 Found 843 hits with Last Name = 'xiang' and Initial = 'r'
Found 843 hits with Last Name = 'xiang' and Initial = 'r' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Lysine-specific demethylase 4D
(Homo sapiens (Human)) | BDBM50574750

(CHEMBL4850758)Show SMILES CC(C)(C)OC(=O)N[C@@H]1CCN(C1)C(c1ccc(cc1)-c1ccncc1)c1cc2OCOc2cc1O |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 293 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Mixed inhibition of human KDM4D expressed in Escherichia coli BL21(DE3) using H3(1-21)K9me3 peptide and varying concentrations of 2-OG as substrate p... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113662
BindingDB Entry DOI: 10.7270/Q24T6P5X |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50021574
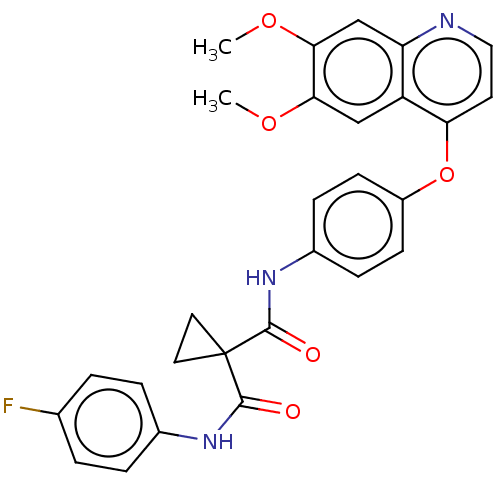
(BMS-907351 | CABOZANTINIB | CHEBI:72317 | Cabomety...)Show SMILES COc1cc2nccc(Oc3ccc(NC(=O)C4(CC4)C(=O)Nc4ccc(F)cc4)cc3)c2cc1OC Show InChI InChI=1S/C28H24FN3O5/c1-35-24-15-21-22(16-25(24)36-2)30-14-11-23(21)37-20-9-7-19(8-10-20)32-27(34)28(12-13-28)26(33)31-18-5-3-17(29)4-6-18/h3-11,14-16H,12-13H2,1-2H3,(H,31,33)(H,32,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Medicine, Nankai University, 94 Weijin Road, Tianjin 300071, China; Tianjin International S& T Cooperation Base, 94 Weijin Road, Tianjin 300071, China.
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 phosphorylation (unknown origin) |
Bioorg Med Chem Lett 27: 3231-3237 (2017)
Article DOI: 10.1016/j.bmcl.2017.06.041
BindingDB Entry DOI: 10.7270/Q2JS9SW3 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM13216

(BMS-354825 | CHEMBL1421 | DASATINIB | N-(2-Chloro-...)Show SMILES Cc1nc(Nc2ncc(s2)C(=O)Nc2c(C)cccc2Cl)cc(n1)N1CCN(CCO)CC1 Show InChI InChI=1S/C22H26ClN7O2S/c1-14-4-3-5-16(23)20(14)28-21(32)17-13-24-22(33-17)27-18-12-19(26-15(2)25-18)30-8-6-29(7-9-30)10-11-31/h3-5,12-13,31H,6-11H2,1-2H3,(H,28,32)(H,24,25,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Src |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50543551
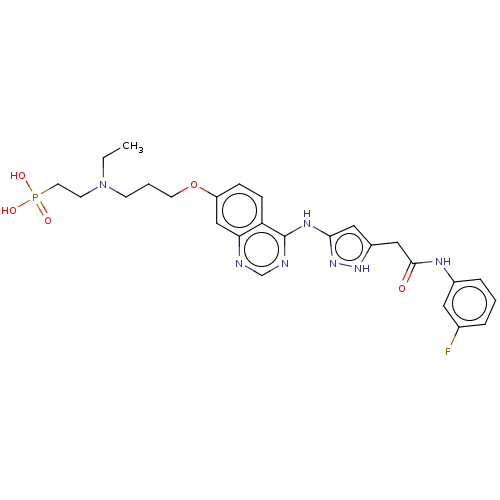
(CHEMBL4633715)Show SMILES CCN(CCCOc1ccc2c(Nc3cc(CC(=O)Nc4cccc(F)c4)[nH]n3)ncnc2c1)CCP(O)(O)=O Show InChI InChI=1S/C26H31FN7O5P/c1-2-34(10-12-40(36,37)38)9-4-11-39-21-7-8-22-23(16-21)28-17-29-26(22)31-24-14-20(32-33-24)15-25(35)30-19-6-3-5-18(27)13-19/h3,5-8,13-14,16-17H,2,4,9-12,15H2,1H3,(H,30,35)(H2,36,37,38)(H2,28,29,31,32,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B in human SW620 cells assessed as reduction in histone H3 phosphorylation on Ser10 by Alexafluor-488 goat anti-rabbit antibody/... |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2020.115351
BindingDB Entry DOI: 10.7270/Q2PC35XX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50244935

(CHEMBL4094507)Show SMILES CN(C)C(=O)c1cc2cnc(Nc3ccc(cc3)C(=O)NCCCCCC(=O)NO)nc2n1C1CCCC1 Show InChI InChI=1S/C27H35N7O4/c1-33(2)26(37)22-16-19-17-29-27(31-24(19)34(22)21-8-5-6-9-21)30-20-13-11-18(12-14-20)25(36)28-15-7-3-4-10-23(35)32-38/h11-14,16-17,21,38H,3-10,15H2,1-2H3,(H,28,36)(H,32,35)(H,29,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
Nankai University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) |
J Med Chem 61: 3166-3192 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00209
BindingDB Entry DOI: 10.7270/Q2KS6TZK |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50004205

(MK-045 | MK-0457 | TOZASERTIB | US9249124, VX680 |...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)[nH]n2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal His6-tagged Aurora A expressed in baculovirus expression system by enzyme coupled assay |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2020.115351
BindingDB Entry DOI: 10.7270/Q2PC35XX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Yes
(Homo sapiens (Human)) | BDBM50086441

(CHEMBL3426225 | US10266537, Compound 3)Show SMILES CCn1nc(C#Cc2cc(ccc2C)C(=O)Nc2ccc(CN3CCN(C)CC3)c(c2)C(F)(F)F)c2c(N)ncnc12 Show InChI InChI=1S/C30H31F3N8O/c1-4-41-28-26(27(34)35-18-36-28)25(38-41)10-8-20-15-21(6-5-19(20)2)29(42)37-23-9-7-22(24(16-23)30(31,32)33)17-40-13-11-39(3)12-14-40/h5-7,9,15-16,18H,4,11-14,17H2,1-3H3,(H,37,42)(H2,34,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Yes |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50244933
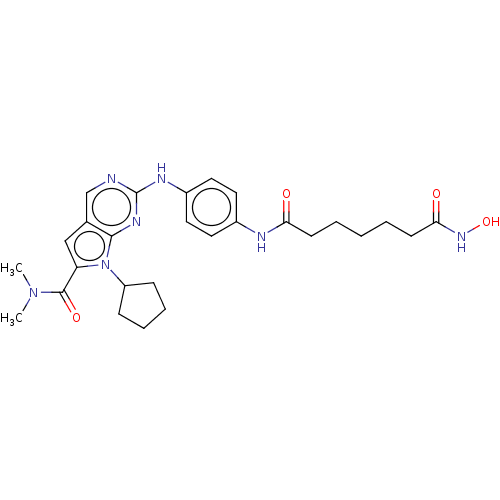
(CHEMBL4069404)Show SMILES CN(C)C(=O)c1cc2cnc(Nc3ccc(NC(=O)CCCCCC(=O)NO)cc3)nc2n1C1CCCC1 Show InChI InChI=1S/C27H35N7O4/c1-33(2)26(37)22-16-18-17-28-27(31-25(18)34(22)21-8-6-7-9-21)30-20-14-12-19(13-15-20)29-23(35)10-4-3-5-11-24(36)32-38/h12-17,21,38H,3-11H2,1-2H3,(H,29,35)(H,32,36)(H,28,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a |
Nankai University
Curated by ChEMBL
| Assay Description
Binding affinity at [3H]QNB and GppNHp radiolabeled muscarinic M2 receptor in rat heart. |
J Med Chem 61: 3166-3192 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00209
BindingDB Entry DOI: 10.7270/Q2KS6TZK |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50086441

