 Found 513 hits with Last Name = 'rana' and Initial = 'p'
Found 513 hits with Last Name = 'rana' and Initial = 'p' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Enoyl-[acyl-carrier-protein] reductase [NADH] FabI
(Escherichia coli) | BDBM8726

(5-chloro-2-(2,4-dichlorophenoxy)phenol | CHEMBL849...)Show InChI InChI=1S/C12H7Cl3O2/c13-7-1-3-11(9(15)5-7)17-12-4-2-8(14)6-10(12)16/h1-6,16H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
| 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Enoyl-[acyl-carrier-protein] reductase [NADH] FabI
(Escherichia coli) | BDBM36537

(2-phenoxylphenol | PT51 | US10071965, Compound PT5...)Show InChI InChI=1S/C12H10O2/c13-11-8-4-5-9-12(11)14-10-6-2-1-3-7-10/h1-9,13H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADH] FabI
(Escherichia coli) | BDBM36538
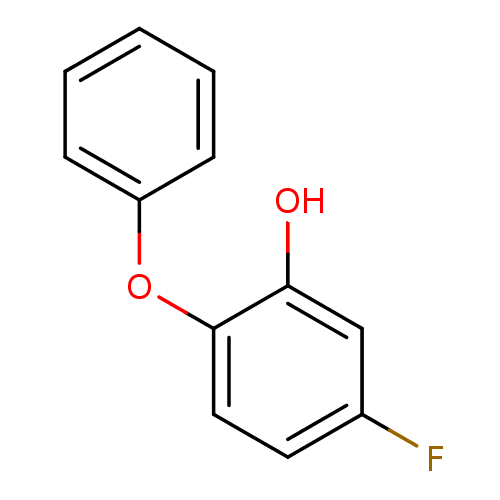
(5-fluoro-2-phenoxylphenol | PT55 | US10071965, Com...)Show InChI InChI=1S/C12H9FO2/c13-9-6-7-12(11(14)8-9)15-10-4-2-1-3-5-10/h1-8,14H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
| | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50080960
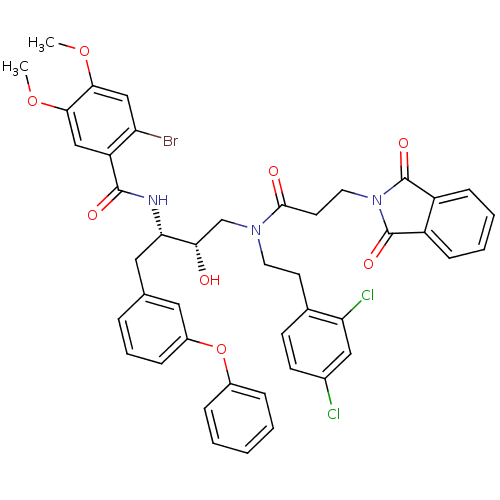
(2-Bromo-N-[(1S,2S)-3-{[2-(2,4-dichloro-phenyl)-eth...)Show SMILES COc1cc(Br)c(cc1OC)C(=O)N[C@@H](Cc1cccc(Oc2ccccc2)c1)[C@@H](O)CN(CCc1ccc(Cl)cc1Cl)C(=O)CCN1C(=O)c2ccccc2C1=O Show InChI InChI=1S/C44H40BrCl2N3O8/c1-56-39-24-34(35(45)25-40(39)57-2)42(53)48-37(22-27-9-8-12-31(21-27)58-30-10-4-3-5-11-30)38(51)26-49(19-17-28-15-16-29(46)23-36(28)47)41(52)18-20-50-43(54)32-13-6-7-14-33(32)44(50)55/h3-16,21,23-25,37-38,51H,17-20,22,26H2,1-2H3,(H,48,53)/t37-,38-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human liver Cathepsin D using (Ac-Glu-Glu(Edans)-Lys-Pro-Ile-Cys-Phe-PheArg-Leu-Gly-Lys(Dabcyl)-Glu-NH2) peptide as substrate by fluoro... |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115879
BindingDB Entry DOI: 10.7270/Q2HQ43KW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Enoyl-[acyl-carrier-protein] reductase [NADH] FabI
(Escherichia coli) | BDBM36539
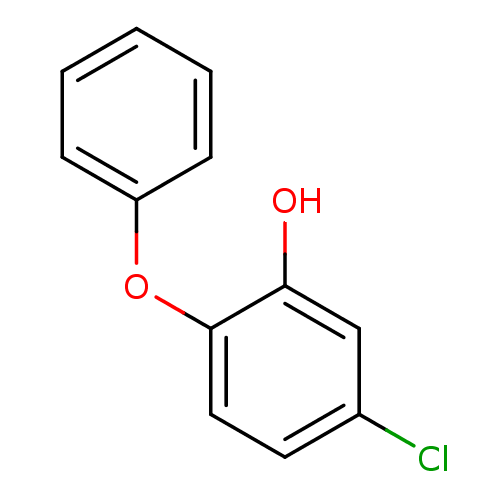
(5-chloro-2-phenoxylphenol | PT52 | US10071965, Com...)Show InChI InChI=1S/C12H9ClO2/c13-9-6-7-12(11(14)8-9)15-10-4-2-1-3-5-10/h1-8,14H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(MOUSE) | BDBM50591643

(CHEMBL5172956)Show SMILES CC(C)[C@H]1N(C)c2cc(OCCCCCCCSC(C)=O)ccc2C[C@H](CO)NC1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112844
BindingDB Entry DOI: 10.7270/Q2KK9GTQ |
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI
(Staphylococcus aureus) | BDBM8726

(5-chloro-2-(2,4-dichlorophenoxy)phenol | CHEMBL849...)Show InChI InChI=1S/C12H7Cl3O2/c13-7-1-3-11(9(15)5-7)17-12-4-2-8(14)6-10(12)16/h1-6,16H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Enoyl-[acyl-carrier-protein] reductase [NADH] FabI
(Escherichia coli) | BDBM36542
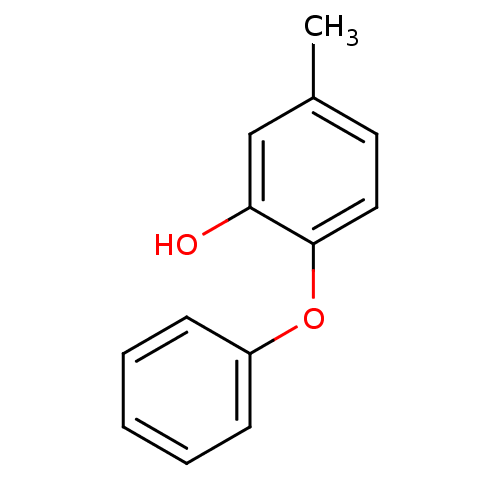
(5-methyl-2-phenoxylphenol | PT53 | US10071965, Com...)Show InChI InChI=1S/C13H12O2/c1-10-7-8-13(12(14)9-10)15-11-5-3-2-4-6-11/h2-9,14H,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(MOUSE) | BDBM50591642
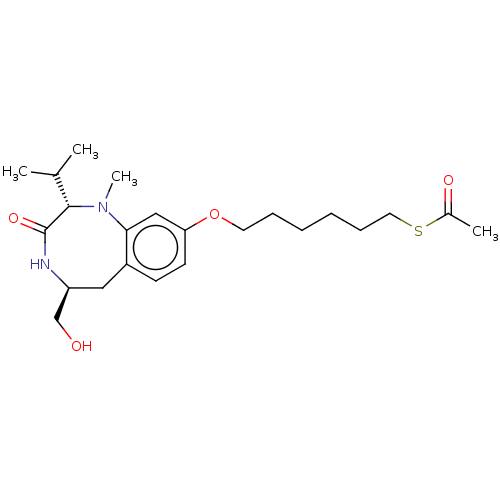
(CHEMBL5205471)Show SMILES CC(C)[C@@H]1N(C)c2cc(OCCCCCCSC(C)=O)ccc2C[C@@H](CO)NC1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112844
BindingDB Entry DOI: 10.7270/Q2KK9GTQ |
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADH]
(Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM8726

