

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimer of Protein farnesyltransferase subunit beta (Homo sapiens (Human)) | BDBM50088001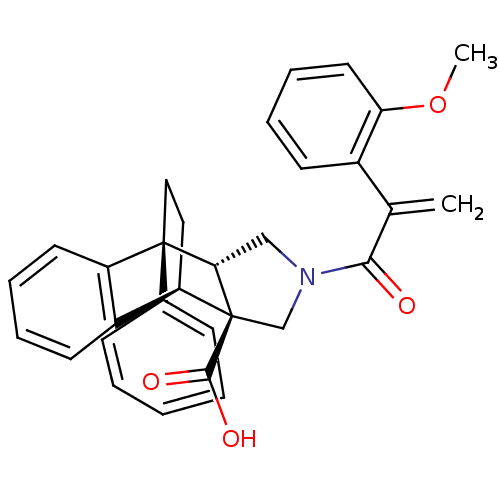 ((+/-)-11-[2-(2-methoxyphenyl)acryloyl]-1-phenyl-(1...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer S.A. Curated by ChEMBL | Assay Description Inhibition of farnesyltransferase by Ras/SPA assay | J Med Chem 43: 1807-16 (2000) BindingDB Entry DOI: 10.7270/Q23N22MR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Protein farnesyltransferase subunit beta (Homo sapiens (Human)) | BDBM50088009 ((+/-)-11-[2-(2-methoxyphenyl)acetyl]-1-phenyl-(1R,...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer S.A. Curated by ChEMBL | Assay Description Inhibition of farnesyltransferase by Ras/SPA assay | J Med Chem 43: 1807-16 (2000) BindingDB Entry DOI: 10.7270/Q23N22MR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Protein farnesyltransferase subunit beta (Homo sapiens (Human)) | BDBM50088004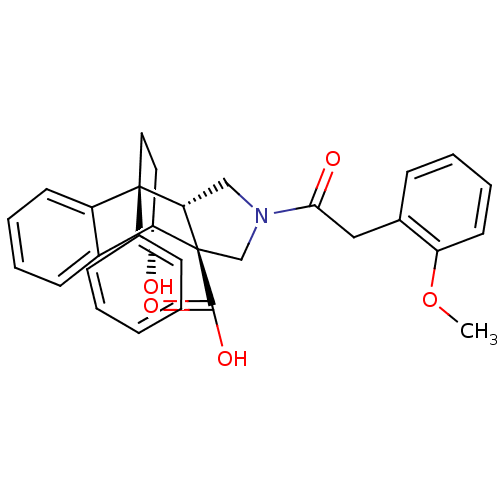 (8-hydroxy-11-[2-(2-methoxyphenyl)acetyl]-1-phenyl-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer S.A. Curated by ChEMBL | Assay Description Inhibition of farnesyltransferase by Ras/SPA assay | J Med Chem 43: 1807-16 (2000) BindingDB Entry DOI: 10.7270/Q23N22MR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Protein farnesyltransferase subunit beta (Homo sapiens (Human)) | BDBM50088008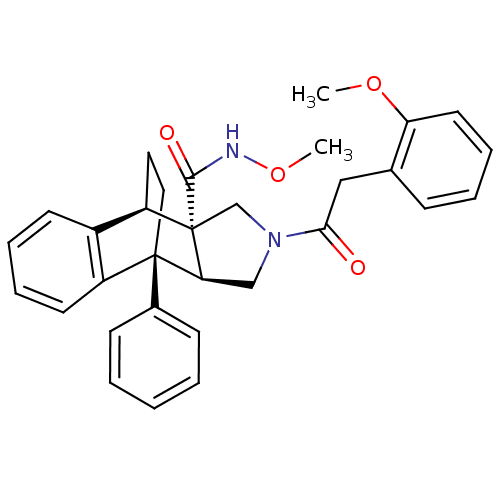 (11-[2-(2-methoxyphenyl)acetyl]-1-phenyl-(1R,8R,9S,...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer S.A. Curated by ChEMBL | Assay Description Inhibition of farnesyltransferase by Ras/SPA assay | J Med Chem 43: 1807-16 (2000) BindingDB Entry DOI: 10.7270/Q23N22MR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Protein farnesyltransferase subunit beta (Homo sapiens (Human)) | BDBM50088014 (1-(2,3-dihydrobenzo[b]furan-5-yl)-11-[2-(2-methoxy...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer S.A. Curated by ChEMBL | Assay Description Inhibition of farnesyltransferase by Ras/SPA assay | J Med Chem 43: 1807-16 (2000) BindingDB Entry DOI: 10.7270/Q23N22MR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Protein farnesyltransferase subunit beta (Homo sapiens (Human)) | BDBM50088013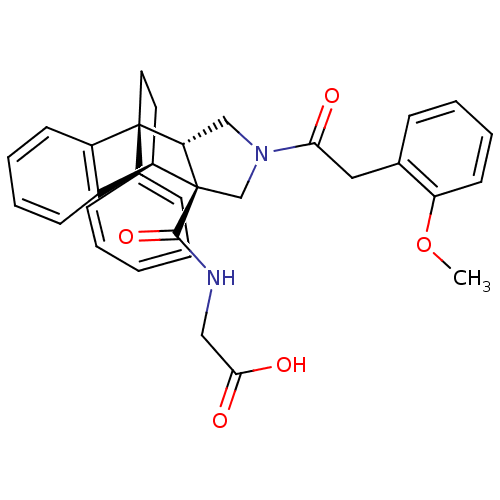 (2-[11-[2-(2-methoxyphenyl)acetyl]-1-phenyl-(1R,8R,...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 738 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer S.A. Curated by ChEMBL | Assay Description Inhibition of farnesyltransferase by Ras/SPA assay | J Med Chem 43: 1807-16 (2000) BindingDB Entry DOI: 10.7270/Q23N22MR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Protein farnesyltransferase subunit beta (Homo sapiens (Human)) | BDBM50088002 (1-(4-carboxymethoxyphenyl)-11-[2-(2-methoxyphenyl)...