(CHEMBL3426225 | US10266537, Compound 3)Show SMILES CCn1nc(C#Cc2cc(ccc2C)C(=O)Nc2ccc(CN3CCN(C)CC3)c(c2)C(F)(F)F)c2c(N)ncnc12 Show InChI InChI=1S/C30H31F3N8O/c1-4-41-28-26(27(34)35-18-36-28)25(38-41)10-8-20-15-21(6-5-19(20)2)29(42)37-23-9-7-22(24(16-23)30(31,32)33)17-40-13-11-39(3)12-14-40/h5-7,9,15-16,18H,4,11-14,17H2,1-3H3,(H,37,42)(H2,34,35,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Src |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50086454
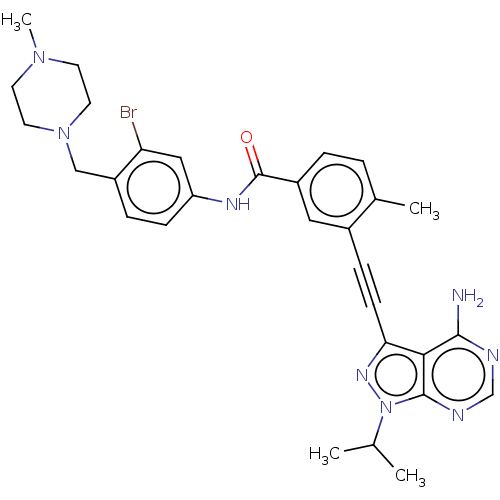
(CHEMBL3425518)Show SMILES CC(C)n1nc(C#Cc2cc(ccc2C)C(=O)Nc2ccc(CN3CCN(C)CC3)c(Br)c2)c2c(N)ncnc12 Show InChI InChI=1S/C30H33BrN8O/c1-19(2)39-29-27(28(32)33-18-34-29)26(36-39)10-8-21-15-22(6-5-20(21)3)30(40)35-24-9-7-23(25(31)16-24)17-38-13-11-37(4)12-14-38/h5-7,9,15-16,18-19H,11-14,17H2,1-4H3,(H,35,40)(H2,32,33,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Src |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Yes
(Homo sapiens (Human)) | BDBM378885

(US10266537, Compound 93)Show SMILES Cc1ccc(NC(=O)c2ccc(C)c(c2)C(F)(F)F)cc1C#Cc1nn([C@H]2CC[C@H](O)CC2)c2ncnc(N)c12 |r,wU:26.27,wD:29.31,(-5.46,5.4,;-3.98,5.8,;-3.58,7.29,;-2.09,7.69,;-1,6.6,;.49,7,;1.57,5.91,;1.18,4.42,;3.06,6.31,;3.46,7.79,;4.95,8.19,;6.04,7.1,;7.53,7.5,;5.64,5.62,;4.15,5.22,;6.73,4.53,;8.22,4.93,;6.33,3.04,;7.82,3.44,;-1.4,5.11,;-2.89,4.71,;-3.29,3.22,;-3.68,1.74,;-4.08,.25,;-3.18,-1,;-4.08,-2.24,;-3.68,-3.73,;-2.2,-4.13,;-1.8,-5.62,;-2.89,-6.7,;-2.49,-8.19,;-4.38,-6.31,;-4.77,-4.82,;-5.55,-1.77,;-6.88,-2.54,;-8.22,-1.77,;-8.22,-.23,;-6.88,.54,;-6.88,2.08,;-5.55,-.23,)| Show InChI InChI=1S/C29H27F3N6O2/c1-16-4-7-20(36-28(40)19-5-3-17(2)23(14-19)29(30,31)32)13-18(16)6-12-24-25-26(33)34-15-35-27(25)38(37-24)21-8-10-22(39)11-9-21/h3-5,7,13-15,21-22,39H,8-11H2,1-2H3,(H,36,40)(H2,33,34,35)/t21-,22- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human full length recombinant YES using poly(Glu,Tyr)4:1 as substrate incubated for 40 mins in presence of [gamma33P-ATP] by radiometri... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2M90DC6 |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50004205

(MK-045 | MK-0457 | TOZASERTIB | US9249124, VX680 |...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)[nH]n2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University
Curated by ChEMBL
| Assay Description
Inhibition of Aurora kinase A (unknown origin) incubated for 30 mins by Kinase Glo luminescent assay |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2020.115351
BindingDB Entry DOI: 10.7270/Q2PC35XX |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50277545

(4-(9-chloro-7-(2-fluoro-6-methoxyphenyl)-5H-benzo[...)Show SMILES COc1cc(Nc2ncc3CN=C(c4cc(Cl)ccc4-c3n2)c2c(F)cccc2OC)ccc1C(O)=O |c:11| Show InChI InChI=1S/C27H20ClFN4O4/c1-36-21-5-3-4-20(29)23(21)25-19-10-15(28)6-8-17(19)24-14(12-30-25)13-31-27(33-24)32-16-7-9-18(26(34)35)22(11-16)37-2/h3-11,13H,12H2,1-2H3,(H,34,35)(H,31,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Aurora A (unknown origin) by radioactive flashplate assay |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2020.115351
BindingDB Entry DOI: 10.7270/Q2PC35XX |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50244900
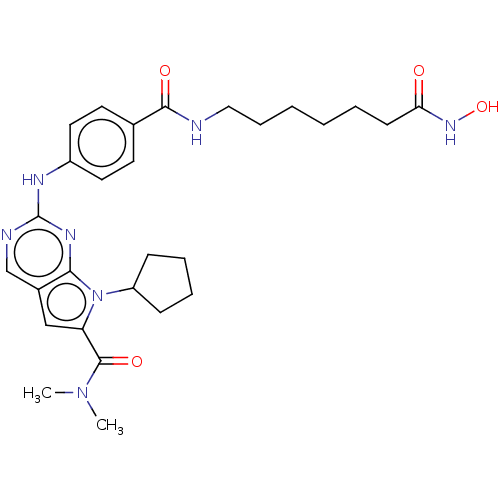
(CHEMBL4068308)Show SMILES CN(C)C(=O)c1cc2cnc(Nc3ccc(cc3)C(=O)NCCCCCCC(=O)NO)nc2n1C1CCCC1 Show InChI InChI=1S/C28H37N7O4/c1-34(2)27(38)23-17-20-18-30-28(32-25(20)35(23)22-9-6-7-10-22)31-21-14-12-19(13-15-21)26(37)29-16-8-4-3-5-11-24(36)33-39/h12-15,17-18,22,39H,3-11,16H2,1-2H3,(H,29,37)(H,33,36)(H,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Nankai University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) |
J Med Chem 61: 3166-3192 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00209
BindingDB Entry DOI: 10.7270/Q2KS6TZK |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50244881
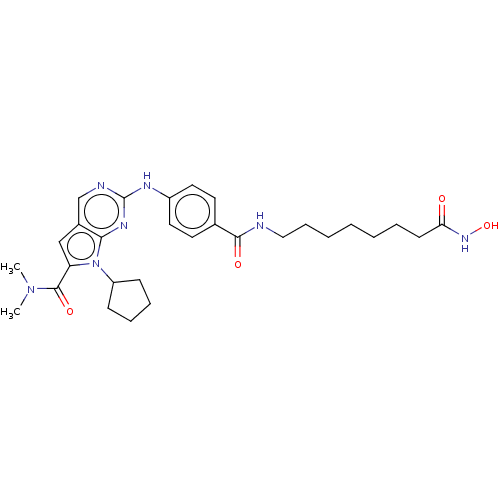
(CHEMBL4102249)Show SMILES CN(C)C(=O)c1cc2cnc(Nc3ccc(cc3)C(=O)NCCCCCCCC(=O)NO)nc2n1C1CCCC1 Show InChI InChI=1S/C29H39N7O4/c1-35(2)28(39)24-18-21-19-31-29(33-26(21)36(24)23-10-7-8-11-23)32-22-15-13-20(14-16-22)27(38)30-17-9-5-3-4-6-12-25(37)34-40/h13-16,18-19,23,40H,3-12,17H2,1-2H3,(H,30,38)(H,34,37)(H,31,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Nankai University
Curated by ChEMBL
| Assay Description
Binding affinity towards [3H]quipazine labeled 5-hydroxytryptamine 3 receptor sites in HG108-15 |
J Med Chem 61: 3166-3192 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00209
BindingDB Entry DOI: 10.7270/Q2KS6TZK |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50244849

