-2-4-6-8-18-41(40)19-9-16-31(33,34)32(35,36)37/h11-12,21-22,26-29,38-39H,2-10,13-20H2,1H3/t22-,26-,27+,28+,29-,30+,41?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.813 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to ERalpha receptor (unknown origin) |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50125055

(CHEMBL3623002)Show SMILES CC(C)CN1[C@H](C)Cc2c([nH]c3ccccc23)[C@H]1c1c(F)cc(\C=C\C(O)=O)cc1F |r| Show InChI InChI=1S/C25H26F2N2O2/c1-14(2)13-29-15(3)10-18-17-6-4-5-7-21(17)28-24(18)25(29)23-19(26)11-16(12-20(23)27)8-9-22(30)31/h4-9,11-12,14-15,25,28H,10,13H2,1-3H3,(H,30,31)/b9-8+/t15-,25-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.832 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at progesterone receptor in human MCF cells assessed as estradiol-induced receptor response |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50125054

(CHEMBL3623003 | US10130617, Example 2 | WO-2014/19...)Show SMILES C[C@@H]1Cc2c([nH]c3ccccc23)[C@H](N1CC(C)(C)F)c1ccc(\C=C\C(O)=O)cc1 |r| Show InChI InChI=1S/C25H27FN2O2/c1-16-14-20-19-6-4-5-7-21(19)27-23(20)24(28(16)15-25(2,3)26)18-11-8-17(9-12-18)10-13-22(29)30/h4-13,16,24,27H,14-15H2,1-3H3,(H,29,30)/b13-10+/t16-,24-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.851 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at ERalpha receptor in human MCF7 cells |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1B
(Homo sapiens (Human)) | BDBM50081185
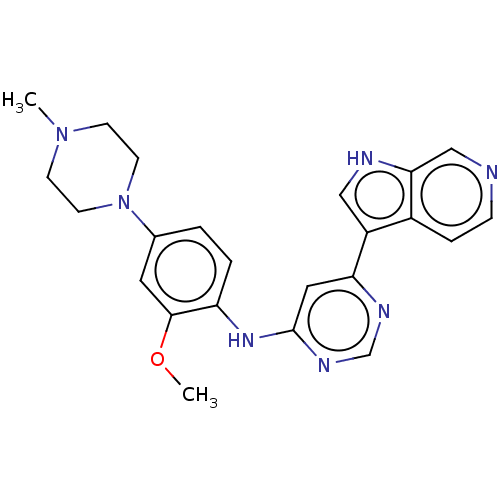
(CHEMBL3421963)Show SMILES COc1cc(ccc1Nc1cc(ncn1)-c1c[nH]c2cnccc12)N1CCN(C)CC1 Show InChI InChI=1S/C23H25N7O/c1-29-7-9-30(10-8-29)16-3-4-19(22(11-16)31-2)28-23-12-20(26-15-27-23)18-13-25-21-14-24-6-5-17(18)21/h3-6,11-15,25H,7-10H2,1-2H3,(H,26,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Dyrk1B (unknown origin) using FITC-Asp-His-Thr-Gly-Phe-Leu-Thr-Glu-Tyr-Val-Ala-Thr-Arg-NH2 as substrate after 60 mins |
J Med Chem 58: 2834-44 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00098
BindingDB Entry DOI: 10.7270/Q23B61VW |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50183849
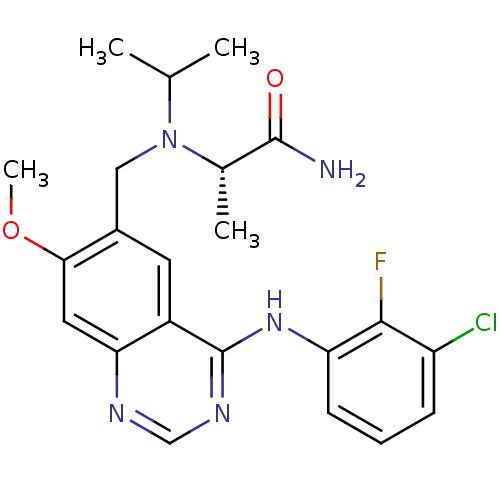
((S)-2-(((4-(3-chloro-2-fluorophenylamino)-7-methox...)Show SMILES COc1cc2ncnc(Nc3cccc(Cl)c3F)c2cc1CN(C(C)C)[C@@H](C)C(N)=O Show InChI InChI=1S/C22H25ClFN5O2/c1-12(2)29(13(3)21(25)30)10-14-8-15-18(9-19(14)31-4)26-11-27-22(15)28-17-7-5-6-16(23)20(17)24/h5-9,11-13H,10H2,1-4H3,(H2,25,30)(H,26,27,28)/t13-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibition of EGFR in presence of 2 uM ATP |
Bioorg Med Chem Lett 16: 2672-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.025
BindingDB Entry DOI: 10.7270/Q25H7FVK |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50373856