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.55E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer S.A. Curated by ChEMBL | Assay Description Inhibition of farnesyltransferase by Ras/SPA assay | J Med Chem 43: 1807-16 (2000) BindingDB Entry DOI: 10.7270/Q23N22MR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Protein farnesyltransferase subunit beta (Homo sapiens (Human)) | BDBM50088003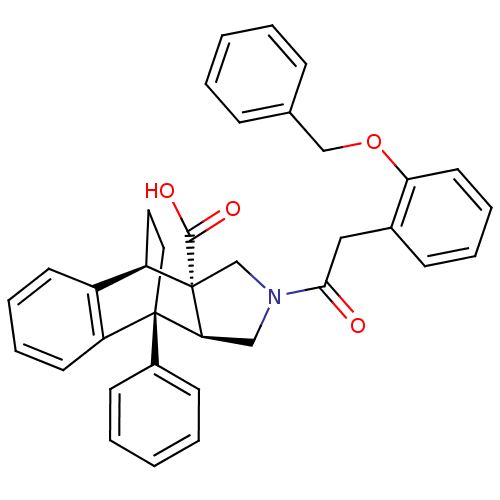 (11-[2-(2-benzyloxyphenyl)acetyl]-1-phenyl-(1R,8R,9...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer S.A. Curated by ChEMBL | Assay Description Inhibition of farnesyltransferase by Ras/SPA assay | J Med Chem 43: 1807-16 (2000) BindingDB Entry DOI: 10.7270/Q23N22MR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Protein farnesyltransferase subunit beta (Homo sapiens (Human)) | BDBM50088005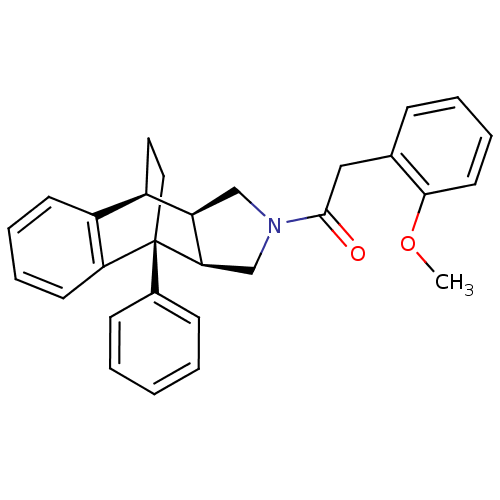 (2-(2-methoxyphenyl)-1-[1-phenyl-(1R,8S,9R,13R)-11-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer S.A. Curated by ChEMBL | Assay Description Inhibition of farnesyltransferase by Ras/SPA assay | J Med Chem 43: 1807-16 (2000) BindingDB Entry DOI: 10.7270/Q23N22MR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Protein farnesyltransferase subunit beta (Homo sapiens (Human)) | BDBM50088006 (6-hydroxy-11-[2-(2-methoxyphenyl)acetyl]-1-phenyl-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer S.A. Curated by ChEMBL | Assay Description Inhibition of farnesyltransferase by Ras/SPA assay | J Med Chem 43: 1807-16 (2000) BindingDB Entry DOI: 10.7270/Q23N22MR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Protein farnesyltransferase subunit beta (Homo sapiens (Human)) | BDBM50088000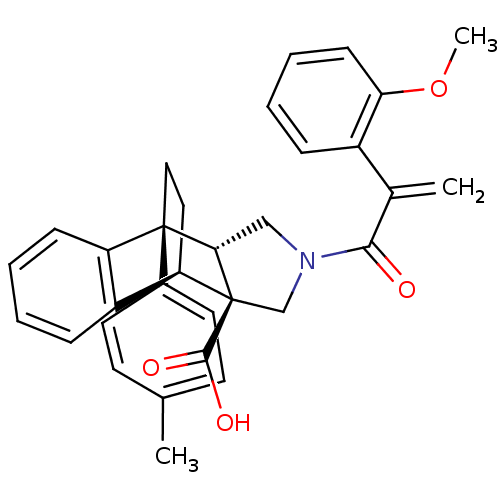 ((+/-)-11-[2-(2-methoxyphenyl)acryloyl]-1-(4-methyl...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer S.A. Curated by ChEMBL | Assay Description Inhibition of Farnesyltransferase | J Med Chem 43: 1807-16 (2000) BindingDB Entry DOI: 10.7270/Q23N22MR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock protein HSP 90-beta (Homo sapiens (Human)) | BDBM50357454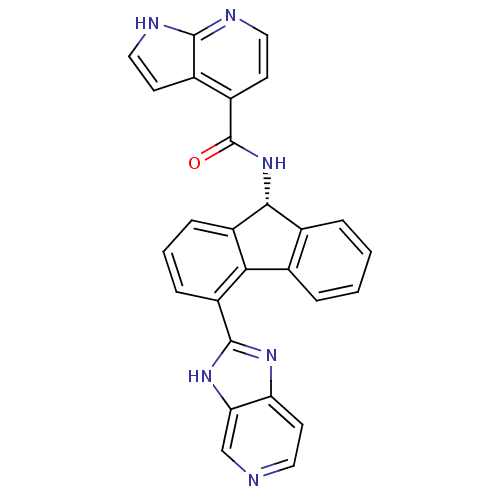 (CHEMBL1917878) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Research and Development Curated by ChEMBL | Assay Description Inhibition of HSP90beta in human SKBR3 cells assessed as down regulation of HER2 expression levels after 24 hrs by FACS analysis | J Med Chem 54: 7206-19 (2011) Article DOI: 10.1021/jm200784m BindingDB Entry DOI: 10.7270/Q2QR4XH4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50453606 (CHEMBL168533) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