(5-chloro-2-(2,4-dichlorophenoxy)phenol | CHEMBL849...)Show InChI InChI=1S/C12H7Cl3O2/c13-7-1-3-11(9(15)5-7)17-12-4-2-8(14)6-10(12)16/h1-6,16H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Enoyl-[acyl-carrier-protein] reductase [NADH]
(Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50443008

(Pyridomycin)Show SMILES CC\C(C)=C1/OC(=O)[C@H](C)[C@H](O)[C@H](Cc2cccnc2)NC(=O)[C@@H](NC(=O)c2ncccc2O)[C@@H](C)OC1=O |r| Show InChI InChI=1S/C27H32N4O8/c1-5-14(2)23-27(37)38-16(4)20(31-25(35)21-19(32)9-7-11-29-21)24(34)30-18(12-17-8-6-10-28-13-17)22(33)15(3)26(36)39-23/h6-11,13,15-16,18,20,22,32-33H,5,12H2,1-4H3,(H,30,34)(H,31,35)/b23-14-/t15-,16-,18+,20+,22+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
| 4.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Enoyl-[acyl-carrier-protein] reductase [NADH]
(Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50443008

(Pyridomycin)Show SMILES CC\C(C)=C1/OC(=O)[C@H](C)[C@H](O)[C@H](Cc2cccnc2)NC(=O)[C@@H](NC(=O)c2ncccc2O)[C@@H](C)OC1=O |r| Show InChI InChI=1S/C27H32N4O8/c1-5-14(2)23-27(37)38-16(4)20(31-25(35)21-19(32)9-7-11-29-21)24(34)30-18(12-17-8-6-10-28-13-17)22(33)15(3)26(36)39-23/h6-11,13,15-16,18,20,22,32-33H,5,12H2,1-4H3,(H,30,34)(H,31,35)/b23-14-/t15-,16-,18+,20+,22+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
| 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ubiquitin carboxyl-terminal hydrolase 7
(Homo sapiens (Human)) | BDBM50538576
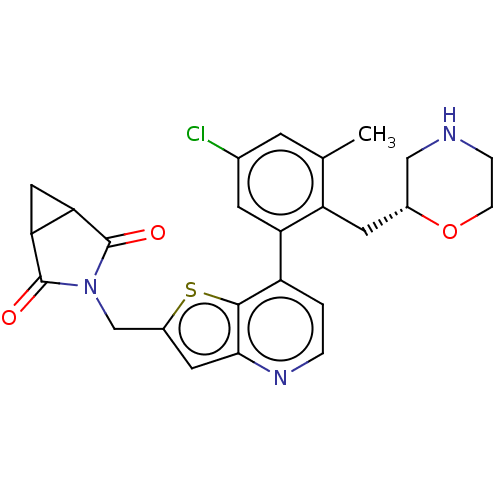
(CHEMBL4634092)Show SMILES Cc1cc(Cl)cc(c1C[C@@H]1CNCCO1)-c1ccnc2cc(CN3C(=O)C4CC4C3=O)sc12 |r| Show InChI InChI=1S/C25H24ClN3O3S/c1-13-6-14(26)7-19(18(13)8-15-11-27-4-5-32-15)17-2-3-28-22-9-16(33-23(17)22)12-29-24(30)20-10-21(20)25(29)31/h2-3,6-7,9,15,20-21,27H,4-5,8,10-12H2,1H3/t15-,20?,21?/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length USP7 (unknown origin) using ubiquintin-rhodamine as substrate preincubated for 30 mins followed by substrate ad... |
J Med Chem 63: 5398-5420 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00245
BindingDB Entry DOI: 10.7270/Q2XK8K2T |
More data for this
Ligand-Target Pair | |
Ubiquitin carboxyl-terminal hydrolase 7
(Homo sapiens (Human)) | BDBM50538571
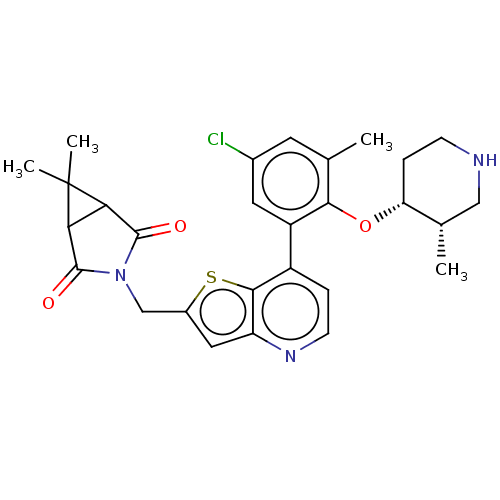
(CHEMBL4635160)Show SMILES C[C@H]1CNCC[C@H]1Oc1c(C)cc(Cl)cc1-c1ccnc2cc(CN3C(=O)C4C(C3=O)C4(C)C)sc12 |r| Show InChI InChI=1S/C28H30ClN3O3S/c1-14-9-16(29)10-19(24(14)35-21-6-7-30-12-15(21)2)18-5-8-31-20-11-17(36-25(18)20)13-32-26(33)22-23(27(32)34)28(22,3)4/h5,8-11,15,21-23,30H,6-7,12-13H2,1-4H3/t15-,21+,22?,23?/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length USP7 (unknown origin) using ubiquintin-rhodamine as substrate preincubated for 30 mins followed by substrate ad... |
J Med Chem 63: 5398-5420 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00245
BindingDB Entry DOI: 10.7270/Q2XK8K2T |
More data for this
Ligand-Target Pair | |
Ubiquitin carboxyl-terminal hydrolase 7
(Homo sapiens (Human)) | BDBM50538560

(CHEMBL4640002)Show SMILES Clc1cc2C[C@@H](Oc2c(c1)-c1ccnc2cc(CN3C(=O)CCC3=O)sc12)C(=O)N1CCNCC1 |r| Show InChI InChI=1S/C25H23ClN4O4S/c26-15-9-14-10-20(25(33)29-7-5-27-6-8-29)34-23(14)18(11-15)17-3-4-28-19-12-16(35-24(17)19)13-30-21(31)1-2-22(30)32/h3-4,9,11-12,20,27H,1-2,5-8,10,13H2/t20-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length USP7 (unknown origin) using ubiquintin-rhodamine as substrate preincubated for 30 mins followed by substrate ad... |
J Med Chem 63: 5398-5420 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00245
BindingDB Entry DOI: 10.7270/Q2XK8K2T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ubiquitin carboxyl-terminal hydrolase 7
(Homo sapiens (Human)) | BDBM50538572

