 Found 393 hits with Last Name = 'maccari' and Initial = 'r'
Found 393 hits with Last Name = 'maccari' and Initial = 'r' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM20878
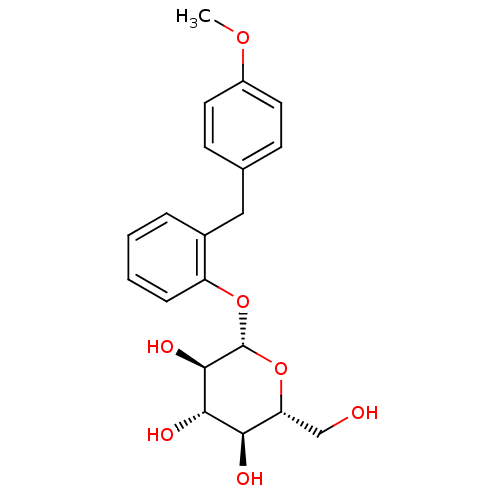
((2R,3S,4S,5R,6S)-2-(hydroxymethyl)-6-{2-[(4-methox...)Show SMILES COc1ccc(Cc2ccccc2O[C@@H]2O[C@H](CO)[C@@H](O)[C@H](O)[C@H]2O)cc1 Show InChI InChI=1S/C20H24O7/c1-25-14-8-6-12(7-9-14)10-13-4-2-3-5-15(13)26-20-19(24)18(23)17(22)16(11-21)27-20/h2-9,16-24H,10-11H2,1H3/t16-,17-,18+,19-,20-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00867
BindingDB Entry DOI: 10.7270/Q2FB570W |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1
(Homo sapiens (Human)) | BDBM50506669
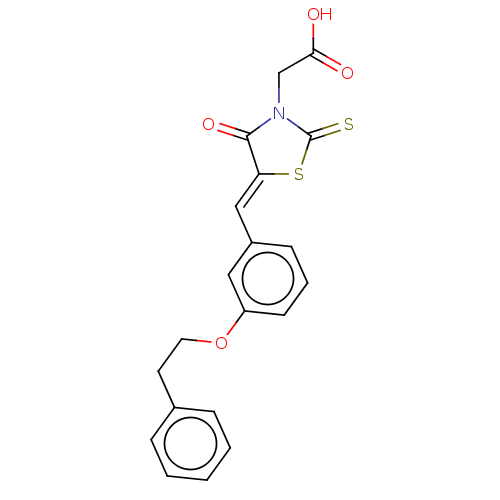
(CHEMBL4459307)Show SMILES OC(=O)CN1C(=S)S\C(=C/c2cccc(OCCc3ccccc3)c2)C1=O Show InChI InChI=1S/C20H17NO4S2/c22-18(23)13-21-19(24)17(27-20(21)26)12-15-7-4-8-16(11-15)25-10-9-14-5-2-1-3-6-14/h1-8,11-12H,9-10,13H2,(H,22,23)/b17-12- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Uncompetitive inhibition of human recombinant aldose reductase expressed in Escherichia coli BL21(DE3)pLysS assessed as dissociation constant of enzy... |
Bioorg Med Chem Lett 28: 3712-3720 (2018)
Article DOI: 10.1016/j.bmcl.2018.10.024
BindingDB Entry DOI: 10.7270/Q2QJ7MKC |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1
(Homo sapiens (Human)) | BDBM50506667
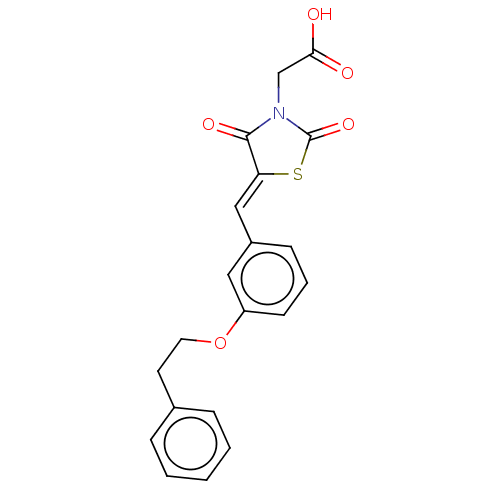
(CHEMBL4448571)Show SMILES OC(=O)CN1C(=O)S\C(=C/c2cccc(OCCc3ccccc3)c2)C1=O Show InChI InChI=1S/C20H17NO5S/c22-18(23)13-21-19(24)17(27-20(21)25)12-15-7-4-8-16(11-15)26-10-9-14-5-2-1-3-6-14/h1-8,11-12H,9-10,13H2,(H,22,23)/b17-12- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Uncompetitive inhibition of human recombinant aldose reductase expressed in Escherichia coli BL21(DE3)pLysS assessed as dissociation constant of enzy... |
Bioorg Med Chem Lett 28: 3712-3720 (2018)
Article DOI: 10.1016/j.bmcl.2018.10.024
BindingDB Entry DOI: 10.7270/Q2QJ7MKC |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 1
(Homo sapiens (Human)) | BDBM50595439

(CHEMBL5178665)Show SMILES CC(C)c1[nH]nc(O[C@@H]2O[C@H](CO)[C@@H](O)[C@H](O)[C@H]2O)c1Cc1ccc(OCCCNCCC(N)=O)cc1C |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 97 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00867
BindingDB Entry DOI: 10.7270/Q2FB570W |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM50351429
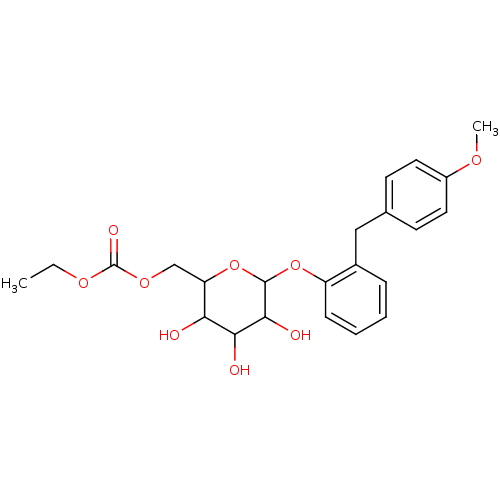
(CHEMBL450044)Show SMILES CCOC(=O)OCC1OC(Oc2ccccc2Cc2ccc(OC)cc2)C(O)C(O)C1O Show InChI InChI=1S/C23H28O9/c1-3-29-23(27)30-13-18-19(24)20(25)21(26)22(32-18)31-17-7-5-4-6-15(17)12-14-8-10-16(28-2)11-9-14/h4-11,18-22,24-26H,3,12-13H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 151 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00867
BindingDB Entry DOI: 10.7270/Q2FB570W |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1
(Homo sapiens (Human)) | BDBM50506669