U266 INSERM-URA D1500 CNRS Curated by ChEMBL | Assay Description Inhibitory potency against protein tyrosine kinase activity associated with EGFR was evaluated using ER 22 cell membrane | J Med Chem 37: 845-59 (1994) BindingDB Entry DOI: 10.7270/Q2QV3KJ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50038199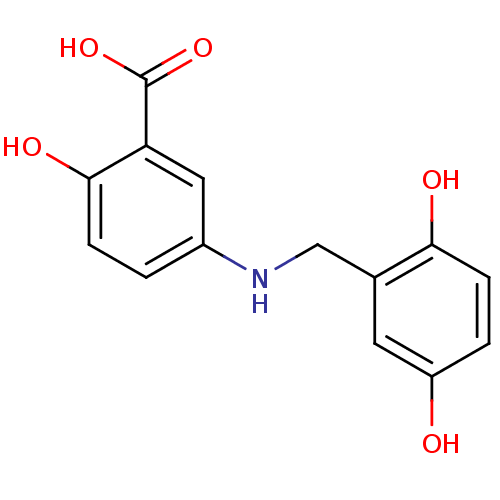 (5-(2,5-Dihydroxy-benzylamino)-2-hydroxy-benzoic ac...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
U266 INSERM-URA D1500 CNRS Curated by ChEMBL | Assay Description Inhibitory potency against protein tyrosine kinase activity associated with EGFR was evaluated using ER 22 cell membrane | J Med Chem 37: 845-59 (1994) BindingDB Entry DOI: 10.7270/Q2QV3KJ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50453600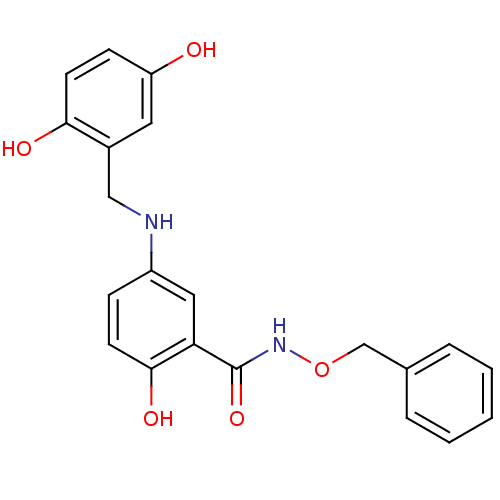 (CHEMBL355294) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
U266 INSERM-URA D1500 CNRS Curated by ChEMBL | Assay Description Inhibitory potency against protein tyrosine kinase activity associated with EGFR was evaluated using ER 22 cell membrane | J Med Chem 37: 845-59 (1994) BindingDB Entry DOI: 10.7270/Q2QV3KJ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Protein farnesyltransferase subunit beta (Homo sapiens (Human)) | BDBM50088001 ((+/-)-11-[2-(2-methoxyphenyl)acryloyl]-1-phenyl-(1...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer S.A. Curated by ChEMBL | Assay Description Inhibition of Farnesyltransferase | J Med Chem 43: 1807-16 (2000) BindingDB Entry DOI: 10.7270/Q23N22MR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50453594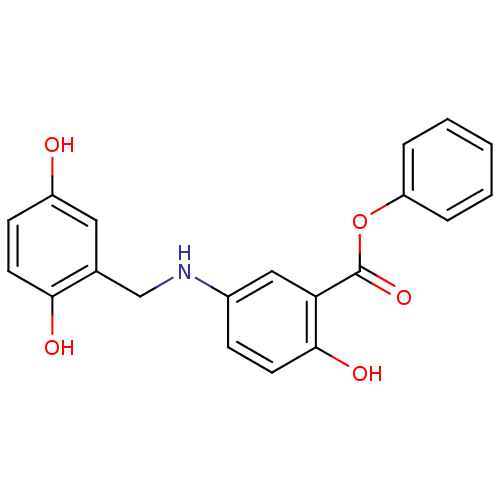 (CHEMBL169970) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
U266 INSERM-URA D1500 CNRS Curated by ChEMBL | Assay Description Inhibitory potency against protein tyrosine kinase activity associated with EGFR was evaluated using ER 22 cell membrane | J Med Chem 37: 845-59 (1994) BindingDB Entry DOI: 10.7270/Q2QV3KJ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50453576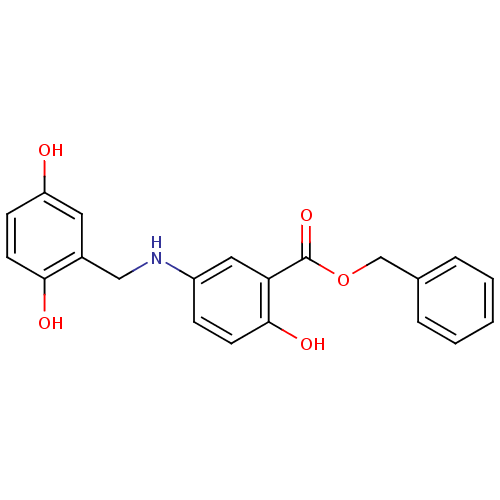 (CHEMBL352583) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
U266 INSERM-URA D1500 CNRS Curated by ChEMBL | Assay Description Inhibitory potency against protein tyrosine kinase activity associated with EGFR was evaluated using ER 22 cell membrane | J Med Chem 37: 845-59 (1994) BindingDB Entry DOI: 10.7270/Q2QV3KJ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50453601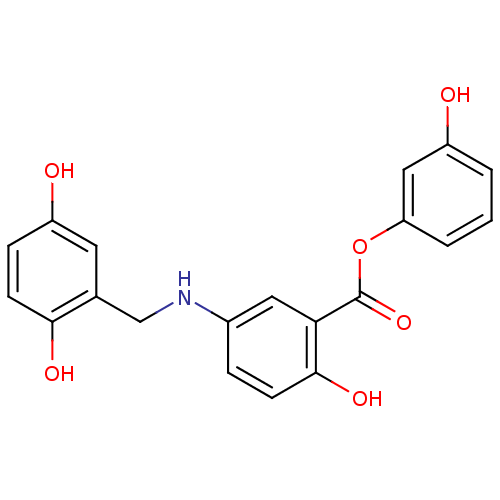 (CHEMBL422335) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
