

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50287776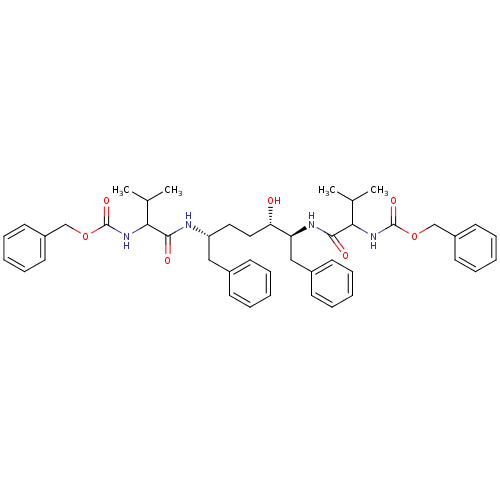 (CHEMBL304545 | {(R)-1-[(S)-(S)-1-Benzyl-5-(2-benzy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | >1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of HIV protease | Bioorg Med Chem Lett 6: 2201-2206 (1996) Article DOI: 10.1016/0960-894X(96)00392-7 BindingDB Entry DOI: 10.7270/Q2125SP0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM199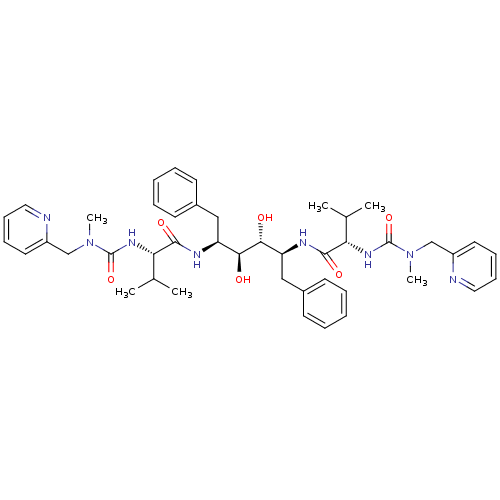 ((2S)-N-[(2S,3R,4S,5S)-3,4-dihydroxy-5-[(2S)-3-meth...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article | n/a | n/a | >1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of HIV protease | Bioorg Med Chem Lett 6: 2201-2206 (1996) Article DOI: 10.1016/0960-894X(96)00392-7 BindingDB Entry DOI: 10.7270/Q2125SP0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50287778 (CHEMBL73308 | Diaminoalcohol analogue) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of HIV protease | Bioorg Med Chem Lett 6: 2201-2206 (1996) Article DOI: 10.1016/0960-894X(96)00392-7 BindingDB Entry DOI: 10.7270/Q2125SP0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50205086 (2-((3-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)benz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of fluorescein labeled ligand from PPARalpha receptor by fluorescence polarization assay | Bioorg Med Chem Lett 17: 2312-6 (2007) Article DOI: 10.1016/j.bmcl.2007.01.060 BindingDB Entry DOI: 10.7270/Q21N80ST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50205080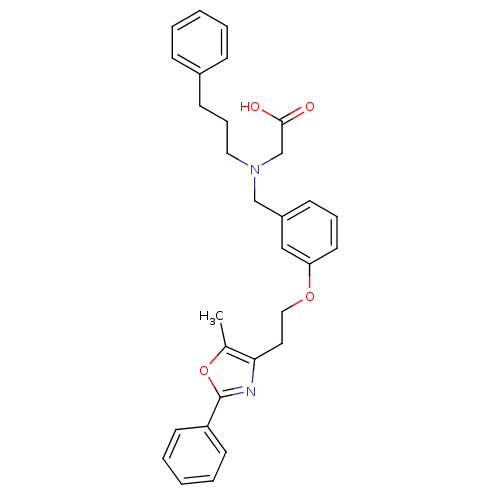 (2-((3-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)benz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of fluorescein labeled ligand from PPARalpha receptor by fluorescence polarization assay | Bioorg Med Chem Lett 17: 2312-6 (2007) Article DOI: 10.1016/j.bmcl.2007.01.060 BindingDB Entry DOI: 10.7270/Q21N80ST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50205086 (2-((3-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)benz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of fluorescein labeled ligand from PPARgamma receptor by fluorescence polarization assay | Bioorg Med Chem Lett 17: 2312-6 (2007) Article DOI: 10.1016/j.bmcl.2007.01.060 BindingDB Entry DOI: 10.7270/Q21N80ST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50205081 (2-((3-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)benz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of fluorescein labeled ligand from PPARalpha receptor by fluorescence polarization assay | Bioorg Med Chem Lett 17: 2312-6 (2007) Article DOI: 10.1016/j.bmcl.2007.01.060 