

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bromodomain-containing protein 4 [368-440] (Homo sapiens (Human)) | BDBM404301 (US10017501, Compound 1020-289) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc US Patent | Assay Description Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores... | US Patent US10017501 (2018) BindingDB Entry DOI: 10.7270/Q2CV4M3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [75-147] (Homo sapiens (Human)) | BDBM404301 (US10017501, Compound 1020-289) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc US Patent | Assay Description Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores... | US Patent US10017501 (2018) BindingDB Entry DOI: 10.7270/Q2CV4M3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [368-440] (Homo sapiens (Human)) | BDBM249309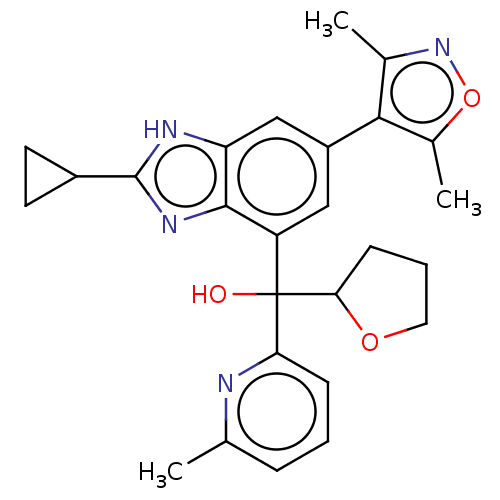 (US10017501, Compound 1020-114 | US9458145, 1020-11...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc US Patent | Assay Description Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores... | US Patent US10017501 (2018) BindingDB Entry DOI: 10.7270/Q2CV4M3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [368-440] (Homo sapiens (Human)) | BDBM249314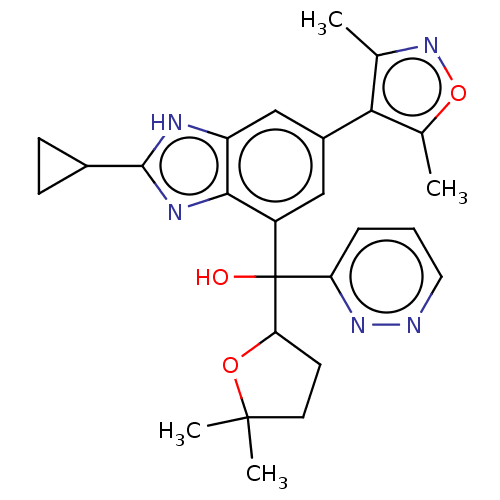 (US10017501, Compound 1020-298 | US9458145, 1020-29...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc US Patent | Assay Description Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores... | US Patent US10017501 (2018) BindingDB Entry DOI: 10.7270/Q2CV4M3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [75-147] (Homo sapiens (Human)) | BDBM249309 (US10017501, Compound 1020-114 | US9458145, 1020-11...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc US Patent | Assay Description Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores... | US Patent US10017501 (2018) BindingDB Entry DOI: 10.7270/Q2CV4M3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [75-147] (Homo sapiens (Human)) | BDBM249305 (US10017501, Compound 1020-103 | US9458145, 1020-10...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc US Patent | Assay Description Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores... | US Patent US10017501 (2018) BindingDB Entry DOI: 10.7270/Q2CV4M3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [75-147] (Homo sapiens (Human)) | BDBM249312 (US10017501, Compound 1020-257 | US9458145, 1020-25...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc US Patent | Assay Description Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores... | US Patent US10017501 (2018) BindingDB Entry DOI: 10.7270/Q2CV4M3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [75-147] (Homo sapiens (Human)) | BDBM249314 (US10017501, Compound 1020-298 | US9458145, 1020-29...