U266 INSERM-URA D1500 CNRS Curated by ChEMBL | Assay Description Inhibitory potency against protein tyrosine kinase activity associated with EGFR was evaluated using ER 22 cell membrane | J Med Chem 37: 845-59 (1994) BindingDB Entry DOI: 10.7270/Q2QV3KJ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50453586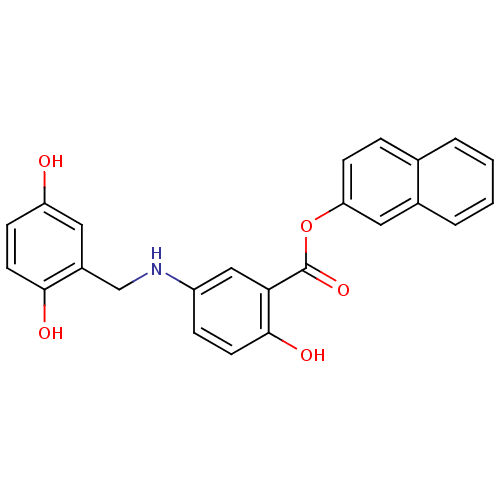 (CHEMBL169514) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
U266 INSERM-URA D1500 CNRS Curated by ChEMBL | Assay Description Inhibitory potency against protein tyrosine kinase activity associated with EGFR was evaluated using ER 22 cell membrane | J Med Chem 37: 845-59 (1994) BindingDB Entry DOI: 10.7270/Q2QV3KJ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50453584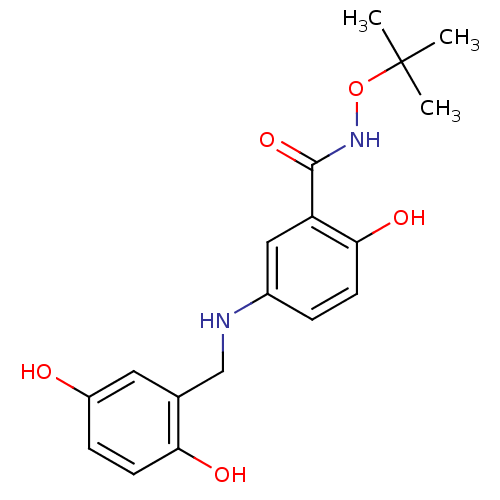 (CHEMBL170996) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
U266 INSERM-URA D1500 CNRS Curated by ChEMBL | Assay Description Inhibitory potency against protein tyrosine kinase activity associated with EGFR was evaluated using ER 22 cell membrane | J Med Chem 37: 845-59 (1994) BindingDB Entry DOI: 10.7270/Q2QV3KJ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50453597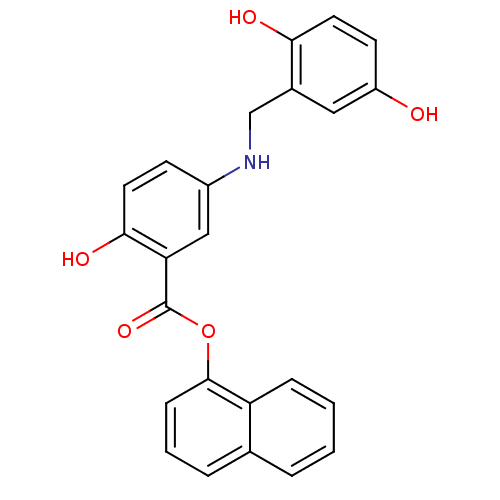 (CHEMBL168915) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
U266 INSERM-URA D1500 CNRS Curated by ChEMBL | Assay Description Inhibitory potency against protein tyrosine kinase activity associated with EGFR was evaluated using ER 22 cell membrane | J Med Chem 37: 845-59 (1994) BindingDB Entry DOI: 10.7270/Q2QV3KJ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50453607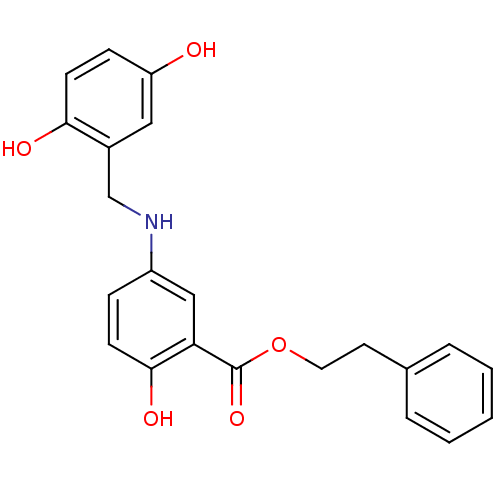 (CHEMBL168366) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
U266 INSERM-URA D1500 CNRS Curated by ChEMBL | Assay Description Inhibitory potency against protein tyrosine kinase activity associated with EGFR was evaluated using ER 22 cell membrane | J Med Chem 37: 845-59 (1994) BindingDB Entry DOI: 10.7270/Q2QV3KJ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50038199 (5-(2,5-Dihydroxy-benzylamino)-2-hydroxy-benzoic ac...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
U266 INSERM-URA D1500 CNRS Curated by ChEMBL | Assay Description Inhibition of EGFR(epidermal growth factor receptor) autophosphorylation in ER22 cell membranes | J Med Chem 37: 845-59 (1994) BindingDB Entry DOI: 10.7270/Q2QV3KJ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Protein farnesyltransferase subunit beta (Homo sapiens (Human)) | BDBM50088009 ((+/-)-11-[2-(2-methoxyphenyl)acetyl]-1-phenyl-(1R,...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer S.A. Curated by ChEMBL | Assay Description Inhibition of Farnesyltransferase | J Med Chem 43: 1807-16 (2000) BindingDB Entry DOI: 10.7270/Q23N22MR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50453607 (CHEMBL168366) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