(CHEMBL4459307)Show SMILES OC(=O)CN1C(=S)S\C(=C/c2cccc(OCCc3ccccc3)c2)C1=O Show InChI InChI=1S/C20H17NO4S2/c22-18(23)13-21-19(24)17(27-20(21)26)12-15-7-4-8-16(11-15)25-10-9-14-5-2-1-3-6-14/h1-8,11-12H,9-10,13H2,(H,22,23)/b17-12- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Uncompetitive inhibition of human recombinant aldose reductase expressed in Escherichia coli BL21(DE3)pLysS assessed as dissociation constant of enzy... |
Bioorg Med Chem Lett 28: 3712-3720 (2018)
Article DOI: 10.1016/j.bmcl.2018.10.024
BindingDB Entry DOI: 10.7270/Q2QJ7MKC |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1
(Homo sapiens (Human)) | BDBM50506667

(CHEMBL4448571)Show SMILES OC(=O)CN1C(=O)S\C(=C/c2cccc(OCCc3ccccc3)c2)C1=O Show InChI InChI=1S/C20H17NO5S/c22-18(23)13-21-19(24)17(27-20(21)25)12-15-7-4-8-16(11-15)26-10-9-14-5-2-1-3-6-14/h1-8,11-12H,9-10,13H2,(H,22,23)/b17-12- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Uncompetitive inhibition of human recombinant aldose reductase expressed in Escherichia coli BL21(DE3)pLysS assessed as dissociation constant of enzy... |
Bioorg Med Chem Lett 28: 3712-3720 (2018)
Article DOI: 10.1016/j.bmcl.2018.10.024
BindingDB Entry DOI: 10.7270/Q2QJ7MKC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50444695
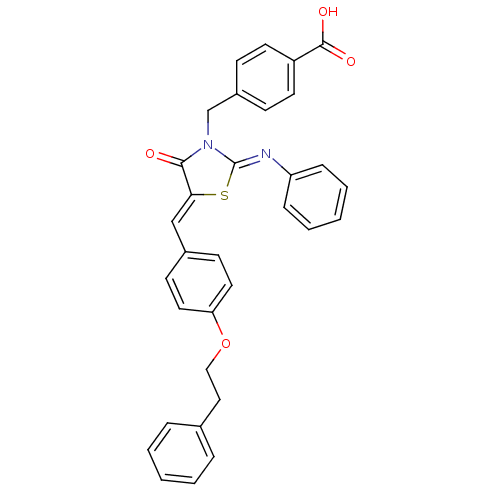
(CHEMBL3098852)Show SMILES OC(=O)c1ccc(CN2\C(S\C(=C/c3ccc(OCCc4ccccc4)cc3)C2=O)=N\c2ccccc2)cc1 Show InChI InChI=1S/C32H26N2O4S/c35-30-29(21-24-13-17-28(18-14-24)38-20-19-23-7-3-1-4-8-23)39-32(33-27-9-5-2-6-10-27)34(30)22-25-11-15-26(16-12-25)31(36)37/h1-18,21H,19-20,22H2,(H,36,37)/b29-21-,33-32- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Mixed type inhibition of human recombinant PTP1B expressed in Escherichia coli TB1 using p-nitrophenylphosphate as substrate by Double reciprocal plo... |
Eur J Med Chem 71: 112-27 (2014)
Article DOI: 10.1016/j.ejmech.2013.11.001
BindingDB Entry DOI: 10.7270/Q2VH5Q95 |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 1
(Homo sapiens (Human)) | BDBM20878