(CHEMBL271705)Show SMILES Clc1cc(Nc2ncnc3n[nH]c(OCCN4CCOCC4)c23)ccc1OCc1ccccn1 Show InChI InChI=1S/C23H24ClN7O3/c24-18-13-16(4-5-19(18)34-14-17-3-1-2-6-25-17)28-21-20-22(27-15-26-21)29-30-23(20)33-12-9-31-7-10-32-11-8-31/h1-6,13,15H,7-12,14H2,(H2,26,27,28,29,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 18: 959-62 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.035
BindingDB Entry DOI: 10.7270/Q21N81ZG |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50190001
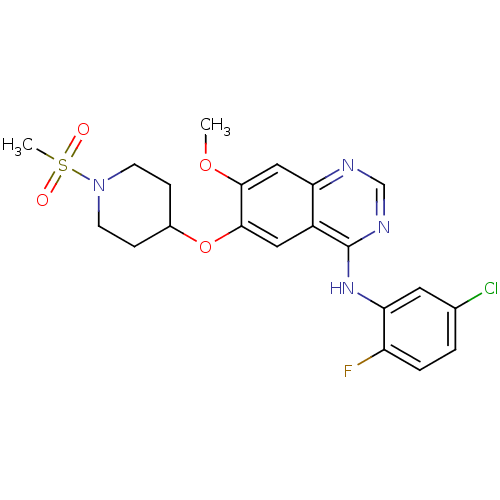
(CHEMBL213007 | N-(5-chloro-2-fluorophenyl)-7-metho...)Show SMILES COc1cc2ncnc(Nc3cc(Cl)ccc3F)c2cc1OC1CCN(CC1)S(C)(=O)=O Show InChI InChI=1S/C21H22ClFN4O4S/c1-30-19-11-17-15(10-20(19)31-14-5-7-27(8-6-14)32(2,28)29)21(25-12-24-17)26-18-9-13(22)3-4-16(18)23/h3-4,9-12,14H,5-8H2,1-2H3,(H,24,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 16: 4908-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.054
BindingDB Entry DOI: 10.7270/Q2R78DTM |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50373853
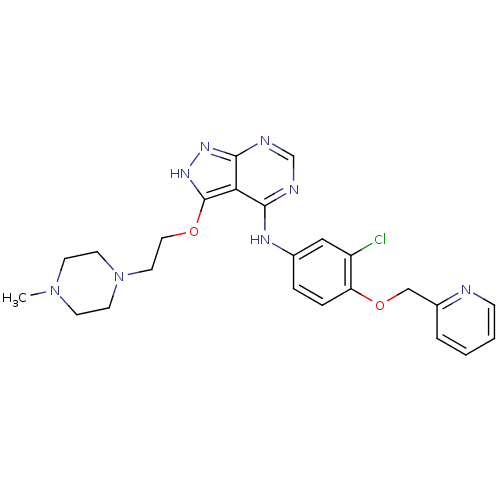
(CHEMBL258282)Show SMILES CN1CCN(CCOc2[nH]nc3ncnc(Nc4ccc(OCc5ccccn5)c(Cl)c4)c23)CC1 Show InChI InChI=1S/C24H27ClN8O2/c1-32-8-10-33(11-9-32)12-13-34-24-21-22(27-16-28-23(21)30-31-24)29-17-5-6-20(19(25)14-17)35-15-18-4-2-3-7-26-18/h2-7,14,16H,8-13,15H2,1H3,(H2,27,28,29,30,31) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of erbB2 |
Bioorg Med Chem Lett 18: 959-62 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.035
BindingDB Entry DOI: 10.7270/Q21N81ZG |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50125054

