

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prothrombin (Homo sapiens (Human)) | BDBM254888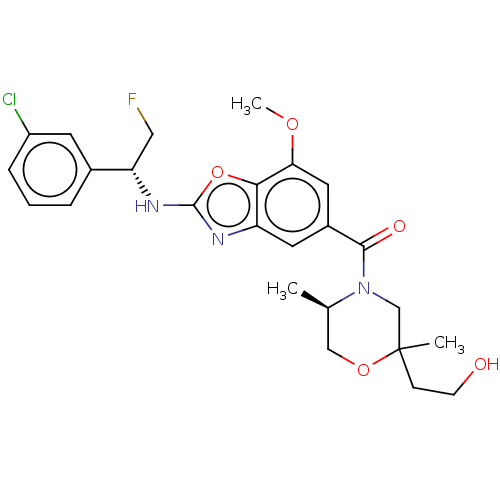 (US9493472, 11) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s... | US Patent US9493472 (2016) BindingDB Entry DOI: 10.7270/Q2B56HPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM254887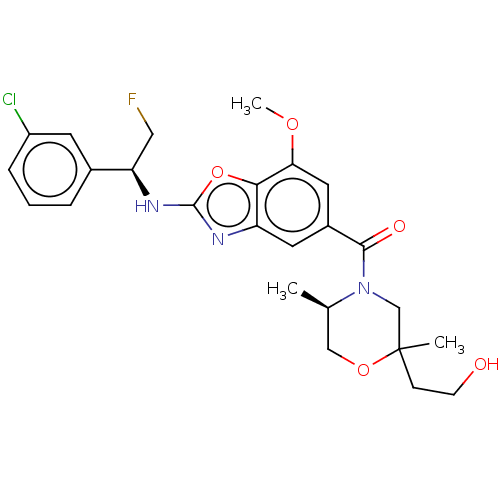 (US9493472, 10) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of purified human plasma thrombin using Boc-Asp(OBzl)-Pro-Arg-AMC substrate incubated for 30 mins by fluorescence based assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01035 BindingDB Entry DOI: 10.7270/Q2Z60SND | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM228279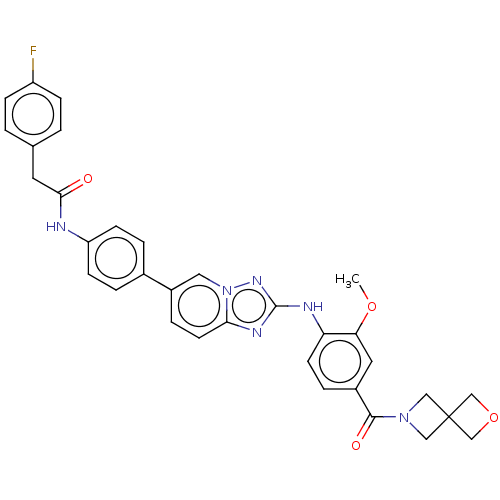 (US9555022, Example 02.17) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Inhibition of GST-tagged recombinant human MPS1 expressed in baculovirus expression system using biotin-Ahx-PWDPDDADITEILG as substrate preincubated ... | J Med Chem 63: 8025-8042 (2020) Article DOI: 10.1021/acs.jmedchem.9b02035 BindingDB Entry DOI: 10.7270/Q2MP56VP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM254889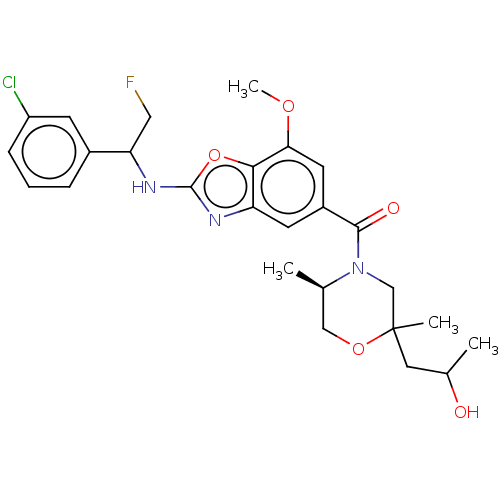 (US9493472, 12) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s... | US Patent US9493472 (2016) BindingDB Entry DOI: 10.7270/Q2B56HPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Mus musculus) | BDBM50591456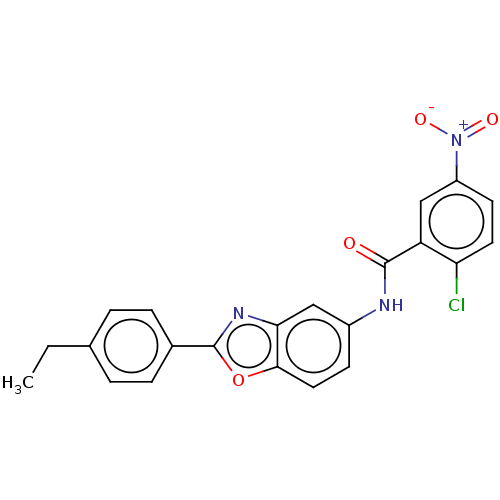 (CHEMBL5207130) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c01379 BindingDB Entry DOI: 10.7270/Q2W099XX | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM228278 (US9555022, Example 02.16) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Inhibition of GST-tagged recombinant human MPS1 expressed in baculovirus expression system using biotin-Ahx-PWDPDDADITEILG as substrate preincubated ... | J Med Chem 63: 8025-8042 (2020) Article DOI: 10.1021/acs.jmedchem.9b02035 BindingDB Entry DOI: 10.7270/Q2MP56VP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM254905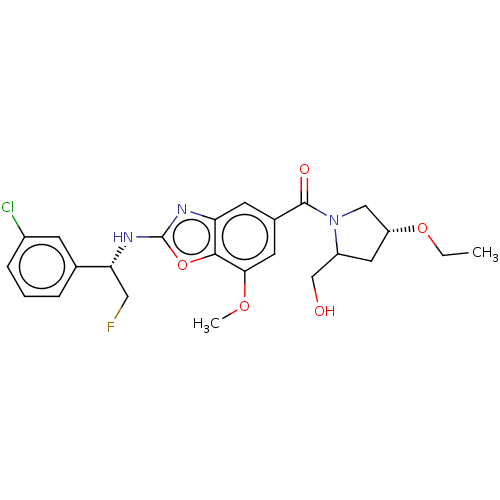 (US9493472, 27) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s... | US Patent US9493472 (2016) BindingDB Entry DOI: 10.7270/Q2B56HPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50591460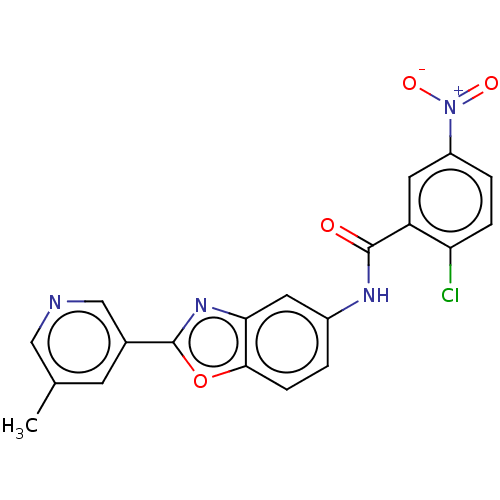 (CHEMBL5191837) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c01379 BindingDB Entry DOI: 10.7270/Q2W099XX | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50591456 (CHEMBL5207130) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c01379 BindingDB Entry DOI: 10.7270/Q2W099XX | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM254904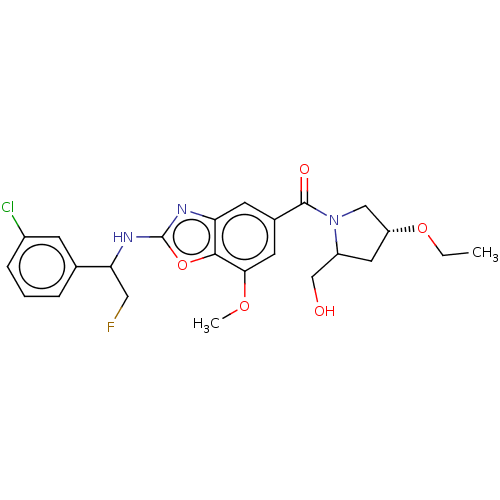 (US9493472, 26) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s... | US Patent US9493472 (2016) BindingDB Entry DOI: 10.7270/Q2B56HPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM254886 (US9493472, 9) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s... | US Patent US9493472 (2016) BindingDB Entry DOI: 10.7270/Q2B56HPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM50542581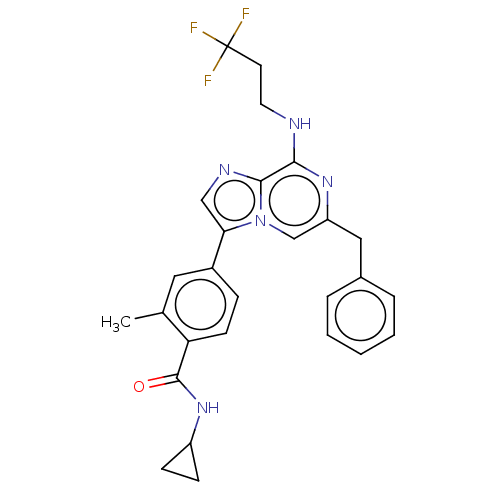 (CHEMBL4642174) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Inhibition of GST-tagged recombinant human MPS1 expressed in baculovirus expression system using biotin-Ahx-PWDPDDADITEILG as substrate preincubated ... | J Med Chem 63: 8025-8042 (2020) Article DOI: 10.1021/acs.jmedchem.9b02035 BindingDB Entry DOI: 10.7270/Q2MP56VP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM228270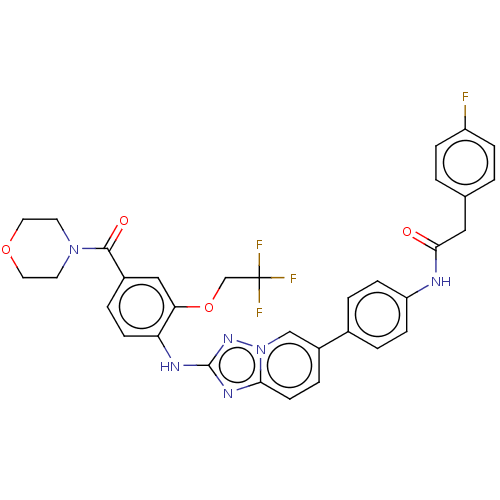 (US9555022, Example 02.09) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Inhibition of GST-tagged recombinant human MPS1 expressed in baculovirus expression system using biotin-Ahx-PWDPDDADITEILG as substrate preincubated ... | J Med Chem 63: 8025-8042 (2020) Article DOI: 10.1021/acs.jmedchem.9b02035 BindingDB Entry DOI: 10.7270/Q2MP56VP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50591479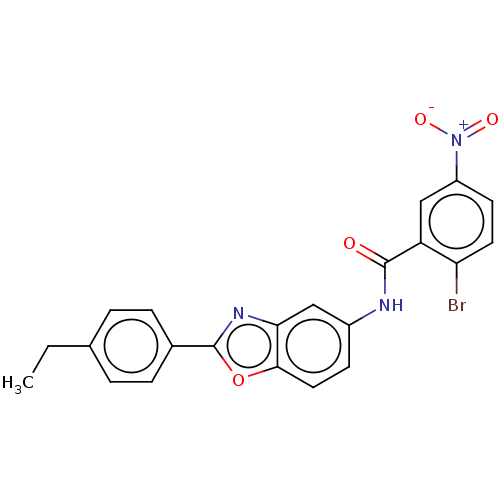 (CHEMBL5187164) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c01379 BindingDB Entry DOI: 10.7270/Q2W099XX | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50591459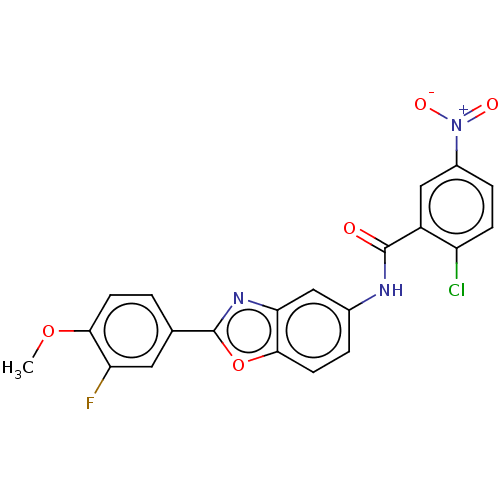 (CHEMBL5179281) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c01379 BindingDB Entry DOI: 10.7270/Q2W099XX | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50591461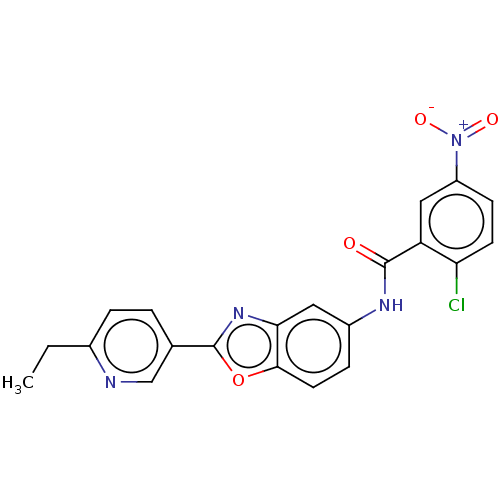 (CHEMBL5206512) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c01379 BindingDB Entry DOI: 10.7270/Q2W099XX | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM258444 (US9512130, 2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Inhibition of MPS1 in human HeLa cells assessed as reduction in spindle assembly checkpoint incubated for 4 hrs by p-histone H3/Hoechst 33342 stainin... | J Med Chem 63: 8025-8042 (2020) Article DOI: 10.1021/acs.jmedchem.9b02035 BindingDB Entry DOI: 10.7270/Q2MP56VP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM50547821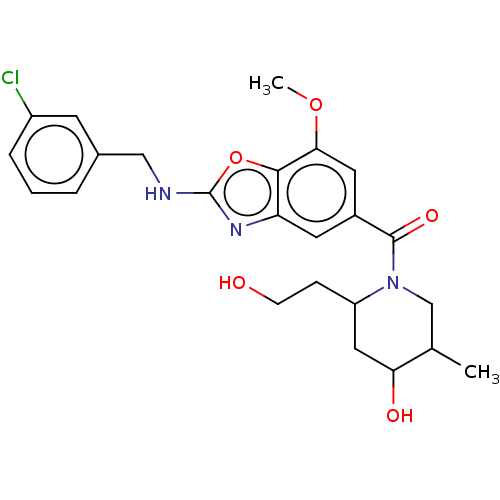 (CHEMBL4761137) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of purified human plasma thrombin using Boc-Asp(OBzl)-Pro-Arg-AMC substrate incubated for 30 mins by fluorescence based assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01035 BindingDB Entry DOI: 10.7270/Q2Z60SND | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50591480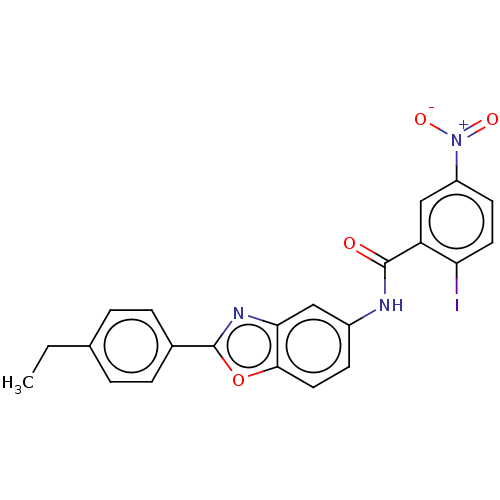 (CHEMBL5207464) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c01379 BindingDB Entry DOI: 10.7270/Q2W099XX | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM50542592 (CHEMBL4645348) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Inhibition of GST-tagged recombinant human MPS1 expressed in baculovirus expression system using biotin-Ahx-PWDPDDADITEILG as substrate preincubated ... | J Med Chem 63: 8025-8042 (2020) Article DOI: 10.1021/acs.jmedchem.9b02035 BindingDB Entry DOI: 10.7270/Q2MP56VP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM254899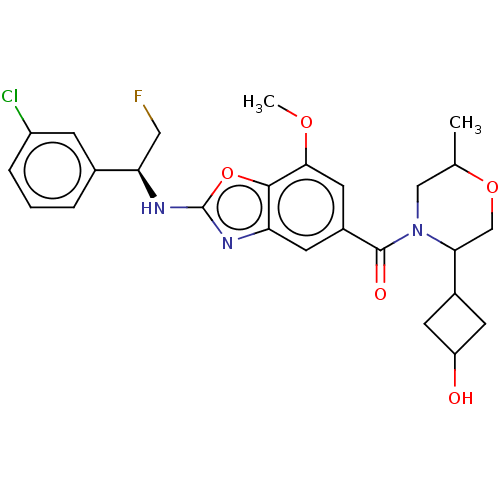 (US9493472, 21) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of purified human plasma thrombin using Boc-Asp(OBzl)-Pro-Arg-AMC substrate incubated for 30 mins by fluorescence based assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01035 BindingDB Entry DOI: 10.7270/Q2Z60SND | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM259464 (US9512126, 1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Inhibition of MPS1 in human HeLa cells assessed as reduction