 Found 63 hits with Last Name = 'codd' and Initial = 'r'
Found 63 hits with Last Name = 'codd' and Initial = 'r' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50083164

((S)-2-[(S)-2-Amino-3-(4-hydroxy-2,6-dimethyl-pheny...)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N1Cc2ccccc2C[C@H]1C(=O)NC12C[C@H]3C[C@H](C[C@H](C3)C1)C2 |TLB:30:31:35:29.28.34,THB:30:29:35:31.36.32| Show InChI InChI=1S/C31H39N3O3/c1-18-7-25(35)8-19(2)26(18)13-27(32)30(37)34-17-24-6-4-3-5-23(24)12-28(34)29(36)33-31-14-20-9-21(15-31)11-22(10-20)16-31/h3-8,20-22,27-28,35H,9-17,32H2,1-2H3,(H,33,36)/t20-,21+,22-,27-,28-,31?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sydney
Curated by ChEMBL
| Assay Description
Antagonist activity at DOR |
Eur J Med Chem 46: 1949-63 (2011)
Article DOI: 10.1016/j.ejmech.2011.01.047
BindingDB Entry DOI: 10.7270/Q22808QQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50323341
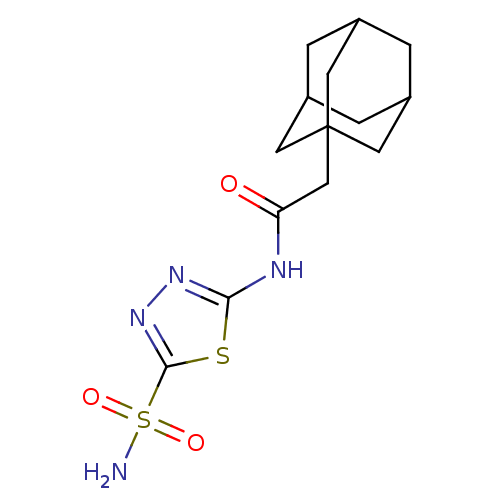
(CHEMBL1209039)Show SMILES NS(=O)(=O)c1nnc(NC(=O)CC23CC4CC(CC(C4)C2)C3)s1 |TLB:11:12:15:19.18.17,THB:13:14:17:21.12.20,13:12:15.14.19:17,20:12:15:19.18.17,20:18:15:21.13.12| Show InChI InChI=1S/C14H20N4O3S2/c15-23(20,21)13-18-17-12(22-13)16-11(19)7-14-4-8-1-9(5-14)3-10(2-8)6-14/h8-10H,1-7H2,(H2,15,20,21)(H,16,17,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sydney
Curated by ChEMBL
| Assay Description
Inhibition of human CA2 |
Eur J Med Chem 46: 1949-63 (2011)
Article DOI: 10.1016/j.ejmech.2011.01.047
BindingDB Entry DOI: 10.7270/Q22808QQ |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50396058

(CHEMBL2170198)Show SMILES NS(=O)(=O)c1nnc(NC(=O)OC23CC4CC(CC(C4)C2)C3)s1 |TLB:11:12:15:19.17.18,THB:17:16:13:19.18.20,17:18:15.16.21:13,20:18:15:21.12.13,20:12:15:19.17.18| Show InChI InChI=1S/C13H18N4O4S2/c14-23(19,20)12-17-16-10(22-12)15-11(18)21-13-4-7-1-8(5-13)3-9(2-7)6-13/h7-9H,1-6H2,(H2,14,19,20)(H,15,16,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sydney
Curated by ChEMBL
| Assay Description
Inhibition of human CA2 |
Eur J Med Chem 46: 1949-63 (2011)
Article DOI: 10.1016/j.ejmech.2011.01.047
BindingDB Entry DOI: 10.7270/Q22808QQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM82104
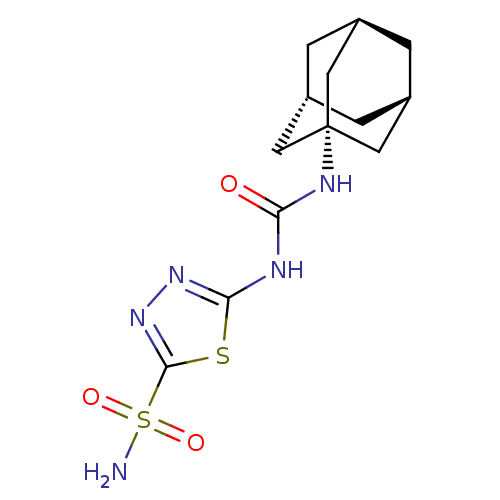
(Investigational agent, 5)Show SMILES NS(=O)(=O)c1nnc(NC(=O)N[C@]23C[C@H]4C[C@H](C[C@H](C4)C2)C3)s1 |r,THB:13:14:20.12.21:17,15:14:20:16.21.17| Show InChI InChI=1S/C13H19N5O3S2/c14-23(20,21)12-18-17-11(22-12)15-10(19)16-13-4-7-1-8(5-13)3-9(2-7)6-13/h7-9H,1-6H2,(H2,14,20,21)(H2,15,16,17,19)/t7-,8+,9-,13- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sydney
Curated by ChEMBL
| Assay Description
Inhibition of human CA2 |
Eur J Med Chem 46: 1949-63 (2011)
Article DOI: 10.1016/j.ejmech.2011.01.047
BindingDB Entry DOI: 10.7270/Q22808QQ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50083164

((S)-2-[(S)-2-Amino-3-(4-hydroxy-2,6-dimethyl-pheny...)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N1Cc2ccccc2C[C@H]1C(=O)NC12C[C@H]3C[C@H](C[C@H](C3)C1)C2 |TLB:30:31:35:29.28.34,THB:30:29:35:31.36.32| Show InChI InChI=1S/C31H39N3O3/c1-18-7-25(35)8-19(2)26(18)13-27(32)30(37)34-17-24-6-4-3-5-23(24)12-28(34)29(36)33-31-14-20-9-21(15-31)11-22(10-20)16-31/h3-8,20-22,27-28,35H,9-17,32H2,1-2H3,(H,33,36)/t20-,21+,22-,27-,28-,31?/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 86 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sydney
Curated by ChEMBL
| Assay Description
Antagonist activity at MOR |
Eur J Med Chem 46: 1949-63 (2011)
Article DOI: 10.1016/j.ejmech.2011.01.047
BindingDB Entry DOI: 10.7270/Q22808QQ |
More data for this
Ligand-Target Pair | |
Non-lysosomal glucosylceramidase
(Homo sapiens (Human)) | BDBM50299749

