

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetyl-CoA carboxylase 1 (Mus musculus) | BDBM50247081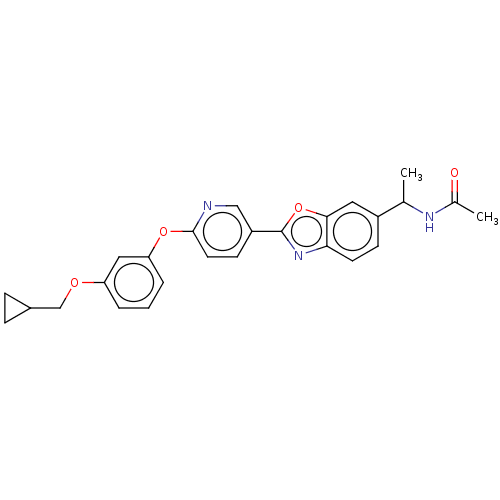 (CHEMBL4060253) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of mouse His-tagged ACC1 expressed in baculovirus infected SF9 cells using acetyl-CoA as substrate incubated for 60 mins followed by subst... | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126749 BindingDB Entry DOI: 10.7270/Q2R214TX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50247130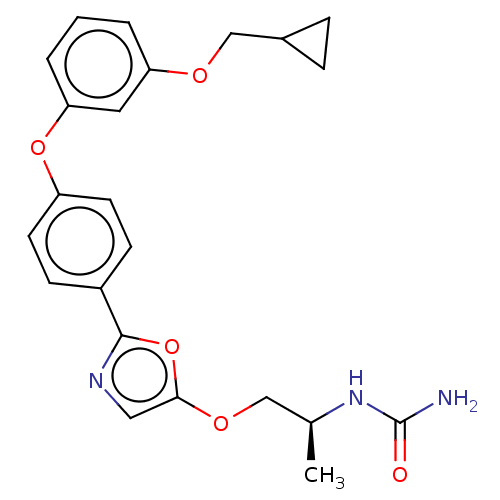 (CHEMBL4073202) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of recombinant human ACC1 expressed in SF-9 cells preincubated for 60 mins followed by addition of substrate solution containing acetyl-Co... | J Med Chem 61: 1098-1117 (2018) Article DOI: 10.1021/acs.jmedchem.7b01547 BindingDB Entry DOI: 10.7270/Q2HQ429W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50247081 (CHEMBL4060253) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of ACC1 in human HCT116 cells assessed as reduction of [14C]acetate uptake preincubated for 60 mins followed by addition of [14C]acetate a... | J Med Chem 61: 1098-1117 (2018) Article DOI: 10.1021/acs.jmedchem.7b01547 BindingDB Entry DOI: 10.7270/Q2HQ429W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50247112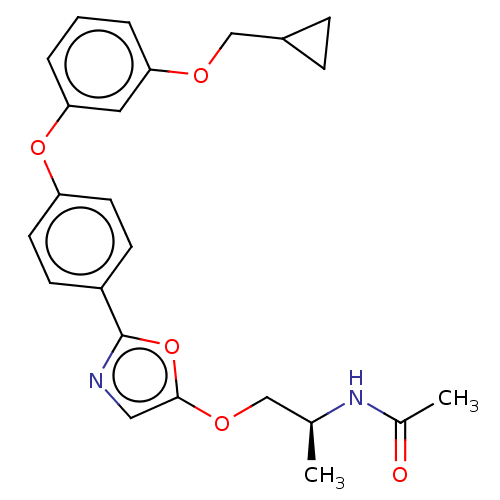 (CHEMBL4085633) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.960 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of recombinant human ACC1 expressed in SF-9 cells preincubated for 60 mins followed by addition of substrate solution containing acetyl-Co... | J Med Chem 61: 1098-1117 (2018) Article DOI: 10.1021/acs.jmedchem.7b01547 BindingDB Entry DOI: 10.7270/Q2HQ429W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50247114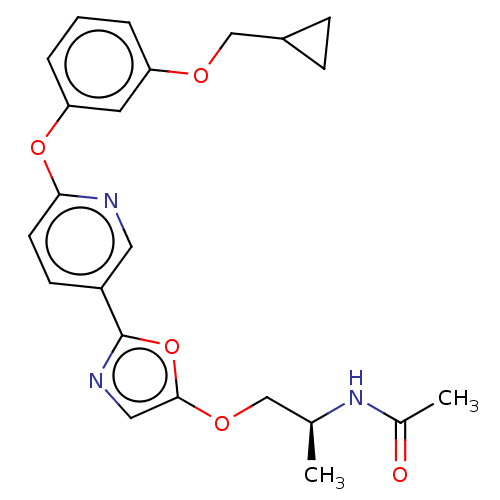 (CHEMBL4086127) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.960 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of recombinant human ACC1 expressed in SF-9 cells preincubated for 60 mins followed by addition of substrate solution containing acetyl-Co... | J Med Chem 61: 1098-1117 (2018) Article DOI: 10.1021/acs.jmedchem.7b01547 BindingDB Entry DOI: 10.7270/Q2HQ429W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50529222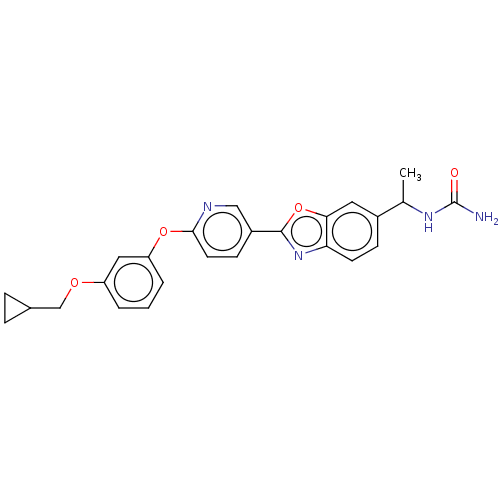 (CHEMBL4528475) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of recombinant human His-tagged ACC1 expressed in baculovirus infected SF9 cells using acetyl-CoA as substrate incubated for 60 mins follo... | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126749 BindingDB Entry DOI: 10.7270/Q2R214TX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50247081 (CHEMBL4060253) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of recombinant human ACC1 expressed in SF-9 cells preincubated for 60 mins followed by addition