in spindle assembly checkpoint incubated for 4 hrs by p-histone H3/Hoechst 33342 stainin... | J Med Chem 63: 8025-8042 (2020) Article DOI: 10.1021/acs.jmedchem.9b02035 BindingDB Entry DOI: 10.7270/Q2MP56VP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM254896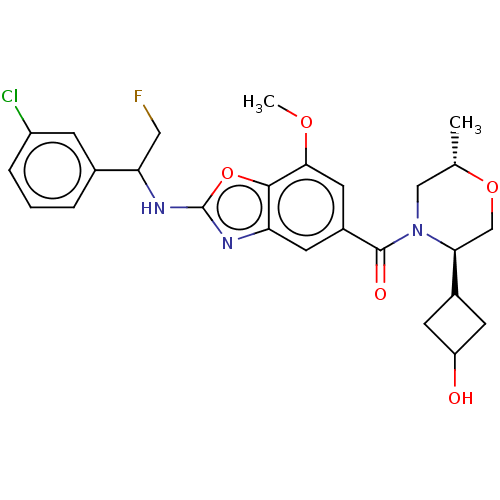 (US9493472, 18) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s... | US Patent US9493472 (2016) BindingDB Entry DOI: 10.7270/Q2B56HPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50591466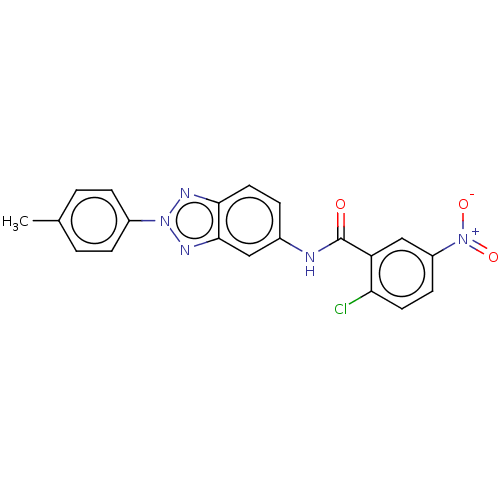 (CHEMBL5204936) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c01379 BindingDB Entry DOI: 10.7270/Q2W099XX | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase TBK1 (Homo sapiens (Human)) | BDBM478724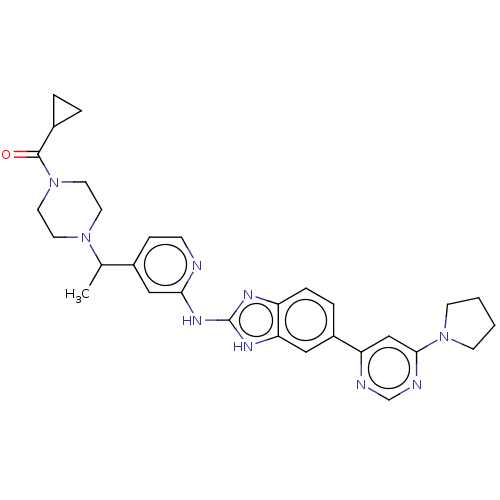 (US10894784, Example 117.02 | cyclopropyl(4-{(1R or...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description Recombinant full-length N-terminally His-tagged human TBK1, expressed in insect cells and purified by Ni-NTA affinity chromatography, was purchased f... | US Patent US10894784 (2021) BindingDB Entry DOI: 10.7270/Q2PV6PFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM254895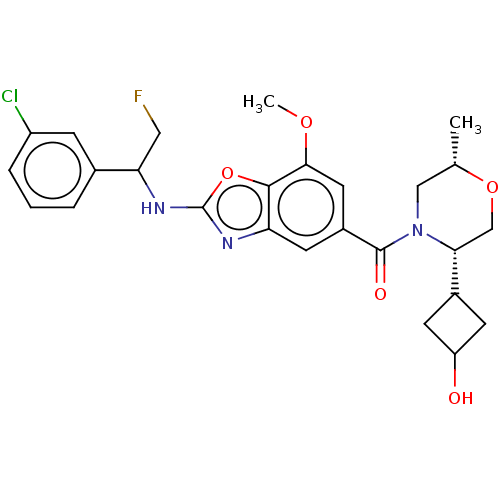 (US9493472, 17) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s... | US Patent US9493472 (2016) BindingDB Entry DOI: 10.7270/Q2B56HPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM254911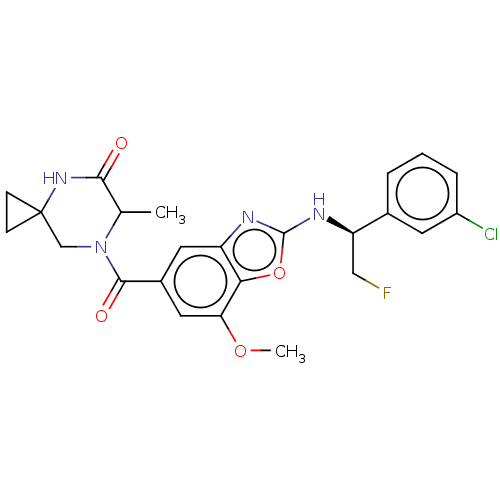 (US9493472, 33) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s... | US Patent US9493472 (2016) BindingDB Entry DOI: 10.7270/Q2B56HPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM50547825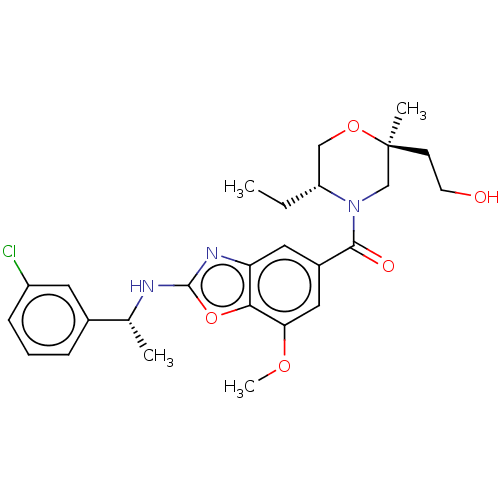 (CHEMBL4749829) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of purified human plasma thrombin using Boc-Asp(OBzl)-Pro-Arg-AMC substrate incubated for 30 mins by fluorescence based assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01035 BindingDB Entry DOI: 10.7270/Q2Z60SND | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM50542591 (CHEMBL4640247) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Inhibition of MPS1 in human HeLa cells assessed as