(CHEMBL4083345)Show SMILES CN(C)C(=O)c1cc2cnc(Nc3ccc(NC(=O)NCCCCCC(=O)NO)cc3)nc2n1C1CCCC1 Show InChI InChI=1S/C27H36N8O4/c1-34(2)25(37)22-16-18-17-29-26(32-24(18)35(22)21-8-5-6-9-21)30-19-11-13-20(14-12-19)31-27(38)28-15-7-3-4-10-23(36)33-39/h11-14,16-17,21,39H,3-10,15H2,1-2H3,(H,33,36)(H2,28,31,38)(H,29,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Nankai University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) |
J Med Chem 61: 3166-3192 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00209
BindingDB Entry DOI: 10.7270/Q2KS6TZK |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase CLK1
(Homo sapiens (Human)) | BDBM50238867
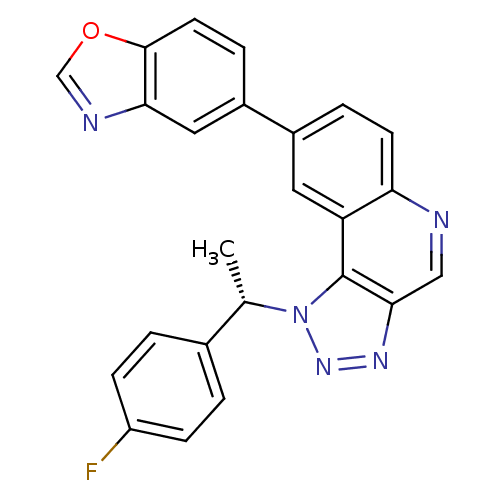
(CHEMBL4083249)Show SMILES C[C@@H](c1ccc(F)cc1)n1nnc2cnc3ccc(cc3c12)-c1ccc2ocnc2c1 |r| Show InChI InChI=1S/C24H16FN5O/c1-14(15-2-6-18(25)7-3-15)30-24-19-10-16(4-8-20(19)26-12-22(24)28-29-30)17-5-9-23-21(11-17)27-13-31-23/h2-14H,1H3/t14-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University and Collaborative Innovation Center for Biotherapy
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CLK1 expressed in insect cells using ERMRPRKRQGSVRRRV peptide as substrate after 40 mins in presence of [gamma-33P]-A... |
J Med Chem 60: 6337-6352 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00665
BindingDB Entry DOI: 10.7270/Q2ZW1P55 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dual specificity protein kinase CLK1
(Homo sapiens (Human)) | BDBM50238867

(CHEMBL4083249)Show SMILES C[C@@H](c1ccc(F)cc1)n1nnc2cnc3ccc(cc3c12)-c1ccc2ocnc2c1 |r| Show InChI InChI=1S/C24H16FN5O/c1-14(15-2-6-18(25)7-3-15)30-24-19-10-16(4-8-20(19)26-12-22(24)28-29-30)17-5-9-23-21(11-17)27-13-31-23/h2-14H,1H3/t14-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University and Collaborative Innovation Center for Biotherapy
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CLK1 expressed in insect cells using ERMRPRKRQGSVRRRV peptide as substrate after 40 mins in presence of [gamma-33P]-A... |
J Med Chem 60: 6337-6352 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00665
BindingDB Entry DOI: 10.7270/Q2ZW1P55 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50086451
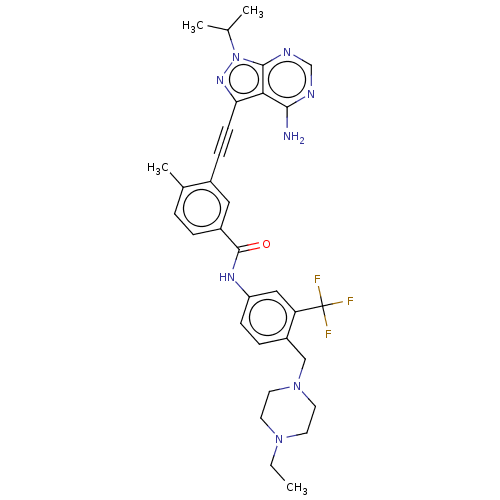
(CHEMBL3426222)Show SMILES CCN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3nn(C(C)C)c4ncnc(N)c34)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C32H35F3N8O/c1-5-41-12-14-42(15-13-41)18-24-8-10-25(17-26(24)32(33,34)35)39-31(44)23-7-6-21(4)22(16-23)9-11-27-28-29(36)37-19-38-30(28)43(40-27)20(2)3/h6-8,10,16-17,19-20H,5,12-15,18H2,1-4H3,(H,39,44)(H2,36,37,38) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Src |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50086455
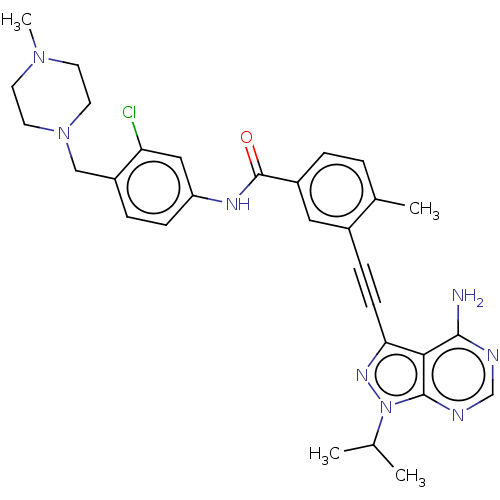
(CHEMBL3426219)Show SMILES CC(C)n1nc(C#Cc2cc(ccc2C)C(=O)Nc2ccc(CN3CCN(C)CC3)c(Cl)c2)c2c(N)ncnc12 Show InChI InChI=1S/C30H33ClN8O/c1-19(2)39-29-27(28(32)33-18-34-29)26(36-39)10-8-21-15-22(6-5-20(21)3)30(40)35-24-9-7-23(25(31)16-24)17-38-13-11-37(4)12-14-38/h5-7,9,15-16,18-19H,11-14,17H2,1-4H3,(H,35,40)(H2,32,33,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Src |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50086457
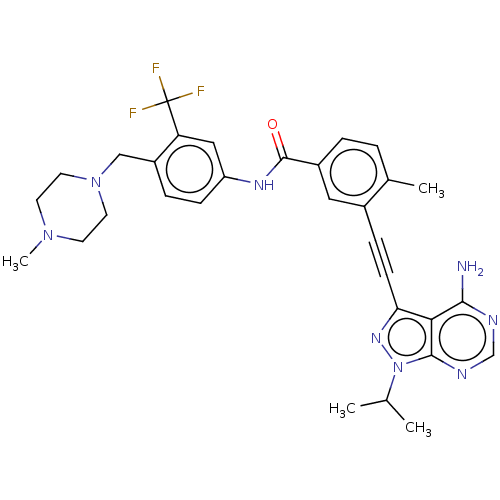
(CHEMBL3426217)Show SMILES CC(C)n1nc(C#Cc2cc(ccc2C)C(=O)Nc2ccc(CN3CCN(C)CC3)c(c2)C(F)(F)F)c2c(N)ncnc12 Show InChI InChI=1S/C31H33F3N8O/c1-19(2)42-29-27(28(35)36-18-37-29)26(39-42)10-8-21-15-22(6-5-20(21)3)30(43)38-24-9-7-23(25(16-24)31(32,33)34)17-41-13-11-40(4)12-14-41/h5-7,9,15-16,18-19H,11-14,17H2,1-4H3,(H,38,43)(H2,35,36,37) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Src |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM378885