(CHEMBL4649132)Show SMILES C[C@H]1CNCC[C@H]1Oc1c(C)cc(Cl)cc1-c1ccnc2cc(Cn3c(=O)ccn(CC(F)(F)F)c3=O)sc12 |r| Show InChI InChI=1S/C27H26ClF3N4O3S/c1-15-9-17(28)10-20(24(15)38-22-4-6-32-12-16(22)2)19-3-7-33-21-11-18(39-25(19)21)13-35-23(36)5-8-34(26(35)37)14-27(29,30)31/h3,5,7-11,16,22,32H,4,6,12-14H2,1-2H3/t16-,22+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length USP7 (unknown origin) using ubiquintin-rhodamine as substrate preincubated for 30 mins followed by substrate ad... |
J Med Chem 63: 5398-5420 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00245
BindingDB Entry DOI: 10.7270/Q2XK8K2T |
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI
(Staphylococcus aureus) | BDBM50481806
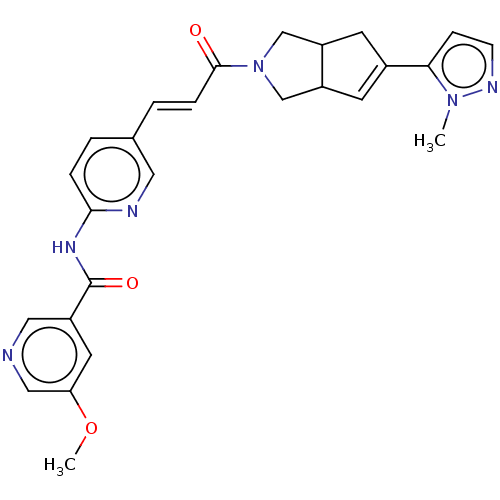
(CHEMBL5269135)Show SMILES NS(=O)(=O)C1C=CC(NC(=O)CCCN2CCOCC2)C=C1 |c:5,21,(4.09,-27.1,;3.32,-25.77,;2.92,-24.27,;4.81,-25.36,;1.78,-25.76,;1.02,-27.09,;-.52,-27.09,;-1.3,-25.76,;-2.84,-25.77,;-3.61,-24.43,;-2.84,-23.1,;-5.15,-24.44,;-5.92,-23.1,;-7.46,-23.1,;-8.23,-21.77,;-7.45,-20.44,;-8.22,-19.11,;-9.76,-19.11,;-10.53,-20.44,;-9.76,-21.78,;-.52,-24.43,;1.02,-24.43,)| Show InChI InChI=1S/C14H23N3O4S/c15-22(19,20)13-5-3-12(4-6-13)16-14(18)2-1-7-17-8-10-21-11-9-17/h3-6,12-13H,1-2,7-11H2,(H,16,18)(H2,15,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Ubiquitin carboxyl-terminal hydrolase 7
(Homo sapiens (Human)) | BDBM50538575
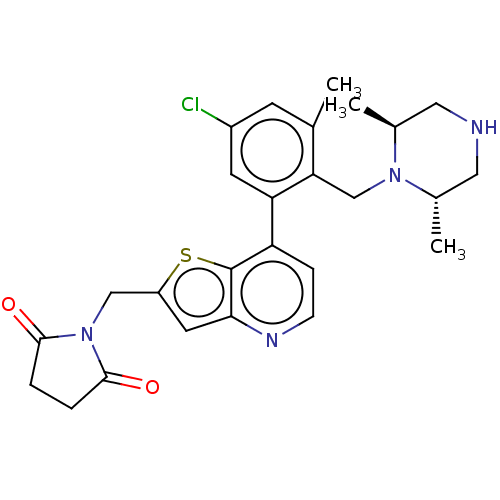
(CHEMBL4638458)Show SMILES C[C@H]1CNC[C@H](C)N1Cc1c(C)cc(Cl)cc1-c1ccnc2cc(CN3C(=O)CCC3=O)sc12 |r| Show InChI InChI=1S/C26H29ClN4O2S/c1-15-8-18(27)9-21(22(15)14-30-16(2)11-28-12-17(30)3)20-6-7-29-23-10-19(34-26(20)23)13-31-24(32)4-5-25(31)33/h6-10,16-17,28H,4-5,11-14H2,1-3H3/t16-,17-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length USP7 (unknown origin) using ubiquintin-rhodamine as substrate preincubated for 30 mins followed by substrate ad... |
J Med Chem 63: 5398-5420 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00245
BindingDB Entry DOI: 10.7270/Q2XK8K2T |
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI
(Staphylococcus aureus) | BDBM50481809
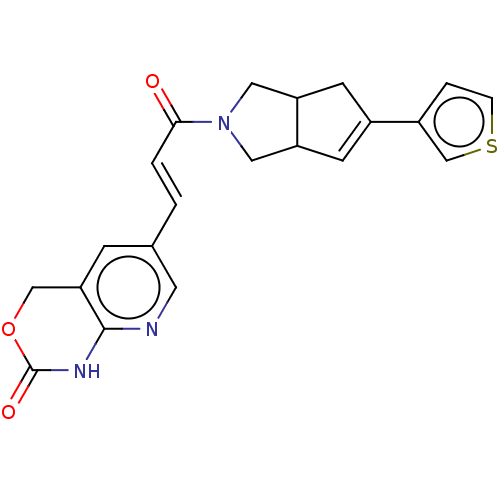
(CHEMBL5268904)Show SMILES COc1cc(\C=N\c2ccc(cc2)S(N)(=O)=O)ccc1C(C)=O Show InChI InChI=1S/C16H16N2O4S/c1-11(19)15-8-3-12(9-16(15)22-2)10-18-13-4-6-14(7-5-13)23(17,20)21/h3-10H,1-2H3,(H2,17,20,21)/b18-10+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI
(Staphylococcus aureus) | BDBM50481808
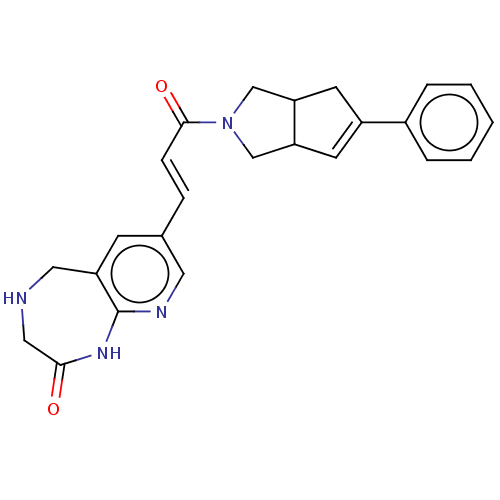
(CHEMBL5273284)Show SMILES NS(=O)(=O)c1ccc(cc1)\N=C\C1=C(Cl)CCC=C1 |c:13,18| Show InChI InChI=1S/C13H13ClN2O2S/c14-13-4-2-1-3-10(13)9-16-11-5-7-12(8-6-11)19(15,17)18/h1,3,5-9H,2,4H2,(H2,15,17,18)/b16-9+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI
(Staphylococcus aureus) | BDBM50481807
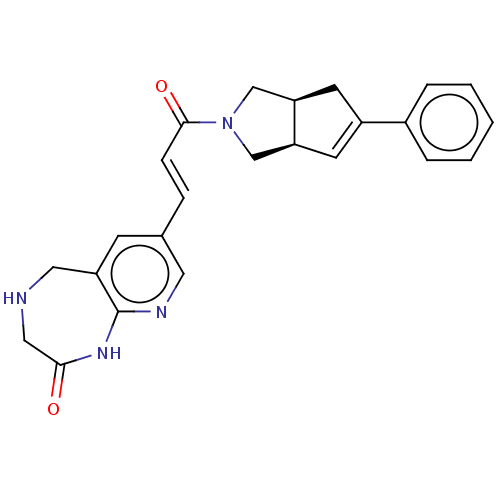
(CHEMBL5291446)Show SMILES NS(=O)(=O)C1C=CC(NC(=O)CCCCN2CCOCC2)C=C1 |c:5,22,(23.8,-27.71,;23.03,-26.38,;22.63,-24.88,;24.52,-25.97,;21.49,-26.38,;20.73,-27.7,;19.19,-27.7,;18.41,-26.38,;16.87,-26.38,;16.1,-25.05,;16.87,-23.71,;14.56,-25.05,;13.79,-23.72,;12.25,-23.72,;11.48,-22.39,;9.94,-22.39,;9.18,-21.05,;7.64,-21.05,;6.87,-22.38,;7.64,-23.72,;9.18,-23.72,;19.19,-25.04,;20.73,-25.04,)| Show InChI InChI=1S/C15H25N3O4S/c16-23(20,21)14-6-4-13(5-7-14)17-15(19)3-1-2-8-18-9-11-22-12-10-18/h4-7,13-14H,1-3,8-12H2,(H,17,19)(H2,16,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Ubiquitin carboxyl-terminal hydrolase 7
(Homo sapiens (Human)) | BDBM50538562