BindingDB Entry DOI: 10.7270/Q21N80ST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50205089 (2-((3-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)benz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of fluorescein labeled ligand from PPARalpha receptor by fluorescence polarization assay | Bioorg Med Chem Lett 17: 2312-6 (2007) Article DOI: 10.1016/j.bmcl.2007.01.060 BindingDB Entry DOI: 10.7270/Q21N80ST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50205072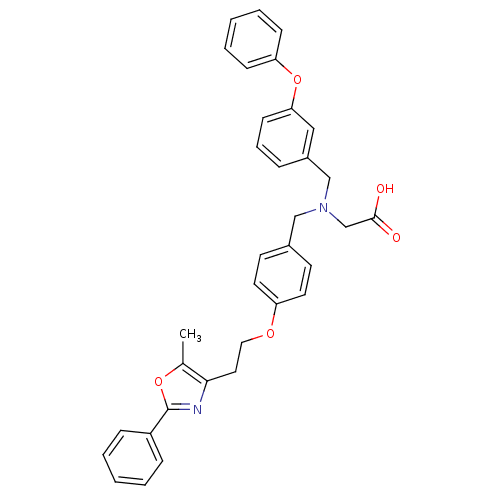 (2-((3-phenoxybenzyl)(4-(2-(5-methyl-2-phenyloxazol...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 88 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of fluorescein labeled ligand from PPARalpha receptor by fluorescence polarization assay | Bioorg Med Chem Lett 17: 2312-6 (2007) Article DOI: 10.1016/j.bmcl.2007.01.060 BindingDB Entry DOI: 10.7270/Q21N80ST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50205075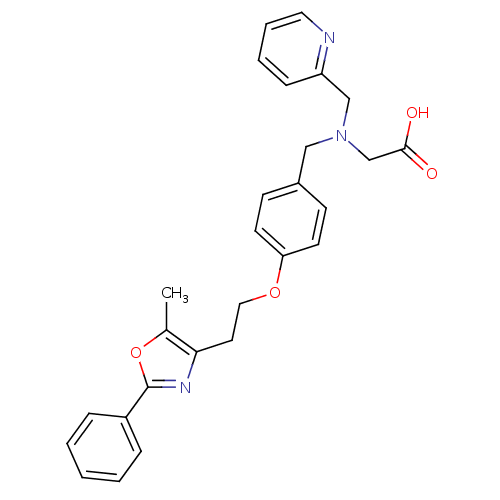 (2-((4-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)benz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of fluorescein labeled ligand from PPARalpha receptor by fluorescence polarization assay | Bioorg Med Chem Lett 17: 2312-6 (2007) Article DOI: 10.1016/j.bmcl.2007.01.060 BindingDB Entry DOI: 10.7270/Q21N80ST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50205088 (2-((3-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)benz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of fluorescein labeled ligand from PPARalpha receptor by fluorescence polarization assay | Bioorg Med Chem Lett 17: 2312-6 (2007) Article DOI: 10.1016/j.bmcl.2007.01.060 BindingDB Entry DOI: 10.7270/Q21N80ST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50205079 (2-((3-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)benz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of fluorescein labeled ligand from PPARalpha receptor by fluorescence polarization assay | Bioorg Med Chem Lett 17: 2312-6 (2007) Article DOI: 10.1016/j.bmcl.2007.01.060 BindingDB Entry DOI: 10.7270/Q21N80ST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50205089 (2-((3-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)benz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of fluorescein labeled ligand from PPARgamma receptor by fluorescence polarization assay | Bioorg Med Chem Lett 17: 2312-6 (2007) Article DOI: 10.1016/j.bmcl.2007.01.060 BindingDB Entry DOI: 10.7270/Q21N80ST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50205081 (2-((3-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)benz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 