(US10266537, Compound 93)Show SMILES Cc1ccc(NC(=O)c2ccc(C)c(c2)C(F)(F)F)cc1C#Cc1nn([C@H]2CC[C@H](O)CC2)c2ncnc(N)c12 |r,wU:26.27,wD:29.31,(-5.46,5.4,;-3.98,5.8,;-3.58,7.29,;-2.09,7.69,;-1,6.6,;.49,7,;1.57,5.91,;1.18,4.42,;3.06,6.31,;3.46,7.79,;4.95,8.19,;6.04,7.1,;7.53,7.5,;5.64,5.62,;4.15,5.22,;6.73,4.53,;8.22,4.93,;6.33,3.04,;7.82,3.44,;-1.4,5.11,;-2.89,4.71,;-3.29,3.22,;-3.68,1.74,;-4.08,.25,;-3.18,-1,;-4.08,-2.24,;-3.68,-3.73,;-2.2,-4.13,;-1.8,-5.62,;-2.89,-6.7,;-2.49,-8.19,;-4.38,-6.31,;-4.77,-4.82,;-5.55,-1.77,;-6.88,-2.54,;-8.22,-1.77,;-8.22,-.23,;-6.88,.54,;-6.88,2.08,;-5.55,-.23,)| Show InChI InChI=1S/C29H27F3N6O2/c1-16-4-7-20(36-28(40)19-5-3-17(2)23(14-19)29(30,31)32)13-18(16)6-12-24-25-26(33)34-15-35-27(25)38(37-24)21-8-10-22(39)11-9-21/h3-5,7,13-15,21-22,39H,8-11H2,1-2H3,(H,36,40)(H2,33,34,35)/t21-,22- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant ABL (27 to end residues) using EAIYAAPFAKKK as substrate incubated for 40 mins in presence of [gamma33P-ATP] by radio... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2M90DC6 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50533659
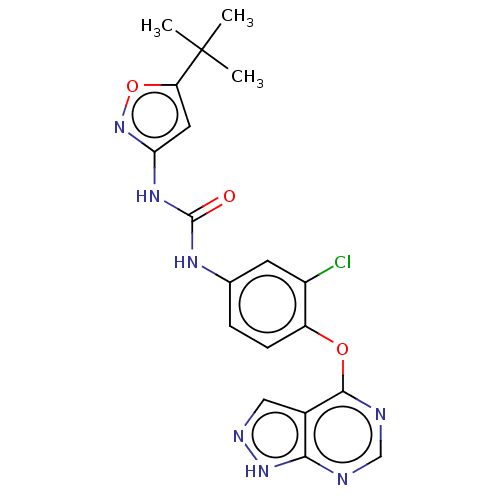
(CHEMBL4444648)Show SMILES CC(C)(C)c1cc(NC(=O)Nc2ccc(Oc3ncnc4[nH]ncc34)c(Cl)c2)no1 Show InChI InChI=1S/C19H18ClN7O3/c1-19(2,3)14-7-15(27-30-14)25-18(28)24-10-4-5-13(12(20)6-10)29-17-11-8-23-26-16(11)21-9-22-17/h4-9H,1-3H3,(H,21,22,23,26)(H2,24,25,27,28) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University/Collaborative Innovation Center of Biotherapy
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal GST-tagged FLT3 (564 to end residues) expressed by baculovirus in Sf21 insect cells measured after 10 mins... |
J Med Chem 59: 8293-305 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00604
BindingDB Entry DOI: 10.7270/Q20Z76SQ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50244953
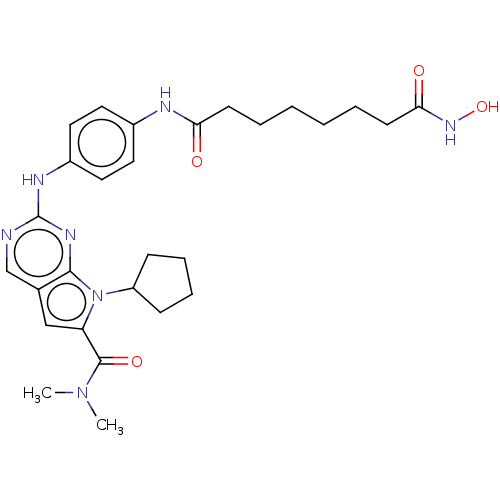
(CHEMBL4063500)Show SMILES CN(C)C(=O)c1cc2cnc(Nc3ccc(NC(=O)CCCCCCC(=O)NO)cc3)nc2n1C1CCCC1 Show InChI InChI=1S/C28H37N7O4/c1-34(2)27(38)23-17-19-18-29-28(32-26(19)35(23)22-9-7-8-10-22)31-21-15-13-20(14-16-21)30-24(36)11-5-3-4-6-12-25(37)33-39/h13-18,22,39H,3-12H2,1-2H3,(H,30,36)(H,33,37)(H,29,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Nankai University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) |
J Med Chem 61: 3166-3192 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00209
BindingDB Entry DOI: 10.7270/Q2KS6TZK |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50244826

(CHEMBL4074698)Show SMILES ONC(=O)CCCCCCC(=O)Nc1ccc(Nc2ncc3ncn(C4CCCC4)c3n2)cc1 Show InChI InChI=1S/C24H31N7O3/c32-21(9-3-1-2-4-10-22(33)30-34)27-17-11-13-18(14-12-17)28-24-25-15-20-23(29-24)31(16-26-20)19-7-5-6-8-19/h11-16,19,34H,1-10H2,(H,27,32)(H,30,33)(H,25,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Nankai University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) |
J Med Chem 61: 3166-3192 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00209
BindingDB Entry DOI: 10.7270/Q2KS6TZK |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50322535

(3-(imidazo[1,2-b]pyridazin-3-ylethynyl)-4-methyl-N...)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3cnc4cccnn34)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C29H27F3N6O/c1-20-5-6-22(16-21(20)8-10-25-18-33-27-4-3-11-34-38(25)27)28(39)35-24-9-7-23(26(17-24)29(30,31)32)19-37-14-12-36(2)13-15-37/h3-7,9,11,16-18H,12-15,19H2,1-2H3,(H,35,39) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Src |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50086453