(CHEMBL4643330)Show SMILES Clc1cc2C[C@@H](Oc2c(c1)-c1ccnc2cc(CN3C(=O)CCC3=O)sc12)c1onc2CCNCc12 |r| Show InChI InChI=1S/C26H21ClN4O4S/c27-14-7-13-8-21(25-18-11-28-5-4-19(18)30-35-25)34-24(13)17(9-14)16-3-6-29-20-10-15(36-26(16)20)12-31-22(32)1-2-23(31)33/h3,6-7,9-10,21,28H,1-2,4-5,8,11-12H2/t21-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length USP7 (unknown origin) using ubiquintin-rhodamine as substrate preincubated for 30 mins followed by substrate ad... |
J Med Chem 63: 5398-5420 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00245
BindingDB Entry DOI: 10.7270/Q2XK8K2T |
More data for this
Ligand-Target Pair | |
Ubiquitin carboxyl-terminal hydrolase 7
(Homo sapiens (Human)) | BDBM50538578
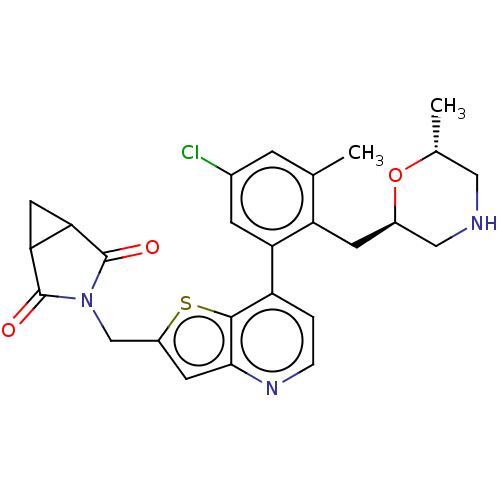
(CHEMBL4640729)Show SMILES C[C@@H]1CNC[C@@H](Cc2c(C)cc(Cl)cc2-c2ccnc3cc(CN4C(=O)C5CC5C4=O)sc23)O1 |r| Show InChI InChI=1S/C26H26ClN3O3S/c1-13-5-15(27)6-20(19(13)7-16-11-28-10-14(2)33-16)18-3-4-29-23-8-17(34-24(18)23)12-30-25(31)21-9-22(21)26(30)32/h3-6,8,14,16,21-22,28H,7,9-12H2,1-2H3/t14-,16-,21?,22?/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length USP7 (unknown origin) using ubiquintin-rhodamine as substrate preincubated for 30 mins followed by substrate ad... |
J Med Chem 63: 5398-5420 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00245
BindingDB Entry DOI: 10.7270/Q2XK8K2T |
More data for this
Ligand-Target Pair | |
Ubiquitin carboxyl-terminal hydrolase 7
(Homo sapiens (Human)) | BDBM50538579

(CHEMBL4647217)Show SMILES Cc1cc(Cl)cc(c1C[C@@H]1CNCCO1)-c1ccnc2cc(CN3C(=O)C4C(C3=O)C4(C)C)sc12 |r| Show InChI InChI=1S/C27H28ClN3O3S/c1-14-8-15(28)9-20(19(14)10-16-12-29-6-7-34-16)18-4-5-30-21-11-17(35-24(18)21)13-31-25(32)22-23(26(31)33)27(22,2)3/h4-5,8-9,11,16,22-23,29H,6-7,10,12-13H2,1-3H3/t16-,22?,23?/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length USP7 (unknown origin) using ubiquintin-rhodamine as substrate preincubated for 30 mins followed by substrate ad... |
J Med Chem 63: 5398-5420 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00245
BindingDB Entry DOI: 10.7270/Q2XK8K2T |
More data for this
Ligand-Target Pair | |
Ubiquitin carboxyl-terminal hydrolase 7
(Homo sapiens (Human)) | BDBM50538582
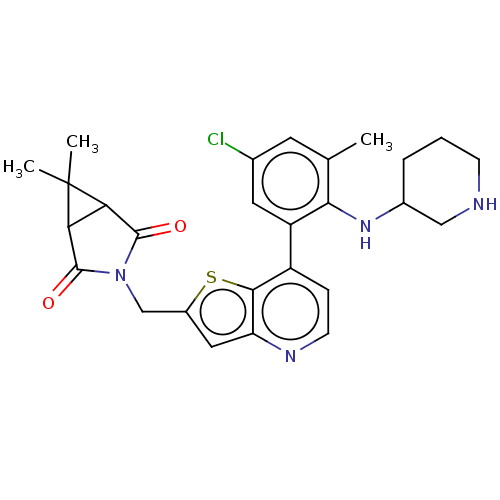
(CHEMBL4638998)Show SMILES Cc1cc(Cl)cc(c1NC1CCCNC1)-c1ccnc2cc(CN3C(=O)C4C(C3=O)C4(C)C)sc12 Show InChI InChI=1S/C27H29ClN4O2S/c1-14-9-15(28)10-19(23(14)31-16-5-4-7-29-12-16)18-6-8-30-20-11-17(35-24(18)20)13-32-25(33)21-22(26(32)34)27(21,2)3/h6,8-11,16,21-22,29,31H,4-5,7,12-13H2,1-3H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length USP7 (unknown origin) using ubiquintin-rhodamine as substrate preincubated for 30 mins followed by substrate ad... |
J Med Chem 63: 5398-5420 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00245
BindingDB Entry DOI: 10.7270/Q2XK8K2T |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM912

((3S,4S)-3-hydroxy-4-[(2S)-2-[(3S,4S)-3-hydroxy-6-m...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)CC(C)C)C(C)C)C(C)C)[C@@H](O)CC(O)=O |r| Show InChI InChI=1S/C34H63N5O9/c1-17(2)12-23(37-33(47)31(21(9)10)39-34(48)30(20(7)8)38-27(42)14-19(5)6)25(40)15-28(43)35-22(11)32(46)36-24(13-18(3)4)26(41)16-29(44)45/h17-26,30-31,40-41H,12-16H2,1-11H3,(H,35,43)(H,36,46)(H,37,47)(H,38,42)(H,39,48)(H,44,45)/t22-,23-,24-,25-,26-,30-,31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Cathepsin D (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115879
BindingDB Entry DOI: 10.7270/Q2HQ43KW |
More data for this
Ligand-Target Pair | |
Ubiquitin carboxyl-terminal hydrolase 7
(Homo sapiens (Human)) | BDBM50538563