U266 INSERM-URA D1500 CNRS Curated by ChEMBL | Assay Description Inhibition of EGFR(epidermal growth factor receptor) autophosphorylation in ER22 cell membranes | J Med Chem 37: 845-59 (1994) BindingDB Entry DOI: 10.7270/Q2QV3KJ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50453572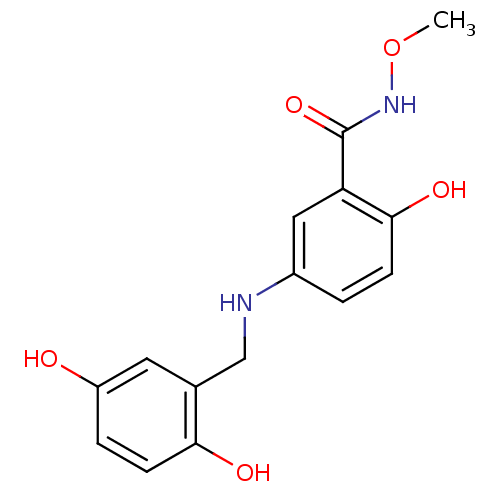 (CHEMBL169657) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
U266 INSERM-URA D1500 CNRS Curated by ChEMBL | Assay Description Inhibitory potency against protein tyrosine kinase activity associated with EGFR was evaluated using ER 22 cell membrane | J Med Chem 37: 845-59 (1994) BindingDB Entry DOI: 10.7270/Q2QV3KJ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50453571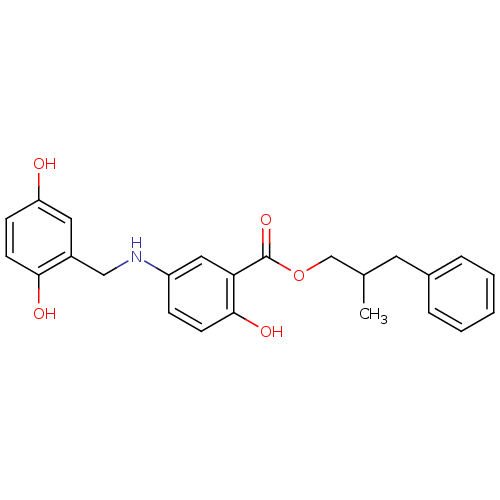 (CHEMBL171054) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
U266 INSERM-URA D1500 CNRS Curated by ChEMBL | Assay Description Inhibitory potency against protein tyrosine kinase activity associated with EGFR was evaluated using ER 22 cell membrane | J Med Chem 37: 845-59 (1994) BindingDB Entry DOI: 10.7270/Q2QV3KJ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50453569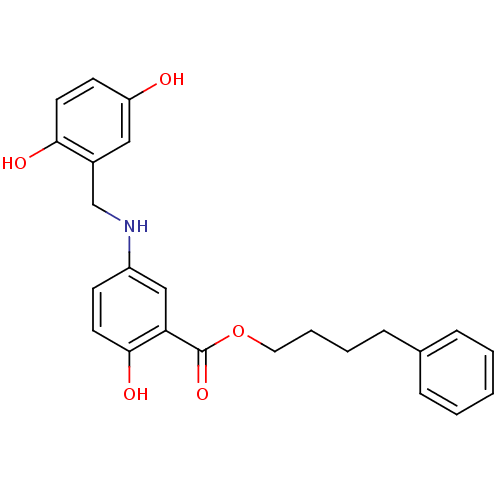 (CHEMBL435902) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
U266 INSERM-URA D1500 CNRS Curated by ChEMBL | Assay Description Inhibitory potency against protein tyrosine kinase activity associated with EGFR was evaluated using ER 22 cell membrane | J Med Chem 37: 845-59 (1994) BindingDB Entry DOI: 10.7270/Q2QV3KJ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50453605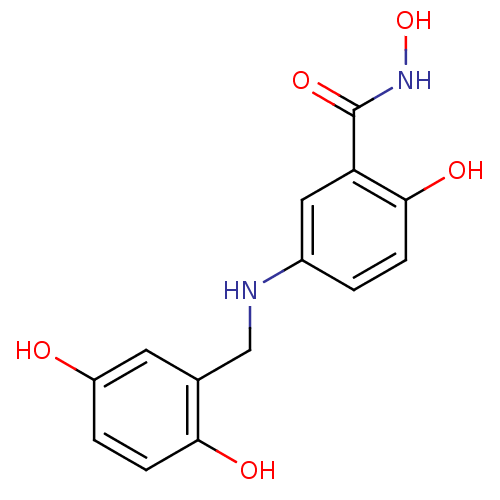 (CHEMBL172449) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
U266 INSERM-URA D1500 CNRS Curated by ChEMBL | Assay Description Inhibitory potency against protein tyrosine kinase activity associated with EGFR was evaluated using ER 22 cell membrane | J Med Chem 37: 845-59 (1994) BindingDB Entry DOI: 10.7270/Q2QV3KJ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50453577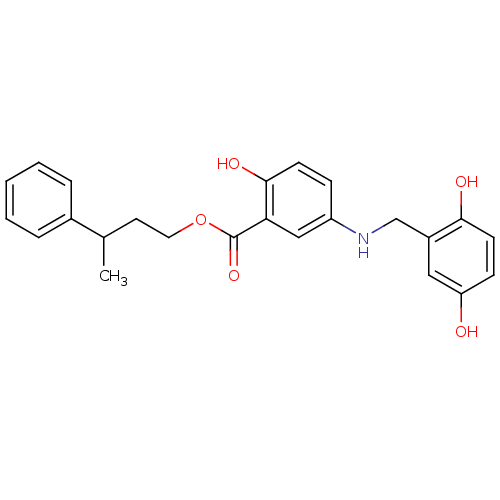 (CHEMBL354128) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