((2R,3R,4R,5S)-1-[5-(Adamantan-1-ylmethoxy)-pentyl]...)Show SMILES OC[C@@H]1[C@@H](O)[C@H](O)[C@@H](O)CN1CCCCCOCC12CC3CC(CC(C3)C1)C2 |r,TLB:21:22:19.20.25:26,THB:21:20:26:27.22.23,23:22:19:25.24.26,23:24:19:27.21.22| Show InChI InChI=1S/C22H39NO5/c24-13-18-20(26)21(27)19(25)12-23(18)4-2-1-3-5-28-14-22-9-15-6-16(10-22)8-17(7-15)11-22/h15-21,24-27H,1-14H2/t15?,16?,17?,18-,19+,20-,21-,22?/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sydney
Curated by ChEMBL
| Assay Description
Inhibition of non-lysosomal glucosylceramidase in cultured melanoma cells |
Eur J Med Chem 46: 1949-63 (2011)
Article DOI: 10.1016/j.ejmech.2011.01.047
BindingDB Entry DOI: 10.7270/Q22808QQ |
More data for this
Ligand-Target Pair | |
Non-lysosomal glucosylceramidase
(Homo sapiens (Human)) | BDBM50299749

((2R,3R,4R,5S)-1-[5-(Adamantan-1-ylmethoxy)-pentyl]...)Show SMILES OC[C@@H]1[C@@H](O)[C@H](O)[C@@H](O)CN1CCCCCOCC12CC3CC(CC(C3)C1)C2 |r,TLB:21:22:19.20.25:26,THB:21:20:26:27.22.23,23:22:19:25.24.26,23:24:19:27.21.22| Show InChI InChI=1S/C22H39NO5/c24-13-18-20(26)21(27)19(25)12-23(18)4-2-1-3-5-28-14-22-9-15-6-16(10-22)8-17(7-15)11-22/h15-21,24-27H,1-14H2/t15?,16?,17?,18-,19+,20-,21-,22?/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sydney
Curated by ChEMBL
| Assay Description
Inhibition of non-lysosomal glucosylceramidase |
Eur J Med Chem 46: 1949-63 (2011)
Article DOI: 10.1016/j.ejmech.2011.01.047
BindingDB Entry DOI: 10.7270/Q22808QQ |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM25737

(12-[(adamantan-1-ylcarbamoyl)amino]dodecanoic acid...)Show SMILES OC(=O)CCCCCCCCCCCNC(=O)NC12CC3CC(CC(C3)C1)C2 |TLB:25:20:27:24.23.26,25:24:20.21.19:27,THB:23:22:19:24.25.26,23:24:19:22.21.27| Show InChI InChI=1S/C23H40N2O3/c26-21(27)10-8-6-4-2-1-3-5-7-9-11-24-22(28)25-23-15-18-12-19(16-23)14-20(13-18)17-23/h18-20H,1-17H2,(H,26,27)(H2,24,25,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sydney
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant soluble epoxide hydrolase |
Eur J Med Chem 46: 1949-63 (2011)
Article DOI: 10.1016/j.ejmech.2011.01.047
BindingDB Entry DOI: 10.7270/Q22808QQ |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50396056
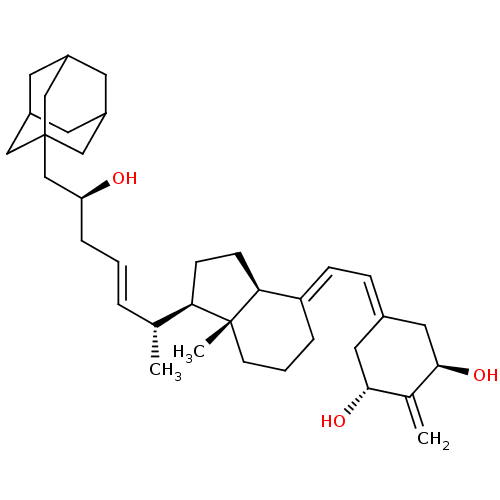
(CHEMBL2170202)Show SMILES [#6]-[#6@H](\[#6]=[#6]\[#6]-[#6@H](-[#8])-[#6]C12[#6]-[#6]-3-[#6]-[#6](-[#6]-[#6](-[#6]-3)-[#6]1)-[#6]2)-[#6@H]1-[#6]-[#6]-[#6@H]2\[#6](-[#6]-[#6]-[#6][C@]12[#6])=[#6]\[#6]=[#6]-1/[#6]-[#6@@H](-[#8])-[#6](=[#6])-[#6@H](-[#8])-[#6]-1 |r,TLB:7:8:11:15.13.14,THB:13:12:9:15.14.16,13:14:11.12.17:9,16:14:11:17.8.9,16:8:11:15.13.14| Show InChI InChI=1S/C36H54O3/c1-23(6-4-8-30(37)22-36-19-26-14-27(20-36)16-28(15-26)21-36)31-11-12-32-29(7-5-13-35(31,32)3)10-9-25-17-33(38)24(2)34(39)18-25/h4,6,9-10,23,26-28,30-34,37-39H,2,5,7-8,11-22H2,1,3H3/b6-4+,29-10+/t23-,26?,27?,28?,30+,31-,32+,33-,34-,35-,36?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sydney
Curated by ChEMBL
| Assay Description
Transactivation of VDR expressed in COS7 cells assessed as reduction in 1,25-dihydroxyvitamin D3-induced transcriptional activity by transient transc... |
Eur J Med Chem 46: 1949-63 (2011)
Article DOI: 10.1016/j.ejmech.2011.01.047
BindingDB Entry DOI: 10.7270/Q22808QQ |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Mus musculus (Mouse)) | BDBM25737