(CHEMBL4648116)Show SMILES Cn1nc2CCNCc2c1[C@H]1Cc2cc(Cl)cc(c2O1)-c1ccnc2cc(CN3C(=O)CCC3=O)sc12 |r| Show InChI InChI=1S/C27H24ClN5O3S/c1-32-25(19-12-29-6-5-20(19)31-32)22-9-14-8-15(28)10-18(26(14)36-22)17-4-7-30-21-11-16(37-27(17)21)13-33-23(34)2-3-24(33)35/h4,7-8,10-11,22,29H,2-3,5-6,9,12-13H2,1H3/t22-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length USP7 (unknown origin) using ubiquintin-rhodamine as substrate preincubated for 30 mins followed by substrate ad... |
J Med Chem 63: 5398-5420 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00245
BindingDB Entry DOI: 10.7270/Q2XK8K2T |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50591637
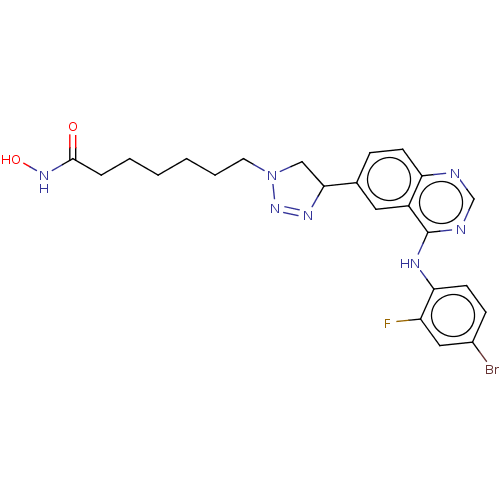
(CHEMBL5178922)Show SMILES ONC(=O)CCCCCCN1CC(N=N1)c1ccc2ncnc(Nc3ccc(Br)cc3F)c2c1 |c:13| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112844
BindingDB Entry DOI: 10.7270/Q2KK9GTQ |
More data for this
Ligand-Target Pair | |
Ubiquitin carboxyl-terminal hydrolase 7
(Homo sapiens (Human)) | BDBM50538570

(CHEMBL4638573)Show SMILES C[C@H]1CNCC[C@H]1Oc1c(C)cc(Cl)cc1-c1ccnc2cc(CN3C(=O)C4CC4C3=O)sc12 |r| Show InChI InChI=1S/C26H26ClN3O3S/c1-13-7-15(27)8-18(23(13)33-22-4-5-28-11-14(22)2)17-3-6-29-21-9-16(34-24(17)21)12-30-25(31)19-10-20(19)26(30)32/h3,6-9,14,19-20,22,28H,4-5,10-12H2,1-2H3/t14-,19?,20?,22+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length USP7 (unknown origin) using ubiquintin-rhodamine as substrate preincubated for 30 mins followed by substrate ad... |
J Med Chem 63: 5398-5420 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00245
BindingDB Entry DOI: 10.7270/Q2XK8K2T |
More data for this
Ligand-Target Pair | |
Ubiquitin carboxyl-terminal hydrolase 7
(Homo sapiens (Human)) | BDBM50538564
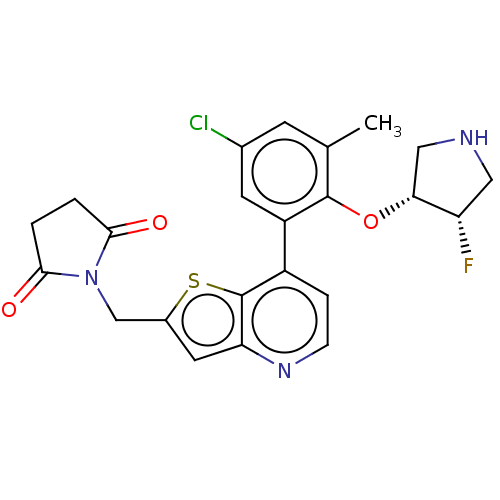
(CHEMBL4641569)Show SMILES Cc1cc(Cl)cc(c1O[C@@H]1CNC[C@@H]1F)-c1ccnc2cc(CN3C(=O)CCC3=O)sc12 |r| Show InChI InChI=1S/C23H21ClFN3O3S/c1-12-6-13(24)7-16(22(12)31-19-10-26-9-17(19)25)15-4-5-27-18-8-14(32-23(15)18)11-28-20(29)2-3-21(28)30/h4-8,17,19,26H,2-3,9-11H2,1H3/t17-,19+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length USP7 (unknown origin) using ubiquintin-rhodamine as substrate preincubated for 30 mins followed by substrate ad... |
J Med Chem 63: 5398-5420 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00245
BindingDB Entry DOI: 10.7270/Q2XK8K2T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cyclin-dependent kinase 4
(Homo sapiens (Human)) | BDBM50591638
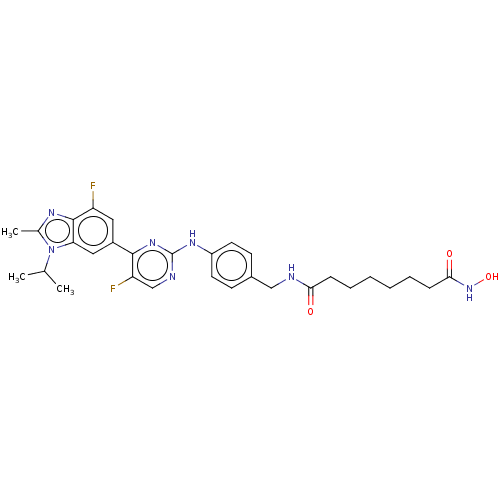
(CHEMBL5190225)Show SMILES CC(C)n1c(C)nc2c(F)cc(cc12)-c1nc(Nc2ccc(CNC(=O)CCCCCCC(=O)NO)cc2)ncc1F | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112844
BindingDB Entry DOI: 10.7270/Q2KK9GTQ |
More data for this
Ligand-Target Pair | |
Ubiquitin carboxyl-terminal hydrolase 7
(Homo sapiens (Human)) | BDBM50538583