U266 INSERM-URA D1500 CNRS Curated by ChEMBL | Assay Description Inhibitory potency against protein tyrosine kinase activity associated with EGFR was evaluated using ER 22 cell membrane | J Med Chem 37: 845-59 (1994) BindingDB Entry DOI: 10.7270/Q2QV3KJ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50453603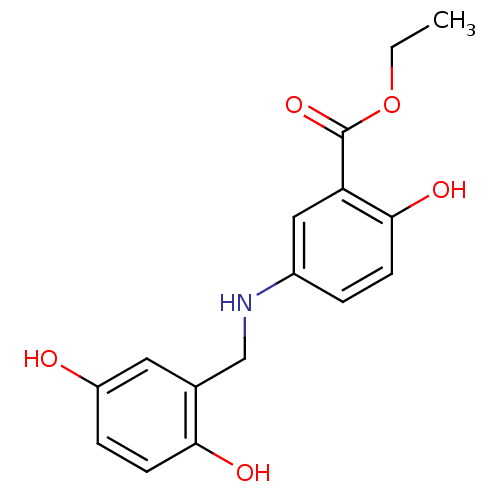 (CHEMBL355857) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
U266 INSERM-URA D1500 CNRS Curated by ChEMBL | Assay Description Inhibitory potency against protein tyrosine kinase activity associated with EGFR was evaluated using ER 22 cell membrane | J Med Chem 37: 845-59 (1994) BindingDB Entry DOI: 10.7270/Q2QV3KJ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50038205 (5-(2,5-Dihydroxy-benzylamino)-2-hydroxy-benzoic ac...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
U266 INSERM-URA D1500 CNRS Curated by ChEMBL | Assay Description Inhibitory potency against protein tyrosine kinase activity associated with EGFR was evaluated using ER 22 cell membrane | J Med Chem 37: 845-59 (1994) BindingDB Entry DOI: 10.7270/Q2QV3KJ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50453606 (CHEMBL168533) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
U266 INSERM-URA D1500 CNRS Curated by ChEMBL | Assay Description Inhibition of EGFR(epidermal growth factor receptor) autophosphorylation in ER22 cell membranes | J Med Chem 37: 845-59 (1994) BindingDB Entry DOI: 10.7270/Q2QV3KJ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50453604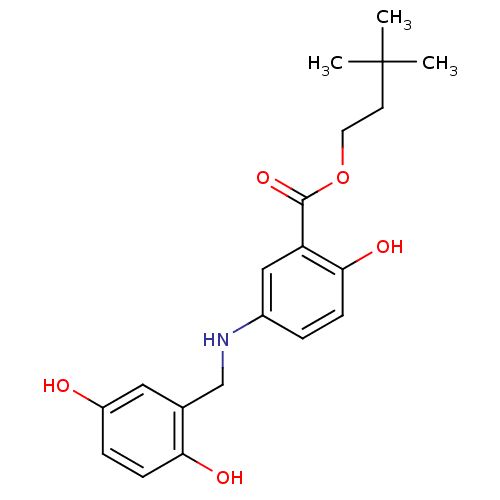 (CHEMBL353028) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
U266 INSERM-URA D1500 CNRS Curated by ChEMBL | Assay Description Inhibitory potency against protein tyrosine kinase activity associated with EGFR was evaluated using ER 22 cell membrane | J Med Chem 37: 845-59 (1994) BindingDB Entry DOI: 10.7270/Q2QV3KJ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50453583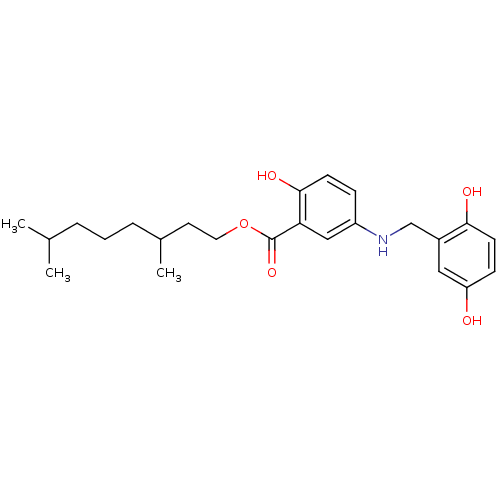 (CHEMBL170997) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
U266 INSERM-URA D1500 CNRS Curated by ChEMBL | Assay Description Inhibition of EGF-stimulated DNA synthesis in ER22 cells, by measuring [3H]Me-dT incorporation into ER 22 cells | J Med Chem 37: 845-59 (1994) BindingDB Entry DOI: 10.7270/Q2QV3KJ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50453578 (CHEMBL368296) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
U266 INSERM-URA D1500 CNRS Curated by ChEMBL | Assay Description Inhibition of EGF-stimulated DNA synthesis in ER22 cells, by measuring [3H]Me-dT incorporation into ER 22 cells | J Med Chem 37: 845-59 (1994) BindingDB Entry DOI: 10.7270/Q2QV3KJ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50453581 (CHEMBL355853) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