((2R,3S,4S,5R,6S)-2-(hydroxymethyl)-6-{2-[(4-methox...)Show SMILES COc1ccc(Cc2ccccc2O[C@@H]2O[C@H](CO)[C@@H](O)[C@H](O)[C@H]2O)cc1 Show InChI InChI=1S/C20H24O7/c1-25-14-8-6-12(7-9-14)10-13-4-2-3-5-15(13)26-20-19(24)18(23)17(22)16(11-21)27-20/h2-9,16-24H,10-11H2,1H3/t16-,17-,18+,19-,20-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 708 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00867
BindingDB Entry DOI: 10.7270/Q2FB570W |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50232100
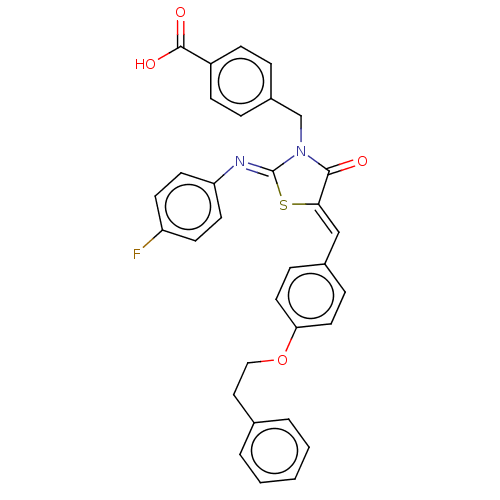
(CHEMBL4061225)Show SMILES OC(=O)c1ccc(CN2C(=O)\C(S\C2=N/c2ccc(F)cc2)=C\c2ccc(OCCc3ccccc3)cc2)cc1 Show InChI InChI=1S/C32H25FN2O4S/c33-26-12-14-27(15-13-26)34-32-35(21-24-6-10-25(11-7-24)31(37)38)30(36)29(40-32)20-23-8-16-28(17-9-23)39-19-18-22-4-2-1-3-5-22/h1-17,20H,18-19,21H2,(H,37,38)/b29-20-,34-32- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Mixed non-competitive inhibition of full length recombinant human PTP1B assessed as enzyme-substrate-inhibitor complex using pNPP as substrate by dou... |
Eur J Med Chem 127: 840-858 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.063
BindingDB Entry DOI: 10.7270/Q21J9D18 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50379825
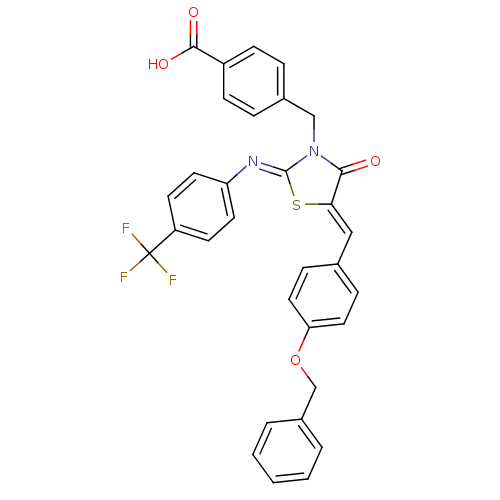
(CHEMBL2011701)Show SMILES OC(=O)c1ccc(CN2C(=O)\C(S\C2=N/c2ccc(cc2)C(F)(F)F)=C\c2ccc(OCc3ccccc3)cc2)cc1 Show InChI InChI=1S/C32H23F3N2O4S/c33-32(34,35)25-12-14-26(15-13-25)36-31-37(19-22-6-10-24(11-7-22)30(39)40)29(38)28(42-31)18-21-8-16-27(17-9-21)41-20-23-4-2-1-3-5-23/h1-18H,19-20H2,(H,39,40)/b28-18-,36-31- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Competitive inhibition of GST-tagged human recombinant PTP1B expressed in Escherichia coli TB1 using p-nitrophenylphosphate as substrate by Line-Weav... |
Eur J Med Chem 50: 332-43 (2012)
Article DOI: 10.1016/j.ejmech.2012.02.012
BindingDB Entry DOI: 10.7270/Q2DN461B |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50444693
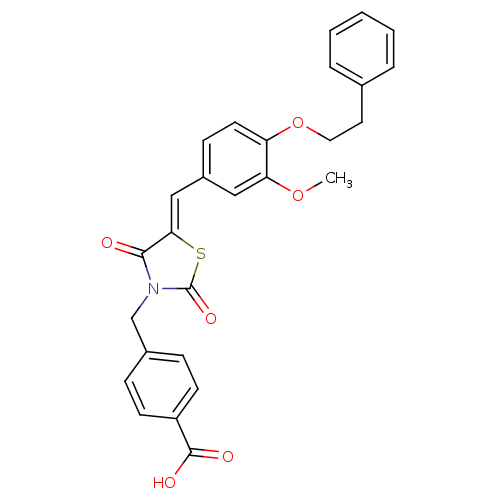
(CHEMBL3098944)Show SMILES COc1cc(\C=C2/SC(=O)N(Cc3ccc(cc3)C(O)=O)C2=O)ccc1OCCc1ccccc1 Show InChI InChI=1S/C27H23NO6S/c1-33-23-15-20(9-12-22(23)34-14-13-18-5-3-2-4-6-18)16-24-25(29)28(27(32)35-24)17-19-7-10-21(11-8-19)26(30)31/h2-12,15-16H,13-14,17H2,1H3,(H,30,31)/b24-16- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Noncompetitive inhibition of human recombinant PTP1B expressed in Escherichia coli TB1 using p-nitrophenylphosphate as substrate by Double reciprocal... |
Eur J Med Chem 71: 112-27 (2014)
Article DOI: 10.1016/j.ejmech.2013.11.001
BindingDB Entry DOI: 10.7270/Q2VH5Q95 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50232102
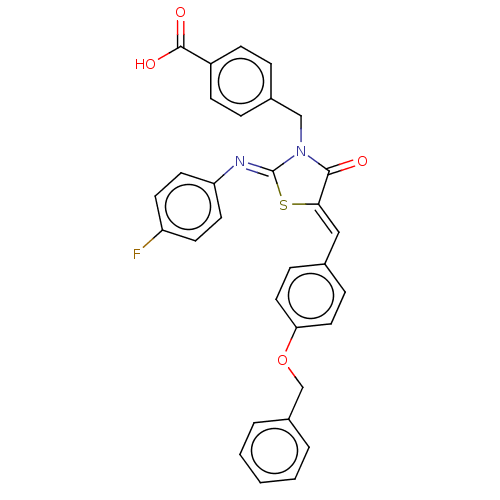
(CHEMBL4080177)Show SMILES OC(=O)c1ccc(CN2C(=O)\C(S\C2=N/c2ccc(F)cc2)=C\c2ccc(OCc3ccccc3)cc2)cc1 Show InChI InChI=1S/C31H23FN2O4S/c32-25-12-14-26(15-13-25)33-31-34(19-22-6-10-24(11-7-22)30(36)37)29(35)28(39-31)18-21-8-16-27(17-9-21)38-20-23-4-2-1-3-5-23/h1-18H,19-20H2,(H,36,37)/b28-18-,33-31- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Competitive inhibition of full length recombinant human PTP1B assessed as enzyme-inhibitor complex using pNPP as substrate by double reciprocal plot ... |
Eur J Med Chem 127: 840-858 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.063
BindingDB Entry DOI: 10.7270/Q21J9D18 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50232101
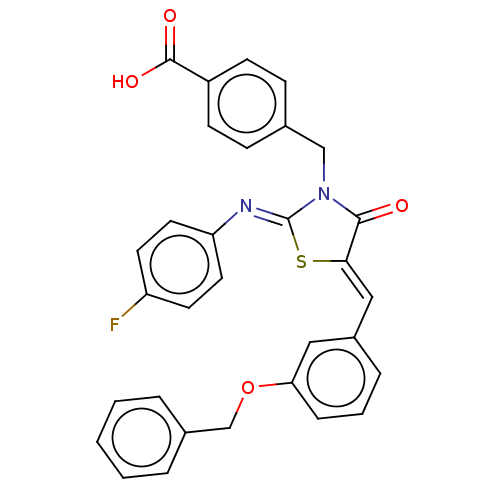
(CHEMBL4098207)Show SMILES OC(=O)c1ccc(CN2C(=O)\C(S\C2=N/c2ccc(F)cc2)=C\c2cccc(OCc3ccccc3)c2)cc1 Show InChI InChI=1S/C31H23FN2O4S/c32-25-13-15-26(16-14-25)33-31-34(19-21-9-11-24(12-10-21)30(36)37)29(35)28(39-31)18-23-7-4-8-27(17-23)38-20-22-5-2-1-3-6-22/h1-18H,19-20H2,(H,36,37)/b28-18-,33-31- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Mixed non-competitive inhibition of full length recombinant human PTP1B assessed as enzyme-substrate-inhibitor complex using pNPP as substrate by dou... |
Eur J Med Chem 127: 840-858 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.063
BindingDB Entry DOI: 10.7270/Q21J9D18 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50444694