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc US Patent | Assay Description Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores... | US Patent US10017501 (2018) BindingDB Entry DOI: 10.7270/Q2CV4M3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [368-440] (Homo sapiens (Human)) | BDBM249312 (US10017501, Compound 1020-257 | US9458145, 1020-25...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc US Patent | Assay Description Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores... | US Patent US10017501 (2018) BindingDB Entry DOI: 10.7270/Q2CV4M3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [75-147] (Homo sapiens (Human)) | BDBM249311 (US10017501, Compound 1020-239 | US9458145, 1020-23...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc US Patent | Assay Description Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores... | US Patent US10017501 (2018) BindingDB Entry DOI: 10.7270/Q2CV4M3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [368-440] (Homo sapiens (Human)) | BDBM249311 (US10017501, Compound 1020-239 | US9458145, 1020-23...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc US Patent | Assay Description Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores... | US Patent US10017501 (2018) BindingDB Entry DOI: 10.7270/Q2CV4M3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [75-147] (Homo sapiens (Human)) | BDBM249306 (US10017501, Compound 1020-104 | US9458145, 1020-10...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc US Patent | Assay Description Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores... | US Patent US10017501 (2018) BindingDB Entry DOI: 10.7270/Q2CV4M3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [75-147] (Homo sapiens (Human)) | BDBM249307 (US10017501, Compound 1020-112 | US9458145, 1020-11...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 10.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc US Patent | Assay Description Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores... | US Patent US10017501 (2018) BindingDB Entry DOI: 10.7270/Q2CV4M3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [75-147] (Homo sapiens (Human)) | BDBM249308 (US10017501, Compound 1020-113 | US9458145, 1020-11...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 10.