134 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of fluorescein labeled ligand from PPARgamma receptor by fluorescence polarization assay | Bioorg Med Chem Lett 17: 2312-6 (2007) Article DOI: 10.1016/j.bmcl.2007.01.060 BindingDB Entry DOI: 10.7270/Q21N80ST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50205074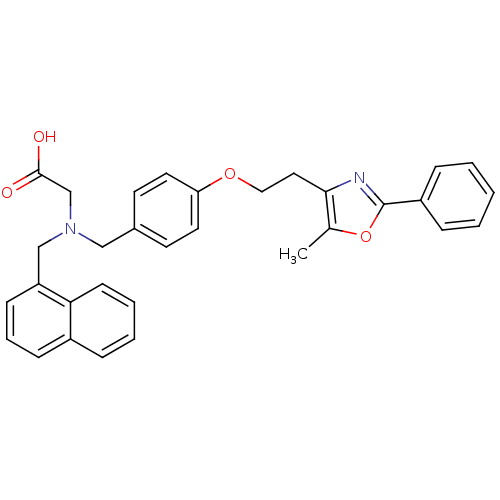 (2-((4-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)benz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of fluorescein labeled ligand from PPARalpha receptor by fluorescence polarization assay | Bioorg Med Chem Lett 17: 2312-6 (2007) Article DOI: 10.1016/j.bmcl.2007.01.060 BindingDB Entry DOI: 10.7270/Q21N80ST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50314811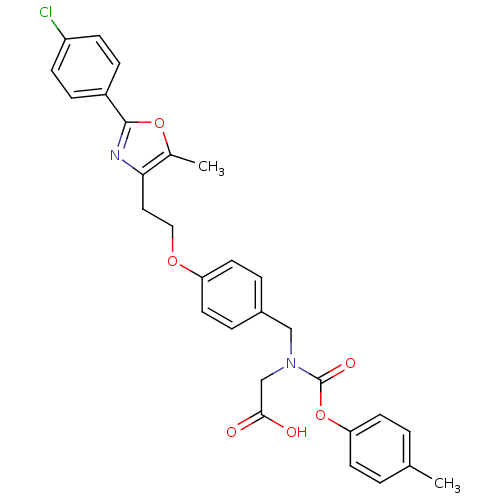 (2-((4-(2-(2-(4-chlorophenyl)-5-methyloxazol-4-yl)e...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 141 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human His-tagged PPARgamma LBD (Q203-Y477) expressed in Escherichia coli BL21 (DE3) by fluorescence polarization assay | J Med Chem 53: 2854-64 (2010) Article DOI: 10.1021/jm9016812 BindingDB Entry DOI: 10.7270/Q2B56JW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50205078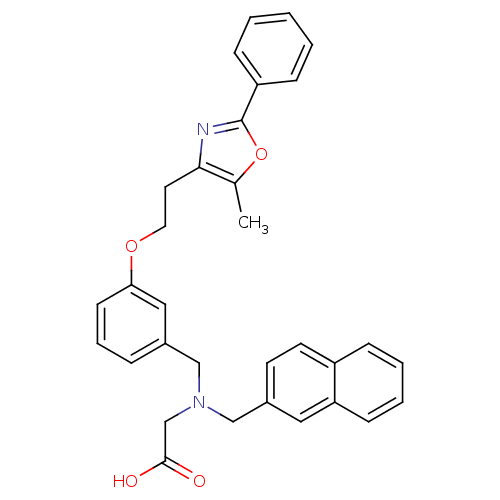 (2-((3-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)benz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 145 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of fluorescein labeled ligand from PPARgamma receptor by fluorescence polarization assay | Bioorg Med Chem Lett 17: 2312-6 (2007) Article DOI: 10.1016/j.bmcl.2007.01.060 BindingDB Entry DOI: 10.7270/Q21N80ST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50205078 (2-((3-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)benz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 149 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of fluorescein labeled ligand from PPARalpha receptor by fluorescence polarization assay | Bioorg Med Chem Lett 17: 2312-6 (2007) Article DOI: 10.1016/j.bmcl.2007.01.060 BindingDB Entry DOI: 10.7270/Q21N80ST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50314813 (2-((3-(2-(2-(4-chlorophenyl)-5-methyloxazol-4-yl)e...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 162 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human His-tagged PPARgamma LBD (Q203-Y477) expressed in Escherichia coli BL21 (DE3) by fluorescence polarization assay | J Med Chem 53: 2854-64 (2010) Article DOI: 10.1021/jm9016812 BindingDB Entry DOI: 10.7270/Q2B56JW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50205083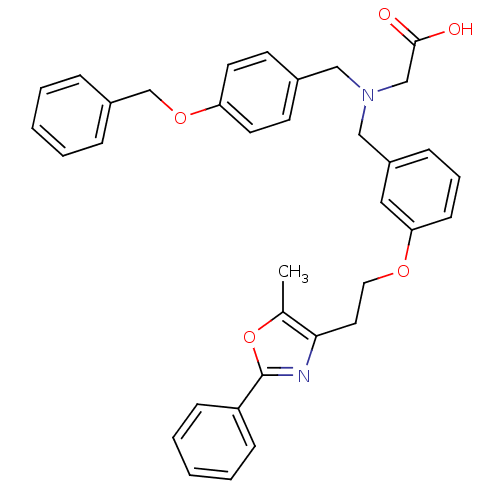 (2-((3-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)benz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 183 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of fluorescein labeled ligand from PPARgamma receptor by fluorescence polarization assay | Bioorg Med Chem Lett 17: 2312-6 (2007) Article DOI: 10.1016/j.bmcl.2007.01.060 BindingDB Entry DOI: 10.7270/Q21N80ST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50150998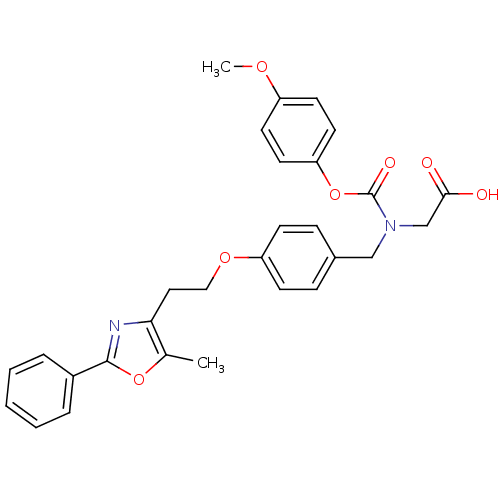 (((4-Methoxy-phenoxycarbonyl)-{4-[2-(5-methyl-2-phe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of human Peroxisome proliferator activated receptor gamma | J Med Chem 48: 2248-50 (2005) Article DOI: 10.1021/jm0496436 BindingDB Entry DOI: 10.7270/Q2SX6CRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50287774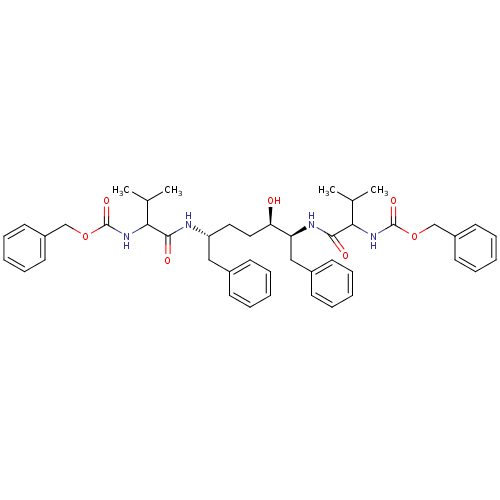 (CHEMBL71514 | {(R)-1-[(S)-(R)-1-Benzyl-5-(2-benzyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of HIV protease | Bioorg Med Chem Lett 6: 2201-2206 (1996) Article DOI: 10.1016/0960-894X(96)00392-7 BindingDB Entry DOI: 10.7270/Q2125SP0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50085044 ((S)-2-(2-Benzoyl-phenylamino)-3-{4-[2-(5-methyl-2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 217 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of fluorescein labeled ligand from PPARgamma receptor by fluorescence polarization assay | Bioorg Med Chem Lett 17: 2312-6 (2007) Article DOI: 10.1016/j.bmcl.2007.01.060 BindingDB Entry DOI: 10.7270/Q21N80ST | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50205073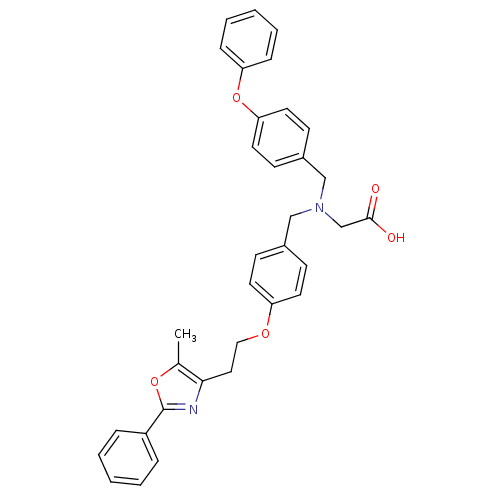 (2-((4-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)benz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 227 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of fluorescein labeled ligand from PPARgamma receptor by fluorescence polarization assay | Bioorg Med Chem Lett 17: 2312-6 (2007) Article DOI: 10.1016/j.bmcl.2007.01.060 BindingDB Entry DOI: 10.7270/Q21N80ST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50314832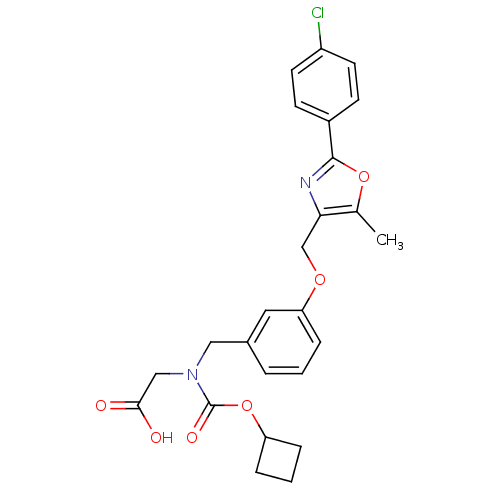 (2-((3-((2-(4-chlorophenyl)-5-methyloxazol-4-yl)met...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 228 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human His-tagged PPARalpha LBD (E196-Y468) expressed in Escherichia coli BL21 (DE3) by fluorescence polarization assay | J Med Chem 53: 2854-64 (2010) Article DOI: 10.1021/jm9016812 BindingDB Entry DOI: 10.7270/Q2B56JW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50205079 (2-((3-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)benz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of