of substrate solution containing acetyl-Co... | J Med Chem 61: 1098-1117 (2018) Article DOI: 10.1021/acs.jmedchem.7b01547 BindingDB Entry DOI: 10.7270/Q2HQ429W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50247081 (CHEMBL4060253) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of recombinant human His-tagged ACC1 expressed in baculovirus infected SF9 cells using acetyl-CoA as substrate incubated for 60 mins follo... | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126749 BindingDB Entry DOI: 10.7270/Q2R214TX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50529224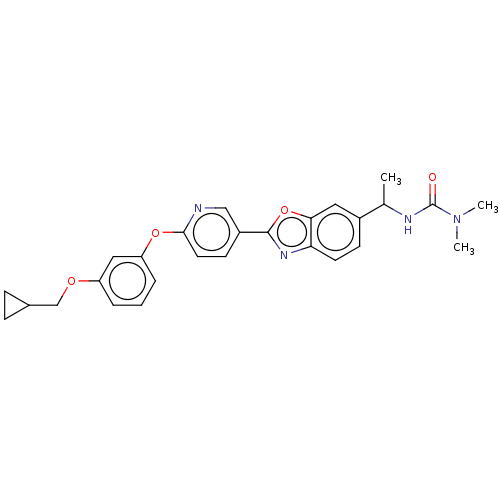 (CHEMBL4435891) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of recombinant human His-tagged ACC1 expressed in baculovirus infected SF9 cells using acetyl-CoA as substrate incubated for 60 mins follo... | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126749 BindingDB Entry DOI: 10.7270/Q2R214TX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50247080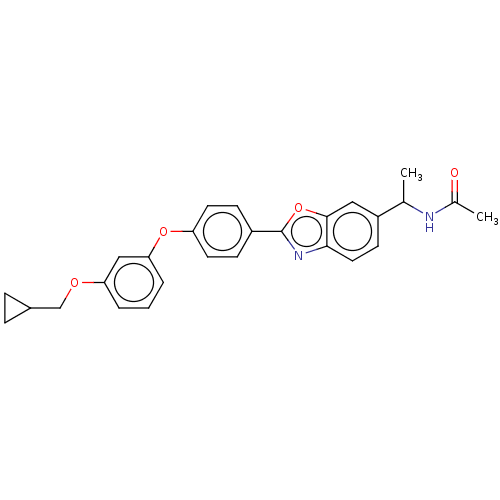 (CHEMBL4098647) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of ACC1 in human HCT116 cells assessed as reduction of [14C]acetate uptake preincubated for 60 mins followed by addition of [14C]acetate a... | J Med Chem 61: 1098-1117 (2018) Article DOI: 10.1021/acs.jmedchem.7b01547 BindingDB Entry DOI: 10.7270/Q2HQ429W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50247114 (CHEMBL4086127) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of ACC1 in human HCT116 cells assessed as reduction of [14C]acetate uptake preincubated for 60 mins followed by addition of [14C]acetate a... | J Med Chem 61: 1098-1117 (2018) Article DOI: 10.1021/acs.jmedchem.7b01547 BindingDB Entry DOI: 10.7270/Q2HQ429W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50247112 (CHEMBL4085633) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of ACC1 in human HCT116 cells assessed as reduction of [14C]acetate uptake preincubated for 60 mins followed by addition of [14C]acetate a... | J Med Chem 61: 1098-1117 (2018) Article DOI: 10.1021/acs.jmedchem.7b01547 BindingDB Entry DOI: 10.7270/Q2HQ429W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50529223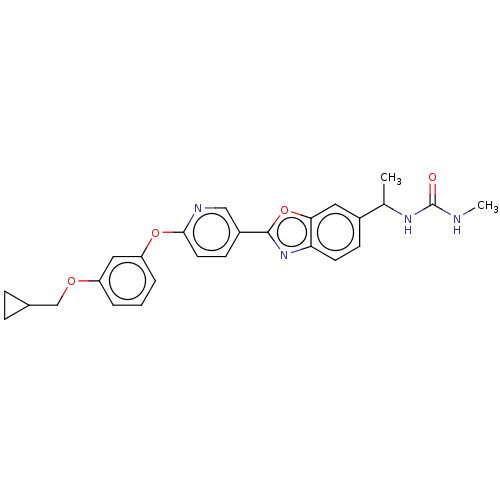 (CHEMBL4577890) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of recombinant human His-tagged ACC1 expressed in baculovirus infected SF9 cells using acetyl-CoA as substrate incubated for 60 mins follo... | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126749 BindingDB Entry DOI: 10.7270/Q2R214TX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Mus musculus) | BDBM50529222 (CHEMBL4528475) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of mouse His-tagged ACC1 expressed in baculovirus infected SF9 cells using acetyl-CoA as substrate incubated for 60 mins followed by subst... | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126749 