(CHEMBL3426220 | US10266537, Compound 14)Show SMILES CC(C)n1nc(C#Cc2cc(ccc2C)C(=O)Nc2cccc(c2)C(F)(F)F)c2c(N)ncnc12 Show InChI InChI=1S/C25H21F3N6O/c1-14(2)34-23-21(22(29)30-13-31-23)20(33-34)10-9-16-11-17(8-7-15(16)3)24(35)32-19-6-4-5-18(12-19)25(26,27)28/h4-8,11-14H,1-3H3,(H,32,35)(H2,29,30,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Src |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50244956

(CHEMBL4091353)Show SMILES CN(C)C(=O)c1cc2cnc(Nc3ccc(NC(=O)CCCCCCCC(=O)NO)cc3)nc2n1C1CCCC1 Show InChI InChI=1S/C29H39N7O4/c1-35(2)28(39)24-18-20-19-30-29(33-27(20)36(24)23-10-8-9-11-23)32-22-16-14-21(15-17-22)31-25(37)12-6-4-3-5-7-13-26(38)34-40/h14-19,23,40H,3-13H2,1-2H3,(H,31,37)(H,34,38)(H,30,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nankai University
Curated by ChEMBL
| Assay Description
Binding affinity towards [3H]quipazine labeled 5-hydroxytryptamine 3 receptor sites in HG108-15 |
J Med Chem 61: 3166-3192 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00209
BindingDB Entry DOI: 10.7270/Q2KS6TZK |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50243924

(CHEMBL4075931)Show SMILES NC(=O)CN1CCC(CC1)n1c2c(cnc3ccc(cc23)-c2ccc(N)nc2)ncc1=O Show InChI InChI=1S/C23H23N7O2/c24-20-4-2-15(10-28-20)14-1-3-18-17(9-14)23-19(11-26-18)27-12-22(32)30(23)16-5-7-29(8-6-16)13-21(25)31/h1-4,9-12,16H,5-8,13H2,(H2,24,28)(H2,25,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nankai University
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal FLAG-tagged recombinant human mTOR (1362 to end residues) |
J Med Chem 61: 881-904 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01402
BindingDB Entry DOI: 10.7270/Q28K7CHW |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50243953

(CHEMBL4101340)Show SMILES Fc1cc2ncc3ncc(=O)n(C4CCN(CC#N)CC4)c3c2cc1-c1cn[nH]c1 Show InChI InChI=1S/C21H18FN7O/c22-17-8-18-16(7-15(17)13-9-26-27-10-13)21-19(11-24-18)25-12-20(30)29(21)14-1-4-28(5-2-14)6-3-23/h7-12,14H,1-2,4-6H2,(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nankai University
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal FLAG-tagged recombinant human mTOR (1362 to end residues) |
J Med Chem 61: 881-904 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01402
BindingDB Entry DOI: 10.7270/Q28K7CHW |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50243948

(CHEMBL4096248)Show SMILES O=c1cnc2cnc3ccc(cc3c2n1C1CCN(CC#N)CC1)-c1cnc2ccccc2c1 Show InChI InChI=1S/C27H22N6O/c28-9-12-32-10-7-21(8-11-32)33-26(34)17-31-25-16-30-24-6-5-18(14-22(24)27(25)33)20-13-19-3-1-2-4-23(19)29-15-20/h1-6,13-17,21H,7-8,10-12H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nankai University
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal FLAG-tagged recombinant human mTOR (1362 to end residues) |
J Med Chem 61: 881-904 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01402
BindingDB Entry DOI: 10.7270/Q28K7CHW |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM378885

(US10266537, Compound 93)Show SMILES Cc1ccc(NC(=O)c2ccc(C)c(c2)C(F)(F)F)cc1C#Cc1nn([C@H]2CC[C@H](O)CC2)c2ncnc(N)c12 |r,wU:26.27,wD:29.31,(-5.46,5.4,;-3.98,5.8,;-3.58,7.29,;-2.09,7.69,;-1,6.6,;.49,7,;1.57,5.91,;1.18,4.42,;3.06,6.31,;3.46,7.79,;4.95,8.19,;6.04,7.1,;7.53,7.5,;5.64,5.62,;4.15,5.22,;6.73,4.53,;8.22,4.93,;6.33,3.04,;7.82,3.44,;-1.4,5.11,;-2.89,4.71,;-3.29,3.22,;-3.68,1.74,;-4.08,.25,;-3.18,-1,;-4.08,-2.24,;-3.68,-3.73,;-2.2,-4.13,;-1.8,-5.62,;-2.89,-6.7,;-2.49,-8.19,;-4.38,-6.31,;-4.77,-4.82,;-5.55,-1.77,;-6.88,-2.54,;-8.22,-1.77,;-8.22,-.23,;-6.88,.54,;-6.88,2.08,;-5.55,-.23,)| Show InChI InChI=1S/C29H27F3N6O2/c1-16-4-7-20(36-28(40)19-5-3-17(2)23(14-19)29(30,31)32)13-18(16)6-12-24-25-26(33)34-15-35-27(25)38(37-24)21-8-10-22(39)11-9-21/h3-5,7,13-15,21-22,39H,8-11H2,1-2H3,(H,36,40)(H2,33,34,35)/t21-,22- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human full length recombinant Src using Cdc2 peptide as substrate incubated for 40 mins in presence of [gamma33P-ATP] by radiometric sc... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2M90DC6 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase HCK
(Homo sapiens (Human)) | BDBM378885

(US10266537, Compound 93)Show SMILES Cc1ccc(NC(=O)c2ccc(C)c(c2)C(F)(F)F)cc1C#Cc1nn([C@H]2CC[C@H](O)CC2)c2ncnc(N)c12 |r,wU:26.27,wD:29.31,(-5.46,5.4,;-3.98,5.8,;-3.58,7.29,;-2.09,7.69,;-1,6.6,;.49,7,;1.57,5.91,;1.18,4.42,;3.06,6.31,;3.46,7.79,;4.95,8.19,;6.04,7.1,;7.53,7.5,;5.64,5.62,;4.15,5.22,;6.73,4.53,;8.22,4.93,;6.33,3.04,;7.82,3.44,;-1.4,5.11,;-2.89,4.71,;-3.29,3.22,;-3.68,1.74,;-4.08,.25,;-3.18,-1,;-4.08,-2.24,;-3.68,-3.73,;-2.2,-4.13,;-1.8,-5.62,;-2.89,-6.7,;-2.49,-8.19,;-4.38,-6.31,;-4.77,-4.82,;-5.55,-1.77,;-6.88,-2.54,;-8.22,-1.77,;-8.22,-.23,;-6.88,.54,;-6.88,2.08,;-5.55,-.23,)| Show InChI InChI=1S/C29H27F3N6O2/c1-16-4-7-20(36-28(40)19-5-3-17(2)23(14-19)29(30,31)32)13-18(16)6-12-24-25-26(33)34-15-35-27(25)38(37-24)21-8-10-22(39)11-9-21/h3-5,7,13-15,21-22,39H,8-11H2,1-2H3,(H,36,40)(H2,33,34,35)/t21-,22- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human HCK (230 to 497 residues) using GGMEDIYFEFMGGKKK as substrate incubated for 40 mins in presence of [gamma33P-ATP] by radiometric ... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2M90DC6 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM378885