fluorescein labeled ligand from PPARgamma receptor by fluorescence polarization assay | Bioorg Med Chem Lett 17: 2312-6 (2007) Article DOI: 10.1016/j.bmcl.2007.01.060 BindingDB Entry DOI: 10.7270/Q21N80ST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50205088 (2-((3-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)benz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of fluorescein labeled ligand from PPARgamma receptor by fluorescence polarization assay | Bioorg Med Chem Lett 17: 2312-6 (2007) Article DOI: 10.1016/j.bmcl.2007.01.060 BindingDB Entry DOI: 10.7270/Q21N80ST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50205076 (2-(((1H-indol-2-yl)methyl)(3-(2-(5-methyl-2-phenyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 246 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of fluorescein labeled ligand from PPARalpha receptor by fluorescence polarization assay | Bioorg Med Chem Lett 17: 2312-6 (2007) Article DOI: 10.1016/j.bmcl.2007.01.060 BindingDB Entry DOI: 10.7270/Q21N80ST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50314812 (2-((4-((2-(4-chlorophenyl)-5-methyloxazol-4-yl)met...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 248 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human His-tagged PPARgamma LBD (Q203-Y477) expressed in Escherichia coli BL21 (DE3) by fluorescence polarization assay | J Med Chem 53: 2854-64 (2010) Article DOI: 10.1021/jm9016812 BindingDB Entry DOI: 10.7270/Q2B56JW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50150998 (((4-Methoxy-phenoxycarbonyl)-{4-[2-(5-methyl-2-phe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of human Peroxisome proliferator activated receptor alpha | J Med Chem 48: 2248-50 (2005) Article DOI: 10.1021/jm0496436 BindingDB Entry DOI: 10.7270/Q2SX6CRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM28681 (5-[(4-{2-[methyl(pyridin-2-yl)amino]ethoxy}phenyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of human Peroxisome proliferator activated receptor gamma | J Med Chem 48: 2248-50 (2005) Article DOI: 10.1021/jm0496436 BindingDB Entry DOI: 10.7270/Q2SX6CRR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM28681 (5-[(4-{2-[methyl(pyridin-2-yl)amino]ethoxy}phenyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 256 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of fluorescein labeled ligand from PPARgamma receptor by fluorescence polarization assay | Bioorg Med Chem Lett 17: 2312-6 (2007) Article DOI: 10.1016/j.bmcl.2007.01.060 BindingDB Entry DOI: 10.7270/Q21N80ST | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM28800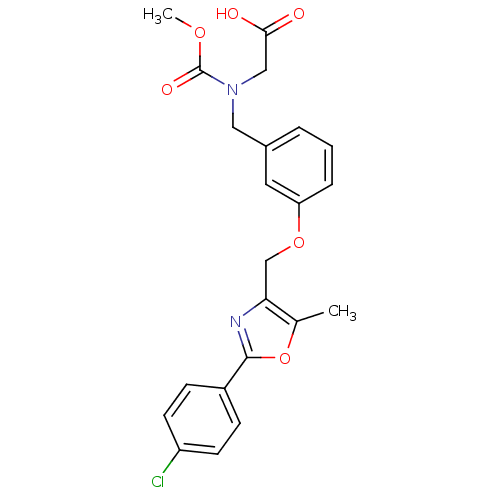 (2-{[(3-{[2-(4-chlorophenyl)-5-methyl-1,3-oxazol-4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human His-tagged PPARalpha LBD (E196-Y468) expressed in Escherichia coli BL21 (DE3) by fluorescence polarization assay | J Med Chem 53: 2854-64 (2010) Article DOI: 10.1021/jm9016812 BindingDB Entry DOI: 10.7270/Q2B56JW0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50205087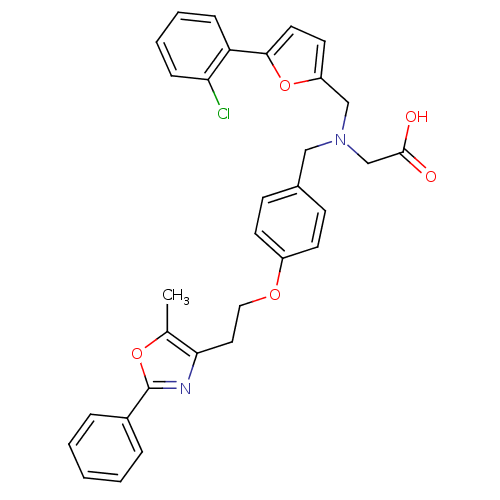 (2-((4-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)benz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of fluorescein labeled ligand from PPARgamma receptor by fluorescence polarization assay | Bioorg Med Chem Lett 17: 2312-6 (2007) Article DOI: 10.1016/j.bmcl.2007.01.060 BindingDB Entry DOI: 10.7270/Q21N80ST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50205085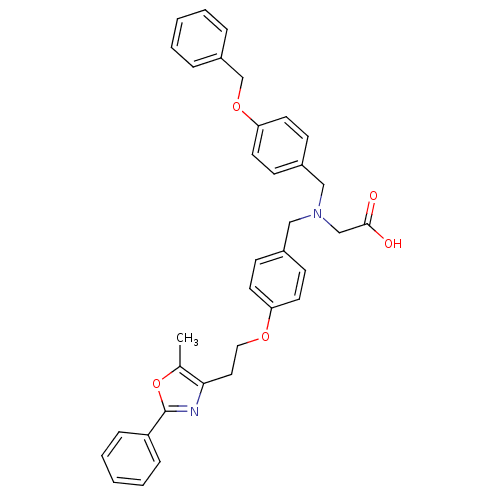 (2-((4-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)benz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 284 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of fluorescein labeled ligand from PPARgamma receptor by fluorescence polarization assay | Bioorg Med Chem Lett 17: 2312-6 (2007) Article DOI: 10.1016/j.bmcl.2007.01.060 BindingDB Entry DOI: 10.7270/Q21N80ST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM28680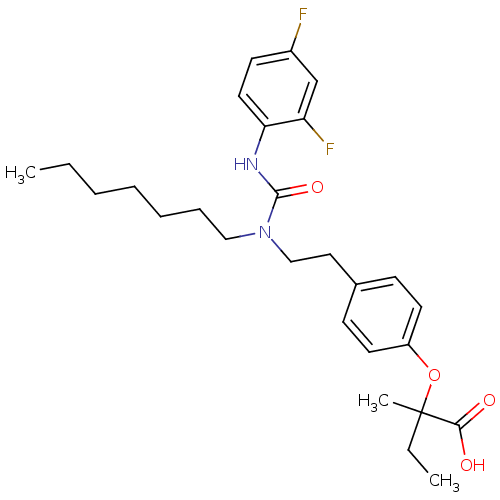 (2-[4-(2-{[(2,4-difluorophenyl)carbamoyl](heptyl)am...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 316 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of human Peroxisome proliferator activated receptor gamma | J Med Chem 48: 2248-50 (2005) Article DOI: 10.1021/jm0496436 BindingDB Entry DOI: 10.7270/Q2SX6CRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50314834 (2-((3-((2-(4-chlorophenyl)-5-methyloxazol-4-yl)met...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 336 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human His-tagged PPARalpha LBD (E196-Y468) expressed in Escherichia coli BL21 (DE3) by fluorescence polarization assay | J Med Chem 53: 2854-64 (2010) Article DOI: 10.1021/jm9016812 BindingDB Entry DOI: 10.7270/Q2B56JW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50314814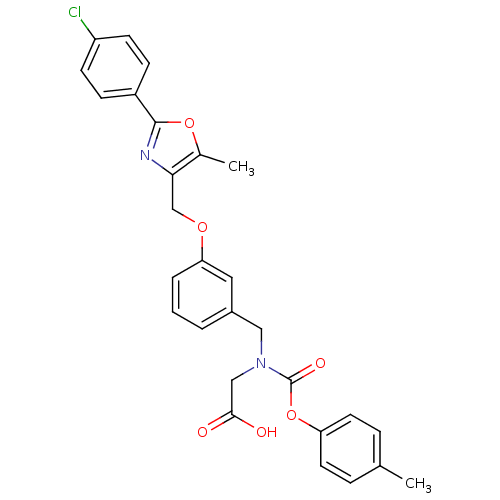 (2-((3-((2-(4-chlorophenyl)-5-methyloxazol-4-yl)met...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 347 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human His-tagged PPARalpha LBD (E196-Y468) expressed in Escherichia coli BL21 (DE3) by fluorescence polarization assay | J Med Chem 53: 2854-64 (2010) Article DOI: 10.1021/jm9016812 BindingDB Entry DOI: 10.7270/Q2B56JW0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50205090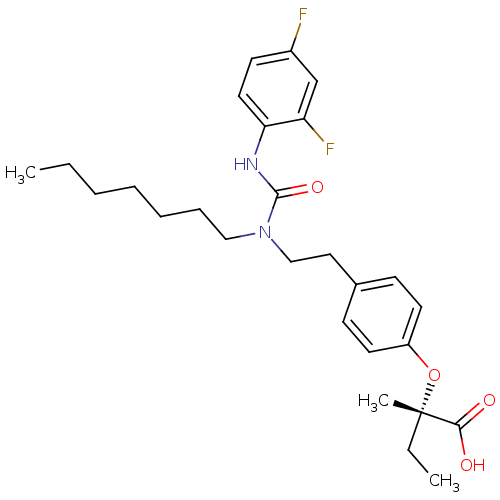 ((2S)-2-(4-[2-(3-[2,4-DIFLUOROPHENYL]-1-HEPTYLUREID...