(12-[(adamantan-1-ylcarbamoyl)amino]dodecanoic acid...)Show SMILES OC(=O)CCCCCCCCCCCNC(=O)NC12CC3CC(CC(C3)C1)C2 |TLB:25:20:27:24.23.26,25:24:20.21.19:27,THB:23:22:19:24.25.26,23:24:19:22.21.27| Show InChI InChI=1S/C23H40N2O3/c26-21(27)10-8-6-4-2-1-3-5-7-9-11-24-22(28)25-23-15-18-12-19(16-23)14-20(13-18)17-23/h18-20H,1-17H2,(H,26,27)(H2,24,25,28) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sydney
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant soluble epoxide hydrolase |
Eur J Med Chem 46: 1949-63 (2011)
Article DOI: 10.1016/j.ejmech.2011.01.047
BindingDB Entry DOI: 10.7270/Q22808QQ |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Rattus norvegicus) | BDBM25737

(12-[(adamantan-1-ylcarbamoyl)amino]dodecanoic acid...)Show SMILES OC(=O)CCCCCCCCCCCNC(=O)NC12CC3CC(CC(C3)C1)C2 |TLB:25:20:27:24.23.26,25:24:20.21.19:27,THB:23:22:19:24.25.26,23:24:19:22.21.27| Show InChI InChI=1S/C23H40N2O3/c26-21(27)10-8-6-4-2-1-3-5-7-9-11-24-22(28)25-23-15-18-12-19(16-23)14-20(13-18)17-23/h18-20H,1-17H2,(H,26,27)(H2,24,25,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sydney
Curated by ChEMBL
| Assay Description
Inhibition of rat recombinant soluble epoxide hydrolase |
Eur J Med Chem 46: 1949-63 (2011)
Article DOI: 10.1016/j.ejmech.2011.01.047
BindingDB Entry DOI: 10.7270/Q22808QQ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM19130

((2E,4E,6R)-7-[4-(dimethylamino)phenyl]-N-hydroxy-4...)Show SMILES CC(C=CC(=O)NO)C=C(C)C(=O)c1ccc(cc1)N(C)C |w:8.7,2.1| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-12,22H,1-4H3,(H,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sydney
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 by fluorometric assay |
Bioorg Med Chem Lett 22: 6200-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.006
BindingDB Entry DOI: 10.7270/Q2BG2Q8K |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1/2/3/8
(Homo sapiens (Human)) | BDBM29589

(Faridak | LBH-589 | LBH-589B | Panobinostat | US10...)Show SMILES Cc1[nH]c2ccccc2c1CCNCc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C21H23N3O2/c1-15-18(19-4-2-3-5-20(19)23-15)12-13-22-14-17-8-6-16(7-9-17)10-11-21(25)24-26/h2-11,22-23,26H,12-14H2,1H3,(H,24,25)/b11-10+ | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Inhibition Class 1 histone deacetylase in human HeLa nuclear extracts using Fluor-de- Lys-green substrate by fluorescence assay |
Bioorg Med Chem Lett 29: 2581-2586 (2019)
Article DOI: 10.1016/j.bmcl.2019.08.002
BindingDB Entry DOI: 10.7270/Q2T43XG1 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM29589

(Faridak | LBH-589 | LBH-589B | Panobinostat | US10...)Show SMILES Cc1[nH]c2ccccc2c1CCNCc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C21H23N3O2/c1-15-18(19-4-2-3-5-20(19)23-15)12-13-22-14-17-8-6-16(7-9-17)10-11-21(25)24-26/h2-11,22-23,26H,12-14H2,1H3,(H,24,25)/b11-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 pre-incubated for 30 mins before substrate addition and measured after 30 mins by fluorescence based assay |
Bioorg Med Chem Lett 29: 2581-2586 (2019)
Article DOI: 10.1016/j.bmcl.2019.08.002
BindingDB Entry DOI: 10.7270/Q2T43XG1 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sydney
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 by fluorometric assay |
Bioorg Med Chem Lett 22: 6200-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.006
BindingDB Entry DOI: 10.7270/Q2BG2Q8K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM29589