(US10266537, Compound 93)Show SMILES Cc1ccc(NC(=O)c2ccc(C)c(c2)C(F)(F)F)cc1C#Cc1nn([C@H]2CC[C@H](O)CC2)c2ncnc(N)c12 |r,wU:26.27,wD:29.31,(-5.46,5.4,;-3.98,5.8,;-3.58,7.29,;-2.09,7.69,;-1,6.6,;.49,7,;1.57,5.91,;1.18,4.42,;3.06,6.31,;3.46,7.79,;4.95,8.19,;6.04,7.1,;7.53,7.5,;5.64,5.62,;4.15,5.22,;6.73,4.53,;8.22,4.93,;6.33,3.04,;7.82,3.44,;-1.4,5.11,;-2.89,4.71,;-3.29,3.22,;-3.68,1.74,;-4.08,.25,;-3.18,-1,;-4.08,-2.24,;-3.68,-3.73,;-2.2,-4.13,;-1.8,-5.62,;-2.89,-6.7,;-2.49,-8.19,;-4.38,-6.31,;-4.77,-4.82,;-5.55,-1.77,;-6.88,-2.54,;-8.22,-1.77,;-8.22,-.23,;-6.88,.54,;-6.88,2.08,;-5.55,-.23,)| Show InChI InChI=1S/C29H27F3N6O2/c1-16-4-7-20(36-28(40)19-5-3-17(2)23(14-19)29(30,31)32)13-18(16)6-12-24-25-26(33)34-15-35-27(25)38(37-24)21-8-10-22(39)11-9-21/h3-5,7,13-15,21-22,39H,8-11H2,1-2H3,(H,36,40)(H2,33,34,35)/t21-,22- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant ABL T315I mutant (27 to end residues) using EAIYAAPFAKKK as substrate incubated for 40 mins in presence of [gamma33P-... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2M90DC6 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50244883
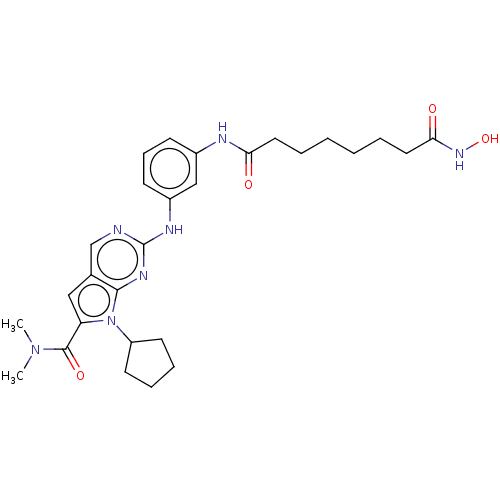
(CHEMBL4067088)Show SMILES CN(C)C(=O)c1cc2cnc(Nc3cccc(NC(=O)CCCCCCC(=O)NO)c3)nc2n1C1CCCC1 Show InChI InChI=1S/C28H37N7O4/c1-34(2)27(38)23-16-19-18-29-28(32-26(19)35(23)22-12-7-8-13-22)31-21-11-9-10-20(17-21)30-24(36)14-5-3-4-6-15-25(37)33-39/h9-11,16-18,22,39H,3-8,12-15H2,1-2H3,(H,30,36)(H,33,37)(H,29,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Nankai University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) |
J Med Chem 61: 3166-3192 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00209
BindingDB Entry DOI: 10.7270/Q2KS6TZK |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50086450
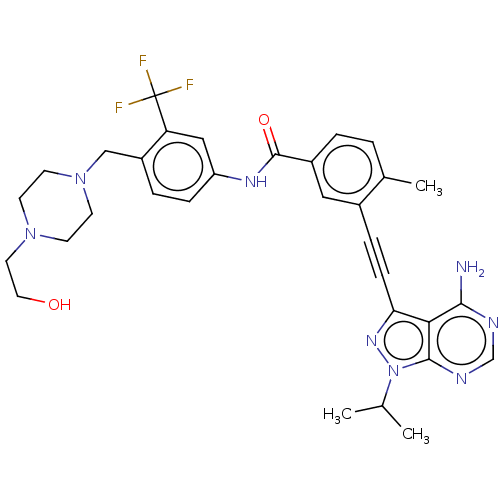
(CHEMBL3426223)Show SMILES CC(C)n1nc(C#Cc2cc(ccc2C)C(=O)Nc2ccc(CN3CCN(CCO)CC3)c(c2)C(F)(F)F)c2c(N)ncnc12 Show InChI InChI=1S/C32H35F3N8O2/c1-20(2)43-30-28(29(36)37-19-38-30)27(40-43)9-7-22-16-23(5-4-21(22)3)31(45)39-25-8-6-24(26(17-25)32(33,34)35)18-42-12-10-41(11-13-42)14-15-44/h4-6,8,16-17,19-20,44H,10-15,18H2,1-3H3,(H,39,45)(H2,36,37,38) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Src |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50086456

(CHEMBL3426218 | US10266537, Compound 17)Show SMILES CC(C)n1nc(C#Cc2cc(ccc2C)C(=O)Nc2ccc(CN3CCN(C)CC3)cc2)c2c(N)ncnc12 Show InChI InChI=1S/C30H34N8O/c1-20(2)38-29-27(28(31)32-19-33-29)26(35-38)12-9-23-17-24(8-5-21(23)3)30(39)34-25-10-6-22(7-11-25)18-37-15-13-36(4)14-16-37/h5-8,10-11,17,19-20H,13-16,18H2,1-4H3,(H,34,39)(H2,31,32,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Src |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50243973
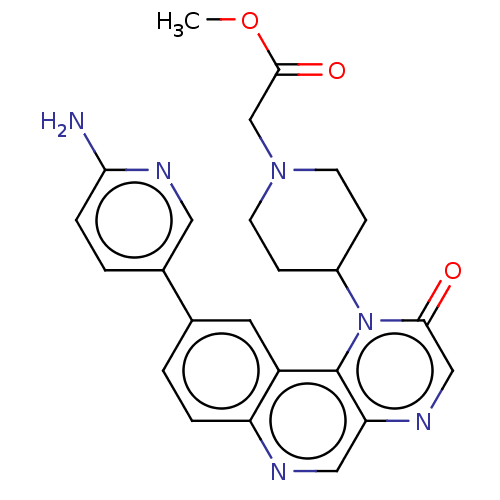
(CHEMBL4064699)Show SMILES COC(=O)CN1CCC(CC1)n1c2c(cnc3ccc(cc23)-c2ccc(N)nc2)ncc1=O Show InChI InChI=1S/C24H24N6O3/c1-33-23(32)14-29-8-6-17(7-9-29)30-22(31)13-27-20-12-26-19-4-2-15(10-18(19)24(20)30)16-3-5-21(25)28-11-16/h2-5,10-13,17H,6-9,14H2,1H3,(H2,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nankai University
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal FLAG-tagged recombinant human mTOR (1362 to end residues) |
J Med Chem 61: 881-904 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01402
BindingDB Entry DOI: 10.7270/Q28K7CHW |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM378885