(CHEMBL3098854)Show SMILES COc1cc(\C=C2/S\C(=N/c3ccccc3)N(Cc3ccc(cc3)C(O)=O)C2=O)ccc1OCCc1ccccc1 Show InChI InChI=1S/C33H28N2O5S/c1-39-29-20-25(14-17-28(29)40-19-18-23-8-4-2-5-9-23)21-30-31(36)35(22-24-12-15-26(16-13-24)32(37)38)33(41-30)34-27-10-6-3-7-11-27/h2-17,20-21H,18-19,22H2,1H3,(H,37,38)/b30-21-,34-33- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Mixed type inhibition of human recombinant PTP1B expressed in Escherichia coli TB1 using p-nitrophenylphosphate as substrate by Double reciprocal plo... |
Eur J Med Chem 71: 112-27 (2014)
Article DOI: 10.1016/j.ejmech.2013.11.001
BindingDB Entry DOI: 10.7270/Q2VH5Q95 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50444692

(CHEMBL3098853)Show SMILES COc1ccc(\C=C2/S\C(=N/c3ccccc3)N(Cc3ccc(cc3)C(O)=O)C2=O)cc1OCCc1ccccc1 Show InChI InChI=1S/C33H28N2O5S/c1-39-28-17-14-25(20-29(28)40-19-18-23-8-4-2-5-9-23)21-30-31(36)35(22-24-12-15-26(16-13-24)32(37)38)33(41-30)34-27-10-6-3-7-11-27/h2-17,20-21H,18-19,22H2,1H3,(H,37,38)/b30-21-,34-33- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant PTP1B expressed in Escherichia coli TB1 using p-nitrophenylphosphate as substrate by Double reciprocal pl... |
Eur J Med Chem 71: 112-27 (2014)
Article DOI: 10.1016/j.ejmech.2013.11.001
BindingDB Entry DOI: 10.7270/Q2VH5Q95 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50232103
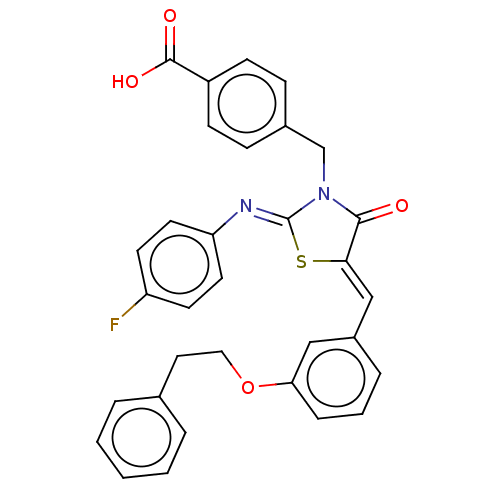
(CHEMBL4089378)Show SMILES OC(=O)c1ccc(CN2C(=O)\C(S\C2=N/c2ccc(F)cc2)=C\c2cccc(OCCc3ccccc3)c2)cc1 Show InChI InChI=1S/C32H25FN2O4S/c33-26-13-15-27(16-14-26)34-32-35(21-23-9-11-25(12-10-23)31(37)38)30(36)29(40-32)20-24-7-4-8-28(19-24)39-18-17-22-5-2-1-3-6-22/h1-16,19-20H,17-18,21H2,(H,37,38)/b29-20-,34-32- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Mixed non-competitive inhibition of full length recombinant human PTP1B assessed as enzyme-substrate-inhibitor complex using pNPP as substrate by dou... |
Eur J Med Chem 127: 840-858 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.063
BindingDB Entry DOI: 10.7270/Q21J9D18 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50444696
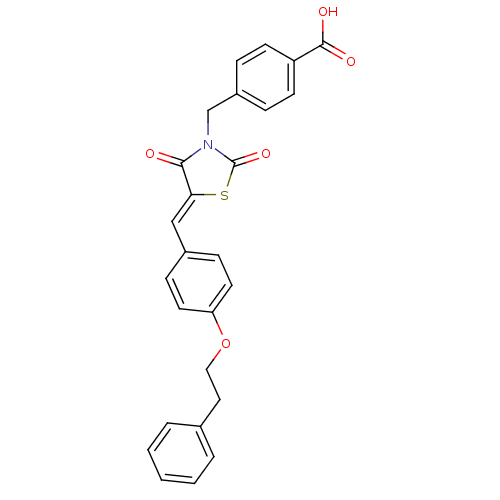
(CHEMBL3098942)Show SMILES OC(=O)c1ccc(CN2C(=O)S\C(=C/c3ccc(OCCc4ccccc4)cc3)C2=O)cc1 Show InChI InChI=1S/C26H21NO5S/c28-24-23(33-26(31)27(24)17-20-6-10-21(11-7-20)25(29)30)16-19-8-12-22(13-9-19)32-15-14-18-4-2-1-3-5-18/h1-13,16H,14-15,17H2,(H,29,30)/b23-16- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Mixed type inhibition of human recombinant PTP1B expressed in Escherichia coli TB1 using p-nitrophenylphosphate as substrate by Double reciprocal plo... |
Eur J Med Chem 71: 112-27 (2014)
Article DOI: 10.1016/j.ejmech.2013.11.001
BindingDB Entry DOI: 10.7270/Q2VH5Q95 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50232093
(CHEMBL4072175)Show SMILES OC(=O)c1ccc(CN2C(=O)\C(S\C2=N/c2ccc(F)cc2)=C\c2cccc(Oc3ccccc3)c2)cc1 Show InChI InChI=1S/C30H21FN2O4S/c31-23-13-15-24(16-14-23)32-30-33(19-20-9-11-22(12-10-20)29(35)36)28(34)27(38-30)18-21-5-4-8-26(17-21)37-25-6-2-1-3-7-25/h1-18H,19H2,(H,35,36)/b27-18-,32-30- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Competitive inhibition of full length recombinant human PTP1B assessed as enzyme-inhibitor complex using pNPP as substrate by double reciprocal plot ... |
Eur J Med Chem 127: 840-858 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.063
BindingDB Entry DOI: 10.7270/Q21J9D18 |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 1
(Homo sapiens (Human)) | BDBM50351429