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc US Patent | Assay Description Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores... | US Patent US10017501 (2018) BindingDB Entry DOI: 10.7270/Q2CV4M3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [75-147] (Homo sapiens (Human)) | BDBM249310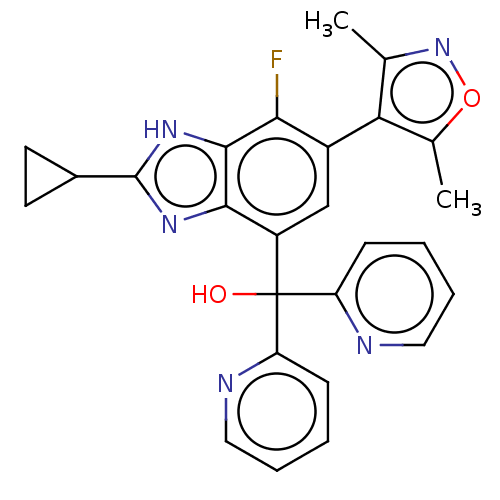 (US10017501, Compound 1020-224 | US9458145, 1020-22...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 10.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc US Patent | Assay Description Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores... | US Patent US10017501 (2018) BindingDB Entry DOI: 10.7270/Q2CV4M3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [368-440] (Homo sapiens (Human)) | BDBM249306 (US10017501, Compound 1020-104 | US9458145, 1020-10...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | 11.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc US Patent | Assay Description Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores... | US Patent US10017501 (2018) BindingDB Entry DOI: 10.7270/Q2CV4M3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [368-440] (Homo sapiens (Human)) | BDBM249305 (US10017501, Compound 1020-103 | US9458145, 1020-10...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 12.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc US Patent | Assay Description Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores... | US Patent US10017501 (2018) BindingDB Entry DOI: 10.7270/Q2CV4M3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [368-440] (Homo sapiens (Human)) | BDBM249308 (US10017501, Compound 1020-113 | US9458145, 1020-11...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 12.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc US Patent | Assay Description Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores... | US Patent US10017501 (2018) BindingDB Entry DOI: 10.7270/Q2CV4M3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [368-440] (Homo sapiens (Human)) | BDBM249307 (US10017501, Compound 1020-112 | US9458145, 1020-11...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 13.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc US Patent | Assay Description Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores... | US Patent US10017501 (2018) BindingDB Entry DOI: 10.7270/Q2CV4M3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [368-440] (Homo sapiens (Human)) | BDBM249310 (US10017501, Compound 1020-224 | US9458145, 1020-22...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 20.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc US Patent | Assay Description Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores... | US Patent US10017501 (2018) BindingDB Entry DOI: 10.7270/Q2CV4M3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50456700 (CHEMBL4205425) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a |