U266 INSERM-URA D1500 CNRS Curated by ChEMBL | Assay Description Inhibition of EGF-stimulated DNA synthesis in ER22 cells, by measuring [3H]Me-dT incorporation into ER 22 cells | J Med Chem 37: 845-59 (1994) BindingDB Entry DOI: 10.7270/Q2QV3KJ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock protein HSP 90-beta (Homo sapiens (Human)) | BDBM50357460 (CHEMBL1917729) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Research and Development Curated by ChEMBL | Assay Description Inhibition of HSP90beta in human SKBR3 cells assessed as down regulation of HER2 expression levels after 24 hrs by FACS analysis | J Med Chem 54: 7206-19 (2011) Article DOI: 10.1021/jm200784m BindingDB Entry DOI: 10.7270/Q2QR4XH4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50453602 (CHEMBL170111) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
U266 INSERM-URA D1500 CNRS Curated by ChEMBL | Assay Description Inhibitory potency against protein tyrosine kinase activity associated with EGFR was evaluated using ER 22 cell membrane | J Med Chem 37: 845-59 (1994) BindingDB Entry DOI: 10.7270/Q2QV3KJ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50038205 (5-(2,5-Dihydroxy-benzylamino)-2-hydroxy-benzoic ac...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
U266 INSERM-URA D1500 CNRS Curated by ChEMBL | Assay Description Inhibition of EGFR(epidermal growth factor receptor) autophosphorylation in ER22 cell membranes | J Med Chem 37: 845-59 (1994) BindingDB Entry DOI: 10.7270/Q2QV3KJ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50453589 (CHEMBL352589) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
U266 INSERM-URA D1500 CNRS Curated by ChEMBL | Assay Description Inhibitory potency against protein tyrosine kinase activity associated with EGFR was evaluated using ER 22 cell membrane | J Med Chem 37: 845-59 (1994) BindingDB Entry DOI: 10.7270/Q2QV3KJ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50453596 (CHEMBL435662) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
U266 INSERM-URA D1500 CNRS Curated by ChEMBL | Assay Description Inhibitory potency against protein tyrosine kinase activity associated with EGFR was evaluated using ER 22 cell membrane | J Med Chem 37: 845-59 (1994) BindingDB Entry DOI: 10.7270/Q2QV3KJ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50453575 (CHEMBL168363) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
U266 INSERM-URA D1500 CNRS Curated by ChEMBL | Assay Description Inhibitory potency against protein tyrosine kinase activity associated with EGFR was evaluated using ER 22 cell membrane | J Med Chem 37: 845-59 (1994) BindingDB Entry DOI: 10.7270/Q2QV3KJ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50453568 (CHEMBL169885) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