BindingDB Entry DOI: 10.7270/Q2R214TX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50247111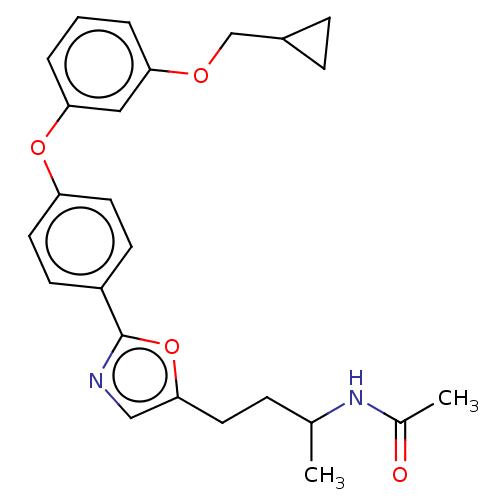 (CHEMBL4090919) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of recombinant human ACC1 expressed in SF-9 cells preincubated for 60 mins followed by addition of substrate solution containing acetyl-Co... | J Med Chem 61: 1098-1117 (2018) Article DOI: 10.1021/acs.jmedchem.7b01547 BindingDB Entry DOI: 10.7270/Q2HQ429W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50247080 (CHEMBL4098647) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of recombinant human ACC1 expressed in SF-9 cells preincubated for 60 mins followed by addition of substrate solution containing acetyl-Co... | J Med Chem 61: 1098-1117 (2018) Article DOI: 10.1021/acs.jmedchem.7b01547 BindingDB Entry DOI: 10.7270/Q2HQ429W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50247113 (CHEMBL4064621) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of recombinant human ACC1 expressed in SF-9 cells preincubated for 60 mins followed by addition of substrate solution containing acetyl-Co... | J Med Chem 61: 1098-1117 (2018) Article DOI: 10.1021/acs.jmedchem.7b01547 BindingDB Entry DOI: 10.7270/Q2HQ429W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50247130 (CHEMBL4073202) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of ACC1 in human HCT116 cells assessed as reduction of [14C]acetate uptake preincubated for 60 mins followed by addition of [14C]acetate a... | J Med Chem 61: 1098-1117 (2018) Article DOI: 10.1021/acs.jmedchem.7b01547 BindingDB Entry DOI: 10.7270/Q2HQ429W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50529222 (CHEMBL4528475) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of ACC1 in human HCT116 cells assessed as reduction in [14C]acetate uptake preincubated for 60 mins followed by [14C]acetate addition and ... | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126749 BindingDB Entry DOI: 10.7270/Q2R214TX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50247153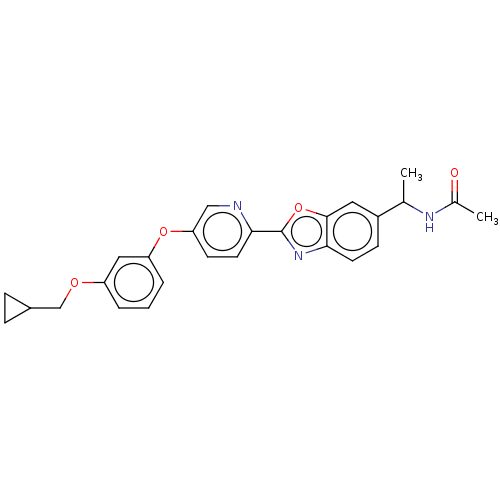 (CHEMBL4082153) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of recombinant human ACC1 expressed in SF-9 cells preincubated for 60 mins followed by addition of substrate solution containing acetyl-Co... | J Med Chem 61: 1098-1117 (2018) Article DOI: 10.1021/acs.jmedchem.7b01547 BindingDB Entry DOI: 10.7270/Q2HQ429W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50247094 (CHEMBL4101119) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of recombinant human ACC1 expressed in SF-9 cells preincubated for 60 mins followed by addition of substrate solution containing acetyl-Co... | J Med Chem 61: 1098-1117 (2018) Article DOI: 10.1021/acs.jmedchem.7b01547 BindingDB Entry DOI: 10.7270/Q2HQ429W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50247111 (CHEMBL4090919) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of ACC1 in human HCT116 cells assessed as reduction of [14C]acetate uptake preincubated for 60 mins followed by addition of [14C]acetate a... | J Med Chem 61: 1098-1117 (2018) Article DOI: 10.1021/acs.jmedchem.7b01547 BindingDB Entry DOI: 10.7270/Q2HQ429W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM50268410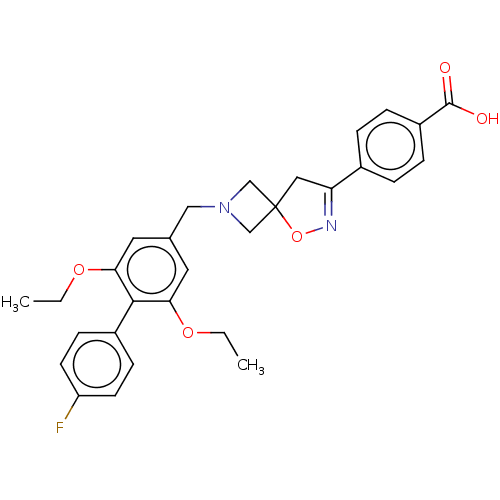 (CHEMBL4097462) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Division Medicinal Chemistry Laboratory, SCOHIA PHARMA, Inc., 26-1, Muraoka-Higashi 2-Chome, Fujisawa, Kanagawa 251-8555, Japan. Electronic address: hideki.hirose@scohia.com. Curated by ChEMBL | Assay Description Antagonist activity at human SSTR5 expressed in CHO cells assessed as inhibition of SST14-induced forskolin-stimulated intracellular cAMP level incub... | Bioorg Med Chem 25: 4175-4193 (2017) Article DOI: 10.1016/j.bmc.2017.06.007 BindingDB Entry DOI: 10.7270/Q2KS6V1W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM50268222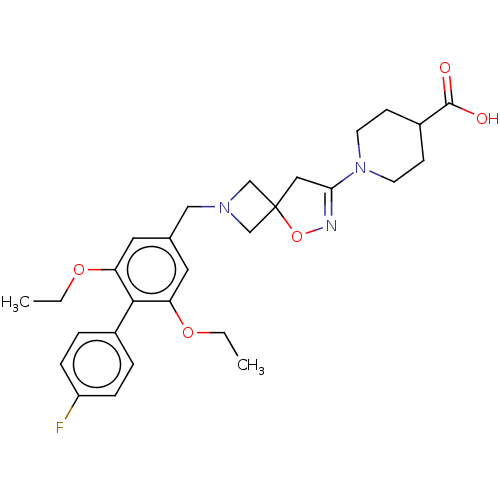 (CHEMBL4063587) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Division Medicinal Chemistry Laboratory, SCOHIA PHARMA, Inc., 26-1, Muraoka-Higashi 2-Chome, Fujisawa, Kanagawa 251-8555, Japan. Electronic address: hideki.hirose@scohia.com. Curated by ChEMBL | Assay Description Antagonist activity at human SSTR5 expressed in CHO cells assessed as inhibition of SST14-induced forskolin-stimulated intracellular cAMP level incub... | Bioorg Med Chem 25: 4175-4193 (2017) Article DOI: 10.1016/j.bmc.2017.06.007 BindingDB Entry DOI: 10.7270/Q2KS6V1W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50247153 (CHEMBL4082153) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of ACC1 in human HCT116 cells assessed as reduction of [14C]acetate uptake preincubated for 60 mins followed by addition of [14C]acetate a... | J Med Chem 61: 1098-1117 (2018) Article DOI: 10.1021/acs.jmedchem.7b01547 BindingDB Entry DOI: 10.7270/Q2HQ429W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50247110 (CHEMBL4083131) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of ACC1 in human HCT116 cells assessed as reduction of [14C]acetate uptake preincubated for 60 mins followed by addition of [14C]acetate a... | J Med Chem 61: 1098-1117 (2018) Article DOI: 10.1021/acs.jmedchem.7b01547 BindingDB Entry DOI: 10.7270/Q2HQ429W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50302337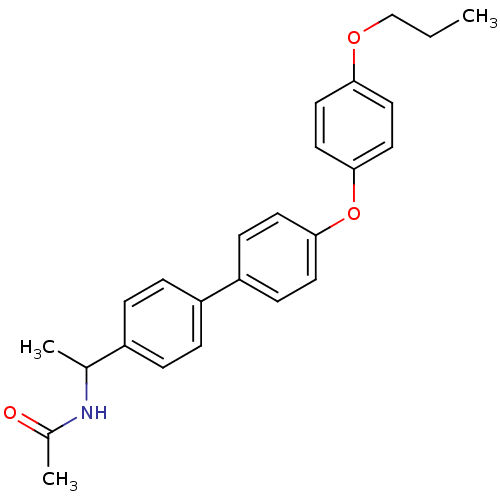 (CHEMBL567935 | N-(1-(4'-(4-propoxyphenoxy)biphenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human N-terminal His6-tagged ACC2 expressed in baculovirus infected Sf9 insect cells using acetyl-CoA as substrate preincub... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116056 BindingDB Entry DOI: 10.7270/Q29C7231 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50247094 (CHEMBL4101119) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of ACC1 in human HCT116 cells assessed as reduction of [14C]acetate uptake preincubated for 60 mins followed by addition of [14C]acetate a... | J Med Chem 61: 1098-1117 (2018) Article DOI: 10.1021/acs.jmedchem.7b01547 BindingDB Entry DOI: 10.7270/Q2HQ429W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50557465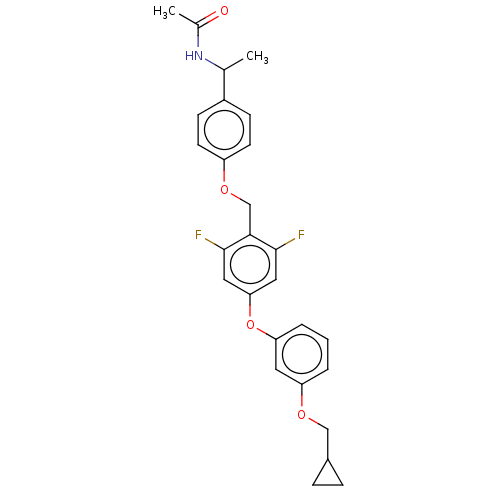 (CHEMBL4742115) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human N-terminal His6-tagged ACC1 expressed in baculovirus infected Sf9 insect cells using acetyl-CoA as substrate preincub... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116056 BindingDB Entry DOI: 10.7270/Q29C7231 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50302337 (CHEMBL567935 | N-(1-(4'-(4-propoxyphenoxy)biphenyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human N-terminal His6-tagged ACC1 expressed in baculovirus infected Sf9 insect cells using acetyl-CoA as substrate preincub... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116056 BindingDB Entry DOI: 10.7270/Q29C7231 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50557466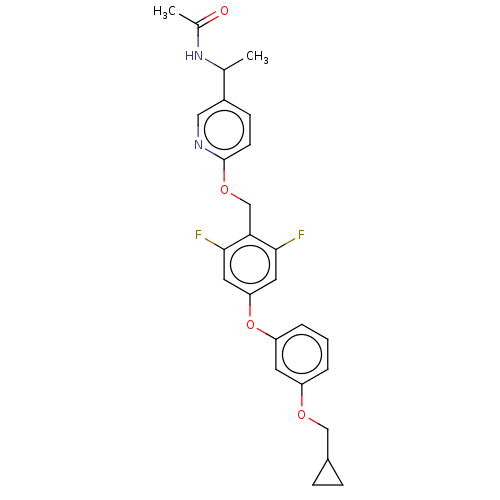 (CHEMBL4762802) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human N-terminal His6-tagged ACC1 expressed in baculovirus infected Sf9 insect cells using acetyl-CoA as substrate preincub... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116056 BindingDB Entry DOI: 10.7270/Q29C7231 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Mus musculus) | BDBM50268457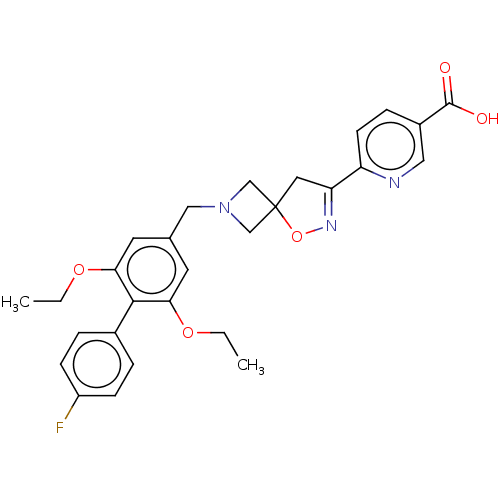 (CHEMBL4074952) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Division Medicinal Chemistry Laboratory, SCOHIA PHARMA, Inc., 26-1, Muraoka-Higashi 2-Chome, Fujisawa, Kanagawa 251-8555, Japan. Electronic address: hideki.hirose@scohia.com. Curated by ChEMBL | Assay Description Antagonist activity at mouse SSTR5 expressed in CHO cells assessed as inhibition of SST14-induced forskolin-stimulated intracellular cAMP level incub... | Bioorg Med Chem 25: 4175-4193 (2017) Article DOI: 10.1016/j.bmc.2017.06.007 BindingDB Entry DOI: 10.7270/Q2KS6V1W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50557470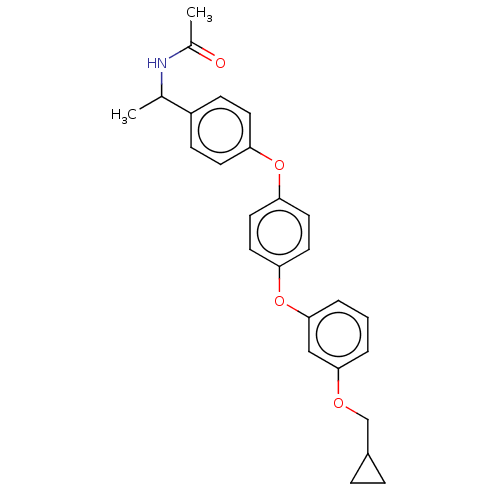 (CHEMBL4746516) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human N-terminal His6-tagged ACC2 expressed in baculovirus infected Sf9 insect cells using acetyl-CoA as substrate preincub... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116056 BindingDB Entry DOI: 10.7270/Q29C7231 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Mus musculus) | BDBM50268448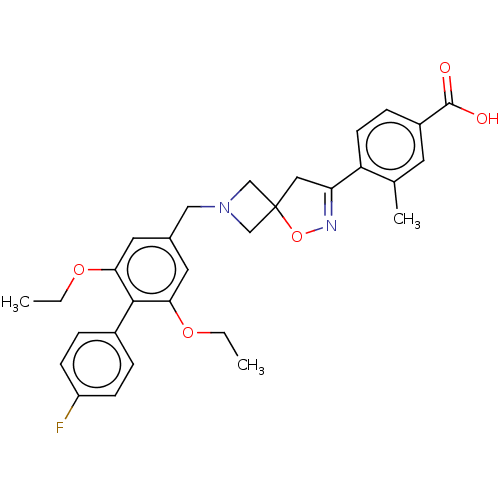 (CHEMBL4092944) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Division Medicinal Chemistry Laboratory, SCOHIA PHARMA, Inc., 26-1, Muraoka-Higashi 2-Chome, Fujisawa, Kanagawa 251-8555, Japan. Electronic address: hideki.hirose@scohia.com. Curated by ChEMBL | Assay Description Antagonist activity at mouse SSTR5 expressed in CHO cells assessed as inhibition of SST14-induced forskolin-stimulated intracellular cAMP level incub... | Bioorg Med Chem 25: 4175-4193 (2017) Article DOI: 10.1016/j.bmc.2017.06.007 BindingDB Entry DOI: 10.7270/Q2KS6V1W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50247078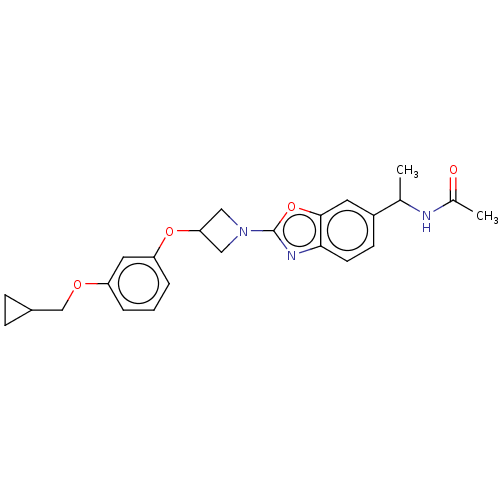 (CHEMBL4086054) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of recombinant human ACC1 expressed in SF-9 cells preincubated for 60 mins followed by addition of substrate solution containing acetyl-Co... | J Med Chem 61: 1098-1117 (2018) Article DOI: 10.1021/acs.jmedchem.7b01547 BindingDB Entry DOI: 10.7270/Q2HQ429W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50247110 (CHEMBL4083131) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of recombinant human ACC1 expressed in SF-9 cells preincubated for 60 mins followed by addition of substrate solution containing acetyl-Co... | J Med Chem 61: 1098-1117 (2018) Article DOI: 10.1021/acs.jmedchem.7b01547 BindingDB Entry DOI: 10.7270/Q2HQ429W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM50268447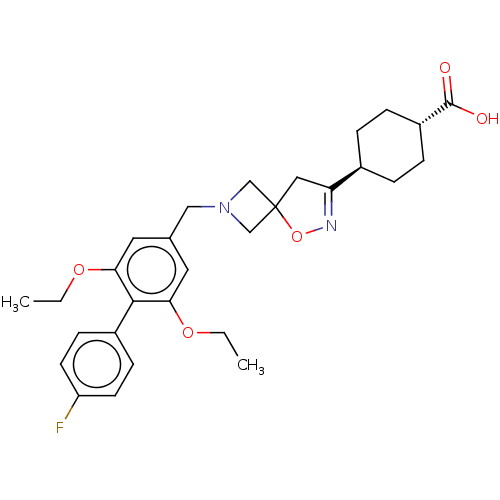 (CHEMBL4072189) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Division Medicinal Chemistry Laboratory, SCOHIA PHARMA, Inc., 26-1, Muraoka-Higashi 2-Chome, Fujisawa, Kanagawa 251-8555, Japan. Electronic address: hideki.hirose@scohia.com. Curated by ChEMBL | Assay Description Antagonist activity at human SSTR5 expressed in CHO cells assessed as inhibition of SST14-induced forskolin-stimulated intracellular cAMP level incub... | Bioorg Med Chem 25: 4175-4193 (2017) Article DOI: 10.1016/j.bmc.2017.06.007 BindingDB Entry DOI: 10.7270/Q2KS6V1W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50557468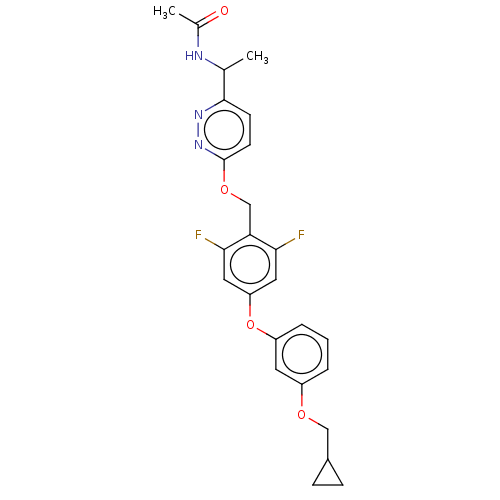 (CHEMBL4764202) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human N-terminal His6-tagged ACC1 expressed in baculovirus infected Sf9 insect cells using acetyl-CoA as substrate preincub... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116056 BindingDB Entry DOI: 10.7270/Q29C7231 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Mus musculus) | BDBM50268449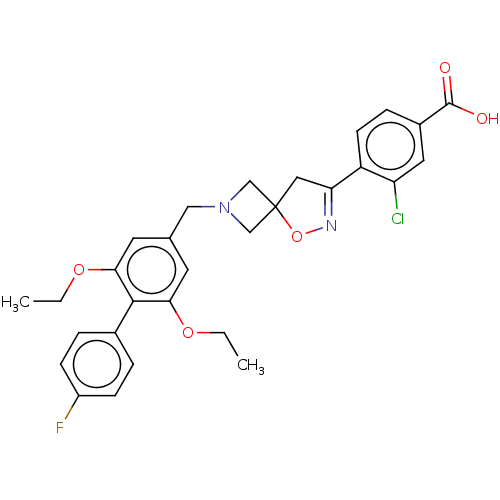 (CHEMBL4093913) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Division Medicinal Chemistry Laboratory, SCOHIA PHARMA, Inc., 26-1, Muraoka-Higashi 2-Chome, Fujisawa, Kanagawa 251-8555, Japan. Electronic address: hideki.hirose@scohia.com. Curated by ChEMBL | Assay Description Antagonist activity at mouse SSTR5 expressed in CHO cells assessed as inhibition of SST14-induced forskolin-stimulated intracellular cAMP level incub... | Bioorg Med Chem 25: 4175-4193 (2017) Article