(CHEMBL4643112)Show SMILES Cc1cc(Cl)cc(c1CC1CCCNC1)-c1ccnc2cc(CN3C(=O)C4C(C3=O)C4(C)C)sc12 Show InChI InChI=1S/C28H30ClN3O2S/c1-15-9-17(29)11-21(20(15)10-16-5-4-7-30-13-16)19-6-8-31-22-12-18(35-25(19)22)14-32-26(33)23-24(27(32)34)28(23,2)3/h6,8-9,11-12,16,23-24,30H,4-5,7,10,13-14H2,1-3H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length USP7 (unknown origin) using ubiquintin-rhodamine as substrate preincubated for 30 mins followed by substrate ad... |
J Med Chem 63: 5398-5420 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00245
BindingDB Entry DOI: 10.7270/Q2XK8K2T |
More data for this
Ligand-Target Pair | |
Ubiquitin carboxyl-terminal hydrolase 7
(Homo sapiens (Human)) | BDBM50538577
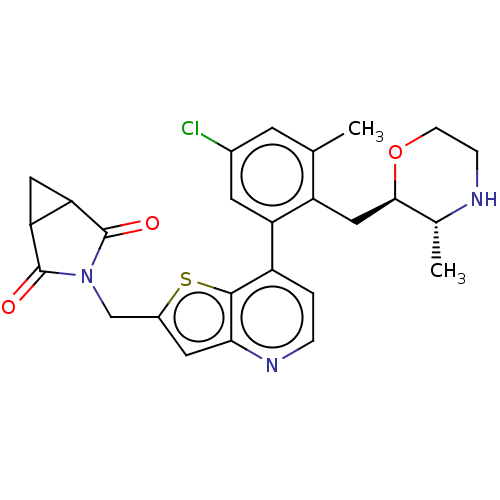
(CHEMBL4648623)Show SMILES C[C@H]1NCCO[C@@H]1Cc1c(C)cc(Cl)cc1-c1ccnc2cc(CN3C(=O)C4CC4C3=O)sc12 |r| Show InChI InChI=1S/C26H26ClN3O3S/c1-13-7-15(27)8-19(18(13)11-23-14(2)28-5-6-33-23)17-3-4-29-22-9-16(34-24(17)22)12-30-25(31)20-10-21(20)26(30)32/h3-4,7-9,14,20-21,23,28H,5-6,10-12H2,1-2H3/t14-,20?,21?,23-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length USP7 (unknown origin) using ubiquintin-rhodamine as substrate preincubated for 30 mins followed by substrate ad... |
J Med Chem 63: 5398-5420 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00245
BindingDB Entry DOI: 10.7270/Q2XK8K2T |
More data for this
Ligand-Target Pair | |
Ubiquitin carboxyl-terminal hydrolase 7
(Homo sapiens (Human)) | BDBM50538567
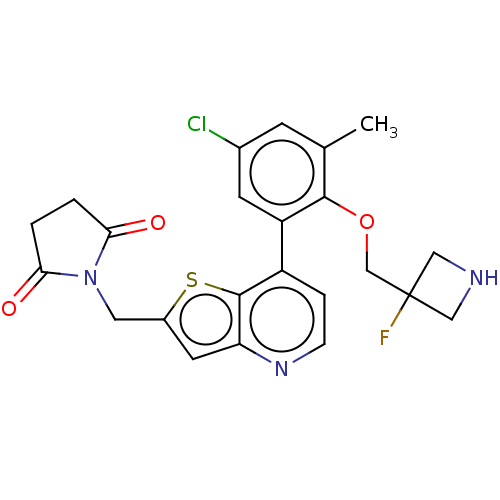
(CHEMBL4633860)Show SMILES Cc1cc(Cl)cc(c1OCC1(F)CNC1)-c1ccnc2cc(CN3C(=O)CCC3=O)sc12 Show InChI InChI=1S/C23H21ClFN3O3S/c1-13-6-14(24)7-17(21(13)31-12-23(25)10-26-11-23)16-4-5-27-18-8-15(32-22(16)18)9-28-19(29)2-3-20(28)30/h4-8,26H,2-3,9-12H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length USP7 (unknown origin) using ubiquintin-rhodamine as substrate preincubated for 30 mins followed by substrate ad... |
J Med Chem 63: 5398-5420 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00245
BindingDB Entry DOI: 10.7270/Q2XK8K2T |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50188961

(CHEMBL3622533)Show SMILES COc1ccc(cn1)-c1nc(N2CCOCC2)c2sc(CN(C)c3ncc(cn3)C(=O)NO)cc2n1 Show InChI InChI=1S/C23H24N8O4S/c1-30(23-25-11-15(12-26-23)22(32)29-33)13-16-9-17-19(36-16)21(31-5-7-35-8-6-31)28-20(27-17)14-3-4-18(34-2)24-10-14/h3-4,9-12,33H,5-8,13H2,1-2H3,(H,29,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112844
BindingDB Entry DOI: 10.7270/Q2KK9GTQ |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50023342
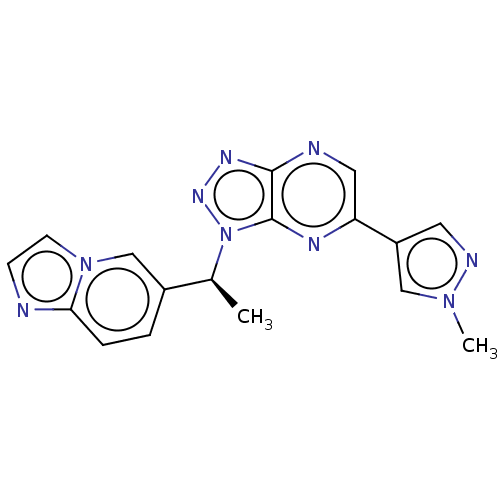
(CHEMBL3334567)Show SMILES C[C@@H](c1ccc2nccn2c1)n1nnc2ncc(nc12)-c1cnn(C)c1 |r| Show InChI InChI=1S/C17H15N9/c1-11(12-3-4-15-18-5-6-25(15)10-12)26-17-16(22-23-26)19-8-14(21-17)13-7-20-24(2)9-13/h3-11H,1-2H3/t11-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of c-Met phosphorylation at Tyr 1234/Tyr1235 residues in human NCI-H1993 cells incubated for 4 hrs by HTRF assay |
ACS Med Chem Lett 10: 1322-1327 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00276
BindingDB Entry DOI: 10.7270/Q27W6GKF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ubiquitin carboxyl-terminal hydrolase 7
(Homo sapiens (Human)) | BDBM50538569