Central South University Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine as substrate by Ellman's method | Eur J Med Chem 138: 738-747 (2017) Article DOI: 10.1016/j.ejmech.2017.07.006 BindingDB Entry DOI: 10.7270/Q2HM5C2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50456689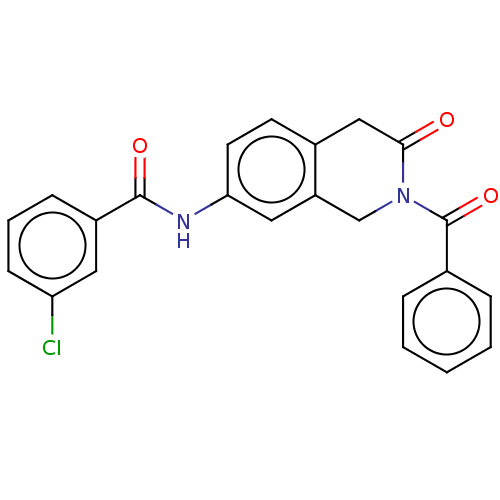 (CHEMBL4205954) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a |
Central South University Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine as substrate by Ellman's method | Eur J Med Chem 138: 738-747 (2017) Article DOI: 10.1016/j.ejmech.2017.07.006 BindingDB Entry DOI: 10.7270/Q2HM5C2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50456692 (CHEMBL4209803) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a |
Central South University Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine as substrate by Ellman's method | Eur J Med Chem 138: 738-747 (2017) Article DOI: 10.1016/j.ejmech.2017.07.006 BindingDB Entry DOI: 10.7270/Q2HM5C2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50456691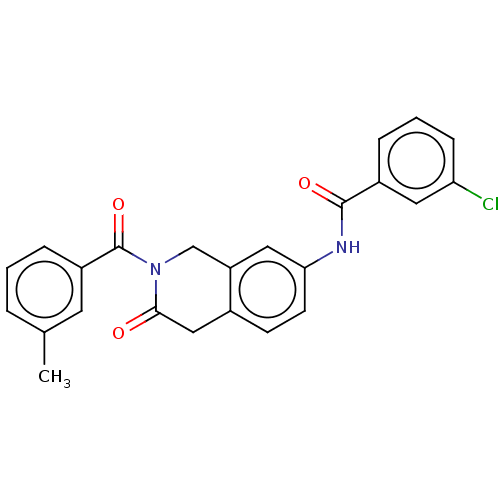 (CHEMBL4210316) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a |
Central South University Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine as substrate by Ellman's method | Eur J Med Chem 138: 738-747 (2017) Article DOI: 10.1016/j.ejmech.2017.07.006 BindingDB Entry DOI: 10.7270/Q2HM5C2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50456690 (CHEMBL4217663) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a |
Central South University Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine as substrate by Ellman's method | Eur J Med Chem 138: 738-747 (2017) Article DOI: 10.1016/j.ejmech.2017.07.006 BindingDB Entry DOI: 10.7270/Q2HM5C2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50456693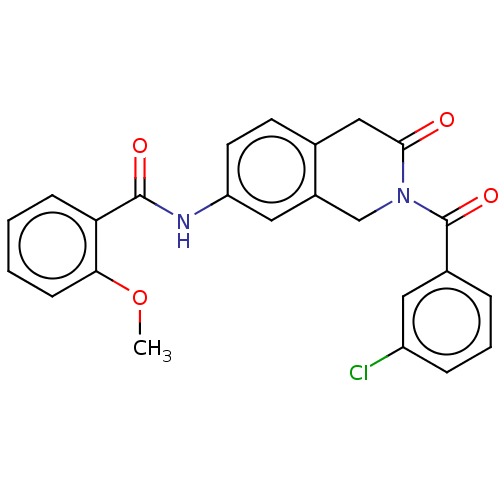 (CHEMBL4214430) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a |
Central South University Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine as substrate by Ellman's method | Eur J Med Chem 138: 738-747 (2017) Article DOI: 10.1016/j.ejmech.2017.07.006 BindingDB Entry DOI: 10.7270/Q2HM5C2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50456699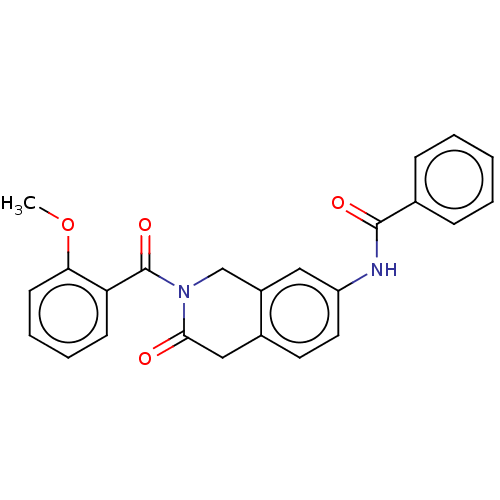 (CHEMBL4213253) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a |