(CHEMBL3623003 | US10130617, Example 2 | WO-2014/19...)Show SMILES C[C@@H]1Cc2c([nH]c3ccccc23)[C@H](N1CC(C)(C)F)c1ccc(\C=C\C(O)=O)cc1 |r| Show InChI InChI=1S/C25H27FN2O2/c1-16-14-20-19-6-4-5-7-21(19)27-23(20)24(28(16)15-25(2,3)26)18-11-8-17(9-12-18)10-13-22(29)30/h4-13,16,24,27H,14-15H2,1-3H3,(H,29,30)/b13-10+/t16-,24-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to ERalpha receptor (unknown origin) |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1B
(Homo sapiens (Human)) | BDBM50081186
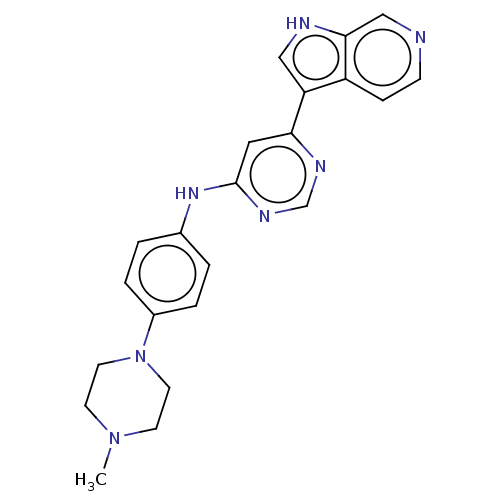
(CHEMBL3421962)Show SMILES CN1CCN(CC1)c1ccc(Nc2cc(ncn2)-c2c[nH]c3cnccc23)cc1 Show InChI InChI=1S/C22H23N7/c1-28-8-10-29(11-9-28)17-4-2-16(3-5-17)27-22-12-20(25-15-26-22)19-13-24-21-14-23-7-6-18(19)21/h2-7,12-15,24H,8-11H2,1H3,(H,25,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Dyrk1B (unknown origin) using FITC-Asp-His-Thr-Gly-Phe-Leu-Thr-Glu-Tyr-Val-Ala-Thr-Arg-NH2 as substrate after 60 mins |
J Med Chem 58: 2834-44 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00098
BindingDB Entry DOI: 10.7270/Q23B61VW |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50029670

(CHEMBL2426277 | US10227342, Example 26)Show SMILES COc1cc(N2CCN(C)CC2)c(NC(=O)C=C)cc1Nc1ncc(Cl)c(n1)-c1cnn2ccccc12 Show InChI InChI=1S/C26H27ClN8O2/c1-4-24(36)30-19-13-20(23(37-3)14-22(19)34-11-9-33(2)10-12-34)31-26-28-16-18(27)25(32-26)17-15-29-35-8-6-5-7-21(17)35/h4-8,13-16H,1,9-12H2,2-3H3,(H,30,36)(H,28,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R (unknown origin) |
J Med Chem 57: 8249-67 (2014)
Article DOI: 10.1021/jm500973a
BindingDB Entry DOI: 10.7270/Q2ZS2Z4C |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50190002

(CHEMBL387265 | N-(3-chloro-2-fluorophenyl)-7-metho...)Show SMILES COc1cc2ncnc(Nc3cccc(Cl)c3F)c2cc1OC1CCN(CC1)S(C)(=O)=O Show InChI InChI=1S/C21H22ClFN4O4S/c1-30-18-11-17-14(10-19(18)31-13-6-8-27(9-7-13)32(2,28)29)21(25-12-24-17)26-16-5-3-4-15(22)20(16)23/h3-5,10-13H,6-9H2,1-2H3,(H,24,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 16: 4908-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.054
BindingDB Entry DOI: 10.7270/Q2R78DTM |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50189995

(CHEMBL213874 | N-(3-chloro-2-fluorophenyl)-7-metho...)Show SMILES COc1cc2ncnc(Nc3cccc(Cl)c3F)c2cc1OCC1CCN(C)CC1 Show InChI InChI=1S/C22H24ClFN4O2/c1-28-8-6-14(7-9-28)12-30-20-10-15-18(11-19(20)29-2)25-13-26-22(15)27-17-5-3-4-16(23)21(17)24/h3-5,10-11,13-14H,6-9,12H2,1-2H3,(H,25,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 16: 4908-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.054
BindingDB Entry DOI: 10.7270/Q2R78DTM |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50190000

(CHEMBL214798 | N-(3-chloro-2-fluorophenyl)-7-metho...)Show SMILES COc1cc2ncnc(Nc3cccc(Cl)c3F)c2cc1OC1CCN(C)CC1 Show InChI InChI=1S/C21H22ClFN4O2/c1-27-8-6-13(7-9-27)29-19-10-14-17(11-18(19)28-2)24-12-25-21(14)26-16-5-3-4-15(22)20(16)23/h3-5,10-13H,6-9H2,1-2H3,(H,24,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 16: 4908-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.054
BindingDB Entry DOI: 10.7270/Q2R78DTM |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50427336
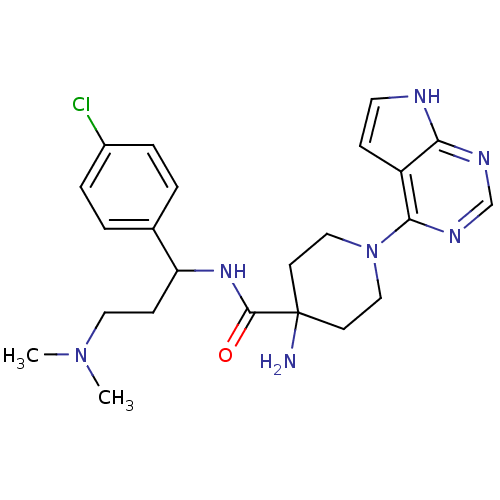
(CHEMBL2325992 | US10654855, Example 11 | US1123609...)Show SMILES CN(C)CCC(NC(=O)C1(N)CCN(CC1)c1ncnc2[nH]ccc12)c1ccc(Cl)cc1 Show InChI InChI=1S/C23H30ClN7O/c1-30(2)12-8-19(16-3-5-17(24)6-4-16)29-22(32)23(25)9-13-31(14-10-23)21-18-7-11-26-20(18)27-15-28-21/h3-7,11,15,19H,8-10,12-14,25H2,1-2H3,(H,29,32)(H,26,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Akt1 (unknown origin) using 5-FAM-labeled peptide as substrate after 1 hr by caliper off-chip incubation mobility shift ass... |
J Med Chem 56: 2059-73 (2013)
Article DOI: 10.1021/jm301762v
BindingDB Entry DOI: 10.7270/Q2QR4ZFN |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50189993