(CHEMBL4644317)Show SMILES C[C@H]1CNCC[C@H]1Oc1c(C)cc(Cl)cc1-c1ccnc2cc(CN3C(=O)CCC3=O)sc12 |r| Show InChI InChI=1S/C25H26ClN3O3S/c1-14-9-16(26)10-19(24(14)32-21-6-7-27-12-15(21)2)18-5-8-28-20-11-17(33-25(18)20)13-29-22(30)3-4-23(29)31/h5,8-11,15,21,27H,3-4,6-7,12-13H2,1-2H3/t15-,21+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length USP7 (unknown origin) using ubiquintin-rhodamine as substrate preincubated for 30 mins followed by substrate ad... |
J Med Chem 63: 5398-5420 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00245
BindingDB Entry DOI: 10.7270/Q2XK8K2T |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50591644
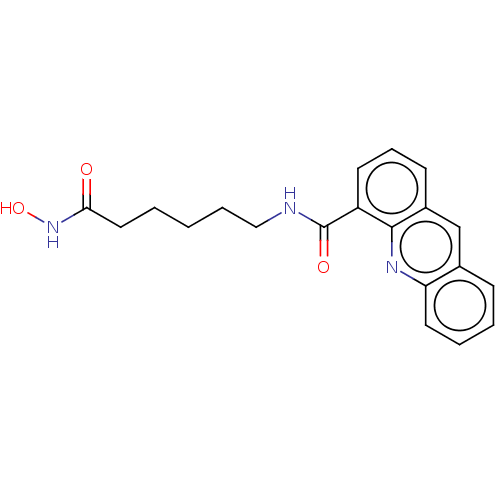
(CHEMBL5206589) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112844
BindingDB Entry DOI: 10.7270/Q2KK9GTQ |
More data for this
Ligand-Target Pair | |
Ubiquitin carboxyl-terminal hydrolase 7
(Homo sapiens (Human)) | BDBM50538581
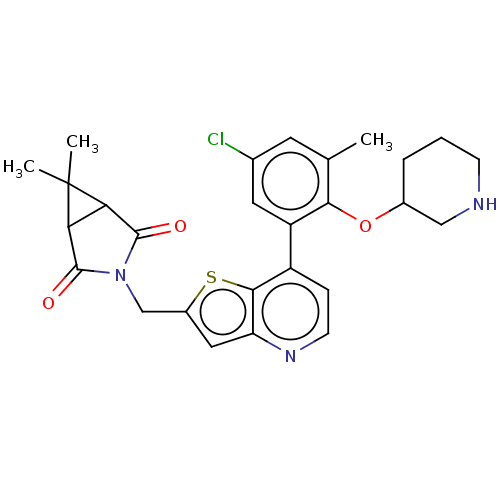
(CHEMBL4642862)Show SMILES Cc1cc(Cl)cc(c1OC1CCCNC1)-c1ccnc2cc(CN3C(=O)C4C(C3=O)C4(C)C)sc12 Show InChI InChI=1S/C27H28ClN3O3S/c1-14-9-15(28)10-19(23(14)34-16-5-4-7-29-12-16)18-6-8-30-20-11-17(35-24(18)20)13-31-25(32)21-22(26(31)33)27(21,2)3/h6,8-11,16,21-22,29H,4-5,7,12-13H2,1-3H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length USP7 (unknown origin) using ubiquintin-rhodamine as substrate preincubated for 30 mins followed by substrate ad... |
J Med Chem 63: 5398-5420 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00245
BindingDB Entry DOI: 10.7270/Q2XK8K2T |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM28031
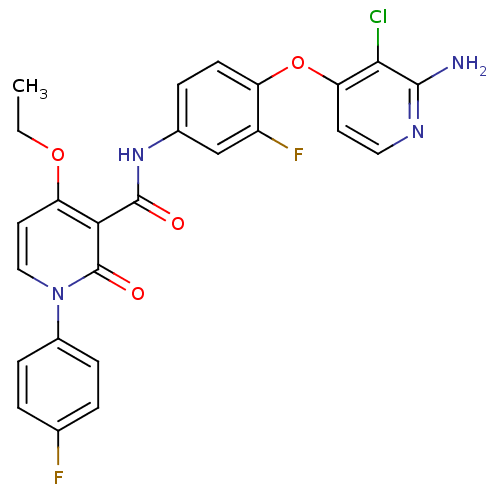
(BMS-777607 | N-{4-[(2-amino-3-chloropyridin-4-yl)o...)Show SMILES CCOc1ccn(-c2ccc(F)cc2)c(=O)c1C(=O)Nc1ccc(Oc2ccnc(N)c2Cl)c(F)c1 Show InChI InChI=1S/C25H19ClF2N4O4/c1-2-35-19-10-12-32(16-6-3-14(27)4-7-16)25(34)21(19)24(33)31-15-5-8-18(17(28)13-15)36-20-9-11-30-23(29)22(20)26/h3-13H,2H2,1H3,(H2,29,30)(H,31,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | >2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of wild type N-terminal NH-tagged and avi-tagged dephosphorylated c-MET (956 to 1390 residues) (unknown origin) expressed in sf21 cells us... |
ACS Med Chem Lett 10: 1322-1327 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00276
BindingDB Entry DOI: 10.7270/Q27W6GKF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50399540

(FORETINIB | US10464902, Foretinib | US10882853, Co...)Show SMILES COc1cc2c(Oc3ccc(NC(=O)C4(CC4)C(=O)Nc4ccc(F)cc4)cc3F)ccnc2cc1OCCCN1CCOCC1 Show InChI InChI=1S/C34H34F2N4O6/c1-43-30-20-25-27(21-31(30)45-16-2-13-40-14-17-44-18-15-40)37-12-9-28(25)46-29-8-7-24(19-26(29)36)39-33(42)34(10-11-34)32(41)38-23-5-3-22(35)4-6-23/h3-9,12,19-21H,2,10-11,13-18H2,1H3,(H,38,41)(H,39,42) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | >2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of wild type N-terminal NH-tagged and avi-tagged dephosphorylated c-MET (956 to 1390 residues) (unknown origin) expressed in sf21 cells us... |
ACS Med Chem Lett 10: 1322-1327 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00276
BindingDB Entry DOI: 10.7270/Q27W6GKF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM28031

(BMS-777607 | N-{4-[(2-amino-3-chloropyridin-4-yl)o...)Show SMILES CCOc1ccn(-c2ccc(F)cc2)c(=O)c1C(=O)Nc1ccc(Oc2ccnc(N)c2Cl)c(F)c1 Show InChI InChI=1S/C25H19ClF2N4O4/c1-2-35-19-10-12-32(16-6-3-14(27)4-7-16)25(34)21(19)24(33)31-15-5-8-18(17(28)13-15)36-20-9-11-30-23(29)22(20)26/h3-13H,2H2,1H3,(H2,29,30)(H,31,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | >2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal NH-tagged and avi-tagged dephosphorylated c-MET D1228V mutant (956 to 1390 residues) (unknown origin) expressed in sf21 cell... |
ACS Med Chem Lett 10: 1322-1327 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00276
BindingDB Entry DOI: 10.7270/Q27W6GKF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50306682

((R)-3-(1-(2,6-dichloro-3-fluorophenyl)ethoxy)-5-(1...)Show SMILES C[C@@H](Oc1cc(cnc1N)-c1cnn(c1)C1CCNCC1)c1c(Cl)ccc(F)c1Cl |r| Show InChI InChI=1S/C21H22Cl2FN5O/c1-12(19-16(22)2-3-17(24)20(19)23)30-18-8-13(9-27-21(18)25)14-10-28-29(11-14)15-4-6-26-7-5-15/h2-3,8-12,15,26H,4-7H2,1H3,(H2,25,27)/t12-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | >2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal NH-tagged and avi-tagged dephosphorylated c-MET D1228V mutant (956 to 1390 residues) (unknown origin) expressed in sf21 cell... |
ACS Med Chem Lett 10: 1322-1327 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00276
BindingDB Entry DOI: 10.7270/Q27W6GKF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50399540

(FORETINIB | US10464902, Foretinib | US10882853, Co...)Show SMILES COc1cc2c(Oc3ccc(NC(=O)C4(CC4)C(=O)Nc4ccc(F)cc4)cc3F)ccnc2cc1OCCCN1CCOCC1 Show InChI InChI=1S/C34H34F2N4O6/c1-43-30-20-25-27(21-31(30)45-16-2-13-40-14-17-44-18-15-40)37-12-9-28(25)46-29-8-7-24(19-26(29)36)39-33(42)34(10-11-34)32(41)38-23-5-3-22(35)4-6-23/h3-9,12,19-21H,2,10-11,13-18H2,1H3,(H,38,41)(H,39,42) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | >2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal NH-tagged and avi-tagged dephosphorylated c-MET D1228V mutant (956 to 1390 residues) (unknown origin) expressed in sf21 cell... |
ACS Med Chem Lett 10: 1322-1327 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00276
BindingDB Entry DOI: 10.7270/Q27W6GKF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50306682