reduction in spindle assembly checkpoint incubated for 4 hrs by p-histone H3/Hoechst 33342 stainin... | J Med Chem 63: 8025-8042 (2020) Article DOI: 10.1021/acs.jmedchem.9b02035 BindingDB Entry DOI: 10.7270/Q2MP56VP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50591456 (CHEMBL5207130) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c01379 BindingDB Entry DOI: 10.7270/Q2W099XX | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM254894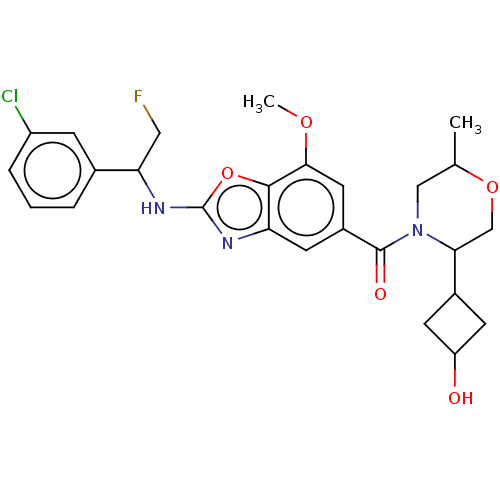 (US9493472, 16) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s... | US Patent US9493472 (2016) BindingDB Entry DOI: 10.7270/Q2B56HPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase TBK1 (Homo sapiens (Human)) | BDBM478844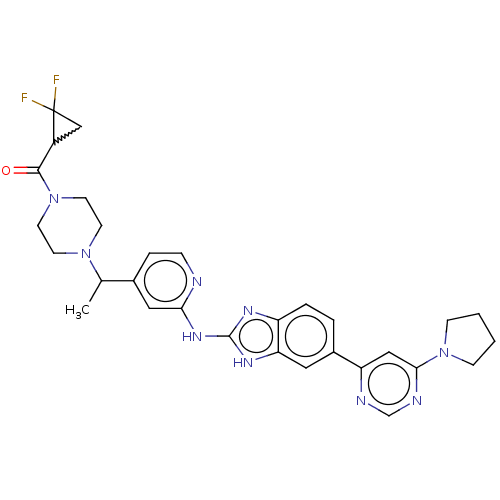 (US10894784, Example 117.05) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.473 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description Recombinant full-length N-terminally His-tagged human TBK1, expressed in insect cells and purified by Ni-NTA affinity chromatography, was purchased f... | US Patent US10894784 (2021) BindingDB Entry DOI: 10.7270/Q2PV6PFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase TBK1 (Homo sapiens (Human)) | BDBM478804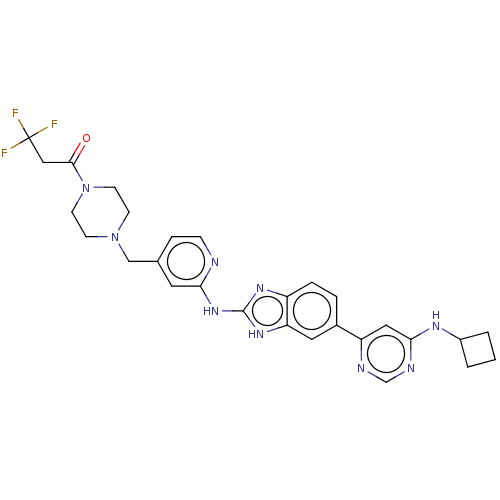 (US10894784, Example 152.02) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description Recombinant full-length N-terminally His-tagged human TBK1, expressed in insect cells and purified by Ni-NTA affinity chromatography, was purchased f... | US Patent US10894784 (2021) BindingDB Entry DOI: 10.7270/Q2PV6PFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase TBK1 (Homo sapiens (Human)) | BDBM478725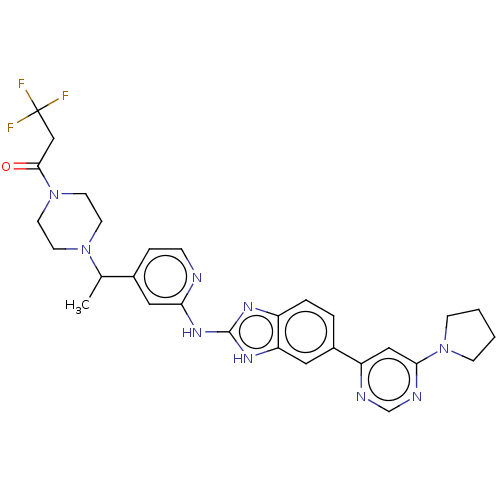 (3,3,3-trifluoro-1-(4-{(1R or 1S)-1-[2-({6-[6-(pyrr...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description Recombinant full-length N-terminally His-tagged human TBK1, expressed in insect cells and purified by Ni-NTA affinity chromatography, was purchased f... | US Patent US10894784 (2021) BindingDB Entry DOI: 10.7270/Q2PV6PFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM50542583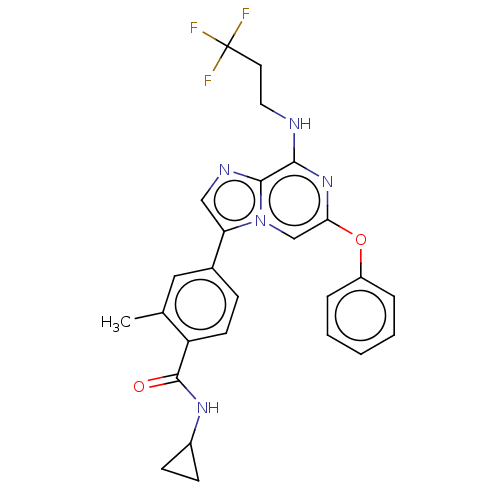 (CHEMBL4634949) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Inhibition of GST-tagged recombinant human MPS1 expressed in baculovirus expression system using biotin-Ahx-PWDPDDADITEILG as substrate preincubated ... | J Med Chem 63: 8025-8042 (2020) Article DOI: 10.1021/acs.jmedchem.9b02035 BindingDB Entry DOI: 10.7270/Q2MP56VP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM259464 (US9512126, 1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Inhibition of GST-tagged recombinant human MPS1 expressed in baculovirus expression system using biotin-Ahx-PWDPDDADITEILG as substrate preincubated ... | J Med Chem 63: 8025-8042 (2020) Article DOI: 10.1021/acs.jmedchem.9b02035 BindingDB Entry DOI: 10.7270/Q2MP56VP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM329340 ((2R)—N-{4-[2-({4-[(3-fluoroazetidin-1-yl)carb...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Inhibition of GST-tagged recombinant human MPS1 expressed in baculovirus expression system using biotin-Ahx-PWDPDDADITEILG as substrate preincubated ... | J Med Chem 63: 8025-8042 (2020) Article DOI: 10.1021/acs.jmedchem.9b02035 BindingDB Entry DOI: 10.7270/Q2MP56VP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM50542592 (CHEMBL4645348) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Inhibition of GST-tagged recombinant human MPS1 expressed in baculovirus expression system using biotin-Ahx-PWDPDDADITEILG as substrate preincubated ... | J Med Chem 63: 8025-8042 (2020) Article DOI: 10.1021/acs.jmedchem.9b02035 BindingDB Entry DOI: 10.7270/Q2MP56VP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM50547824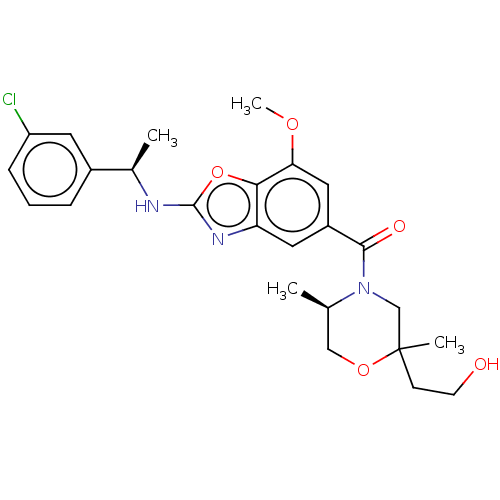 (CHEMBL4754786) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of purified human plasma thrombin using Boc-Asp(OBzl)-Pro-Arg-AMC substrate incubated for 30 mins by fluorescence based assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01035 BindingDB Entry DOI: 10.7270/Q2Z60SND | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM50542591 (CHEMBL4640247) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Inhibition of GST-tagged recombinant human MPS1 expressed in baculovirus expression system using biotin-Ahx-PWDPDDADITEILG as substrate