(US10266537, Compound 93)Show SMILES Cc1ccc(NC(=O)c2ccc(C)c(c2)C(F)(F)F)cc1C#Cc1nn([C@H]2CC[C@H](O)CC2)c2ncnc(N)c12 |r,wU:26.27,wD:29.31,(-5.46,5.4,;-3.98,5.8,;-3.58,7.29,;-2.09,7.69,;-1,6.6,;.49,7,;1.57,5.91,;1.18,4.42,;3.06,6.31,;3.46,7.79,;4.95,8.19,;6.04,7.1,;7.53,7.5,;5.64,5.62,;4.15,5.22,;6.73,4.53,;8.22,4.93,;6.33,3.04,;7.82,3.44,;-1.4,5.11,;-2.89,4.71,;-3.29,3.22,;-3.68,1.74,;-4.08,.25,;-3.18,-1,;-4.08,-2.24,;-3.68,-3.73,;-2.2,-4.13,;-1.8,-5.62,;-2.89,-6.7,;-2.49,-8.19,;-4.38,-6.31,;-4.77,-4.82,;-5.55,-1.77,;-6.88,-2.54,;-8.22,-1.77,;-8.22,-.23,;-6.88,.54,;-6.88,2.08,;-5.55,-.23,)| Show InChI InChI=1S/C29H27F3N6O2/c1-16-4-7-20(36-28(40)19-5-3-17(2)23(14-19)29(30,31)32)13-18(16)6-12-24-25-26(33)34-15-35-27(25)38(37-24)21-8-10-22(39)11-9-21/h3-5,7,13-15,21-22,39H,8-11H2,1-2H3,(H,36,40)(H2,33,34,35)/t21-,22- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant RET (658 to end residues) using KKKSPGEYVNIEFG as substrate incubated for 40 mins in presence of [gamma33P-ATP] by ra... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2M90DC6 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50243960

(CHEMBL4083691)Show SMILES NC(=O)N1CCC(CC1)n1c2c(cnc3ccc(cc23)-c2ccc(N)nc2)ncc1=O Show InChI InChI=1S/C22H21N7O2/c23-19-4-2-14(10-27-19)13-1-3-17-16(9-13)21-18(11-25-17)26-12-20(30)29(21)15-5-7-28(8-6-15)22(24)31/h1-4,9-12,15H,5-8H2,(H2,23,27)(H2,24,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nankai University
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal FLAG-tagged recombinant human mTOR (1362 to end residues) |
J Med Chem 61: 881-904 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01402
BindingDB Entry DOI: 10.7270/Q28K7CHW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lyn
(Homo sapiens (Human)) | BDBM378885

(US10266537, Compound 93)Show SMILES Cc1ccc(NC(=O)c2ccc(C)c(c2)C(F)(F)F)cc1C#Cc1nn([C@H]2CC[C@H](O)CC2)c2ncnc(N)c12 |r,wU:26.27,wD:29.31,(-5.46,5.4,;-3.98,5.8,;-3.58,7.29,;-2.09,7.69,;-1,6.6,;.49,7,;1.57,5.91,;1.18,4.42,;3.06,6.31,;3.46,7.79,;4.95,8.19,;6.04,7.1,;7.53,7.5,;5.64,5.62,;4.15,5.22,;6.73,4.53,;8.22,4.93,;6.33,3.04,;7.82,3.44,;-1.4,5.11,;-2.89,4.71,;-3.29,3.22,;-3.68,1.74,;-4.08,.25,;-3.18,-1,;-4.08,-2.24,;-3.68,-3.73,;-2.2,-4.13,;-1.8,-5.62,;-2.89,-6.7,;-2.49,-8.19,;-4.38,-6.31,;-4.77,-4.82,;-5.55,-1.77,;-6.88,-2.54,;-8.22,-1.77,;-8.22,-.23,;-6.88,.54,;-6.88,2.08,;-5.55,-.23,)| Show InChI InChI=1S/C29H27F3N6O2/c1-16-4-7-20(36-28(40)19-5-3-17(2)23(14-19)29(30,31)32)13-18(16)6-12-24-25-26(33)34-15-35-27(25)38(37-24)21-8-10-22(39)11-9-21/h3-5,7,13-15,21-22,39H,8-11H2,1-2H3,(H,36,40)(H2,33,34,35)/t21-,22- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human full length recombinant LYN using poly(Glu,Tyr)4:1 as substrate incubated for 40 mins in presence of [gamma33P-ATP] by radiometri... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2M90DC6 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL2
(Homo sapiens (Human)) | BDBM378885

(US10266537, Compound 93)Show SMILES Cc1ccc(NC(=O)c2ccc(C)c(c2)C(F)(F)F)cc1C#Cc1nn([C@H]2CC[C@H](O)CC2)c2ncnc(N)c12 |r,wU:26.27,wD:29.31,(-5.46,5.4,;-3.98,5.8,;-3.58,7.29,;-2.09,7.69,;-1,6.6,;.49,7,;1.57,5.91,;1.18,4.42,;3.06,6.31,;3.46,7.79,;4.95,8.19,;6.04,7.1,;7.53,7.5,;5.64,5.62,;4.15,5.22,;6.73,4.53,;8.22,4.93,;6.33,3.04,;7.82,3.44,;-1.4,5.11,;-2.89,4.71,;-3.29,3.22,;-3.68,1.74,;-4.08,.25,;-3.18,-1,;-4.08,-2.24,;-3.68,-3.73,;-2.2,-4.13,;-1.8,-5.62,;-2.89,-6.7,;-2.49,-8.19,;-4.38,-6.31,;-4.77,-4.82,;-5.55,-1.77,;-6.88,-2.54,;-8.22,-1.77,;-8.22,-.23,;-6.88,.54,;-6.88,2.08,;-5.55,-.23,)| Show InChI InChI=1S/C29H27F3N6O2/c1-16-4-7-20(36-28(40)19-5-3-17(2)23(14-19)29(30,31)32)13-18(16)6-12-24-25-26(33)34-15-35-27(25)38(37-24)21-8-10-22(39)11-9-21/h3-5,7,13-15,21-22,39H,8-11H2,1-2H3,(H,36,40)(H2,33,34,35)/t21-,22- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant ARG (38 to end residues) using EAIYAAPFAKKK as substrate incubated for 40 mins in presence of [gamma33P-ATP] by radio... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2M90DC6 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D3
(Homo sapiens (Human)) | BDBM50244937
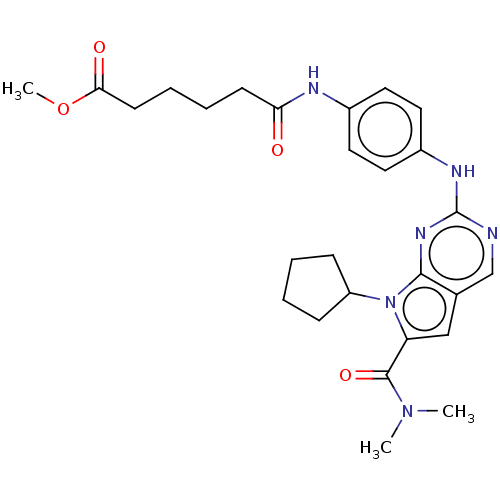
(CHEMBL4104221)Show SMILES COC(=O)CCCCC(=O)Nc1ccc(Nc2ncc3cc(C(=O)N(C)C)n(C4CCCC4)c3n2)cc1 Show InChI InChI=1S/C27H34N6O4/c1-32(2)26(36)22-16-18-17-28-27(31-25(18)33(22)21-8-4-5-9-21)30-20-14-12-19(13-15-20)29-23(34)10-6-7-11-24(35)37-3/h12-17,21H,4-11H2,1-3H3,(H,29,34)(H,28,30,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Nankai University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human full length N-terminal GST-tagged CDK4/Cyclin-D3 co-expressed in baculovirus infected sf21 cells using Rb substrate i... |
J Med Chem 61: 3166-3192 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00209
BindingDB Entry DOI: 10.7270/Q2KS6TZK |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Fyn
(Homo sapiens (Human)) | BDBM50086441