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 348 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of fluorescein labeled ligand from PPARalpha receptor by fluorescence polarization assay | Bioorg Med Chem Lett 17: 2312-6 (2007) Article DOI: 10.1016/j.bmcl.2007.01.060 BindingDB Entry DOI: 10.7270/Q21N80ST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50314833 (2-((3-((2-(4-chlorophenyl)-5-methyloxazol-4-yl)met...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 351 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human His-tagged PPARalpha LBD (E196-Y468) expressed in Escherichia coli BL21 (DE3) by fluorescence polarization assay | J Med Chem 53: 2854-64 (2010) Article DOI: 10.1021/jm9016812 BindingDB Entry DOI: 10.7270/Q2B56JW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50314820 (2-((3-((2-(4-fluorophenyl)-5-methyloxazol-4-yl)met...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 362 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human His-tagged PPARalpha LBD (E196-Y468) expressed in Escherichia coli BL21 (DE3) by fluorescence polarization assay | J Med Chem 53: 2854-64 (2010) Article DOI: 10.1021/jm9016812 BindingDB Entry DOI: 10.7270/Q2B56JW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50314823 (2-((3-((5-methyl-2-p-tolyloxazol-4-yl)methoxy)benz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 372 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human His-tagged PPARalpha LBD (E196-Y468) expressed in Escherichia coli BL21 (DE3) by fluorescence polarization assay | J Med Chem 53: 2854-64 (2010) Article DOI: 10.1021/jm9016812 BindingDB Entry DOI: 10.7270/Q2B56JW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50314822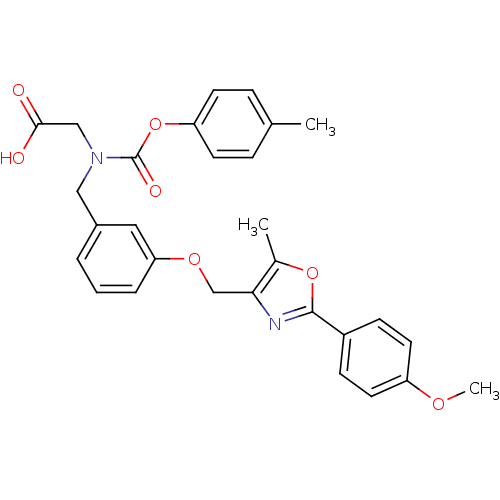 (2-((3-((2-(4-methoxyphenyl)-5-methyloxazol-4-yl)me...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 375 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human His-tagged PPARalpha LBD (E196-Y468) expressed in Escherichia coli BL21 (DE3) by fluorescence polarization assay | J Med Chem 53: 2854-64 (2010) Article DOI: 10.1021/jm9016812 BindingDB Entry DOI: 10.7270/Q2B56JW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50287775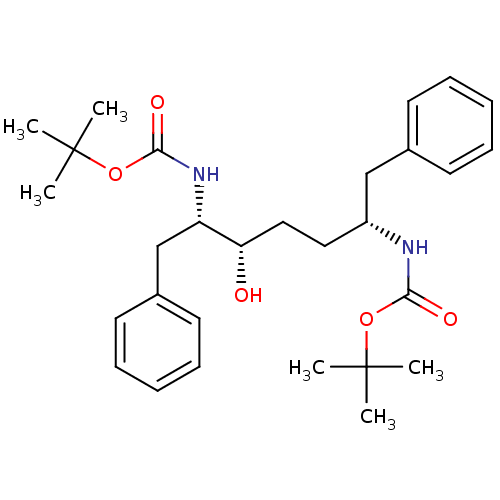 (((1R,4S,5S)-1-Benzyl-5-tert-butoxycarbonylamino-4-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of HIV protease | Bioorg Med Chem Lett 6: 2201-2206 (1996) Article DOI: 10.1016/0960-894X(96)00392-7 BindingDB Entry DOI: 10.7270/Q2125SP0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50314819 (2-((3-((2-(3-chlorophenyl)-5-methyloxazol-4-yl)met...