preincubated ... | J Med Chem 63: 8025-8042 (2020) Article DOI: 10.1021/acs.jmedchem.9b02035 BindingDB Entry DOI: 10.7270/Q2MP56VP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM50542581 (CHEMBL4642174) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Inhibition of GST-tagged recombinant human MPS1 expressed in baculovirus expression system using biotin-Ahx-PWDPDDADITEILG as substrate preincubated ... | J Med Chem 63: 8025-8042 (2020) Article DOI: 10.1021/acs.jmedchem.9b02035 BindingDB Entry DOI: 10.7270/Q2MP56VP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM254907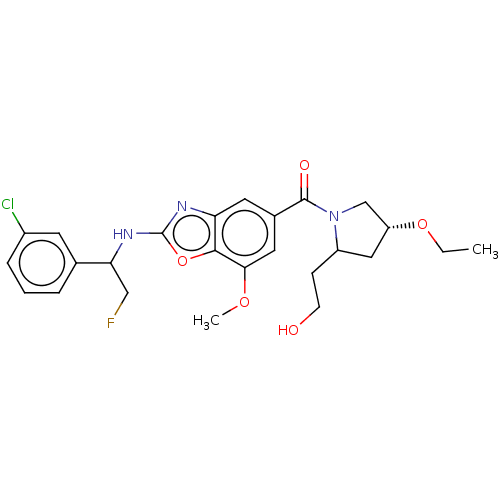 (US9493472, 29 | US9493472, 30 | US9493472, 31) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s... | US Patent US9493472 (2016) BindingDB Entry DOI: 10.7270/Q2B56HPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase TBK1 (Homo sapiens (Human)) | BDBM478853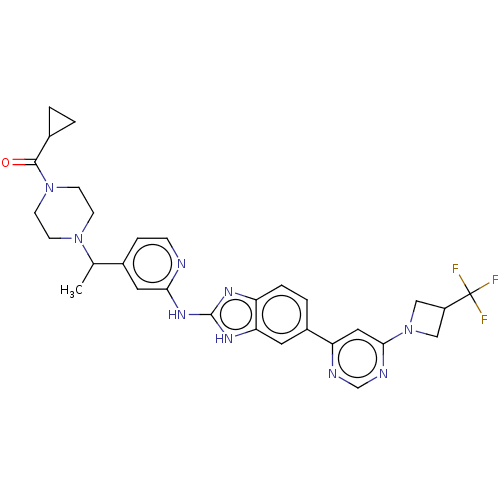 (US10894784, Example 164.03) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.558 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description Recombinant full-length N-terminally His-tagged human TBK1, expressed in insect cells and purified by Ni-NTA affinity chromatography, was purchased f... | US Patent US10894784 (2021) BindingDB Entry DOI: 10.7270/Q2PV6PFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase TBK1 (Homo sapiens (Human)) | BDBM478542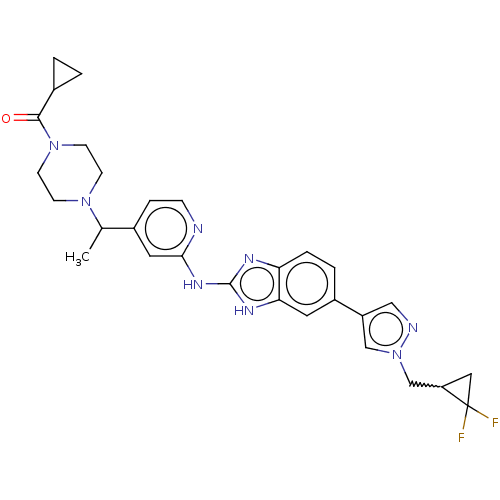 (US10894784, Example 55.03 | cyclopropyl{4-[(1R or ...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.564 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description Recombinant full-length N-terminally His-tagged human TBK1, expressed in insect cells and purified by Ni-NTA affinity chromatography, was purchased f... | US Patent US10894784 (2021) BindingDB Entry DOI: 10.7270/Q2PV6PFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase TBK1 (Homo sapiens (Human)) | BDBM478855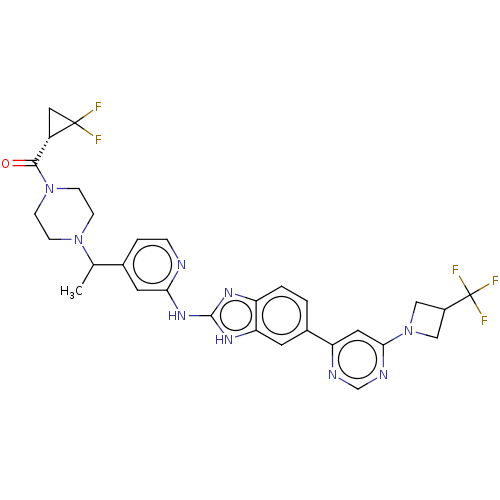 (US10894784, Example 164.05) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.566 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description Recombinant full-length N-terminally His-tagged human TBK1, expressed