(2-(4-(4-(3-chloro-2-fluorophenylamino)-7-methoxyqu...)Show SMILES COc1cc2ncnc(Nc3cccc(Cl)c3F)c2cc1OC1CCN(CC(N)=O)CC1 Show InChI InChI=1S/C22H23ClFN5O3/c1-31-18-10-17-14(9-19(18)32-13-5-7-29(8-6-13)11-20(25)30)22(27-12-26-17)28-16-4-2-3-15(23)21(16)24/h2-4,9-10,12-13H,5-8,11H2,1H3,(H2,25,30)(H,26,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 16: 4908-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.054
BindingDB Entry DOI: 10.7270/Q2R78DTM |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50222489

((R)-N-(2-(4-(3-chloro-4-(pyridin-2-ylmethoxy)pheny...)Show SMILES C[C@H](CN(C)C(=O)CO)Oc1cccc2ncnc(Nc3ccc(OCc4ccccn4)c(Cl)c3)c12 Show InChI InChI=1S/C26H26ClN5O4/c1-17(13-32(2)24(34)14-33)36-23-8-5-7-21-25(23)26(30-16-29-21)31-18-9-10-22(20(27)12-18)35-15-19-6-3-4-11-28-19/h3-12,16-17,33H,13-15H2,1-2H3,(H,29,30,31)/t17-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 17: 6326-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.073
BindingDB Entry DOI: 10.7270/Q2CF9PTZ |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50371358
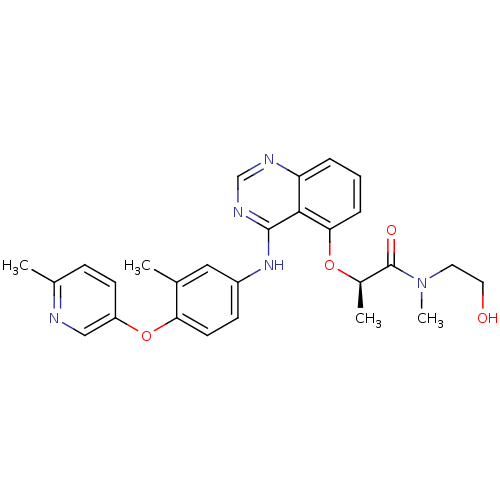
(CHEMBL257478)Show SMILES C[C@@H](Oc1cccc2ncnc(Nc3ccc(Oc4ccc(C)nc4)c(C)c3)c12)C(=O)N(C)CCO Show InChI InChI=1S/C27H29N5O4/c1-17-14-20(9-11-23(17)36-21-10-8-18(2)28-15-21)31-26-25-22(29-16-30-26)6-5-7-24(25)35-19(3)27(34)32(4)12-13-33/h5-11,14-16,19,33H,12-13H2,1-4H3,(H,29,30,31)/t19-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibition of ebrB2 |
Bioorg Med Chem Lett 18: 674-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.052
BindingDB Entry DOI: 10.7270/Q2736RSH |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50373862

(CHEMBL402553)Show SMILES Clc1cc(Nc2ncnc3n[nH]c(OCCN4CCCC4)c23)ccc1OCc1ccccn1 Show InChI InChI=1S/C23H24ClN7O2/c24-18-13-16(6-7-19(18)33-14-17-5-1-2-8-25-17)28-21-20-22(27-15-26-21)29-30-23(20)32-12-11-31-9-3-4-10-31/h1-2,5-8,13,15H,3-4,9-12,14H2,(H2,26,27,28,29,30) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of erbB2 |
Bioorg Med Chem Lett 18: 959-62 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.035
BindingDB Entry DOI: 10.7270/Q21N81ZG |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50222495