(CHEMBL450044)Show SMILES CCOC(=O)OCC1OC(Oc2ccccc2Cc2ccc(OC)cc2)C(O)C(O)C1O Show InChI InChI=1S/C23H28O9/c1-3-29-23(27)30-13-18-19(24)20(25)21(26)22(32-18)31-17-7-5-4-6-15(17)12-14-8-10-16(28-2)11-9-14/h4-11,18-22,24-26H,3,12-13H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00867
BindingDB Entry DOI: 10.7270/Q2FB570W |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50506663

(CHEMBL4483785)Show SMILES COc1cc(\C=C2/S\C(=N/c3ccccc3)N(CC(O)=O)C2=O)ccc1OCc1ccccc1 Show InChI InChI=1S/C26H22N2O5S/c1-32-22-14-19(12-13-21(22)33-17-18-8-4-2-5-9-18)15-23-25(31)28(16-24(29)30)26(34-23)27-20-10-6-3-7-11-20/h2-15H,16-17H2,1H3,(H,29,30)/b23-15-,27-26- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Mixed non-competitive inhibition of human full-length GST-fused PTP1B expressed in bacterial expression system assessed as assessed as dissociation c... |
Bioorg Med Chem Lett 28: 3712-3720 (2018)
Article DOI: 10.1016/j.bmcl.2018.10.024
BindingDB Entry DOI: 10.7270/Q2QJ7MKC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50232094
(CHEMBL4062661)Show SMILES OC(=O)c1ccc(CN2C(=O)\C(S\C2=N/c2ccc(F)cc2)=C\c2ccc(Oc3ccccc3)cc2)cc1 Show InChI InChI=1S/C30H21FN2O4S/c31-23-12-14-24(15-13-23)32-30-33(19-21-6-10-22(11-7-21)29(35)36)28(34)27(38-30)18-20-8-16-26(17-9-20)37-25-4-2-1-3-5-25/h1-18H,19H2,(H,35,36)/b27-18-,32-30- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Competitive inhibition of full length recombinant human PTP1B assessed as enzyme-inhibitor complex using pNPP as substrate by double reciprocal plot ... |
Eur J Med Chem 127: 840-858 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.063
BindingDB Entry DOI: 10.7270/Q21J9D18 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50506669

(CHEMBL4459307)Show SMILES OC(=O)CN1C(=S)S\C(=C/c2cccc(OCCc3ccccc3)c2)C1=O Show InChI InChI=1S/C20H17NO4S2/c22-18(23)13-21-19(24)17(27-20(21)26)12-15-7-4-8-16(11-15)25-10-9-14-5-2-1-3-6-14/h1-8,11-12H,9-10,13H2,(H,22,23)/b17-12- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Non-competitive inhibition of human full-length GST-fused PTP1B expressed in bacterial expression system assessed as dissociation constant of enzyme-... |
Bioorg Med Chem Lett 28: 3712-3720 (2018)
Article DOI: 10.1016/j.bmcl.2018.10.024
BindingDB Entry DOI: 10.7270/Q2QJ7MKC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50506669

(CHEMBL4459307)Show SMILES OC(=O)CN1C(=S)S\C(=C/c2cccc(OCCc3ccccc3)c2)C1=O Show InChI InChI=1S/C20H17NO4S2/c22-18(23)13-21-19(24)17(27-20(21)26)12-15-7-4-8-16(11-15)25-10-9-14-5-2-1-3-6-14/h1-8,11-12H,9-10,13H2,(H,22,23)/b17-12- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Non-competitive inhibition of human full-length GST-fused PTP1B expressed in bacterial expression system assessed as dissociation constant of enzyme-... |
Bioorg Med Chem Lett 28: 3712-3720 (2018)
Article DOI: 10.1016/j.bmcl.2018.10.024
BindingDB Entry DOI: 10.7270/Q2QJ7MKC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50506667

(CHEMBL4448571)Show SMILES OC(=O)CN1C(=O)S\C(=C/c2cccc(OCCc3ccccc3)c2)C1=O Show InChI InChI=1S/C20H17NO5S/c22-18(23)13-21-19(24)17(27-20(21)25)12-15-7-4-8-16(11-15)26-10-9-14-5-2-1-3-6-14/h1-8,11-12H,9-10,13H2,(H,22,23)/b17-12- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.31E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Non-competitive inhibition of human full-length GST-fused PTP1B expressed in bacterial expression system assessed as dissociation constant of enzyme-... |
Bioorg Med Chem Lett 28: 3712-3720 (2018)
Article DOI: 10.1016/j.bmcl.2018.10.024
BindingDB Entry DOI: 10.7270/Q2QJ7MKC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50506667

(CHEMBL4448571)Show SMILES OC(=O)CN1C(=O)S\C(=C/c2cccc(OCCc3ccccc3)c2)C1=O Show InChI InChI=1S/C20H17NO5S/c22-18(23)13-21-19(24)17(27-20(21)25)12-15-7-4-8-16(11-15)26-10-9-14-5-2-1-3-6-14/h1-8,11-12H,9-10,13H2,(H,22,23)/b17-12- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.31E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Non-competitive inhibition of human full-length GST-fused PTP1B expressed in bacterial expression system assessed as dissociation constant of enzyme-... |
Bioorg Med Chem Lett 28: 3712-3720 (2018)
Article DOI: 10.1016/j.bmcl.2018.10.024
BindingDB Entry DOI: 10.7270/Q2QJ7MKC |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM50595439

(CHEMBL5178665)Show SMILES CC(C)c1[nH]nc(O[C@@H]2O[C@H](CO)[C@@H](O)[C@H](O)[C@H]2O)c1Cc1ccc(OCCCNCCC(N)=O)cc1C |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 1.36E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00867
BindingDB Entry DOI: 10.7270/Q2FB570W |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50506663

(CHEMBL4483785)Show SMILES COc1cc(\C=C2/S\C(=N/c3ccccc3)N(CC(O)=O)C2=O)ccc1OCc1ccccc1 Show InChI InChI=1S/C26H22N2O5S/c1-32-22-14-19(12-13-21(22)33-17-18-8-4-2-5-9-18)15-23-25(31)28(16-24(29)30)26(34-23)27-20-10-6-3-7-11-20/h2-15H,16-17H2,1H3,(H,29,30)/b23-15-,27-26- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.06E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Mixed non-competitive inhibition of human full-length GST-fused PTP1B expressed in bacterial expression system assessed as assessed as dissociation c... |
Bioorg Med Chem Lett 28: 3712-3720 (2018)
Article DOI: 10.1016/j.bmcl.2018.10.024
BindingDB Entry DOI: 10.7270/Q2QJ7MKC |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM50595437