(Faridak | LBH-589 | LBH-589B | Panobinostat | US10...)Show SMILES Cc1[nH]c2ccccc2c1CCNCc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C21H23N3O2/c1-15-18(19-4-2-3-5-20(19)23-15)12-13-22-14-17-8-6-16(7-9-17)10-11-21(25)24-26/h2-11,22-23,26H,12-14H2,1H3,(H,24,25)/b11-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 136 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC2 pre-incubated for 30 mins before substrate addition and measured after 30 mins by fluorescence based assay |
Bioorg Med Chem Lett 29: 2581-2586 (2019)
Article DOI: 10.1016/j.bmcl.2019.08.002
BindingDB Entry DOI: 10.7270/Q2T43XG1 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1/2/3/8
(Homo sapiens (Human)) | BDBM25150
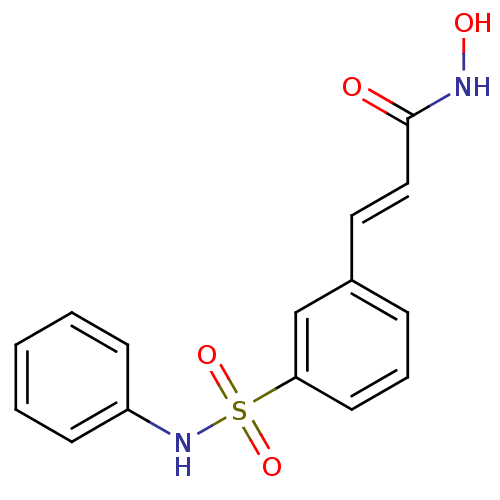
((2E)-N-hydroxy-3-[3-(phenylsulfamoyl)phenyl]prop-2...)Show InChI InChI=1S/C15H14N2O4S/c18-15(16-19)10-9-12-5-4-8-14(11-12)22(20,21)17-13-6-2-1-3-7-13/h1-11,17,19H,(H,16,18)/b10-9+ | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 189 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Inhibition Class 1 histone deacetylase in human HeLa nuclear extracts using Fluor-de- Lys-green substrate by fluorescence assay |
Bioorg Med Chem Lett 29: 2581-2586 (2019)
Article DOI: 10.1016/j.bmcl.2019.08.002
BindingDB Entry DOI: 10.7270/Q2T43XG1 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1/2/3/8
(Homo sapiens (Human)) | BDBM50522146
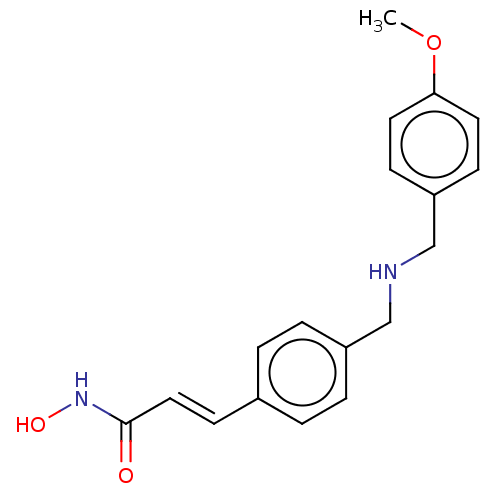
(CHEMBL4469317)Show InChI InChI=1S/C18H20N2O3/c1-23-17-9-6-16(7-10-17)13-19-12-15-4-2-14(3-5-15)8-11-18(21)20-22/h2-11,19,22H,12-13H2,1H3,(H,20,21)/b11-8+ | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 195 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Inhibition Class 1 histone deacetylase in human HeLa nuclear extracts using Fluor-de- Lys-green substrate by fluorescence assay |
Bioorg Med Chem Lett 29: 2581-2586 (2019)
Article DOI: 10.1016/j.bmcl.2019.08.002
BindingDB Entry DOI: 10.7270/Q2T43XG1 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1/2/3/8
(Homo sapiens (Human)) | BDBM50522150
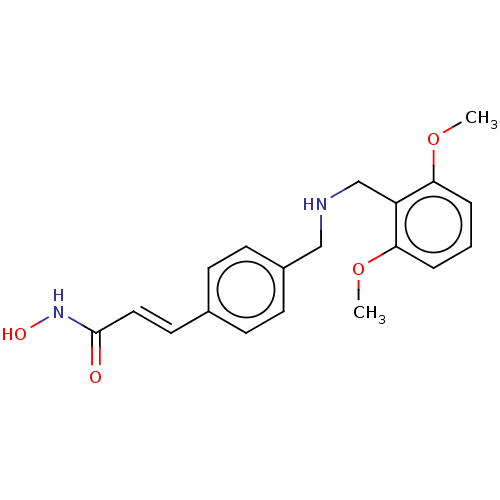
(CHEMBL4466520)Show InChI InChI=1S/C19H22N2O4/c1-24-17-4-3-5-18(25-2)16(17)13-20-12-15-8-6-14(7-9-15)10-11-19(22)21-23/h3-11,20,23H,12-13H2,1-2H3,(H,21,22)/b11-10+ | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 235 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Inhibition Class 1 histone deacetylase in human HeLa nuclear extracts using Fluor-de- Lys-green substrate by fluorescence assay |
Bioorg Med Chem Lett 29: 2581-2586 (2019)
Article DOI: 10.1016/j.bmcl.2019.08.002
BindingDB Entry DOI: 10.7270/Q2T43XG1 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1/2/3/8
(Homo sapiens (Human)) | BDBM50522151
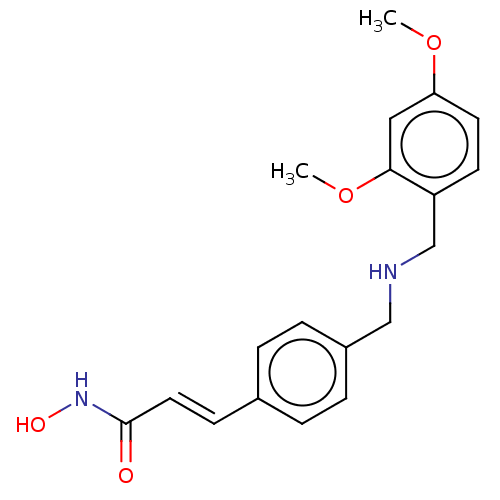
(CHEMBL4571280)Show InChI InChI=1S/C19H22N2O4/c1-24-17-9-8-16(18(11-17)25-2)13-20-12-15-5-3-14(4-6-15)7-10-19(22)21-23/h3-11,20,23H,12-13H2,1-2H3,(H,21,22)/b10-7+ | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 258 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Inhibition Class 1 histone deacetylase in human HeLa nuclear extracts using Fluor-de- Lys-green substrate by fluorescence assay |
Bioorg Med Chem Lett 29: 2581-2586 (2019)
Article DOI: 10.1016/j.bmcl.2019.08.002
BindingDB Entry DOI: 10.7270/Q2T43XG1 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1/2/3/8
(Homo sapiens (Human)) | BDBM50522148
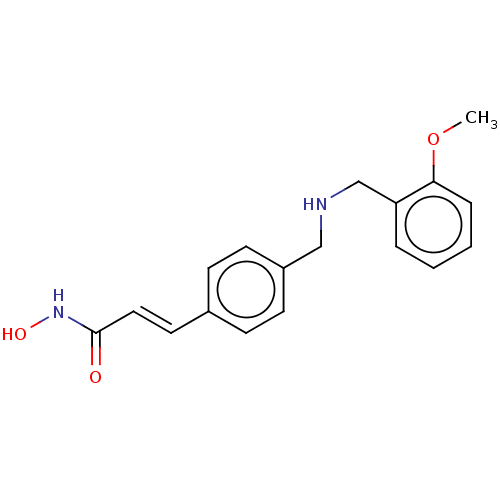
(CHEMBL4454331)Show InChI InChI=1S/C18H20N2O3/c1-23-17-5-3-2-4-16(17)13-19-12-15-8-6-14(7-9-15)10-11-18(21)20-22/h2-11,19,22H,12-13H2,1H3,(H,20,21)/b11-10+ | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 278 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Inhibition Class 1 histone deacetylase in human HeLa nuclear extracts using Fluor-de- Lys-green substrate by fluorescence assay |
Bioorg Med Chem Lett 29: 2581-2586 (2019)
Article DOI: 10.1016/j.bmcl.2019.08.002
BindingDB Entry DOI: 10.7270/Q2T43XG1 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1/2/3/8
(Homo sapiens (Human)) | BDBM50522145
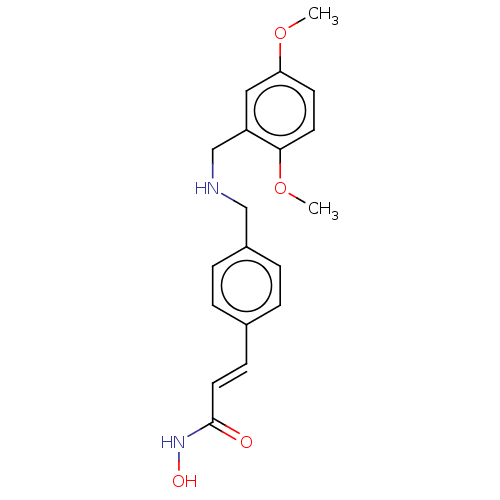
(CHEMBL4463774)Show InChI InChI=1S/C19H22N2O4/c1-24-17-8-9-18(25-2)16(11-17)13-20-12-15-5-3-14(4-6-15)7-10-19(22)21-23/h3-11,20,23H,12-13H2,1-2H3,(H,21,22)/b10-7+ | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 292 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Inhibition Class 1 histone deacetylase in human HeLa nuclear extracts using Fluor-de- Lys-green substrate by fluorescence assay |
Bioorg Med Chem Lett 29: 2581-2586 (2019)
Article DOI: 10.1016/j.bmcl.2019.08.002
BindingDB Entry DOI: 10.7270/Q2T43XG1 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1/2/3/8
(Homo sapiens (Human)) | BDBM50522149
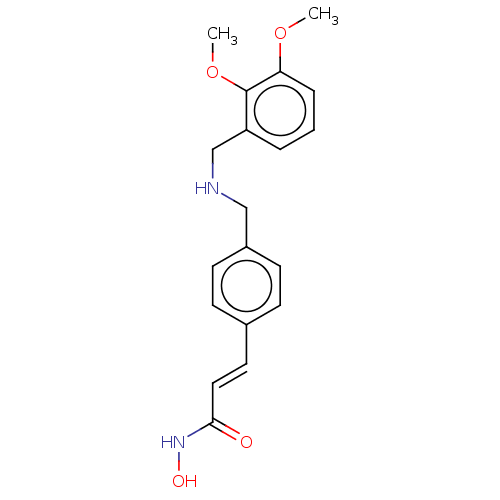
(CHEMBL4516302)Show InChI InChI=1S/C19H22N2O4/c1-24-17-5-3-4-16(19(17)25-2)13-20-12-15-8-6-14(7-9-15)10-11-18(22)21-23/h3-11,20,23H,12-13H2,1-2H3,(H,21,22)/b11-10+ | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 296 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Inhibition Class 1 histone deacetylase in human HeLa nuclear extracts using Fluor-de- Lys-green substrate by fluorescence assay |
Bioorg Med Chem Lett 29: 2581-2586 (2019)
Article DOI: 10.1016/j.bmcl.2019.08.002
BindingDB Entry DOI: 10.7270/Q2T43XG1 |
More data for this
Ligand-Target Pair | |
Non-lysosomal glucosylceramidase
(Homo sapiens (Human)) | BDBM18355