((S)-1-(2-((4-(3-chloro-4-(pyridin-2-ylmethoxy)phen...)Show SMILES OCC(=O)N1CCC[C@H]1COc1cccc2ncnc(Nc3ccc(OCc4ccccn4)c(Cl)c3)c12 Show InChI InChI=1S/C27H26ClN5O4/c28-21-13-18(9-10-23(21)36-15-19-5-1-2-11-29-19)32-27-26-22(30-17-31-27)7-3-8-24(26)37-16-20-6-4-12-33(20)25(35)14-34/h1-3,5,7-11,13,17,20,34H,4,6,12,14-16H2,(H,30,31,32)/t20-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ErbB2 kinase |
Bioorg Med Chem Lett 17: 6326-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.073
BindingDB Entry DOI: 10.7270/Q2CF9PTZ |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50125077

(CHEMBL3623001)Show SMILES CC(C)CN1[C@H](C)Cc2c([nH]c3ccccc23)[C@H]1c1ccc(\C=C\C(O)=O)cc1 |r| Show InChI InChI=1S/C25H28N2O2/c1-16(2)15-27-17(3)14-21-20-6-4-5-7-22(20)26-24(21)25(27)19-11-8-18(9-12-19)10-13-23(28)29/h4-13,16-17,25-26H,14-15H2,1-3H3,(H,28,29)/b13-10+/t17-,25-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at ERalpha receptor in human MCF7 cells |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1B
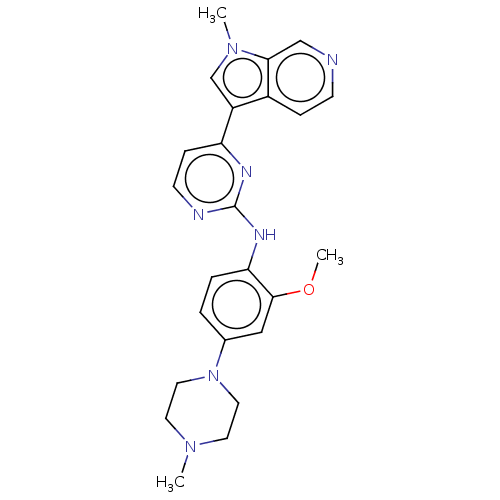
(Homo sapiens (Human)) | BDBM50081174
(CHEMBL3421968)Show SMILES COc1cc(ccc1Nc1nccc(n1)-c1cn(C)c2cnccc12)N1CCN(C)CC1 Show InChI InChI=1S/C24H27N7O/c1-29-10-12-31(13-11-29)17-4-5-21(23(14-17)32-3)28-24-26-9-7-20(27-24)19-16-30(2)22-15-25-8-6-18(19)22/h4-9,14-16H,10-13H2,1-3H3,(H,26,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Dyrk1B (unknown origin) using FITC-Asp-His-Thr-Gly-Phe-Leu-Thr-Glu-Tyr-Val-Ala-Thr-Arg-NH2 as substrate after 60 mins |
J Med Chem 58: 2834-44 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00098
BindingDB Entry DOI: 10.7270/Q23B61VW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50373869

(CHEMBL258270)Show SMILES CC(=O)N1CCN(CCOc2[nH]nc3ncnc(Nc4ccc(OCc5ccccn5)c(Cl)c4)c23)CC1 Show InChI InChI=1S/C25H27ClN8O3/c1-17(35)34-10-8-33(9-11-34)12-13-36-25-22-23(28-16-29-24(22)31-32-25)30-18-5-6-21(20(26)14-18)37-15-19-4-2-3-7-27-19/h2-7,14,16H,8-13,15H2,1H3,(H2,28,29,30,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 18: 959-62 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.035
BindingDB Entry DOI: 10.7270/Q21N81ZG |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50179758
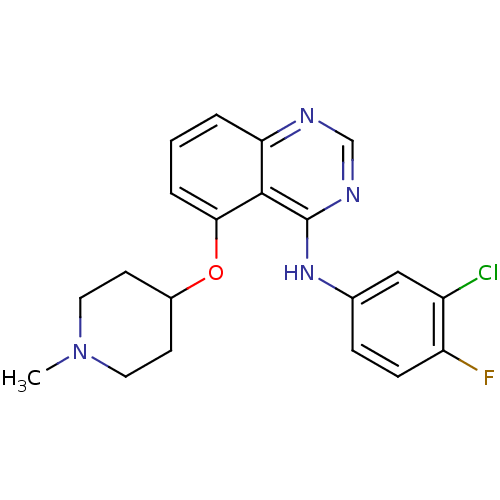
(CHEMBL203725 | N-(3-chloro-4-fluorophenyl)-5-(1-me...)Show SMILES CN1CCC(CC1)Oc1cccc2ncnc(Nc3ccc(F)c(Cl)c3)c12 Show InChI InChI=1S/C20H20ClFN4O/c1-26-9-7-14(8-10-26)27-18-4-2-3-17-19(18)20(24-12-23-17)25-13-5-6-16(22)15(21)11-13/h2-6,11-12,14H,7-10H2,1H3,(H,23,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibitory activity against EGFR |
Bioorg Med Chem Lett 16: 1633-7 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.028
BindingDB Entry DOI: 10.7270/Q2P84BGH |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1B
(Homo sapiens (Human)) | BDBM50081188
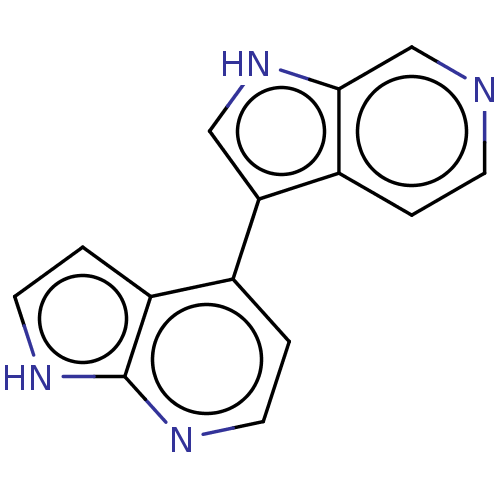
(CHEMBL3421981)Show InChI InChI=1S/C14H10N4/c1-4-15-8-13-10(1)12(7-18-13)9-2-5-16-14-11(9)3-6-17-14/h1-8,18H,(H,16,17) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Dyrk1B (unknown origin) using FITC-Asp-His-Thr-Gly-Phe-Leu-Thr-Glu-Tyr-Val-Ala-Thr-Arg-NH2 as substrate after 60 mins |
J Med Chem 58: 2834-44 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00098
BindingDB Entry DOI: 10.7270/Q23B61VW |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50373864