(CHEMBL5202047)Show SMILES CCOc1ccc(Cc2cc(ccc2Cl)[C@H]2O[C@@H](SC)[C@H](O)[C@@H](O)[C@@H]2O)cc1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00867
BindingDB Entry DOI: 10.7270/Q2FB570W |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM50438460
(CHEMBL2414617)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)c1ccc(Cl)c(Cc2ccc(s2)-c2ccc(F)nc2)c1 |r| Show InChI InChI=1S/C22H21ClFNO5S/c23-15-4-1-11(22-21(29)20(28)19(27)16(10-26)30-22)7-13(15)8-14-3-5-17(31-14)12-2-6-18(24)25-9-12/h1-7,9,16,19-22,26-29H,8,10H2/t16-,19-,20+,21-,22+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00867
BindingDB Entry DOI: 10.7270/Q2FB570W |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM50595438

(CHEMBL5182632)Show SMILES COc1ccc(Cc2cc(ccc2Cl)[C@H]2O[C@@H](SC)[C@H](O)[C@@H](O)[C@@H]2O)cc1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00867
BindingDB Entry DOI: 10.7270/Q2FB570W |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM50595436

(CHEMBL5177502)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)c1ccc(Cl)c(Cc2ccc(OC3CCOC3)cc2)c1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00867
BindingDB Entry DOI: 10.7270/Q2FB570W |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 1
(Homo sapiens (Human)) | BDBM50595438

(CHEMBL5182632)Show SMILES COc1ccc(Cc2cc(ccc2Cl)[C@H]2O[C@@H](SC)[C@H](O)[C@@H](O)[C@@H]2O)cc1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00867
BindingDB Entry DOI: 10.7270/Q2FB570W |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM50559517

(Remogliflozin | Remogliflozin a)Show SMILES CC(C)Oc1ccc(Cc2c(O[C@@H]3O[C@H](CO)[C@@H](O)[C@H](O)[C@H]3O)nn(C(C)C)c2C)cc1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00867
BindingDB Entry DOI: 10.7270/Q2FB570W |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1
(Homo sapiens (Human)) | BDBM50334180
(2-(4-oxo-5-(3-phenoxybenzylidene)-2-thioxothiazoli...)Show SMILES OC(=O)CN1C(=S)S\C(=C/c2cccc(Oc3ccccc3)c2)C1=O Show InChI InChI=1S/C18H13NO4S2/c20-16(21)11-19-17(22)15(25-18(19)24)10-12-5-4-8-14(9-12)23-13-6-2-1-3-7-13/h1-10H,11H2,(H,20,21)/b15-10- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant aldose reductase expressed in Escherichia coli BL21 (DE3) pLysS assessed as reduction in NADPH oxidation using L-idos... |
Bioorg Med Chem Lett 28: 3712-3720 (2018)
Article DOI: 10.1016/j.bmcl.2018.10.024
BindingDB Entry DOI: 10.7270/Q2QJ7MKC |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1
(Homo sapiens (Human)) | BDBM16313
(2-(2-{[(4-bromo-2-fluorophenyl)methyl]carbamothioy...)Show InChI InChI=1S/C16H12BrF2NO3S/c17-10-2-1-9(13(19)5-10)7-20-16(24)12-4-3-11(18)6-14(12)23-8-15(21)22/h1-6H,7-8H2,(H,20,24)(H,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Messina
Curated by ChEMBL
| Assay Description
Inhibition of AR (unknown origin) |
J Med Chem 58: 2047-67 (2015)
Article DOI: 10.1021/jm500907a
BindingDB Entry DOI: 10.7270/Q2D79D4X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sodium/glucose cotransporter 1
(Homo sapiens (Human)) | BDBM50595437

(CHEMBL5202047)Show SMILES CCOc1ccc(Cc2cc(ccc2Cl)[C@H]2O[C@@H](SC)[C@H](O)[C@@H](O)[C@@H]2O)cc1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00867
BindingDB Entry DOI: 10.7270/Q2FB570W |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1
(Homo sapiens (Human)) | BDBM50506665
(CHEMBL4554837)Show SMILES COc1cc(\C=C2/SC(=S)N(CC(O)=O)C2=O)ccc1OCc1ccccc1 Show InChI InChI=1S/C20H17NO5S2/c1-25-16-9-14(7-8-15(16)26-12-13-5-3-2-4-6-13)10-17-19(24)21(11-18(22)23)20(27)28-17/h2-10H,11-12H2,1H3,(H,22,23)/b17-10- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant aldose reductase expressed in Escherichia coli BL21 (DE3) pLysS assessed as reduction in NADPH oxidation using L-idos... |
Bioorg Med Chem Lett 28: 3712-3720 (2018)
Article DOI: 10.1016/j.bmcl.2018.10.024
BindingDB Entry DOI: 10.7270/Q2QJ7MKC |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1
(Homo sapiens (Human)) | BDBM50149769

((Z)-2-(5-(4-(benzyloxy)benzylidene)-4-oxo-2-thioxo...)Show SMILES OC(=O)CN1C(=S)S\C(=C/c2ccc(OCc3ccccc3)cc2)C1=O Show InChI InChI=1S/C19H15NO4S2/c21-17(22)11-20-18(23)16(26-19(20)25)10-13-6-8-15(9-7-13)24-12-14-4-2-1-3-5-14/h1-10H,11-12H2,(H,21,22)/b16-10- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant aldose reductase expressed in Escherichia coli BL21 (DE3) pLysS assessed as reduction in NADPH oxidation using L-idos... |
Bioorg Med Chem Lett 28: 3712-3720 (2018)
Article DOI: 10.1016/j.bmcl.2018.10.024
BindingDB Entry DOI: 10.7270/Q2QJ7MKC |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1
(Homo sapiens (Human)) | BDBM50149743