(CHEMBL3426225 | US10266537, Compound 3)Show SMILES CCn1nc(C#Cc2cc(ccc2C)C(=O)Nc2ccc(CN3CCN(C)CC3)c(c2)C(F)(F)F)c2c(N)ncnc12 Show InChI InChI=1S/C30H31F3N8O/c1-4-41-28-26(27(34)35-18-36-28)25(38-41)10-8-20-15-21(6-5-19(20)2)29(42)37-23-9-7-22(24(16-23)30(31,32)33)17-40-13-11-39(3)12-14-40/h5-7,9,15-16,18H,4,11-14,17H2,1-3H3,(H,37,42)(H2,34,35,36) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Fyn |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50533653
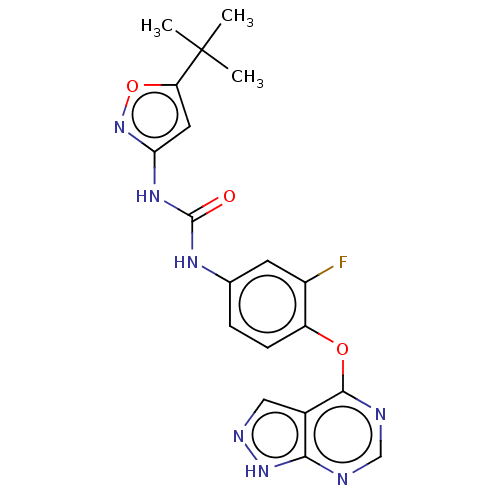
(CHEMBL4519741)Show SMILES CC(C)(C)c1cc(NC(=O)Nc2ccc(Oc3ncnc4[nH]ncc34)c(F)c2)no1 Show InChI InChI=1S/C19H18FN7O3/c1-19(2,3)14-7-15(27-30-14)25-18(28)24-10-4-5-13(12(20)6-10)29-17-11-8-23-26-16(11)21-9-22-17/h4-9H,1-3H3,(H,21,22,23,26)(H2,24,25,27,28) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University/Collaborative Innovation Center of Biotherapy
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal GST-tagged FLT3 (564 to end residues) expressed by baculovirus in Sf21 insect cells measured after 10 mins... |
J Med Chem 59: 8293-305 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00604
BindingDB Entry DOI: 10.7270/Q20Z76SQ |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50086586

(CHEMBL3426234 | US10266537, Compound 29)Show SMILES CCn1nc(C#Cc2cccc(c2)C(=O)Nc2ccc(CN3CCN(C)CC3)c(c2)C(F)(F)F)c2c(N)ncnc12 Show InChI InChI=1S/C29H29F3N8O/c1-3-40-27-25(26(33)34-18-35-27)24(37-40)10-7-19-5-4-6-20(15-19)28(41)36-22-9-8-21(23(16-22)29(30,31)32)17-39-13-11-38(2)12-14-39/h4-6,8-9,15-16,18H,3,11-14,17H2,1-2H3,(H,36,41)(H2,33,34,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Src |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase CLK1
(Homo sapiens (Human)) | BDBM50238882
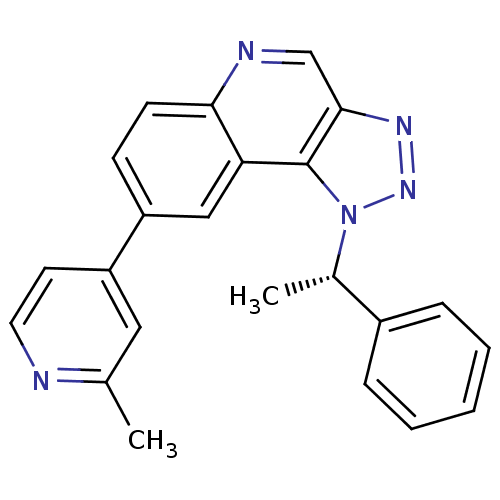
(CHEMBL4094600)Show SMILES C[C@@H](c1ccccc1)n1nnc2cnc3ccc(cc3c12)-c1ccnc(C)c1 |r| Show InChI InChI=1S/C23H19N5/c1-15-12-19(10-11-24-15)18-8-9-21-20(13-18)23-22(14-25-21)26-27-28(23)16(2)17-6-4-3-5-7-17/h3-14,16H,1-2H3/t16-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University and Collaborative Innovation Center for Biotherapy
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CLK1 expressed in insect cells using ERMRPRKRQGSVRRRV peptide as substrate after 40 mins in presence of [gamma-33P]-A... |
J Med Chem 60: 6337-6352 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00665
BindingDB Entry DOI: 10.7270/Q2ZW1P55 |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase CLK1
(Homo sapiens (Human)) | BDBM50238882

(CHEMBL4094600)Show SMILES C[C@@H](c1ccccc1)n1nnc2cnc3ccc(cc3c12)-c1ccnc(C)c1 |r| Show InChI InChI=1S/C23H19N5/c1-15-12-19(10-11-24-15)18-8-9-21-20(13-18)23-22(14-25-21)26-27-28(23)16(2)17-6-4-3-5-7-17/h3-14,16H,1-2H3/t16-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University and Collaborative Innovation Center for Biotherapy
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CLK1 expressed in insect cells using ERMRPRKRQGSVRRRV peptide as substrate after 40 mins in presence of [gamma-33P]-A... |
J Med Chem 60: 6337-6352 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00665
BindingDB Entry DOI: 10.7270/Q2ZW1P55 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50533672
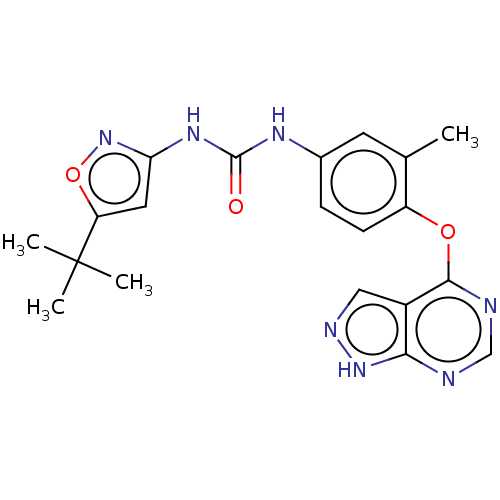
(CHEMBL4448466)Show SMILES Cc1cc(NC(=O)Nc2cc(on2)C(C)(C)C)ccc1Oc1ncnc2[nH]ncc12 Show InChI InChI=1S/C20H21N7O3/c1-11-7-12(24-19(28)25-16-8-15(30-27-16)20(2,3)4)5-6-14(11)29-18-13-9-23-26-17(13)21-10-22-18/h5-10H,1-4H3,(H,21,22,23,26)(H2,24,25,27,28) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University/Collaborative Innovation Center of Biotherapy
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal GST-tagged FLT3 (564 to end residues) expressed by baculovirus in Sf21 insect cells measured after 10 mins... |
J Med Chem 59: 8293-305 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00604
BindingDB Entry DOI: 10.7270/Q20Z76SQ |
More data for this
Ligand-Target Pair | |
Cyclin-T1/Cyclin-dependent kinase 9
(Homo sapiens (Human)) | BDBM50519562

(CHEMBL4592737)Show SMILES CN(C)C(=O)c1cc2cnc(Nc3ccc(cc3F)C(=O)NCCCCN=C=S)nc2n1C1CCCC1 Show InChI InChI=1S/C26H30FN7O2S/c1-33(2)25(36)22-14-18-15-30-26(32-23(18)34(22)19-7-3-4-8-19)31-21-10-9-17(13-20(21)27)24(35)29-12-6-5-11-28-16-37/h9-10,13-15,19H,3-8,11-12H2,1-2H3,(H,29,35)(H,30,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Nankai University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full-length human CDK9/cyclinT1 using KTFCGTPEYLAPEVRREPRILSEEEQEMFRDFDYIADWC peptide as substrate measured after 40 mins i... |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.07.038
BindingDB Entry DOI: 10.7270/Q25B05VW |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data