DOI: 10.1016/j.bmc.2017.06.007 BindingDB Entry DOI: 10.7270/Q2KS6V1W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Mus musculus) | BDBM50268410 (CHEMBL4097462) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Division Medicinal Chemistry Laboratory, SCOHIA PHARMA, Inc., 26-1, Muraoka-Higashi 2-Chome, Fujisawa, Kanagawa 251-8555, Japan. Electronic address: hideki.hirose@scohia.com. Curated by ChEMBL | Assay Description Antagonist activity at mouse SSTR5 expressed in CHO cells assessed as inhibition of SST14-induced forskolin-stimulated intracellular cAMP level incub... | Bioorg Med Chem 25: 4175-4193 (2017) Article DOI: 10.1016/j.bmc.2017.06.007 BindingDB Entry DOI: 10.7270/Q2KS6V1W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Mus musculus) | BDBM50247081 (CHEMBL4060253) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of mouse His-tagged ACC2 expressed in baculovirus infected SF9 cells using acetyl-CoA as substrate incubated for 60 mins followed by subst... | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126749 BindingDB Entry DOI: 10.7270/Q2R214TX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50557467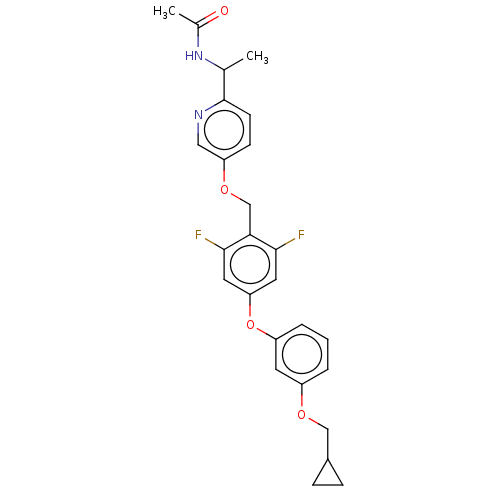 (CHEMBL4755102) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human N-terminal His6-tagged ACC1 expressed in baculovirus infected Sf9 insect cells using acetyl-CoA as substrate preincub... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116056 BindingDB Entry DOI: 10.7270/Q29C7231 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Mus musculus) | BDBM50268447 (CHEMBL4072189) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Division Medicinal Chemistry Laboratory, SCOHIA PHARMA, Inc., 26-1, Muraoka-Higashi 2-Chome, Fujisawa, Kanagawa 251-8555, Japan. Electronic address: hideki.hirose@scohia.com. Curated by ChEMBL | Assay Description Antagonist activity at mouse SSTR5 expressed in CHO cells assessed as inhibition of SST14-induced forskolin-stimulated intracellular cAMP level incub... | Bioorg Med Chem 25: 4175-4193 (2017) Article DOI: 10.1016/j.bmc.2017.06.007 BindingDB Entry DOI: 10.7270/Q2KS6V1W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50557470 (CHEMBL4746516) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human N-terminal His6-tagged ACC1 expressed in baculovirus infected Sf9 insect cells using acetyl-CoA as substrate preincub... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116056 BindingDB Entry DOI: 10.7270/Q29C7231 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM50268449 (CHEMBL4093913) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Division Medicinal Chemistry Laboratory, SCOHIA PHARMA, Inc., 26-1, Muraoka-Higashi 2-Chome, Fujisawa, Kanagawa 251-8555, Japan. Electronic address: hideki.hirose@scohia.com. Curated by ChEMBL | Assay Description Antagonist activity at human SSTR5 expressed in CHO cells