U266 INSERM-URA D1500 CNRS Curated by ChEMBL | Assay Description Inhibition of EGF-stimulated DNA synthesis in ER22 cells, by measuring [3H]Me-dT incorporation into ER 22 cells | J Med Chem 37: 845-59 (1994) BindingDB Entry DOI: 10.7270/Q2QV3KJ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50453569 (CHEMBL435902) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
U266 INSERM-URA D1500 CNRS Curated by ChEMBL | Assay Description Inhibition of EGF-stimulated DNA synthesis in ER22 cells, by measuring [3H]Me-dT incorporation into ER 22 cells | J Med Chem 37: 845-59 (1994) BindingDB Entry DOI: 10.7270/Q2QV3KJ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50453595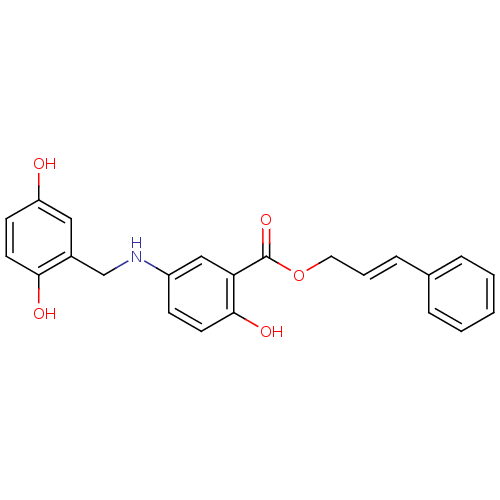 (CHEMBL172333) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
U266 INSERM-URA D1500 CNRS Curated by ChEMBL | Assay Description Inhibitory potency against protein tyrosine kinase activity associated with EGFR was evaluated using ER 22 cell membrane | J Med Chem 37: 845-59 (1994) BindingDB Entry DOI: 10.7270/Q2QV3KJ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock protein HSP 90-beta (Homo sapiens (Human)) | BDBM50357458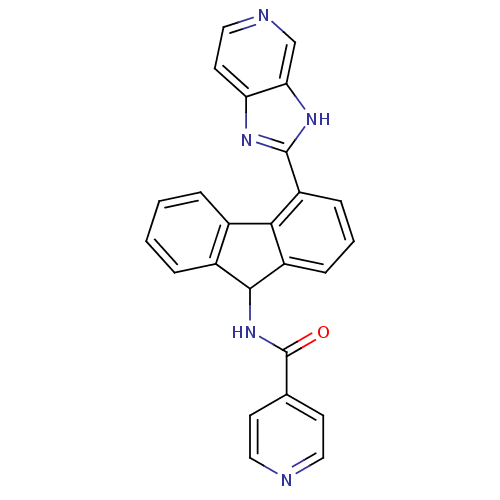 (CHEMBL1917727) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Research and Development Curated by ChEMBL | Assay Description Inhibition of HSP90beta in human SKBR3 cells assessed as down regulation of HER2 expression levels after 24 hrs by FACS analysis | J Med Chem 54: 7206-19 (2011) Article DOI: 10.1021/jm200784m BindingDB Entry DOI: 10.7270/Q2QR4XH4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Heat shock protein HSP 90-beta (Homo sapiens (Human)) | BDBM50357459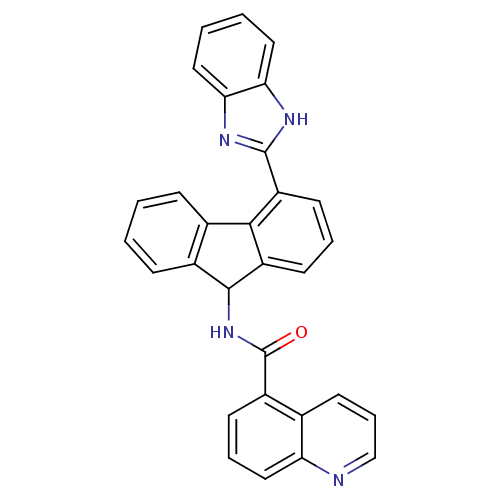 (CHEMBL1917728) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Research and Development Curated by ChEMBL | Assay Description Inhibition of HSP90beta in human SKBR3 cells assessed as down regulation of HER2 expression levels after 24 hrs by FACS analysis | J Med Chem 54: 7206-19 (2011) Article DOI: 10.1021/jm200784m BindingDB Entry DOI: 10.7270/Q2QR4XH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50453596 (CHEMBL435662) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
U266 INSERM-URA D1500 CNRS Curated by ChEMBL | Assay Description Inhibition of EGF-stimulated DNA synthesis in ER22 cells, by measuring [3H]Me-dT incorporation into ER 22 cells | J Med Chem 37: 845-59 (1994) BindingDB Entry DOI: 10.7270/Q2QV3KJ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 109 total ) | Next | Last >> |