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 384 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human His-tagged PPARgamma LBD (Q203-Y477) expressed in Escherichia coli BL21 (DE3) by fluorescence polarization assay | J Med Chem 53: 2854-64 (2010) Article DOI: 10.1021/jm9016812 BindingDB Entry DOI: 10.7270/Q2B56JW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50205083 (2-((3-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)benz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 387 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of fluorescein labeled ligand from PPARalpha receptor by fluorescence polarization assay | Bioorg Med Chem Lett 17: 2312-6 (2007) Article DOI: 10.1016/j.bmcl.2007.01.060 BindingDB Entry DOI: 10.7270/Q21N80ST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50314831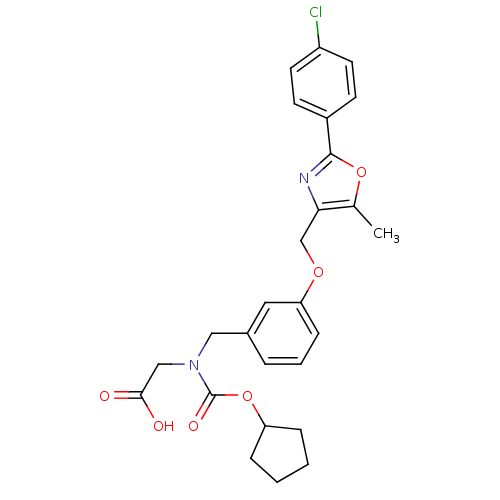 (2-((3-((2-(4-chlorophenyl)-5-methyloxazol-4-yl)met...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 408 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human His-tagged PPARalpha LBD (E196-Y468) expressed in Escherichia coli BL21 (DE3) by fluorescence polarization assay | J Med Chem 53: 2854-64 (2010) Article DOI: 10.1021/jm9016812 BindingDB Entry DOI: 10.7270/Q2B56JW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50314818 (2-((3-((2-(2-chlorophenyl)-5-methyloxazol-4-yl)met...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human His-tagged PPARalpha LBD (E196-Y468) expressed in Escherichia coli BL21 (DE3) by fluorescence polarization assay | J Med Chem 53: 2854-64 (2010) Article DOI: 10.1021/jm9016812 BindingDB Entry DOI: 10.7270/Q2B56JW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50314819 (2-((3-((2-(3-chlorophenyl)-5-methyloxazol-4-yl)met...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 422 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human His-tagged PPARalpha LBD (E196-Y468) expressed in Escherichia coli BL21 (DE3) by fluorescence polarization assay | J Med Chem 53: 2854-64 (2010) Article DOI: 10.1021/jm9016812 BindingDB Entry DOI: 10.7270/Q2B56JW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50314821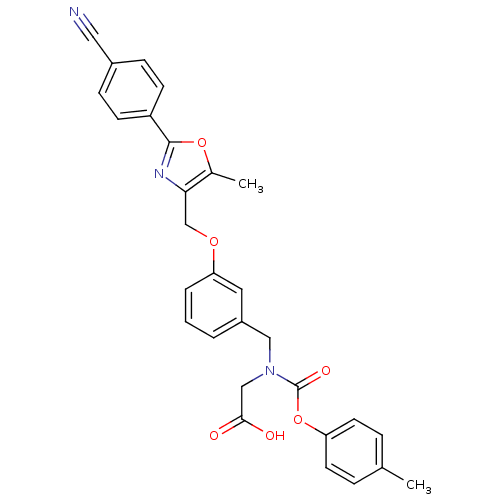 (2-((3-((2-(4-cyanophenyl)-5-methyloxazol-4-yl)meth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 473 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human His-tagged PPARalpha LBD (E196-Y468) expressed in Escherichia coli BL21 (DE3) by fluorescence polarization assay | J Med Chem 53: 2854-64 (2010) Article DOI: 10.1021/jm9016812 BindingDB Entry DOI: 10.7270/Q2B56JW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 251 total ) | Next | Last >> |