((2R,3R,4R,5S)-1-butyl-2-(hydroxymethyl)piperidine-...)Show InChI InChI=1S/C10H21NO4/c1-2-3-4-11-5-8(13)10(15)9(14)7(11)6-12/h7-10,12-15H,2-6H2,1H3/t7-,8+,9-,10-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sydney
Curated by ChEMBL
| Assay Description
Inhibition of non-lysosomal glucosylceramidase in cultured melanoma cells |
Eur J Med Chem 46: 1949-63 (2011)
Article DOI: 10.1016/j.ejmech.2011.01.047
BindingDB Entry DOI: 10.7270/Q22808QQ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1/2/3/8
(Homo sapiens (Human)) | BDBM50522142
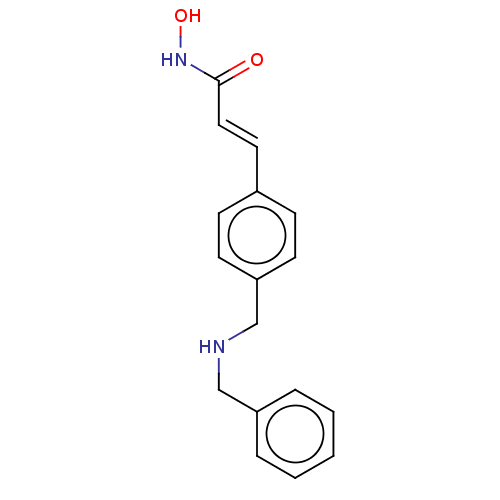
(CHEMBL566831)Show InChI InChI=1S/C17H18N2O2/c20-17(19-21)11-10-14-6-8-16(9-7-14)13-18-12-15-4-2-1-3-5-15/h1-11,18,21H,12-13H2,(H,19,20)/b11-10+ | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Inhibition Class 1 histone deacetylase in human HeLa nuclear extracts using Fluor-de- Lys-green substrate by fluorescence assay |
Bioorg Med Chem Lett 29: 2581-2586 (2019)
Article DOI: 10.1016/j.bmcl.2019.08.002
BindingDB Entry DOI: 10.7270/Q2T43XG1 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1/2/3/8
(Homo sapiens (Human)) | BDBM50522144
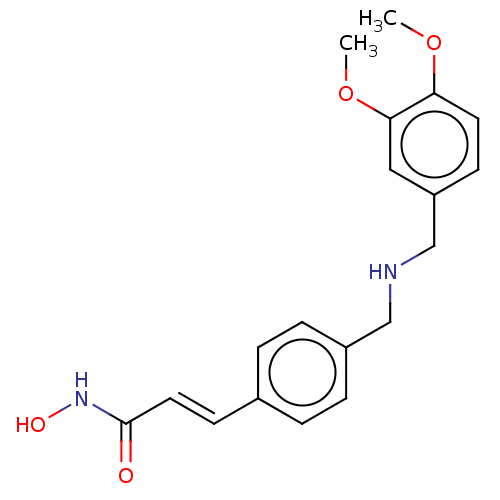
(CHEMBL4466864)Show InChI InChI=1S/C19H22N2O4/c1-24-17-9-7-16(11-18(17)25-2)13-20-12-15-5-3-14(4-6-15)8-10-19(22)21-23/h3-11,20,23H,12-13H2,1-2H3,(H,21,22)/b10-8+ | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 371 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Inhibition Class 1 histone deacetylase in human HeLa nuclear extracts using Fluor-de- Lys-green substrate by fluorescence assay |
Bioorg Med Chem Lett 29: 2581-2586 (2019)
Article DOI: 10.1016/j.bmcl.2019.08.002
BindingDB Entry DOI: 10.7270/Q2T43XG1 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50522146