assessed as inhibition of SST14-induced forskolin-stimulated intracellular cAMP level incub... | Bioorg Med Chem 25: 4175-4193 (2017) Article DOI: 10.1016/j.bmc.2017.06.007 BindingDB Entry DOI: 10.7270/Q2KS6V1W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Mus musculus) | BDBM50268415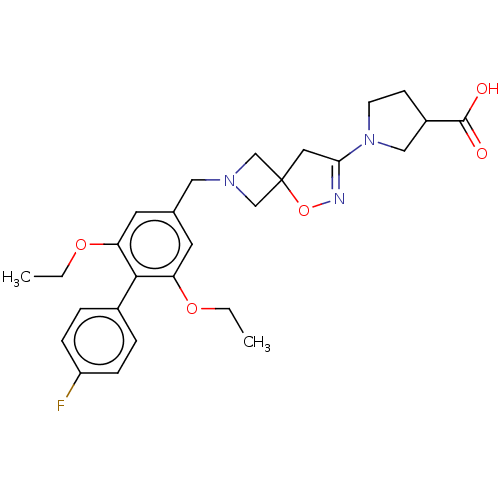 (CHEMBL4088598) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Division Medicinal Chemistry Laboratory, SCOHIA PHARMA, Inc., 26-1, Muraoka-Higashi 2-Chome, Fujisawa, Kanagawa 251-8555, Japan. Electronic address: hideki.hirose@scohia.com. Curated by ChEMBL | Assay Description Antagonist activity at mouse SSTR5 expressed in CHO cells assessed as inhibition of SST14-induced forskolin-stimulated intracellular cAMP level incub... | Bioorg Med Chem 25: 4175-4193 (2017) Article DOI: 10.1016/j.bmc.2017.06.007 BindingDB Entry DOI: 10.7270/Q2KS6V1W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic initiation factor 4A-III (Homo sapiens (Human)) | BDBM50283464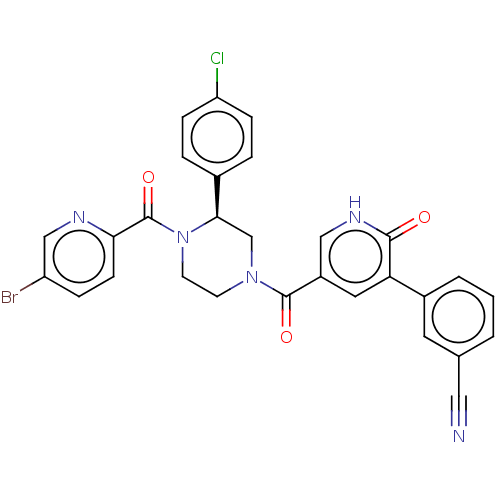 (CHEMBL4162764) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Inhibition of MLN51-induced full length recombinant human N-terminal His6/SUMO-tagged eIF4A3 RNA dependent ATPase activity expressed in Escherichia c... | ACS Med Chem Lett 8: 1077-1082 (2017) Article DOI: 10.1021/acsmedchemlett.7b00283 BindingDB Entry DOI: 10.7270/Q2QJ7KT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50247078 (CHEMBL4086054) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of ACC1 in human HCT116 cells assessed as reduction of [14C]acetate uptake preincubated for 60 mins followed by addition of [14C]acetate a... | J Med Chem 61: 1098-1117 (2018) Article DOI: 10.1021/acs.jmedchem.7b01547 BindingDB Entry DOI: 10.7270/Q2HQ429W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50247113 (CHEMBL4064621) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of ACC1 in human HCT116 cells assessed as reduction of [14C]acetate uptake preincubated for 60 mins followed by addition of [14C]acetate a... | J Med Chem 61: 1098-1117 (2018) Article DOI: 10.1021/acs.jmedchem.7b01547 BindingDB Entry DOI: 10.7270/Q2HQ429W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Mus musculus) | BDBM50268222 (CHEMBL4063587) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Division Medicinal Chemistry Laboratory, SCOHIA PHARMA, Inc., 26-1, Muraoka-Higashi 2-Chome, Fujisawa, Kanagawa 251-8555, Japan. Electronic address: hideki.hirose@scohia.com. Curated by ChEMBL | Assay Description Antagonist activity at mouse SSTR5 expressed in CHO cells assessed as inhibition of SST14-induced forskolin-stimulated intracellular cAMP level incub... | Bioorg Med Chem 25: 4175-4193 (2017) Article DOI: 10.1016/j.bmc.2017.06.007 BindingDB Entry DOI: 10.7270/Q2KS6V1W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 172 total ) | Next | Last >> |