

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Replicase polyprotein 1ab (2019-nCoV) | BDBM581847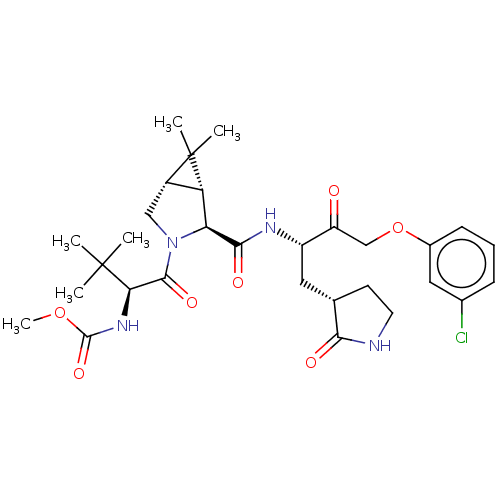 (WO2022208262, Example 16) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022208262 | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of compounds to prevent SARS-CoV-2 coronavirus-induced cell death or cytopathic effect can be assessed via cell viability, using an assa... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R49VMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50528182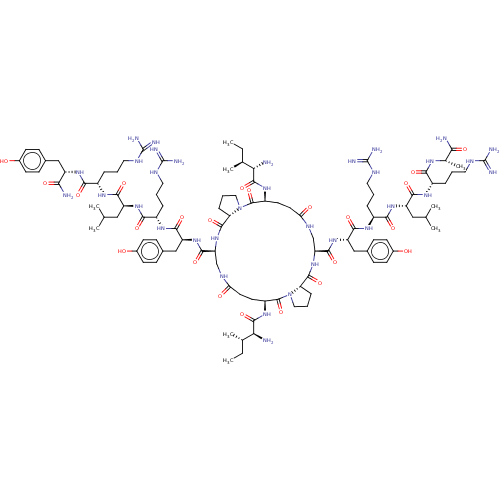 (CHEMBL4454036) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.257 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Displacement of (sCy5)-[Lys2 Arg4]-BVD15 from GFP-tagged Y1R in human HEK293T cells assessed as inhibitory constant incubated for 5 mins followed by ... | J Med Chem 63: 5274-5286 (2020) Article DOI: 10.1021/acs.jmedchem.0c00027 BindingDB Entry DOI: 10.7270/Q2RB782R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50528179 (CHEMBL4476074) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.275 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Displacement of (sCy5)-[Lys2 Arg4]-BVD15 from GFP-tagged Y1R in human HEK293T cells assessed as inhibitory constant incubated for 5 mins followed by ... | J Med Chem 63: 5274-5286 (2020) Article DOI: 10.1021/acs.jmedchem.0c00027 BindingDB Entry DOI: 10.7270/Q2RB782R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50528186 (CHEMBL4445317) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.363 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Displacement of (sCy5)-[Lys2 Arg4]-BVD15 from GFP-tagged Y1R in human HEK293T cells assessed as inhibitory constant incubated for 5 mins followed by ... | J Med Chem 63: 5274-5286 (2020) Article DOI: 10.1021/acs.jmedchem.0c00027 BindingDB Entry DOI: 10.7270/Q2RB782R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM581848 (WO2022208262, Example 17) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022208262 | <0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of compounds to prevent SARS-CoV-2 coronavirus-induced cell death or cytopathic effect can be assessed via cell viability, using an assa... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R49VMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50528181 (CHEMBL4519035) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.575 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Displacement of (sCy5)-[Lys2 Arg4]-BVD15 from GFP-tagged Y1R in human HEK293T cells assessed as inhibitory constant incubated for 5 mins followed by ... | J Med Chem 63: 5274-5286 (2020) Article DOI: 10.1021/acs.jmedchem.0c00027 BindingDB Entry DOI: 10.7270/Q2RB782R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50409214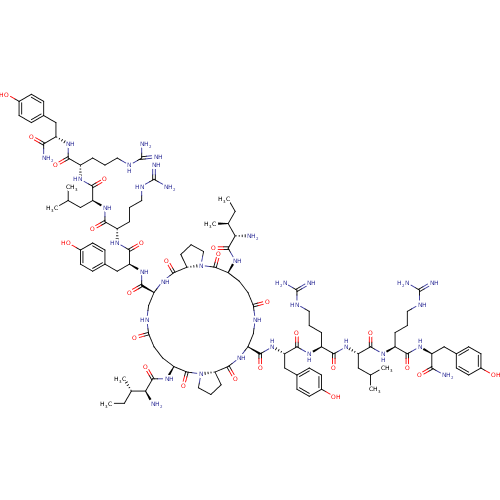 (CHEMBL2110365 | GR-231118) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Displacement of (sCy5)-[Lys2 Arg4]-BVD15 from GFP-tagged Y1R in human HEK293T cells assessed as inhibitory constant incubated for 5 mins followed by ... | J Med Chem 63: 5274-5286 (2020) Article DOI: 10.1021/acs.jmedchem.0c00027 BindingDB Entry DOI: 10.7270/Q2RB782R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50166728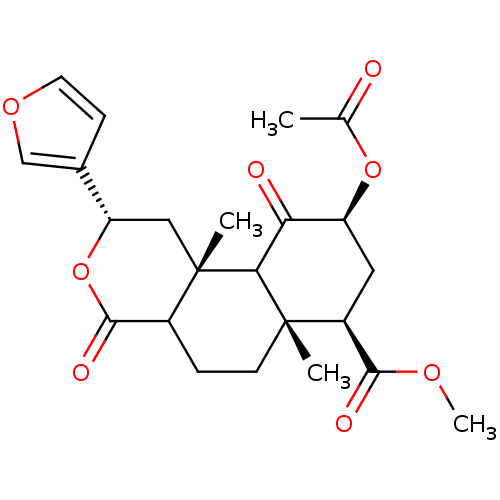 ((3S,4aR,6S,8R,8aR)-6-Acetoxy-3-furan-3-yl-4a,8a-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McLean Hospital Curated by ChEMBL | Assay Description Inhibition of [3H]diprenorphine binding to human Opioid receptor kappa 1 expressed in chinese hamster ovary cells | Bioorg Med Chem Lett 15: 2761-5 (2005) Article DOI: 10.1016/j.bmcl.2005.03.113 BindingDB Entry DOI: 10.7270/Q2TD9Z3J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50159165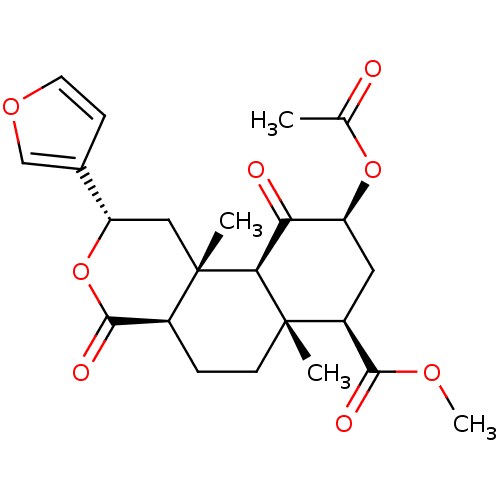 ((2S,4aR,6aR,7R,9S,10aS,10bR)-9-(acetyloxy)-2-(fura...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McLean Hospital Curated by ChEMBL | Assay Description Inhibition of [3H]diprenorphine binding to human KOR expressed in CHO cells | Bioorg Med Chem Lett 16: 4679-85 (2006) Article DOI: 10.1016/j.bmcl.2006.05.093 BindingDB Entry DOI: 10.7270/Q2GX4B55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50159165 ((2S,4aR,6aR,7R,9S,10aS,10bR)-9-(acetyloxy)-2-(fura...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McLean Hospital Curated by ChEMBL | Assay Description Inhibition of [3H]diprenorphine binding to human KOR expressed in CHO cells | Bioorg Med Chem Lett 16: 4679-85 (2006) Article DOI: 10.1016/j.bmcl.2006.05.093 BindingDB Entry DOI: 10.7270/Q2GX4B55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000296 (CHEMBL441765 | CHEMBL482811 | U-50488H | US1149237...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McLean Hospital Curated by ChEMBL | Assay Description Inhibition of [3H]diprenorphine binding to human KOR expressed in CHO cells | Bioorg Med Chem Lett 16: 4679-85 (2006) Article DOI: 10.1016/j.bmcl.2006.05.093 BindingDB Entry DOI: 10.7270/Q2GX4B55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000296 (CHEMBL441765 | CHEMBL482811 | U-50488H | US1149237...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McLean Hospital Curated by ChEMBL | Assay Description Inhibition of [3H]diprenorphine binding to human Opioid receptor kappa 1 expressed in chinese hamster ovary cells | Bioorg Med Chem Lett 15: 2761-5 (2005) Article DOI: 10.1016/j.bmcl.2005.03.113 BindingDB Entry DOI: 10.7270/Q2TD9Z3J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM581851 (WO2022208262, Example 24) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022208262 | 1.41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of compounds to prevent SARS-CoV-2 coronavirus-induced cell death or cytopathic effect can be assessed via cell viability, using an assa... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R49VMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM535166 (WO2022013684, Example 18) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022013684 | 1.55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The proteolytic activity of the main protease, 3CLpro, of SARS-CoV-2 was monitored using a continuous fluorescence resonance energy transfer (FRET) a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43SW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50189154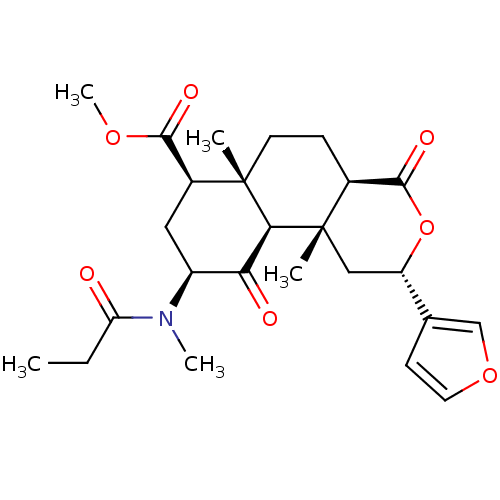 ((2S,4aR,6aR,7R,9S,10aS,10bR)-methyl 2-(furan-3-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McLean Hospital Curated by ChEMBL | Assay Description Inhibition of [3H]diprenorphine binding to human KOR expressed in CHO cells | Bioorg Med Chem Lett 16: 4679-85 (2006) Article DOI: 10.1016/j.bmcl.2006.05.093 BindingDB Entry DOI: 10.7270/Q2GX4B55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM581852 (WO2022208262, Example 25) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022208262 | 1.76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of compounds to prevent SARS-CoV-2 coronavirus-induced cell death or cytopathic effect can be assessed via cell viability, using an assa... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R49VMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM581846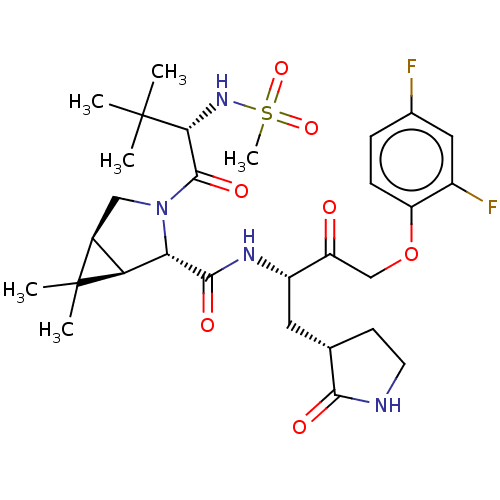 (WO2022208262, Example 13) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022208262 | 1.78 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of compounds to prevent SARS-CoV-2 coronavirus-induced cell death or cytopathic effect can be assessed via cell viability, using an assa... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R49VMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM581850 (WO2022208262, Example 23) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022208262 | 1.78 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of compounds to prevent SARS-CoV-2 coronavirus-induced cell death or cytopathic effect can be assessed via cell viability, using an assa... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R49VMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM581839 (WO2022208262, Example 1 | WO2022208262, Example 35) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022208262 | 2.02 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of compounds to prevent SARS-CoV-2 coronavirus-induced cell death or cytopathic effect can be assessed via cell viability, using an assa... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R49VMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM581857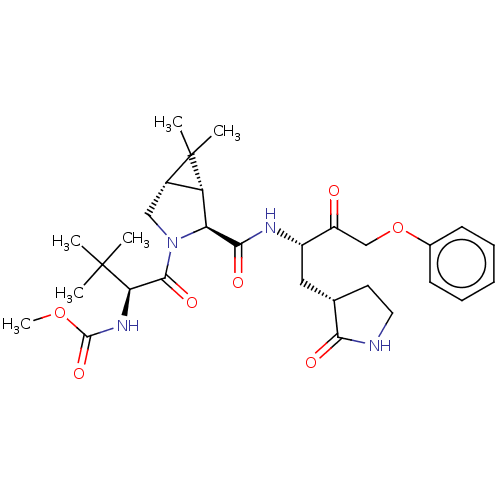 (WO2022208262, Example 30) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022208262 | 2.15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of compounds to prevent SARS-CoV-2 coronavirus-induced cell death or cytopathic effect can be assessed via cell viability, using an assa... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R49VMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM581855 (WO2022208262, Example 28) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022208262 | 2.15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of compounds to prevent SARS-CoV-2 coronavirus-induced cell death or cytopathic effect can be assessed via cell viability, using an assa... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R49VMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50528185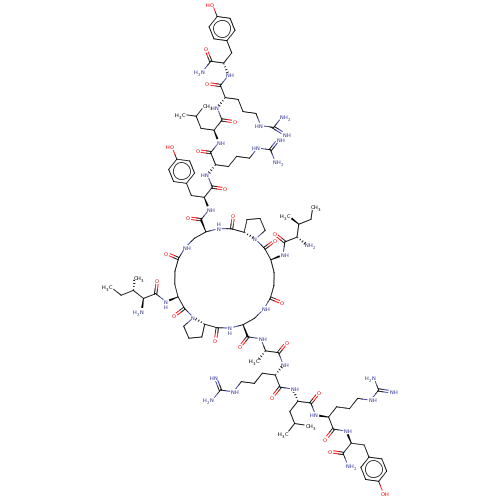 (CHEMBL4573545) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Displacement of (sCy5)-[Lys2 Arg4]-BVD15 from GFP-tagged Y1R in human HEK293T cells assessed as inhibitory constant incubated for 5 mins followed by ... | J Med Chem 63: 5274-5286 (2020) Article DOI: 10.1021/acs.jmedchem.0c00027 BindingDB Entry DOI: 10.7270/Q2RB782R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50189134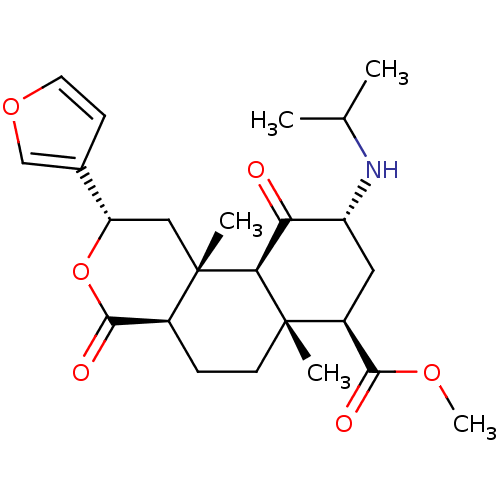 ((2S,4aR,6aR,7R,9R,10aS,10bR)-methyl 2-(furan-3-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McLean Hospital Curated by ChEMBL | Assay Description Inhibition of [3H]diprenorphine binding to human KOR expressed in CHO cells | Bioorg Med Chem Lett 16: 4679-85 (2006) Article DOI: 10.1016/j.bmcl.2006.05.093 BindingDB Entry DOI: 10.7270/Q2GX4B55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM581858 (WO2022208262, Example 32) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022208262 | 2.42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of compounds to prevent SARS-CoV-2 coronavirus-induced cell death or cytopathic effect can be assessed via cell viability, using an assa... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R49VMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM581839 (WO2022208262, Example 1 | WO2022208262, Example 35) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022208262 | 2.76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of compounds to prevent SARS-CoV-2 coronavirus-induced cell death or cytopathic effect can be assessed via cell viability, using an assa... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R49VMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM581860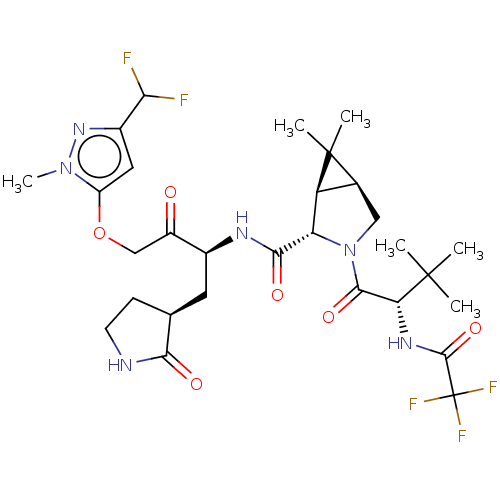 (WO2022208262, Example 8) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022208262 | 2.87 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of compounds to prevent SARS-CoV-2 coronavirus-induced cell death or cytopathic effect can be assessed via cell viability, using an assa... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R49VMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM581842 (WO2022208262, Example 7) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022208262 | 2.88 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of compounds to prevent SARS-CoV-2 coronavirus-induced cell death or cytopathic effect can be assessed via cell viability, using an assa... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R49VMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM581859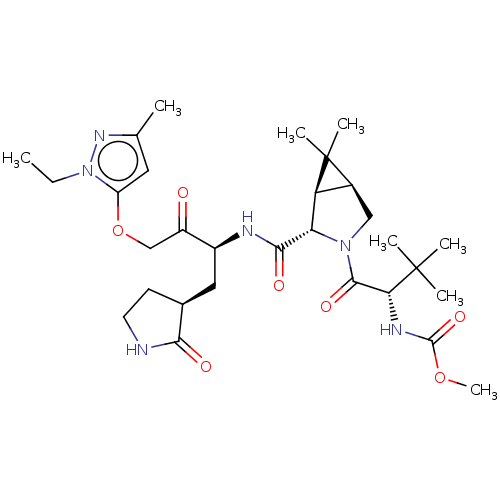 (WO2022208262, Example 33) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022208262 | 3.04 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of compounds to prevent SARS-CoV-2 coronavirus-induced cell death or cytopathic effect can be assessed via cell viability, using an assa... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R49VMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50189162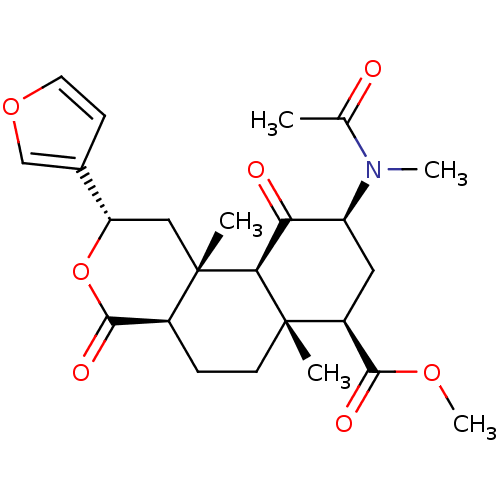 ((2S,4aR,6aR,7R,9S,10aS,10bR)-methyl 2-(furan-3-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McLean Hospital Curated by ChEMBL | Assay Description Inhibition of [3H]diprenorphine binding to human KOR expressed in CHO cells | Bioorg Med Chem Lett 16: 4679-85 (2006) Article DOI: 10.1016/j.bmcl.2006.05.093 BindingDB Entry DOI: 10.7270/Q2GX4B55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50166715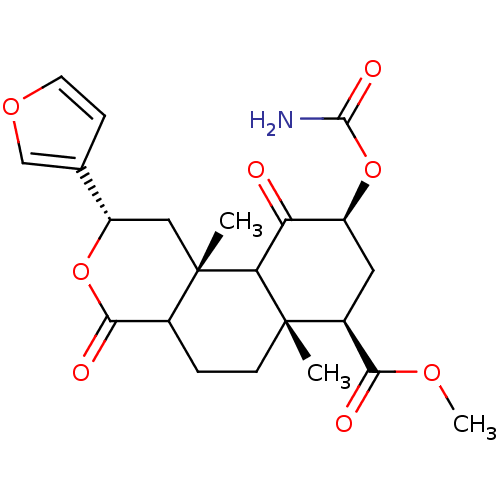 ((3S,4aR,6S,8R,8aR)-6-Carbamoyloxy-3-furan-3-yl-4a,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McLean Hospital Curated by ChEMBL | Assay Description Inhibition of [3H]diprenorphine binding to human Opioid receptor kappa 1 expressed in chinese hamster ovary cells | Bioorg Med Chem Lett 15: 2761-5 (2005) Article DOI: 10.1016/j.bmcl.2005.03.113 BindingDB Entry DOI: 10.7270/Q2TD9Z3J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM581840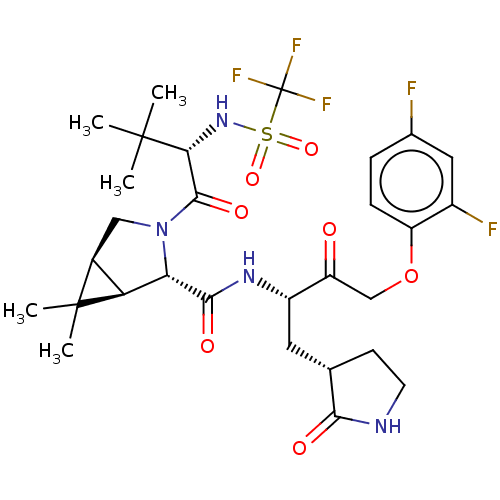 (WO2022208262, Example 3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022208262 | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of compounds to prevent SARS-CoV-2 coronavirus-induced cell death or cytopathic effect can be assessed via cell viability, using an assa... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R49VMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM581863 (WO2022208262, Example 39) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022208262 | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of compounds to prevent SARS-CoV-2 coronavirus-induced cell death or cytopathic effect can be assessed via cell viability, using an assa... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R49VMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM581862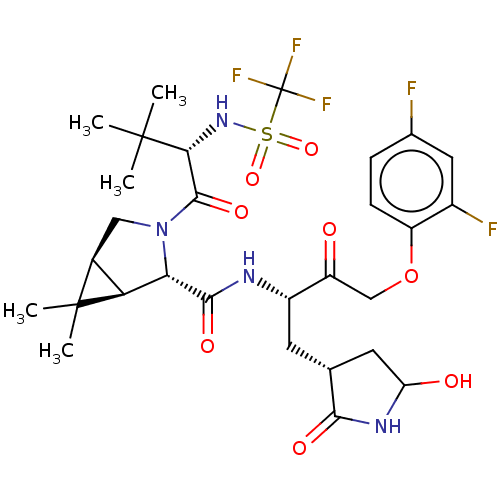 (WO2022208262, Example 38) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022208262 | 4.12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of compounds to prevent SARS-CoV-2 coronavirus-induced cell death or cytopathic effect can be assessed via cell viability, using an assa... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R49VMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM581864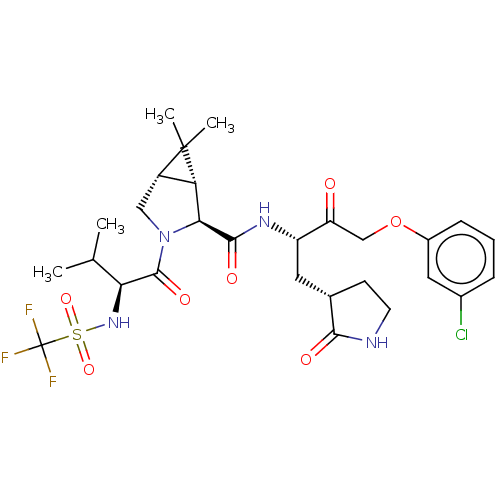 (WO2022208262, Example 40) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022208262 | 4.22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of compounds to prevent SARS-CoV-2 coronavirus-induced cell death or cytopathic effect can be assessed via cell viability, using an assa... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R49VMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50166724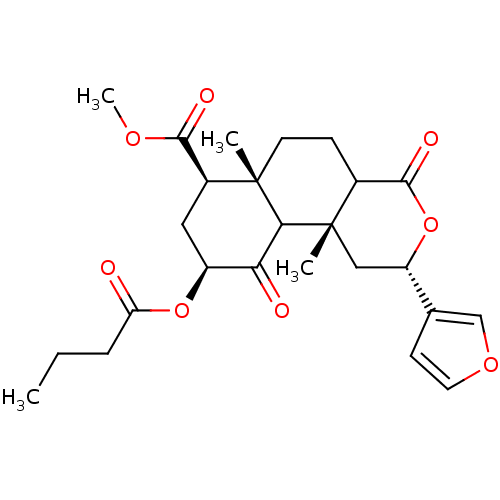 ((3S,4aR,6S,8R,8aR)-6-Butyryloxy-3-furan-3-yl-4a,8a...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McLean Hospital Curated by ChEMBL | Assay Description Inhibition of [3H]diprenorphine binding to human Opioid receptor kappa 1 expressed in chinese hamster ovary cells | Bioorg Med Chem Lett 15: 2761-5 (2005) Article DOI: 10.1016/j.bmcl.2005.03.113 BindingDB Entry DOI: 10.7270/Q2TD9Z3J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50189138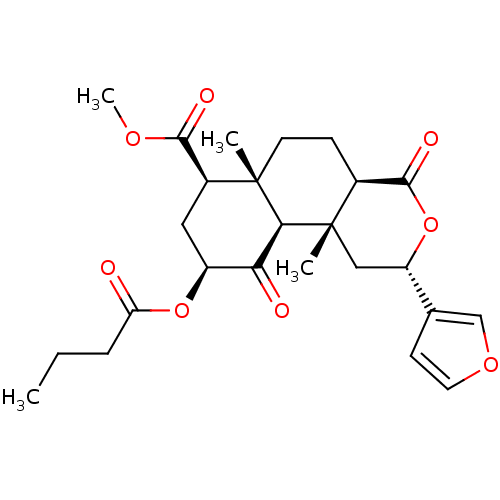 ((2S,4aR,6aR,7R,9S,10aS,10bR)-methyl 9-(butyryloxy)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McLean Hospital Curated by ChEMBL | Assay Description Inhibition of [3H]diprenorphine binding to human KOR expressed in CHO cells | Bioorg Med Chem Lett 16: 4679-85 (2006) Article DOI: 10.1016/j.bmcl.2006.05.093 BindingDB Entry DOI: 10.7270/Q2GX4B55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM581865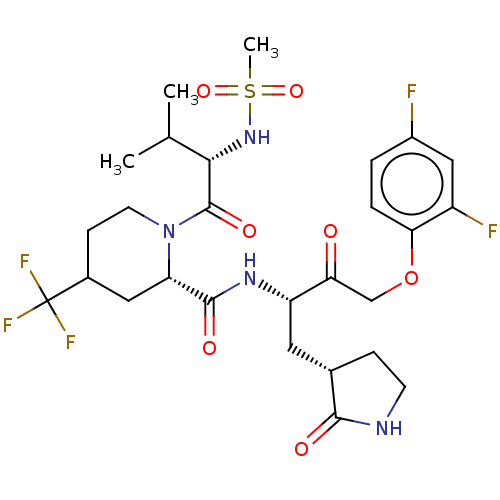 (WO2022208262, Example 41) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022208262 | 5.45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of compounds to prevent SARS-CoV-2 coronavirus-induced cell death or cytopathic effect can be assessed via cell viability, using an assa... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R49VMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM535159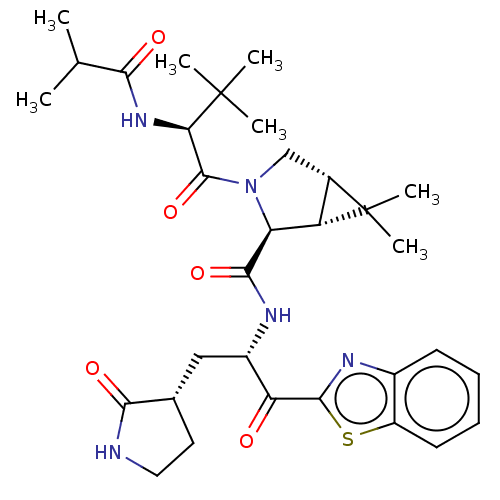 (WO2022013684, Example 14) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022013684 | 6.02 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The proteolytic activity of the main protease, 3CLpro, of SARS-CoV-2 was monitored using a continuous fluorescence resonance energy transfer (FRET) a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43SW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM535160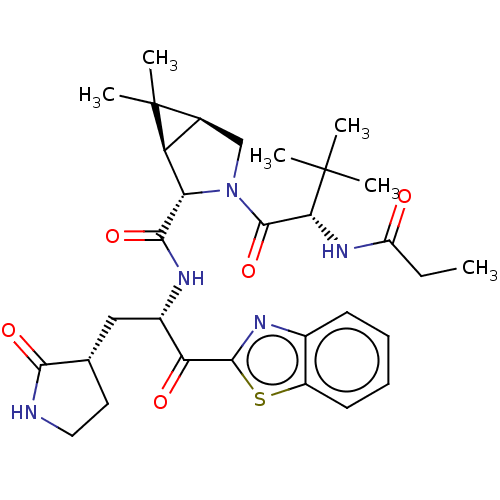 (WO2022013684, Example 15) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022013684 | 6.14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The proteolytic activity of the main protease, 3CLpro, of SARS-CoV-2 was monitored using a continuous fluorescence resonance energy transfer (FRET) a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43SW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM581849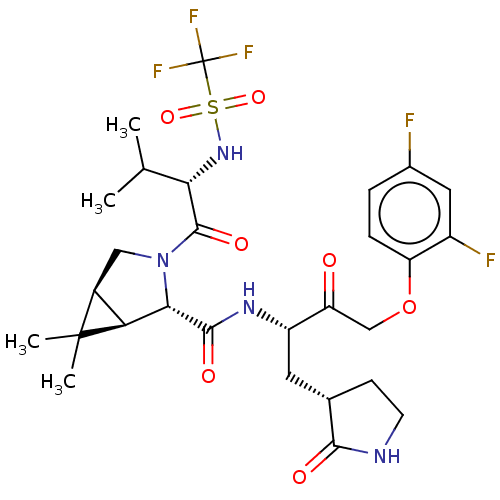 (WO2022208262, Example 4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022208262 | 6.34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of compounds to prevent SARS-CoV-2 coronavirus-induced cell death or cytopathic effect can be assessed via cell viability, using an assa... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R49VMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 4 (Homo sapiens (Human)) | BDBM50528183 (CHEMBL4540843) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Displacement of (sCy5)-[Lys2 Arg4]-BVD15 from GFP-tagged Y4R in human HEK293T cells assessed as inhibitory constant incubated for 5 mins followed by ... | J Med Chem 63: 5274-5286 (2020) Article DOI: 10.1021/acs.jmedchem.0c00027 BindingDB Entry DOI: 10.7270/Q2RB782R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50189142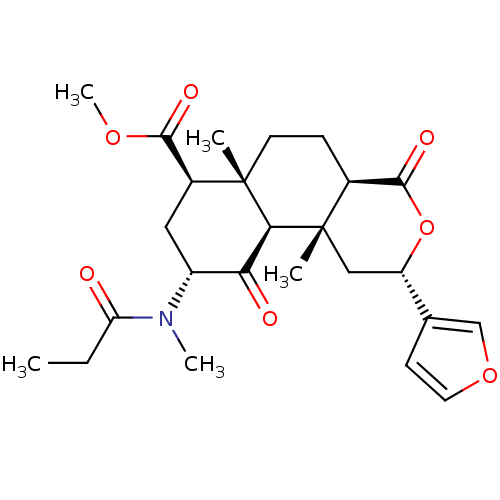 ((2S,4aR,6aR,7R,9R,10aS,10bR)-methyl 2-(furan-3-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McLean Hospital Curated by ChEMBL | Assay Description Inhibition of [3H]diprenorphine binding to human KOR expressed in CHO cells | Bioorg Med Chem Lett 16: 4679-85 (2006) Article DOI: 10.1016/j.bmcl.2006.05.093 BindingDB Entry DOI: 10.7270/Q2GX4B55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM581866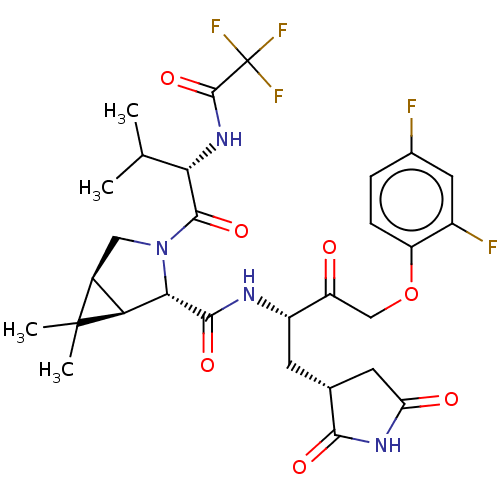 (WO2022208262, Example 42) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022208262 | 7.05 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of compounds to prevent SARS-CoV-2 coronavirus-induced cell death or cytopathic effect can be assessed via cell viability, using an assa... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R49VMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50166729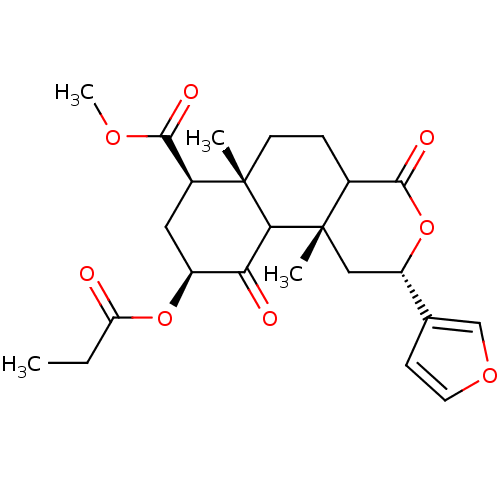 ((3S,4aR,6S,8R,8aR)-3-Furan-3-yl-4a,8a-dimethyl-1,5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McLean Hospital Curated by ChEMBL | Assay Description Inhibition of [3H]diprenorphine binding to human Opioid receptor kappa 1 expressed in chinese hamster ovary cells | Bioorg Med Chem Lett 15: 2761-5 (2005) Article DOI: 10.1016/j.bmcl.2005.03.113 BindingDB Entry DOI: 10.7270/Q2TD9Z3J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50170672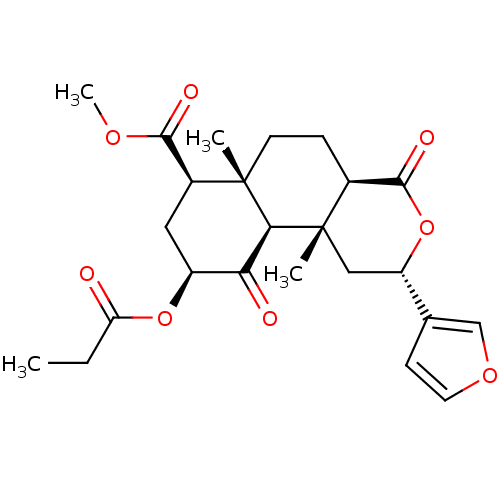 ((2S,4aR,6aR,7R,9S,10aS,10bR)-methyl 2-(furan-3-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McLean Hospital Curated by ChEMBL | Assay Description Inhibition of [3H]diprenorphine binding to human KOR expressed in CHO cells | Bioorg Med Chem Lett 16: 4679-85 (2006) Article DOI: 10.1016/j.bmcl.2006.05.093 BindingDB Entry DOI: 10.7270/Q2GX4B55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM535126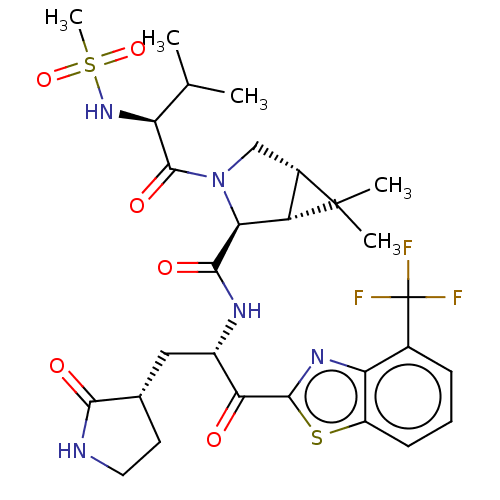 ((1R,2S,5S)-6,6-Dimethyl-3-[N-(methylsulfonyl)-L-va...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022013684 | 7.74 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The proteolytic activity of the main protease, 3CLpro, of SARS-CoV-2 was monitored using a continuous fluorescence resonance energy transfer (FRET) a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43SW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM581867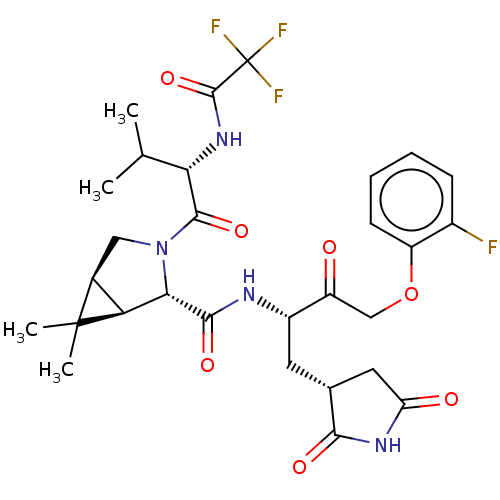 (WO2022208262, Example 48) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022208262 | 7.87 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of compounds to prevent SARS-CoV-2 coronavirus-induced cell death or cytopathic effect can be assessed via cell viability, using an assa... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R49VMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50189165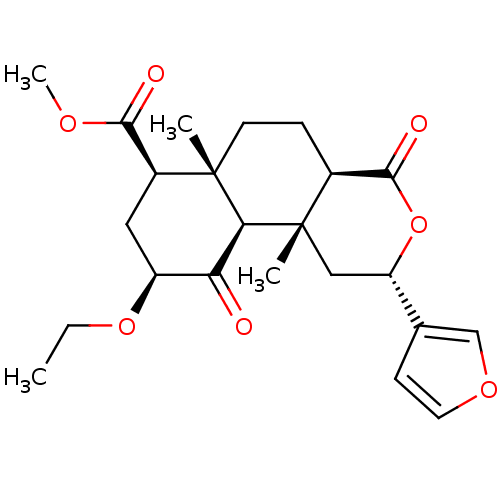 ((2S,4aR,6aR,7R,9S,10aS,10bR)-methyl 9-ethoxy-2-(fu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McLean Hospital Curated by ChEMBL | Assay Description Inhibition of [3H]diprenorphine binding to human KOR expressed in CHO cells | Bioorg Med Chem Lett 16: 4679-85 (2006) Article DOI: 10.1016/j.bmcl.2006.05.093 BindingDB Entry DOI: 10.7270/Q2GX4B55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50166723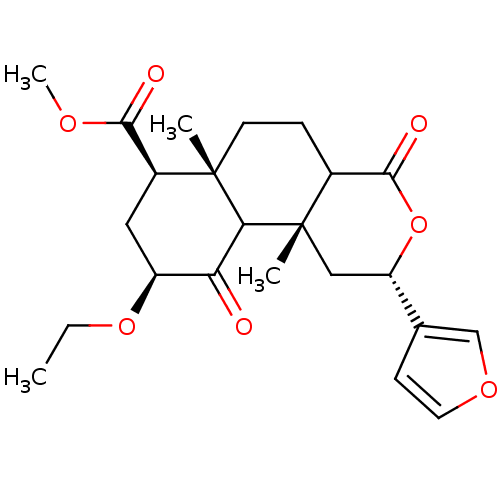 ((3S,4aR,6S,8R,8aR)-6-Ethoxy-3-furan-3-yl-4a,8a-dim...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McLean Hospital Curated by ChEMBL | Assay Description Inhibition of [3H]diprenorphine binding to human Opioid receptor kappa 1 expressed in chinese hamster ovary cells | Bioorg Med Chem Lett 15: 2761-5 (2005) Article DOI: 10.1016/j.bmcl.2005.03.113 BindingDB Entry DOI: 10.7270/Q2TD9Z3J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50528184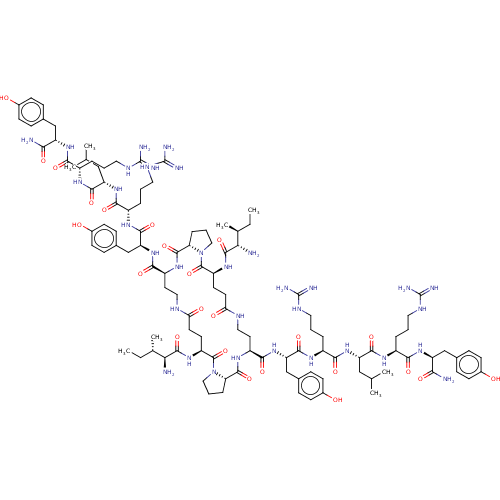 (CHEMBL4534028) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Displacement of (sCy5)-[Lys2 Arg4]-BVD15 from GFP-tagged Y1R in human HEK293T cells assessed as inhibitory constant incubated for 5 mins followed by ... | J Med Chem 63: 5274-5286 (2020) Article DOI: 10.1021/acs.jmedchem.0c00027 BindingDB Entry DOI: 10.7270/Q2RB782R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1132 total ) | Next | Last >> |