Central South University Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine as substrate by Ellman's method | Eur J Med Chem 138: 738-747 (2017) Article DOI: 10.1016/j.ejmech.2017.07.006 BindingDB Entry DOI: 10.7270/Q2HM5C2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50456696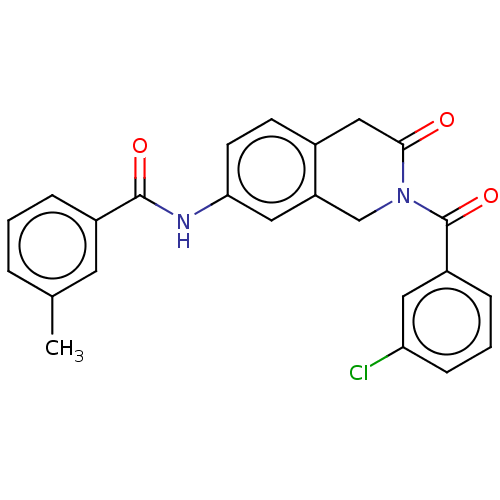 (CHEMBL4214707) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a |
Central South University Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine as substrate by Ellman's method | Eur J Med Chem 138: 738-747 (2017) Article DOI: 10.1016/j.ejmech.2017.07.006 BindingDB Entry DOI: 10.7270/Q2HM5C2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50456682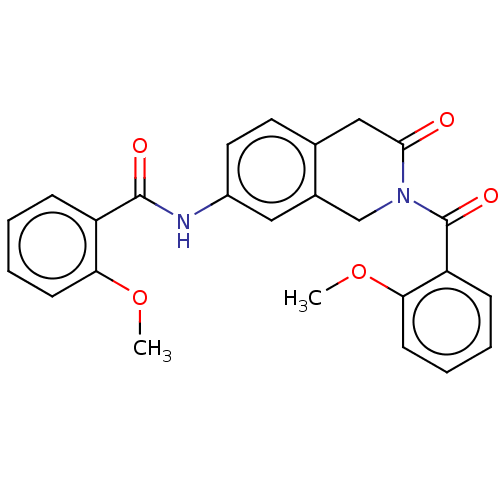 (CHEMBL4210041) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0220 | n/a | n/a | n/a | n/a | n/a | n/a |
Central South University Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine as substrate by Ellman's method | Eur J Med Chem 138: 738-747 (2017) Article DOI: 10.1016/j.ejmech.2017.07.006 BindingDB Entry DOI: 10.7270/Q2HM5C2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50456688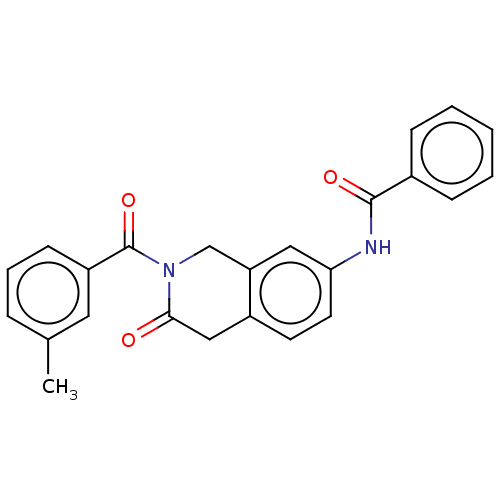 (CHEMBL4218191) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0260 | n/a | n/a | n/a | n/a | n/a | n/a |
Central South University Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine as substrate by Ellman's method | Eur J Med Chem 138: 738-747 (2017) Article DOI: 10.1016/j.ejmech.2017.07.006 BindingDB Entry DOI: 10.7270/Q2HM5C2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50456694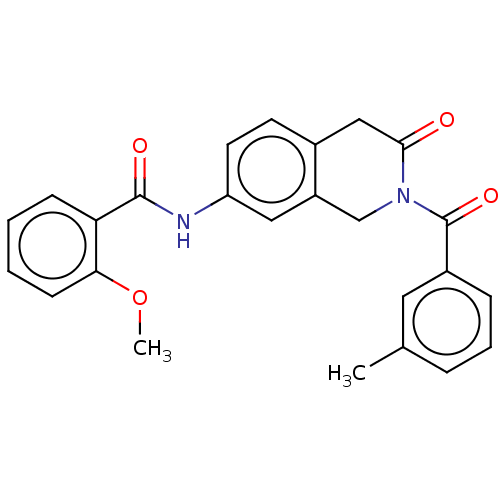 (CHEMBL4205144) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0260 | n/a | n/a | n/a | n/a | n/a | n/a |
Central South University Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine as substrate by Ellman's method | Eur J Med Chem 138: 738-747 (2017) Article DOI: 10.1016/j.ejmech.2017.07.006 BindingDB Entry DOI: 10.7270/Q2HM5C2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50456687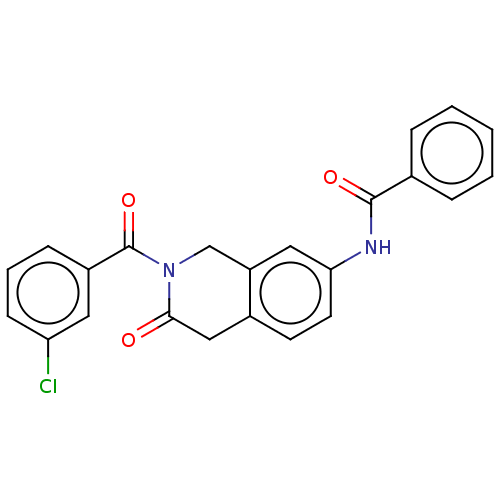 (CHEMBL4208866) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0280 | n/a | n/a | n/a | n/a | n/a | n/a |
Central South University Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine as substrate by Ellman's method | Eur J Med Chem 138: 738-747 (2017) Article DOI: 10.1016/j.ejmech.2017.07.006 BindingDB Entry DOI: 10.7270/Q2HM5C2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50456698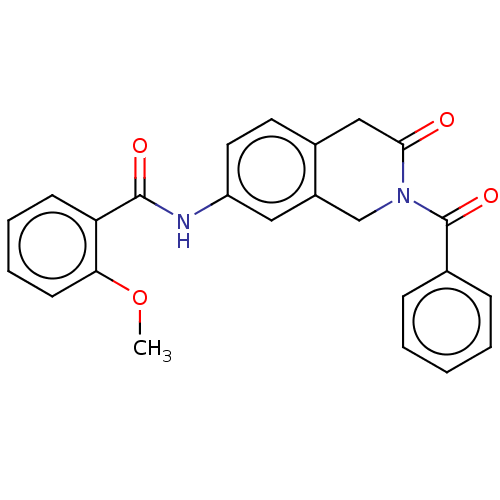 (CHEMBL4209518) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0290 | n/a | n/a | n/a | n/a | n/a | n/a |
Central South University Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine as substrate by Ellman's method | Eur J Med Chem 138: 738-747 (2017) Article DOI: 10.1016/j.ejmech.2017.07.006 BindingDB Entry DOI: 10.7270/Q2HM5C2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50456697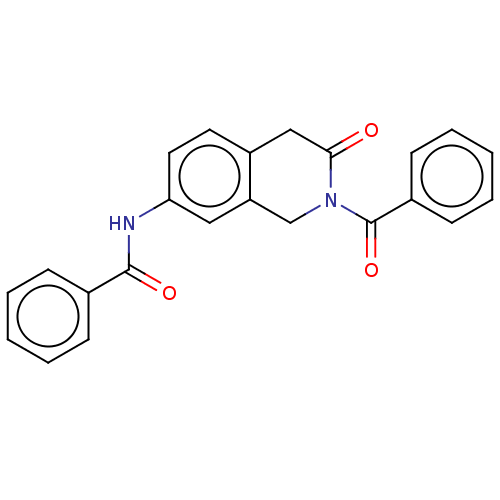 (CHEMBL4213792) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a |