(CHEMBL402339)Show SMILES COc1cc(Nc2ncnc3n[nH]c(OCCN4CCC(O)CC4)c23)ccc1OCc1cccc(F)c1 Show InChI InChI=1S/C26H29FN6O4/c1-35-22-14-19(5-6-21(22)37-15-17-3-2-4-18(27)13-17)30-24-23-25(29-16-28-24)31-32-26(23)36-12-11-33-9-7-20(34)8-10-33/h2-6,13-14,16,20,34H,7-12,15H2,1H3,(H2,28,29,30,31,32) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of erbB2 |
Bioorg Med Chem Lett 18: 959-62 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.035
BindingDB Entry DOI: 10.7270/Q21N81ZG |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50427335
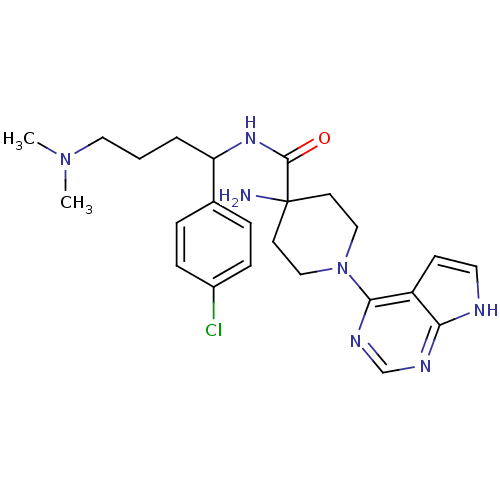
(CHEMBL2325993 | US10654855, Example 25 | US1123609...)Show SMILES CN(C)CCCC(NC(=O)C1(N)CCN(CC1)c1ncnc2[nH]ccc12)c1ccc(Cl)cc1 Show InChI InChI=1S/C24H32ClN7O/c1-31(2)13-3-4-20(17-5-7-18(25)8-6-17)30-23(33)24(26)10-14-32(15-11-24)22-19-9-12-27-21(19)28-16-29-22/h5-9,12,16,20H,3-4,10-11,13-15,26H2,1-2H3,(H,30,33)(H,27,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Akt1 (unknown origin) using 5-FAM-labeled peptide as substrate after 1 hr by caliper off-chip incubation mobility shift ass... |
J Med Chem 56: 2059-73 (2013)
Article DOI: 10.1021/jm301762v
BindingDB Entry DOI: 10.7270/Q2QR4ZFN |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50189990

(CHEMBL215786 | N-(3-chloro-4-fluorophenyl)-7-metho...)Show SMILES COc1cc2ncnc(Nc3ccc(F)c(Cl)c3)c2cc1OCC1CCN(C)CC1 Show InChI InChI=1S/C22H24ClFN4O2/c1-28-7-5-14(6-8-28)12-30-21-10-16-19(11-20(21)29-2)25-13-26-22(16)27-15-3-4-18(24)17(23)9-15/h3-4,9-11,13-14H,5-8,12H2,1-2H3,(H,25,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 16: 4908-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.054
BindingDB Entry DOI: 10.7270/Q2R78DTM |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50189991