((Z)-2-(5-(3-(benzyloxy)benzylidene)-4-oxo-2-thioxo...)Show SMILES OC(=O)CN1C(=S)S\C(=C/c2cccc(OCc3ccccc3)c2)C1=O Show InChI InChI=1S/C19H15NO4S2/c21-17(22)11-20-18(23)16(26-19(20)25)10-14-7-4-8-15(9-14)24-12-13-5-2-1-3-6-13/h1-10H,11-12H2,(H,21,22)/b16-10- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant aldose reductase expressed in Escherichia coli BL21 (DE3) pLysS assessed as reduction in NADPH oxidation using L-idos... |
Bioorg Med Chem Lett 28: 3712-3720 (2018)
Article DOI: 10.1016/j.bmcl.2018.10.024
BindingDB Entry DOI: 10.7270/Q2QJ7MKC |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1
(Homo sapiens (Human)) | BDBM50506669

(CHEMBL4459307)Show SMILES OC(=O)CN1C(=S)S\C(=C/c2cccc(OCCc3ccccc3)c2)C1=O Show InChI InChI=1S/C20H17NO4S2/c22-18(23)13-21-19(24)17(27-20(21)26)12-15-7-4-8-16(11-15)25-10-9-14-5-2-1-3-6-14/h1-8,11-12H,9-10,13H2,(H,22,23)/b17-12- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant aldose reductase expressed in Escherichia coli BL21 (DE3) pLysS assessed as reduction in NADPH oxidation using L-idos... |
Bioorg Med Chem Lett 28: 3712-3720 (2018)
Article DOI: 10.1016/j.bmcl.2018.10.024
BindingDB Entry DOI: 10.7270/Q2QJ7MKC |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1
(Homo sapiens (Human)) | BDBM50334181
(2-(4-oxo-5-(4-phenoxybenzylidene)-2-thioxothiazoli...)Show SMILES OC(=O)CN1C(=S)S\C(=C/c2ccc(Oc3ccccc3)cc2)C1=O Show InChI InChI=1S/C18H13NO4S2/c20-16(21)11-19-17(22)15(25-18(19)24)10-12-6-8-14(9-7-12)23-13-4-2-1-3-5-13/h1-10H,11H2,(H,20,21)/b15-10- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant aldose reductase expressed in Escherichia coli BL21 (DE3) pLysS assessed as reduction in NADPH oxidation using L-idos... |
Bioorg Med Chem Lett 28: 3712-3720 (2018)
Article DOI: 10.1016/j.bmcl.2018.10.024
BindingDB Entry DOI: 10.7270/Q2QJ7MKC |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1
(Homo sapiens (Human)) | BDBM50016233
(CHEMBL3262475)Show SMILES NC(=O)COc1cccc(\C=C2/SC(=S)N(CC(O)=O)C2=O)c1 Show InChI InChI=1S/C14H12N2O5S2/c15-11(17)7-21-9-3-1-2-8(4-9)5-10-13(20)16(6-12(18)19)14(22)23-10/h1-5H,6-7H2,(H2,15,17)(H,18,19)/b10-5- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant aldose reductase expressed in Escherichia coli BL21 (DE3) pLysS assessed as reduction in NADPH oxidation using L-idos... |
Bioorg Med Chem Lett 28: 3712-3720 (2018)
Article DOI: 10.1016/j.bmcl.2018.10.024
BindingDB Entry DOI: 10.7270/Q2QJ7MKC |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1 [K65Q]
(Bos taurus (Cattle)) | BDBM50334175
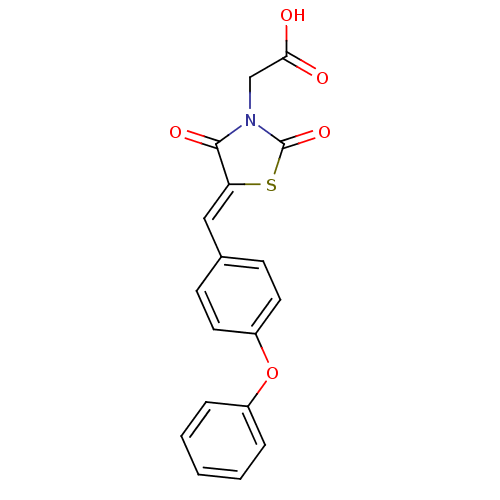
(2-(2,4-dioxo-5-(4-phenoxybenzylidene)thiazolidin-3...)Show SMILES OC(=O)CN1C(=O)S\C(=C/c2ccc(Oc3ccccc3)cc2)C1=O Show InChI InChI=1S/C18H13NO5S/c20-16(21)11-19-17(22)15(25-18(19)23)10-12-6-8-14(9-7-12)24-13-4-2-1-3-5-13/h1-10H,11H2,(H,20,21)/b15-10- | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Messina
Curated by ChEMBL
| Assay Description
Inhibition of bovine lens ALR2 |
Eur J Med Chem 46: 2797-806 (2011)
Article DOI: 10.1016/j.ejmech.2011.03.068
BindingDB Entry DOI: 10.7270/Q2CF9QFF |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1
(Homo sapiens (Human)) | BDBM50334182