(CHEMBL4469317)Show InChI InChI=1S/C18H20N2O3/c1-23-17-9-6-16(7-10-17)13-19-12-15-4-2-14(3-5-15)8-11-18(21)20-22/h2-11,19,22H,12-13H2,1H3,(H,20,21)/b11-8+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 381 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 pre-incubated for 30 mins before substrate addition and measured after 30 mins by fluorescence based assay |
Bioorg Med Chem Lett 29: 2581-2586 (2019)
Article DOI: 10.1016/j.bmcl.2019.08.002
BindingDB Entry DOI: 10.7270/Q2T43XG1 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1/2/3/8
(Homo sapiens (Human)) | BDBM50522143
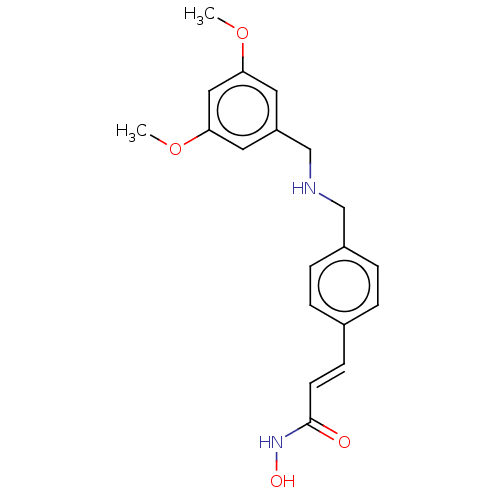
(CHEMBL4518387)Show InChI InChI=1S/C19H22N2O4/c1-24-17-9-16(10-18(11-17)25-2)13-20-12-15-5-3-14(4-6-15)7-8-19(22)21-23/h3-11,20,23H,12-13H2,1-2H3,(H,21,22)/b8-7+ | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 415 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Inhibition Class 1 histone deacetylase in human HeLa nuclear extracts using Fluor-de- Lys-green substrate by fluorescence assay |
Bioorg Med Chem Lett 29: 2581-2586 (2019)
Article DOI: 10.1016/j.bmcl.2019.08.002
BindingDB Entry DOI: 10.7270/Q2T43XG1 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1/2/3/8
(Homo sapiens (Human)) | BDBM50522147
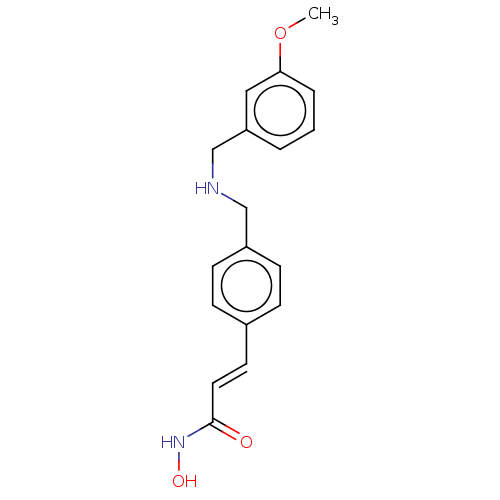
(CHEMBL4573804)Show InChI InChI=1S/C18H20N2O3/c1-23-17-4-2-3-16(11-17)13-19-12-15-7-5-14(6-8-15)9-10-18(21)20-22/h2-11,19,22H,12-13H2,1H3,(H,20,21)/b10-9+ | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Inhibition Class 1 histone deacetylase in human HeLa nuclear extracts using Fluor-de- Lys-green substrate by fluorescence assay |
Bioorg Med Chem Lett 29: 2581-2586 (2019)
Article DOI: 10.1016/j.bmcl.2019.08.002
BindingDB Entry DOI: 10.7270/Q2T43XG1 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50522151

(CHEMBL4571280)Show InChI InChI=1S/C19H22N2O4/c1-24-17-9-8-16(18(11-17)25-2)13-20-12-15-5-3-14(4-6-15)7-10-19(22)21-23/h3-11,20,23H,12-13H2,1-2H3,(H,21,22)/b10-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 449 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 pre-incubated for 30 mins before substrate addition and measured after 30 mins by fluorescence based assay |
Bioorg Med Chem Lett 29: 2581-2586 (2019)
Article DOI: 10.1016/j.bmcl.2019.08.002
BindingDB Entry DOI: 10.7270/Q2T43XG1 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50522147

(CHEMBL4573804)Show InChI InChI=1S/C18H20N2O3/c1-23-17-4-2-3-16(11-17)13-19-12-15-7-5-14(6-8-15)9-10-18(21)20-22/h2-11,19,22H,12-13H2,1H3,(H,20,21)/b10-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 463 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 pre-incubated for 30 mins before substrate addition and measured after 30 mins by fluorescence based assay |
Bioorg Med Chem Lett 29: 2581-2586 (2019)
Article DOI: 10.1016/j.bmcl.2019.08.002
BindingDB Entry DOI: 10.7270/Q2T43XG1 |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50000541

((+-)-1-(1-Benzo[b]thien-2-ylethyl)-1-hydroxyurea |...)Show InChI InChI=1S/C11H12N2O2S/c1-7(13(15)11(12)14)10-6-8-4-2-3-5-9(8)16-10/h2-7,15H,1H3,(H2,12,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of 5-Lipoxygenase (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01541
BindingDB Entry DOI: 10.7270/Q2M61PTP |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50522149