(CHEMBL214857 | N-(3-chloro-2-fluorophenyl)-7-metho...)Show SMILES COCCN1CCC(CC1)Oc1cc2c(Nc3cccc(Cl)c3F)ncnc2cc1OC Show InChI InChI=1S/C23H26ClFN4O3/c1-30-11-10-29-8-6-15(7-9-29)32-21-12-16-19(13-20(21)31-2)26-14-27-23(16)28-18-5-3-4-17(24)22(18)25/h3-5,12-15H,6-11H2,1-2H3,(H,26,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 16: 4908-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.054
BindingDB Entry DOI: 10.7270/Q2R78DTM |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM256836
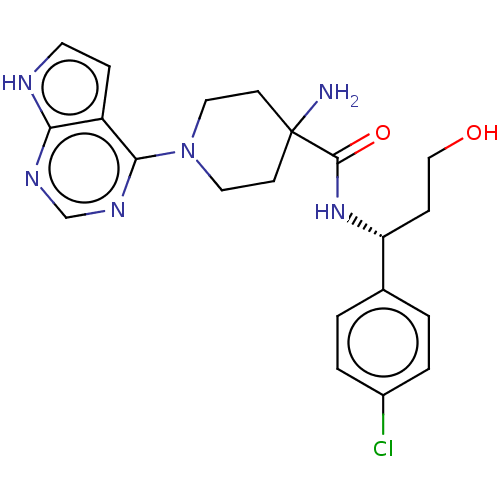
(BDBM50427349 | US10654855, Example 15 | US11236095...)Show SMILES NC1(CCN(CC1)c1ncnc2[nH]ccc12)C(=O)N[C@H](CCO)c1ccc(Cl)cc1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Akt1 (unknown origin) using 5-FAM-labeled peptide as substrate after 1 hr by caliper off-chip incubation mobility shift ass... |
J Med Chem 56: 2059-73 (2013)
Article DOI: 10.1021/jm301762v
BindingDB Entry DOI: 10.7270/Q2QR4ZFN |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50373852

(CHEMBL257430)Show SMILES Clc1cc(Nc2ncnc3n[nH]c(OCCN4CCNCC4)c23)ccc1OCc1ccccn1 Show InChI InChI=1S/C23H25ClN8O2/c24-18-13-16(4-5-19(18)34-14-17-3-1-2-6-26-17)29-21-20-22(28-15-27-21)30-31-23(20)33-12-11-32-9-7-25-8-10-32/h1-6,13,15,25H,7-12,14H2,(H2,27,28,29,30,31) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of erbB2 |
Bioorg Med Chem Lett 18: 959-62 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.035
BindingDB Entry DOI: 10.7270/Q21N81ZG |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50373857
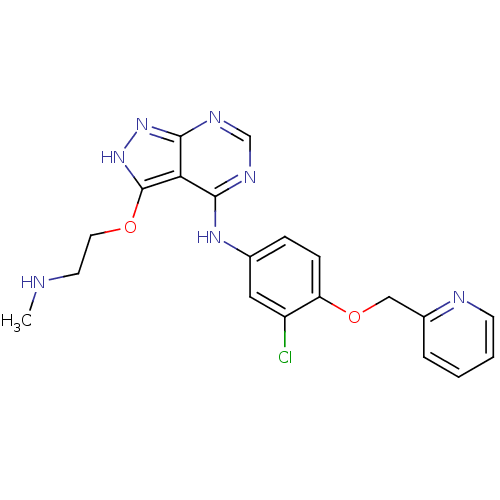
(CHEMBL255135)Show SMILES CNCCOc1[nH]nc2ncnc(Nc3ccc(OCc4ccccn4)c(Cl)c3)c12 Show InChI InChI=1S/C20H20ClN7O2/c1-22-8-9-29-20-17-18(24-12-25-19(17)27-28-20)26-13-5-6-16(15(21)10-13)30-11-14-4-2-3-7-23-14/h2-7,10,12,22H,8-9,11H2,1H3,(H2,24,25,26,27,28) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of erbB2 |
Bioorg Med Chem Lett 18: 959-62 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.035
BindingDB Entry DOI: 10.7270/Q21N81ZG |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50222493