(2-(5-(3-methoxybenzylidene)-4-oxo-2-thioxothiazoli...)Show InChI InChI=1S/C13H11NO4S2/c1-18-9-4-2-3-8(5-9)6-10-12(17)14(7-11(15)16)13(19)20-10/h2-6H,7H2,1H3,(H,15,16)/b10-6- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant aldose reductase expressed in Escherichia coli BL21 (DE3) pLysS assessed as reduction in NADPH oxidation using L-idos... |
Bioorg Med Chem Lett 28: 3712-3720 (2018)
Article DOI: 10.1016/j.bmcl.2018.10.024
BindingDB Entry DOI: 10.7270/Q2QJ7MKC |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1
(Homo sapiens (Human)) | BDBM50049730
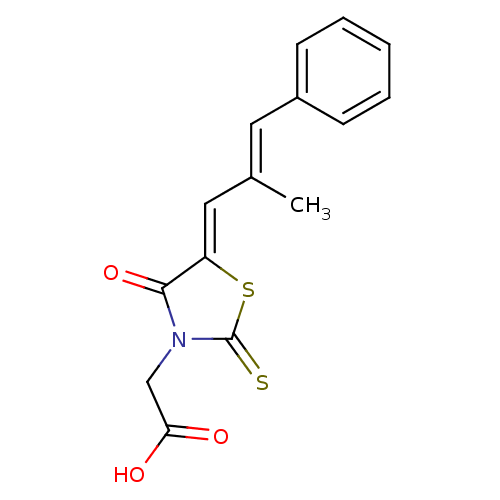
(2-(5-(2-methyl-3-phenylallylidene)-4-oxo-2-thioxot...)Show InChI InChI=1S/C15H13NO3S2/c1-10(7-11-5-3-2-4-6-11)8-12-14(19)16(9-13(17)18)15(20)21-12/h2-8H,9H2,1H3,(H,17,18)/b10-7+,12-8- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 102 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant aldose reductase expressed in Escherichia coli BL21 (DE3) pLysS assessed as reduction in NADPH oxidation using L-idos... |
Bioorg Med Chem Lett 28: 3712-3720 (2018)
Article DOI: 10.1016/j.bmcl.2018.10.024
BindingDB Entry DOI: 10.7270/Q2QJ7MKC |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1
(Homo sapiens (Human)) | BDBM50506664
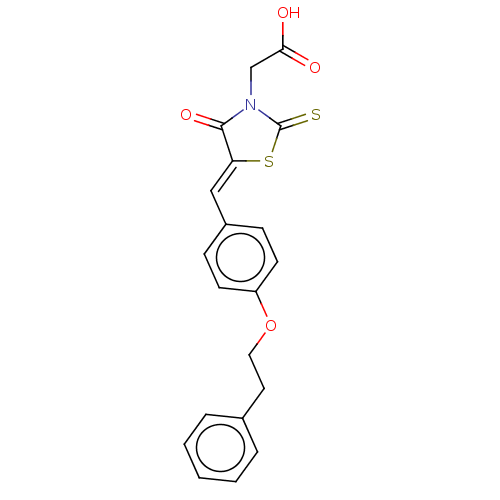
(CHEMBL4436037)Show SMILES OC(=O)CN1C(=S)S\C(=C/c2ccc(OCCc3ccccc3)cc2)C1=O Show InChI InChI=1S/C20H17NO4S2/c22-18(23)13-21-19(24)17(27-20(21)26)12-15-6-8-16(9-7-15)25-11-10-14-4-2-1-3-5-14/h1-9,12H,10-11,13H2,(H,22,23)/b17-12- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 104 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant aldose reductase expressed in Escherichia coli BL21 (DE3) pLysS assessed as reduction in NADPH oxidation using L-idos... |
Bioorg Med Chem Lett 28: 3712-3720 (2018)
Article DOI: 10.1016/j.bmcl.2018.10.024
BindingDB Entry DOI: 10.7270/Q2QJ7MKC |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1 [K65Q]
(Bos taurus (Cattle)) | BDBM50334181

(2-(4-oxo-5-(4-phenoxybenzylidene)-2-thioxothiazoli...)Show SMILES OC(=O)CN1C(=S)S\C(=C/c2ccc(Oc3ccccc3)cc2)C1=O Show InChI InChI=1S/C18H13NO4S2/c20-16(21)11-19-17(22)15(25-18(19)24)10-12-6-8-14(9-7-12)23-13-4-2-1-3-5-13/h1-10H,11H2,(H,20,21)/b15-10- | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of bovine lens aldose reductase assessed as inhibition of NDAPH oxidation by non-linear regression analysis |
Bioorg Med Chem Lett 21: 200-3 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.041
BindingDB Entry DOI: 10.7270/Q2ZP46C4 |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1 [K65Q]
(Bos taurus (Cattle)) | BDBM50016230
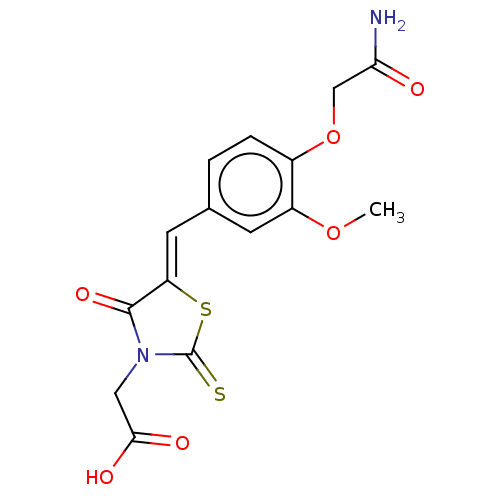
(CHEMBL3262478)Show SMILES COc1cc(\C=C2/SC(=S)N(CC(O)=O)C2=O)ccc1OCC(N)=O Show InChI InChI=1S/C15H14N2O6S2/c1-22-10-4-8(2-3-9(10)23-7-12(16)18)5-11-14(21)17(6-13(19)20)15(24)25-11/h2-5H,6-7H2,1H3,(H2,16,18)(H,19,20)/b11-5- | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of bovine aldose reductase assessed as oxidation of NADPH |
Eur J Med Chem 81: 1-14 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.003
BindingDB Entry DOI: 10.7270/Q2833TJC |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1
(Homo sapiens (Human)) | BDBM50506668
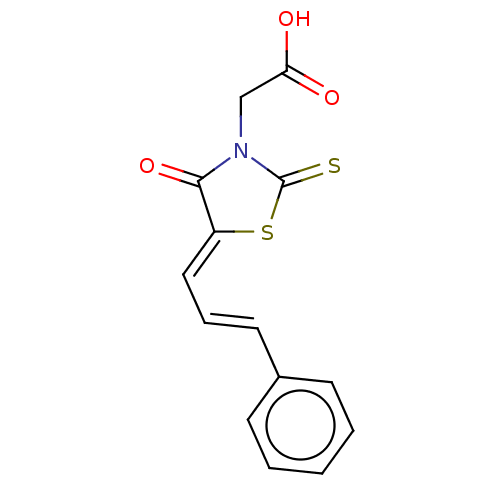
(CHEMBL1384494)Show InChI InChI=1S/C14H11NO3S2/c16-12(17)9-15-13(18)11(20-14(15)19)8-4-7-10-5-2-1-3-6-10/h1-8H,9H2,(H,16,17)/b7-4+,11-8- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 125 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant aldose reductase expressed in Escherichia coli BL21 (DE3) pLysS assessed as reduction in NADPH oxidation using L-idos... |
Bioorg Med Chem Lett 28: 3712-3720 (2018)
Article DOI: 10.1016/j.bmcl.2018.10.024
BindingDB Entry DOI: 10.7270/Q2QJ7MKC |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data