((R)-3-(1-(2,6-dichloro-3-fluorophenyl)ethoxy)-5-(1...)Show SMILES C[C@@H](Oc1cc(cnc1N)-c1cnn(c1)C1CCNCC1)c1c(Cl)ccc(F)c1Cl |r| Show InChI InChI=1S/C21H22Cl2FN5O/c1-12(19-16(22)2-3-17(24)20(19)23)30-18-8-13(9-27-21(18)25)14-10-28-29(11-14)15-4-6-26-7-5-15/h2-3,8-12,15,26H,4-7H2,1H3,(H2,25,27)/t12-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | >2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of wild type N-terminal NH-tagged and avi-tagged dephosphorylated c-MET (956 to 1390 residues) (unknown origin) expressed in sf21 cells us... |
ACS Med Chem Lett 10: 1322-1327 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00276
BindingDB Entry DOI: 10.7270/Q27W6GKF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50023342

(CHEMBL3334567)Show SMILES C[C@@H](c1ccc2nccn2c1)n1nnc2ncc(nc12)-c1cnn(C)c1 |r| Show InChI InChI=1S/C17H15N9/c1-11(12-3-4-15-18-5-6-25(15)10-12)26-17-16(22-23-26)19-8-14(21-17)13-7-20-24(2)9-13/h3-11H,1-2H3/t11-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | >2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of wild type N-terminal NH-tagged and avi-tagged dephosphorylated c-MET (956 to 1390 residues) (unknown origin) expressed in sf21 cells us... |
ACS Med Chem Lett 10: 1322-1327 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00276
BindingDB Entry DOI: 10.7270/Q27W6GKF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Flap endonuclease 1
(Homo sapiens (Human)) | BDBM50157830
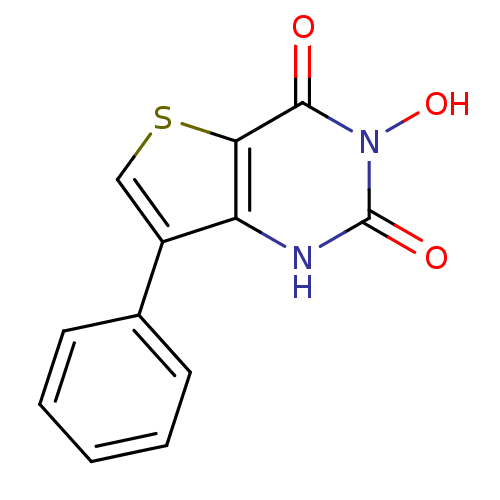
(3-Hydroxy-7-phenyl-1H-thieno[3,2-d]pyrimidine-2,4-...)Show InChI InChI=1S/C12H8N2O3S/c15-11-10-9(13-12(16)14(11)17)8(6-18-10)7-4-2-1-3-5-7/h1-6,17H,(H,13,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
MRC Technology
Curated by ChEMBL
| Assay Description
Inhibition of FEN1 (unknown origin) |
Bioorg Med Chem Lett 25: 4104-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.024
BindingDB Entry DOI: 10.7270/Q28S4RQ2 |
More data for this
Ligand-Target Pair | |
Ubiquitin carboxyl-terminal hydrolase 7
(Homo sapiens (Human)) | BDBM50538568
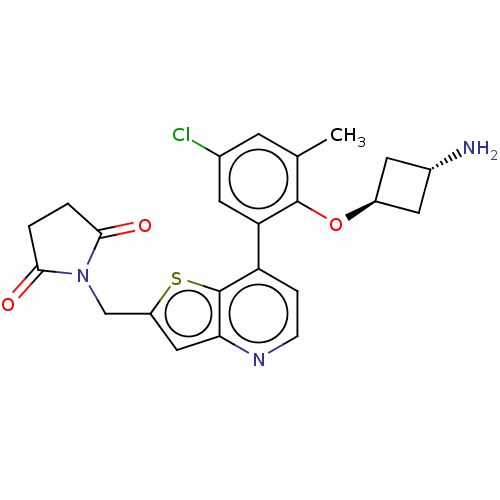
(CHEMBL4640563)Show SMILES Cc1cc(Cl)cc(c1O[C@H]1C[C@H](N)C1)-c1ccnc2cc(CN3C(=O)CCC3=O)sc12 |r,wU:9.9,wD:11.12,(39.39,-38.53,;38.06,-37.76,;36.71,-38.54,;35.38,-37.77,;34.03,-38.54,;35.38,-36.22,;36.71,-35.44,;38.05,-36.21,;39.38,-35.44,;40.72,-36.2,;42.2,-35.79,;42.6,-37.28,;43.94,-38.04,;41.12,-37.68,;36.7,-33.9,;38.04,-33.13,;38.04,-31.58,;36.69,-30.81,;35.35,-31.6,;33.88,-31.14,;32.99,-32.39,;31.45,-32.4,;30.69,-33.74,;29.16,-33.91,;28.12,-32.78,;28.85,-35.42,;30.19,-36.18,;31.33,-35.14,;32.84,-35.45,;33.91,-33.62,;35.36,-33.14,)| Show InChI InChI=1S/C23H22ClN3O3S/c1-12-6-13(24)7-18(22(12)30-15-8-14(25)9-15)17-4-5-26-19-10-16(31-23(17)19)11-27-20(28)2-3-21(27)29/h4-7,10,14-15H,2-3,8-9,11,25H2,1H3/t14-,15- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length USP7 (unknown origin) using ubiquintin-rhodamine as substrate preincubated for 30 mins followed by substrate ad... |
J Med Chem 63: 5398-5420 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00245
BindingDB Entry DOI: 10.7270/Q2XK8K2T |
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADH]
(Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50443004
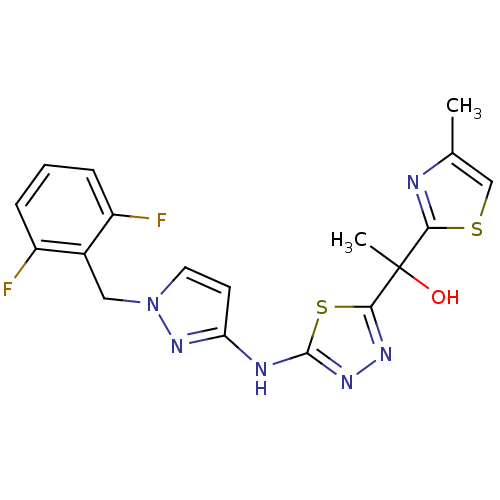
(CHEMBL3088173)Show SMILES Cc1csc(n1)C(C)(O)c1nnc(Nc2ccn(Cc3c(F)cccc3F)n2)s1 Show InChI InChI=1S/C18H16F2N6OS2/c1-10-9-28-15(21-10)18(2,27)16-23-24-17(29-16)22-14-6-7-26(25-14)8-11-12(19)4-3-5-13(11)20/h3-7,9,27H,8H2,1-2H3,(H,22,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cathepsin D
(Homo sapiens (Human)) | BDBM50555173

(CHEMBL4754691)Show SMILES OC(=O)C(F)(F)F.CNS(=O)(=O)c1ccc(C)c(NC(=O)[C@@H](CC2CCCCC2)NC(=N)NC(=O)Cc2ccc(OC)c(OC)c2)c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human liver Cathepsin D preincubated for 10 mins followed by substrate addition and further incubated for 2 hrs in dark by fluorimetric... |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115879
BindingDB Entry DOI: 10.7270/Q2HQ43KW |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data