((R)-N-(1-(4-(3-chloro-4-(pyridin-2-ylmethoxy)pheny...)Show SMILES C[C@H](COc1cccc2ncnc(Nc3ccc(OCc4ccccn4)c(Cl)c3)c12)N(C)C(=O)CO Show InChI InChI=1S/C26H26ClN5O4/c1-17(32(2)24(34)13-33)14-35-23-8-5-7-21-25(23)26(30-16-29-21)31-18-9-10-22(20(27)12-18)36-15-19-6-3-4-11-28-19/h3-12,16-17,33H,13-15H2,1-2H3,(H,29,30,31)/t17-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 17: 6326-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.073
BindingDB Entry DOI: 10.7270/Q2CF9PTZ |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50427361
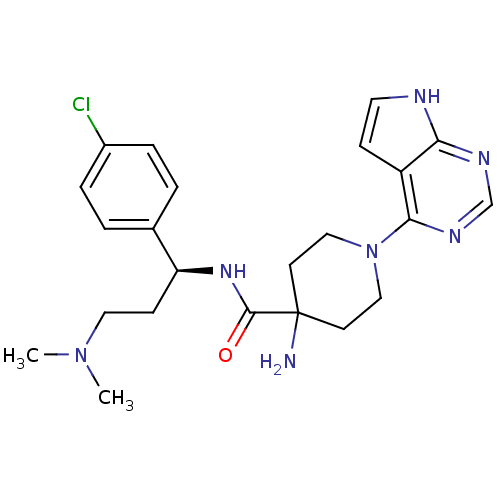
(CHEMBL2325727 | US10654855, Example 11A | US112360...)Show SMILES CN(C)CC[C@H](NC(=O)C1(N)CCN(CC1)c1ncnc2[nH]ccc12)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C23H30ClN7O/c1-30(2)12-8-19(16-3-5-17(24)6-4-16)29-22(32)23(25)9-13-31(14-10-23)21-18-7-11-26-20(18)27-15-28-21/h3-7,11,15,19H,8-10,12-14,25H2,1-2H3,(H,29,32)(H,26,27,28)/t19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Akt1 (unknown origin) using 5-FAM-labeled peptide as substrate after 1 hr by caliper off-chip incubation mobility shift ass... |
J Med Chem 56: 2059-73 (2013)
Article DOI: 10.1021/jm301762v
BindingDB Entry DOI: 10.7270/Q2QR4ZFN |
More data for this
Ligand-Target Pair | |
Estrogen receptor
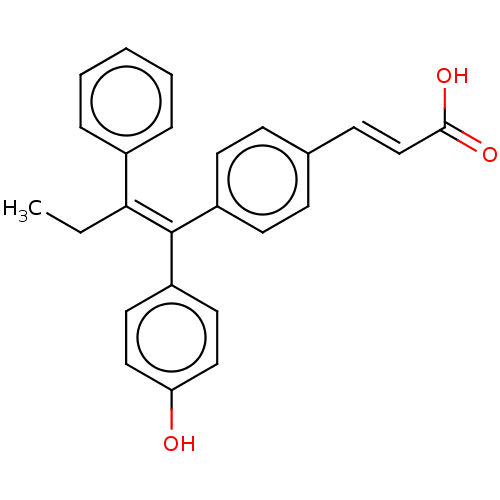
(Homo sapiens (Human)) | BDBM50041611
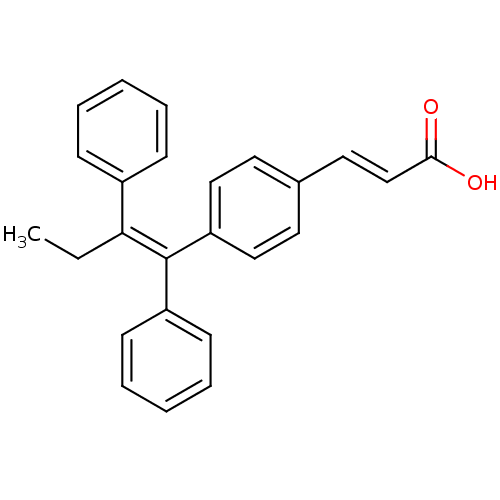
((2E)-3-{4-[(1E)-1,2-DIPHENYLBUT-1-ENYL]PHENYL}ACRY...)Show SMILES CC\C(=C(/c1ccccc1)c1ccc(\C=C\C(O)=O)cc1)c1ccccc1 Show InChI InChI=1S/C25H22O2/c1-2-23(20-9-5-3-6-10-20)25(21-11-7-4-8-12-21)22-16-13-19(14-17-22)15-18-24(26)27/h3-18H,2H2,1H3,(H,26,27)/b18-15+,25-23- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to ERalpha receptor (unknown origin) |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50125054

(CHEMBL3623003 | US10130617, Example 2 | WO-2014/19...)Show SMILES C[C@@H]1Cc2c([nH]c3ccccc23)[C@H](N1CC(C)(C)F)c1ccc(\C=C\C(O)=O)cc1 |r| Show InChI InChI=1S/C25H27FN2O2/c1-16-14-20-19-6-4-5-7-21(19)27-23(20)24(28(16)15-25(2,3)26)18-11-8-17(9-12-18)10-13-22(29)30/h4-13,16,24,27H,14-15H2,1-3H3,(H,29,30)/b13-10+/t16-,24-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at progesterone receptor in human MCF cells assessed as estradiol-induced receptor response |
J Med Chem 58: 8128-40 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00984
BindingDB Entry DOI: 10.7270/Q2F76FCN |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data