(CHEMBL4516302)Show InChI InChI=1S/C19H22N2O4/c1-24-17-5-3-4-16(19(17)25-2)13-20-12-15-8-6-14(7-9-15)10-11-18(22)21-23/h3-11,20,23H,12-13H2,1-2H3,(H,21,22)/b11-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 602 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 pre-incubated for 30 mins before substrate addition and measured after 30 mins by fluorescence based assay |
Bioorg Med Chem Lett 29: 2581-2586 (2019)
Article DOI: 10.1016/j.bmcl.2019.08.002
BindingDB Entry DOI: 10.7270/Q2T43XG1 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50522148

(CHEMBL4454331)Show InChI InChI=1S/C18H20N2O3/c1-23-17-5-3-2-4-16(17)13-19-12-15-8-6-14(7-9-15)10-11-18(21)20-22/h2-11,19,22H,12-13H2,1H3,(H,20,21)/b11-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 610 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 pre-incubated for 30 mins before substrate addition and measured after 30 mins by fluorescence based assay |
Bioorg Med Chem Lett 29: 2581-2586 (2019)
Article DOI: 10.1016/j.bmcl.2019.08.002
BindingDB Entry DOI: 10.7270/Q2T43XG1 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50522145

(CHEMBL4463774)Show InChI InChI=1S/C19H22N2O4/c1-24-17-8-9-18(25-2)16(11-17)13-20-12-15-5-3-14(4-6-15)7-10-19(22)21-23/h3-11,20,23H,12-13H2,1-2H3,(H,21,22)/b10-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 620 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 pre-incubated for 30 mins before substrate addition and measured after 30 mins by fluorescence based assay |
Bioorg Med Chem Lett 29: 2581-2586 (2019)
Article DOI: 10.1016/j.bmcl.2019.08.002
BindingDB Entry DOI: 10.7270/Q2T43XG1 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50522150

(CHEMBL4466520)Show InChI InChI=1S/C19H22N2O4/c1-24-17-4-3-5-18(25-2)16(17)13-20-12-15-8-6-14(7-9-15)10-11-19(22)21-23/h3-11,20,23H,12-13H2,1-2H3,(H,21,22)/b11-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 636 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 pre-incubated for 30 mins before substrate addition and measured after 30 mins by fluorescence based assay |
Bioorg Med Chem Lett 29: 2581-2586 (2019)
Article DOI: 10.1016/j.bmcl.2019.08.002
BindingDB Entry DOI: 10.7270/Q2T43XG1 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50522147

(CHEMBL4573804)Show InChI InChI=1S/C18H20N2O3/c1-23-17-4-2-3-16(11-17)13-19-12-15-7-5-14(6-8-15)9-10-18(21)20-22/h2-11,19,22H,12-13H2,1H3,(H,20,21)/b10-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 819 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC2 pre-incubated for 30 mins before substrate addition and measured after 30 mins by fluorescence based assay |
Bioorg Med Chem Lett 29: 2581-2586 (2019)
Article DOI: 10.1016/j.bmcl.2019.08.002
BindingDB Entry DOI: 10.7270/Q2T43XG1 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50522143

(CHEMBL4518387)Show InChI InChI=1S/C19H22N2O4/c1-24-17-9-16(10-18(11-17)25-2)13-20-12-15-5-3-14(4-6-15)7-8-19(22)21-23/h3-11,20,23H,12-13H2,1-2H3,(H,21,22)/b8-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 837 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 pre-incubated for 30 mins before substrate addition and measured after 30 mins by fluorescence based assay |
Bioorg Med Chem Lett 29: 2581-2586 (2019)
Article DOI: 10.1016/j.bmcl.2019.08.002
BindingDB Entry DOI: 10.7270/Q2T43XG1 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50522146

(CHEMBL4469317)Show InChI InChI=1S/C18H20N2O3/c1-23-17-9-6-16(7-10-17)13-19-12-15-4-2-14(3-5-15)8-11-18(21)20-22/h2-11,19,22H,12-13H2,1H3,(H,20,21)/b11-8+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 926 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC2 pre-incubated for 30 mins before substrate addition and measured after 30 mins by fluorescence based assay |
Bioorg Med Chem Lett 29: 2581-2586 (2019)
Article DOI: 10.1016/j.bmcl.2019.08.002
BindingDB Entry DOI: 10.7270/Q2T43XG1 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50522149

(CHEMBL4516302)Show InChI InChI=1S/C19H22N2O4/c1-24-17-5-3-4-16(19(17)25-2)13-20-12-15-8-6-14(7-9-15)10-11-18(22)21-23/h3-11,20,23H,12-13H2,1-2H3,(H,21,22)/b11-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 950 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC2 pre-incubated for 30 mins before substrate addition and measured after 30 mins by fluorescence based assay |
Bioorg Med Chem Lett 29: 2581-2586 (2019)
Article DOI: 10.1016/j.bmcl.2019.08.002
BindingDB Entry DOI: 10.7270/Q2T43XG1 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50522151

(CHEMBL4571280)Show InChI InChI=1S/C19H22N2O4/c1-24-17-9-8-16(18(11-17)25-2)13-20-12-15-5-3-14(4-6-15)7-10-19(22)21-23/h3-11,20,23H,12-13H2,1-2H3,(H,21,22)/b10-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 994 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC2 pre-incubated for 30 mins before substrate addition and measured after 30 mins by fluorescence based assay |
Bioorg Med Chem Lett 29: 2581-2586 (2019)
Article DOI: 10.1016/j.bmcl.2019.08.002
BindingDB Entry DOI: 10.7270/Q2T43XG1 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50522150

(CHEMBL4466520)Show InChI InChI=1S/C19H22N2O4/c1-24-17-4-3-5-18(25-2)16(17)13-20-12-15-8-6-14(7-9-15)10-11-19(22)21-23/h3-11,20,23H,12-13H2,1-2H3,(H,21,22)/b11-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC2 pre-incubated for 30 mins before substrate addition and measured after 30 mins by fluorescence based assay |
Bioorg Med Chem Lett 29: 2581-2586 (2019)
Article DOI: 10.1016/j.bmcl.2019.08.002
BindingDB Entry DOI: 10.7270/Q2T43XG1 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50522144