Central South University Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine as substrate by Ellman's method | Eur J Med Chem 138: 738-747 (2017) Article DOI: 10.1016/j.ejmech.2017.07.006 BindingDB Entry DOI: 10.7270/Q2HM5C2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50384412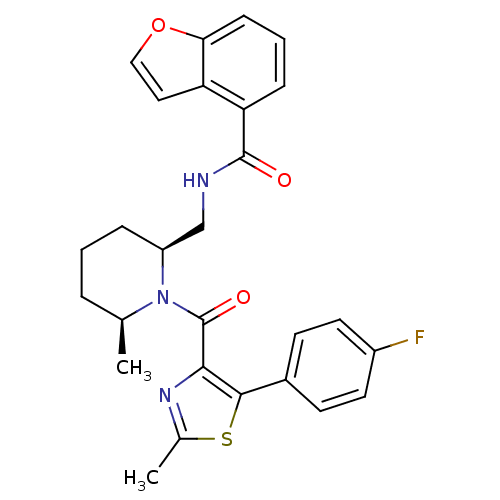 (CHEMBL2031504) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Scripps Florida Curated by ChEMBL | Assay Description Antagonist activity at OX1 receptor expressed in CHO cells assessed as inhibition of OXA-stimulated intracellular calcium mobilization after 30 mins ... | Bioorg Med Chem Lett 22: 3890-4 (2012) Article DOI: 10.1016/j.bmcl.2012.04.122 BindingDB Entry DOI: 10.7270/Q2M046GG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50384423 (CHEMBL2031485 | US9896452, Example 62) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Scripps Florida Curated by ChEMBL | Assay Description Antagonist activity at OX1 receptor expressed in CHO cells assessed as inhibition of OXA-stimulated intracellular calcium mobilization after 30 mins ... | Bioorg Med Chem Lett 22: 3890-4 (2012) Article DOI: 10.1016/j.bmcl.2012.04.122 BindingDB Entry DOI: 10.7270/Q2M046GG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50384437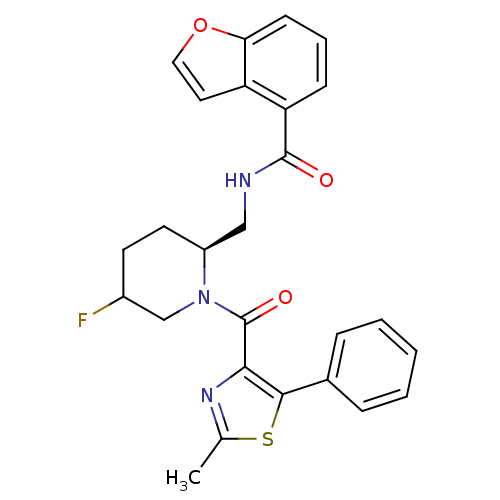 (CHEMBL2031501) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Scripps Florida Curated by ChEMBL | Assay Description Antagonist activity at OX1 receptor expressed in CHO cells assessed as inhibition of OXA-stimulated intracellular calcium mobilization after 30 mins ... | Bioorg Med Chem Lett 22: 3890-4 (2012) Article DOI: 10.1016/j.bmcl.2012.04.122 BindingDB Entry DOI: 10.7270/Q2M046GG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM373153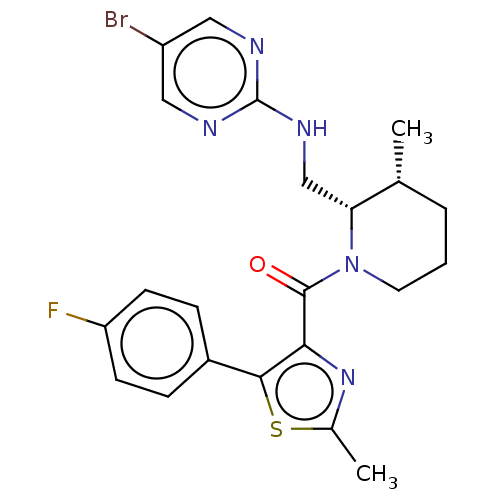 (US9896452, Example 15 | rac-((2S,3R)-2-(((5-bromop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mississippi | Assay Description Measurement of [Ca2+]i using a FLIPR: CHO-OX1 or CHO-OX2 cells are seeded into black-walled clear-base 384-well plates (Costar) at a density of 20,00... | J Med Chem 48: 2906-15 (2005) BindingDB Entry DOI: 10.7270/Q22J6F5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM373241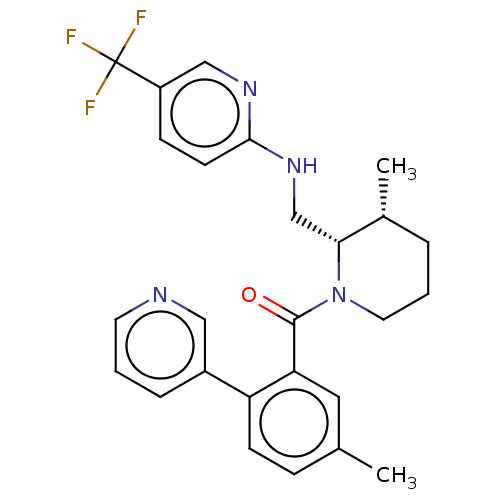 (US9896452, Example 104 | rac-((2S,3R)-3-methyl-2-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mississippi | Assay Description Measurement of [Ca2+]i using a FLIPR: CHO-OX1 or