in insect cells and purified by Ni-NTA affinity chromatography, was purchased f... | US Patent US10894784 (2021) BindingDB Entry DOI: 10.7270/Q2PV6PFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50591465 (CHEMBL5201278) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c01379 BindingDB Entry DOI: 10.7270/Q2W099XX | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM259482 (US9512126, 23) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Inhibition of GST-tagged recombinant human MPS1 expressed in baculovirus expression system using biotin-Ahx-PWDPDDADITEILG as substrate preincubated ... | J Med Chem 63: 8025-8042 (2020) Article DOI: 10.1021/acs.jmedchem.9b02035 BindingDB Entry DOI: 10.7270/Q2MP56VP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase A1/Isoform 2 of MAP kinase-interacting serine/threonine-protein kinase 1 (MNK1a) [T344D] (Homo sapiens (Human)) | BDBM218770 (US9296757, 92) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | 7.5 | 22 |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description For the assay 50 nL of a 100 fold concentrated solution of the test compound in DMSO was pipetted into a black low volume 384 well microtiter plate (... | US Patent US9296757 (2016) BindingDB Entry DOI: 10.7270/Q2XK8DD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM50512456 (CHEMBL4469414) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Inhibition of GST-tagged recombinant human MPS1 expressed in baculovirus expression system using biotin-Ahx-PWDPDDADITEILG as substrate preincubated ... | J Med Chem 63: 8025-8042 (2020) Article DOI: 10.1021/acs.jmedchem.9b02035 BindingDB Entry DOI: 10.7270/Q2MP56VP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase TBK1 (Homo sapiens (Human)) | BDBM478573 (US10894784, Example 55.03.01 | US10894784, Example...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description Recombinant full-length N-terminally His-tagged human TBK1, expressed in insect cells and purified by Ni-NTA affinity chromatography, was purchased f... | US Patent US10894784 (2021) BindingDB Entry DOI: 10.7270/Q2PV6PFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 8833 total ) | Next | Last >> |