(CHEMBL4466864)Show InChI InChI=1S/C19H22N2O4/c1-24-17-9-7-16(11-18(17)25-2)13-20-12-15-5-3-14(4-6-15)8-10-19(22)21-23/h3-11,20,23H,12-13H2,1-2H3,(H,21,22)/b10-8+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 pre-incubated for 30 mins before substrate addition and measured after 30 mins by fluorescence based assay |
Bioorg Med Chem Lett 29: 2581-2586 (2019)
Article DOI: 10.1016/j.bmcl.2019.08.002
BindingDB Entry DOI: 10.7270/Q2T43XG1 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50522143

(CHEMBL4518387)Show InChI InChI=1S/C19H22N2O4/c1-24-17-9-16(10-18(11-17)25-2)13-20-12-15-5-3-14(4-6-15)7-8-19(22)21-23/h3-11,20,23H,12-13H2,1-2H3,(H,21,22)/b8-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC2 pre-incubated for 30 mins before substrate addition and measured after 30 mins by fluorescence based assay |
Bioorg Med Chem Lett 29: 2581-2586 (2019)
Article DOI: 10.1016/j.bmcl.2019.08.002
BindingDB Entry DOI: 10.7270/Q2T43XG1 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50522148

(CHEMBL4454331)Show InChI InChI=1S/C18H20N2O3/c1-23-17-5-3-2-4-16(17)13-19-12-15-8-6-14(7-9-15)10-11-18(21)20-22/h2-11,19,22H,12-13H2,1H3,(H,20,21)/b11-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.49E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC2 pre-incubated for 30 mins before substrate addition and measured after 30 mins by fluorescence based assay |
Bioorg Med Chem Lett 29: 2581-2586 (2019)
Article DOI: 10.1016/j.bmcl.2019.08.002
BindingDB Entry DOI: 10.7270/Q2T43XG1 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50522145

(CHEMBL4463774)Show InChI InChI=1S/C19H22N2O4/c1-24-17-8-9-18(25-2)16(11-17)13-20-12-15-5-3-14(4-6-15)7-10-19(22)21-23/h3-11,20,23H,12-13H2,1-2H3,(H,21,22)/b10-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC2 pre-incubated for 30 mins before substrate addition and measured after 30 mins by fluorescence based assay |
Bioorg Med Chem Lett 29: 2581-2586 (2019)
Article DOI: 10.1016/j.bmcl.2019.08.002
BindingDB Entry DOI: 10.7270/Q2T43XG1 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50328678
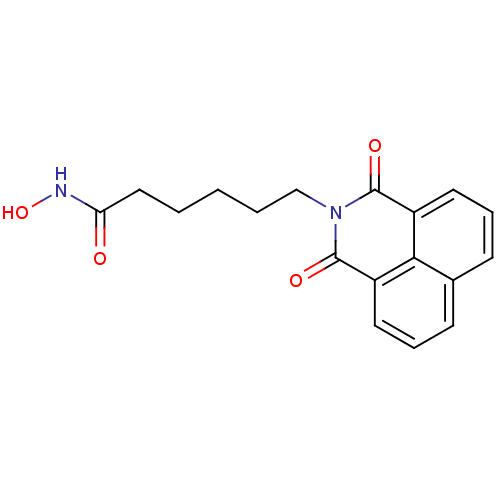
(6-(1,3-Dioxo-1H,3H-benzo[de]isoquinolin-2-yl)-hexa...)Show InChI InChI=1S/C18H18N2O4/c21-15(19-24)10-2-1-3-11-20-17(22)13-8-4-6-12-7-5-9-14(16(12)13)18(20)23/h4-9,24H,1-3,10-11H2,(H,19,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.82E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
| Assay Description
A FLUOR DE LYS fluorometric activity assay kit (HDAC source: HeLa cell nuclear extract) and a FLUOR DE LYS HDAC1 fluorometric drug discovery assay ki... |
Chembiochem 18: 368-373 (2017)
Article DOI: 10.1002/cbic.201600636
BindingDB Entry DOI: 10.7270/Q2RV0MH7 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50522144

(CHEMBL4466864)Show InChI InChI=1S/C19H22N2O4/c1-24-17-9-7-16(11-18(17)25-2)13-20-12-15-5-3-14(4-6-15)8-10-19(22)21-23/h3-11,20,23H,12-13H2,1-2H3,(H,21,22)/b10-8+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC2 pre-incubated for 30 mins before substrate addition and measured after 30 mins by fluorescence based assay |
Bioorg Med Chem Lett 29: 2581-2586 (2019)
Article DOI: 10.1016/j.bmcl.2019.08.002
BindingDB Entry DOI: 10.7270/Q2T43XG1 |
More data for this
Ligand-Target Pair | |
Lipoxygenase
(Solanum tuberosum (potato)) | BDBM22334

(BW A4C | BW4C | BWA4C | BWA4C, 10 | CHEMBL314360 |...)Show InChI InChI=1S/C17H17NO3/c1-14(19)18(20)12-6-8-15-7-5-11-17(13-15)21-16-9-3-2-4-10-16/h2-11,13,20H,12H2,1H3/b8-6+ | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.71E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
| Assay Description
A colorimetric 5-LO assay kit (Lipoxygenase Inhibitor Screening Assay Kit) and potato 5-LO isolate were purchased from Cayman Chemicals (Ann Arbor, M... |
Chembiochem 18: 368-373 (2017)
Article DOI: 10.1002/cbic.201600636
BindingDB Entry DOI: 10.7270/Q2RV0MH7 |
More data for this
Ligand-Target Pair | |
Non-lysosomal glucosylceramidase
(Homo sapiens (Human)) | BDBM18351

((2R,3R,4R,5S)-2-(Hydroxymethyl)piperidine-3,4,5-tr...)Show InChI InChI=1S/C6H13NO4/c8-2-3-5(10)6(11)4(9)1-7-3/h3-11H,1-2H2/t3-,4+,5-,6-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sydney
Curated by ChEMBL
| Assay Description
Inhibition of non-lysosomal glucosylceramidase in cultured melanoma cells |
Eur J Med Chem 46: 1949-63 (2011)
Article DOI: 10.1016/j.ejmech.2011.01.047
BindingDB Entry DOI: 10.7270/Q22808QQ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data