CHO-OX2 cells are seeded into black-walled clear-base 384-well plates (Costar) at a density of 20,00... | J Med Chem 48: 2906-15 (2005) BindingDB Entry DOI: 10.7270/Q22J6F5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM373415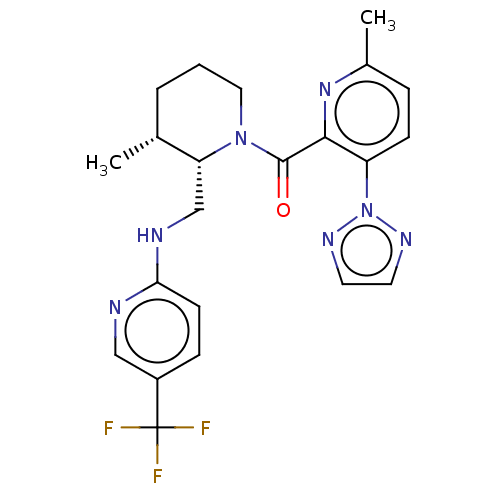 (((2S,3R)-3-Methyl-2-(((5-(trifluoromethyl)pyridin-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mississippi | Assay Description Measurement of [Ca2+]i using a FLIPR: CHO-OX1 or CHO-OX2 cells are seeded into black-walled clear-base 384-well plates (Costar) at a density of 20,00... | J Med Chem 48: 2906-15 (2005) BindingDB Entry DOI: 10.7270/Q22J6F5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM373416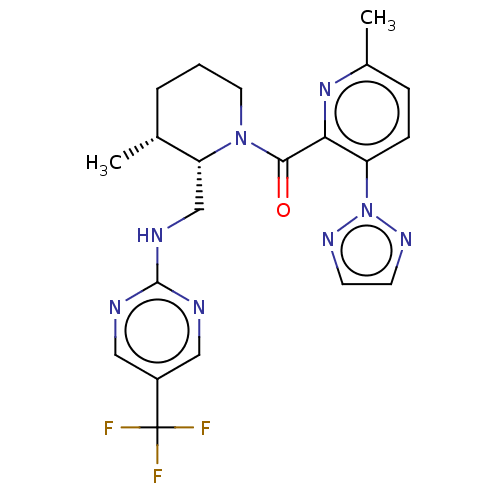 (((2S,3R)-3-Methyl-2-(((5-(trifluoromethyl)pyrimidi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mississippi | Assay Description Measurement of [Ca2+]i using a FLIPR: CHO-OX1 or CHO-OX2 cells are seeded into black-walled clear-base 384-well plates (Costar) at a density of 20,00... | J Med Chem 48: 2906-15 (2005) BindingDB Entry DOI: 10.7270/Q22J6F5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM373429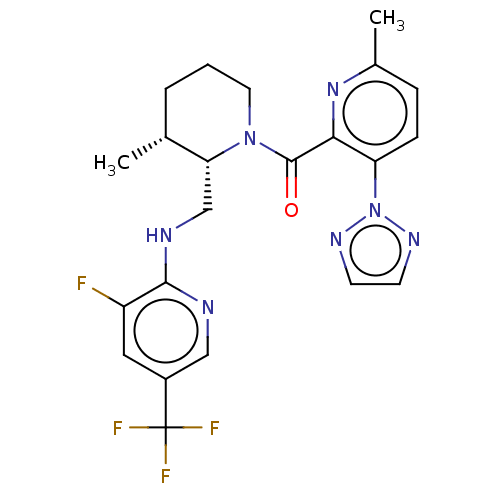 (((2S,3R)-2-(((3-Fluoro-5-(trifluoromethyl)pyridin-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mississippi | Assay Description Measurement of [Ca2+]i using a FLIPR: CHO-OX1 or CHO-OX2 cells are seeded into black-walled clear-base 384-well plates (Costar) at a density of 20,00... | J Med Chem 48: 2906-15 (2005) BindingDB Entry DOI: 10.7270/Q22J6F5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM373342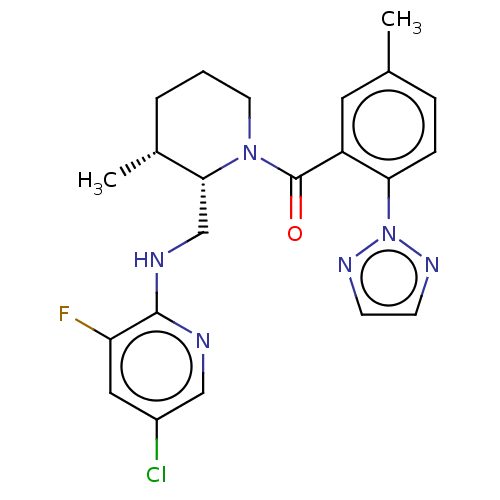 (((2S,3R)-2-(((5-Chloro-3-fluoropyridin-2-yl)amino)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mississippi | Assay Description Measurement of [Ca2+]i using a FLIPR: CHO-OX1 or CHO-OX2 cells are seeded into black-walled clear-base 384-well plates (Costar) at a density of 20,00... | J Med Chem 48: 2906-15 (2005) BindingDB Entry DOI: 10.7270/Q22J6F5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM373466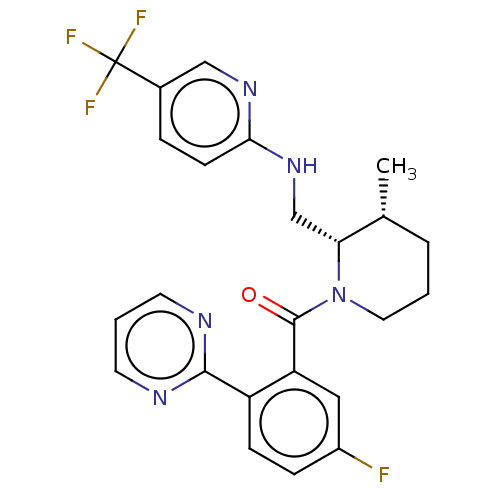 ((5-Fluoro-2-(pyrimidin-2-yl)phenyl)((2S,3R)-3-meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mississippi | Assay Description Measurement of [Ca2+]i using a FLIPR: CHO-OX1 or CHO-OX2 cells are seeded into black-walled clear-base 384-well plates (Costar) at a density of 20,00... | J Med Chem 48: 2906-15 (2005) BindingDB Entry DOI: 10.7270/Q22J6F5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM373507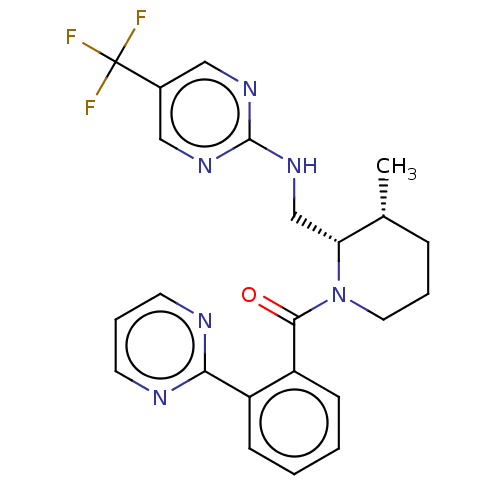 (((2S,3R)-3-methyl-2-(((5-(trifluoromethyl)pyrimidi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mississippi | Assay Description Measurement of [Ca2+]i using a FLIPR: CHO-OX1 or CHO-OX2 cells are seeded into black-walled clear-base 384-well plates (Costar) at a density of 20,00... | J Med Chem 48: 2906-15 (2005) BindingDB Entry DOI: 10.7270/Q22J6F5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM373402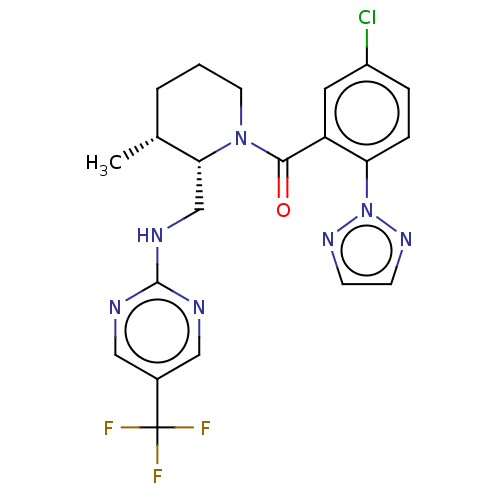 ((5-Chloro-2-(2H-1,2,3-triazol-2-yl)phenyl)((2S,3R)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mississippi | Assay Description Measurement of [Ca2+]i using a FLIPR: CHO-OX1 or CHO-OX2 cells are seeded into black-walled clear-base 384-well plates (Costar) at a density of 20,00... | J Med Chem 48: 2906-15 (2005) BindingDB Entry DOI: 10.7270/Q22J6F5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM373342 (((2S,3R)-2-(((5-Chloro-3-fluoropyridin-2-yl)amino)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mississippi | Assay Description Measurement of [Ca2+]i using a FLIPR: CHO-OX1 or CHO-OX2 cells are seeded into black-walled clear-base 384-well plates (Costar) at a density of 20,00... | J Med Chem 48: 2906-15 (2005) BindingDB Entry DOI: 10.7270/Q22J6F5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM373177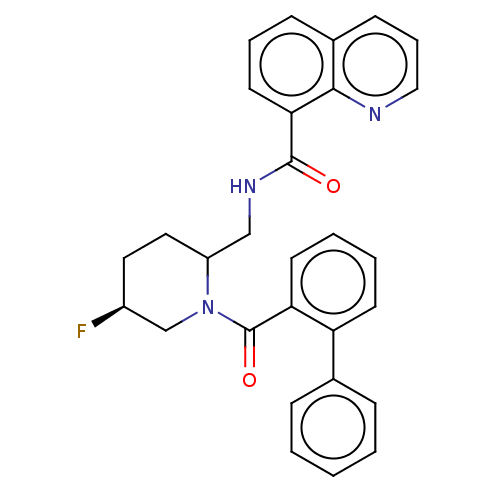 (US9896452, Example 37 | rac-N-(((5S)-1-(biphenylca...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mississippi | Assay Description Measurement of [Ca2+]i using a FLIPR: CHO-OX1 or CHO-OX2 cells are seeded into black-walled clear-base 384-well plates (Costar) at a density of 20,00... | J Med Chem 48: 2906-15 (2005) BindingDB Entry DOI: 10.7270/Q22J6F5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM373192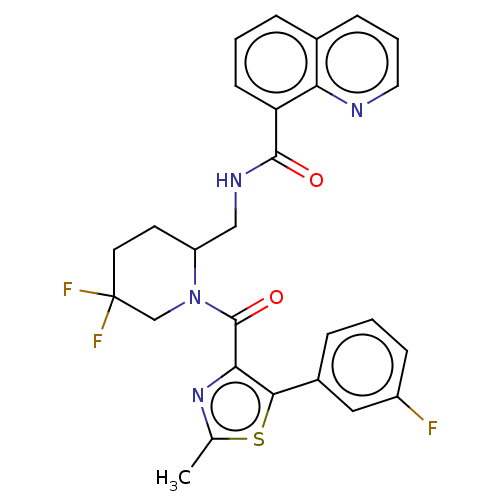 (US9896452, Example 52 | rac-N-((5,5-difluoro-1-(5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mississippi | Assay Description Measurement of [Ca2+]i using a FLIPR: CHO-OX1 or CHO-OX2 cells are seeded into black-walled clear-base 384-well plates (Costar) at a density of 20,00... | J Med Chem 48: 2906-15 (2005) BindingDB Entry DOI: 10.7270/Q22J6F5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM373198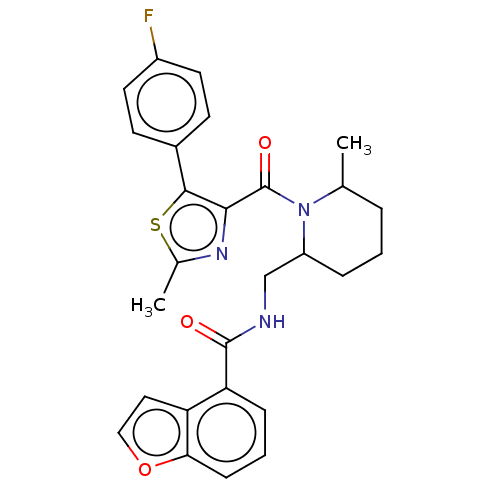 (US9896452, Example 58 | rac-N-((1-(5-(4-fluorophen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mississippi | Assay Description Measurement of [Ca2+]i using a FLIPR: CHO-OX1 or CHO-OX2 cells are seeded into black-walled clear-base 384-well plates (Costar) at a density of 20,00... | J Med Chem 48: 2906-15 (2005) BindingDB Entry DOI: 10.7270/Q22J6F5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1292 total ) | Next | Last >> |