

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Botulinum neurotoxin type A2 [1-425] (Clostridium botulinum) | BDBM23274 ((2E)-3-(2,4-dichlorophenyl)-N-hydroxyprop-2-enamid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Absolute Science, Inc. | Assay Description Reactions between recombinant BoNTA-LC and fluorescent peptide substrate were carried out in 96-well microplates. Reaction progress was measured cont... | Bioorg Med Chem 17: 3072-9 (2009) Article DOI: 10.1016/j.bmc.2009.03.013 BindingDB Entry DOI: 10.7270/Q2G44NMS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Botulinum neurotoxin type A2 [1-425] (Clostridium botulinum) | BDBM28913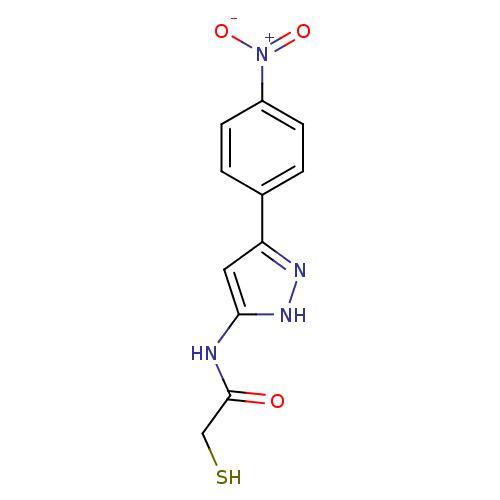 (2-mercaptoacetamide, 12l | N-[3-(4-nitrophenyl)-1H...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Absolute Science, Inc. | Assay Description Reactions between recombinant BoNTA-LC and fluorescent peptide substrate were carried out in 96-well microplates. Reaction progress was measured cont... | Bioorg Med Chem 17: 3072-9 (2009) Article DOI: 10.1016/j.bmc.2009.03.013 BindingDB Entry DOI: 10.7270/Q2G44NMS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A2 [1-425] (Clostridium botulinum) | BDBM28906 (2-mercaptoacetamide, 12a | N-[3-(4-chlorophenyl)-1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Absolute Science, Inc. | Assay Description Reactions between recombinant BoNTA-LC and fluorescent peptide substrate were carried out in 96-well microplates. Reaction progress was measured cont... | Bioorg Med Chem 17: 3072-9 (2009) Article DOI: 10.1016/j.bmc.2009.03.013 BindingDB Entry DOI: 10.7270/Q2G44NMS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Botulinum neurotoxin type A2 [1-425] (Clostridium botulinum) | BDBM28917 (2-mercaptoacetamide, 12g | N-[3-(3-chlorophenyl)-1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Absolute Science, Inc. | Assay Description Reactions between recombinant BoNTA-LC and fluorescent peptide substrate were carried out in 96-well microplates. Reaction progress was measured cont... | Bioorg Med Chem 17: 3072-9 (2009) Article DOI: 10.1016/j.bmc.2009.03.013 BindingDB Entry DOI: 10.7270/Q2G44NMS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A2 [1-425] (Clostridium botulinum) | BDBM28911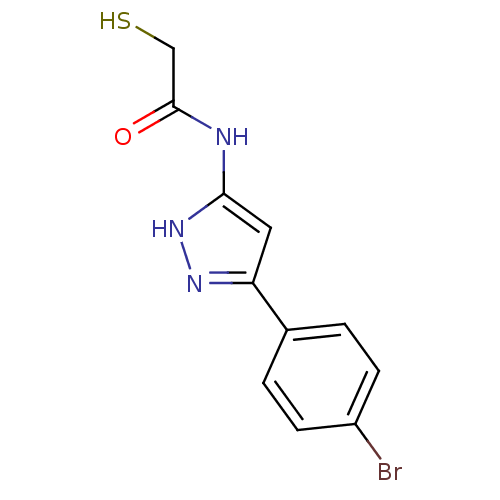 (2-mercaptoacetamide, 12i | N-[3-(4-bromophenyl)-1H...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.60E+3 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Absolute Science, Inc. | Assay Description Reactions between recombinant BoNTA-LC and fluorescent peptide substrate were carried out in 96-well microplates. Reaction progress was measured cont... | Bioorg Med Chem 17: 3072-9 (2009) Article DOI: 10.1016/j.bmc.2009.03.013 BindingDB Entry DOI: 10.7270/Q2G44NMS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A2 [1-425] (Clostridium botulinum) | BDBM28918 (2-mercaptoacetamide, 12f | 2-sulfanyl-N-{3-[2-(tri...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.20E+3 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Absolute Science, Inc. | Assay Description Reactions between recombinant BoNTA-LC and fluorescent peptide substrate were carried out in 96-well microplates. Reaction progress was measured cont... | Bioorg Med Chem 17: 3072-9 (2009) Article DOI: 10.1016/j.bmc.2009.03.013 BindingDB Entry DOI: 10.7270/Q2G44NMS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A2 [1-425] (Clostridium botulinum) | BDBM28912 (2-mercaptoacetamide, 12d | 2-sulfanyl-N-{3-[4-(tri...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Absolute Science, Inc. | Assay Description Reactions between recombinant BoNTA-LC and fluorescent peptide substrate were carried out in 96-well microplates. Reaction progress was measured cont... | Bioorg Med Chem 17: 3072-9 (2009) Article DOI: 10.1016/j.bmc.2009.03.013 BindingDB Entry DOI: 10.7270/Q2G44NMS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A2 [1-425] (Clostridium botulinum) | BDBM28919 (2-mercaptoacetamide, 12c | 2-sulfanyl-N-{3-[3-(tri...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Absolute Science, Inc. | Assay Description Reactions between recombinant BoNTA-LC and fluorescent peptide substrate were carried out in 96-well microplates. Reaction progress was measured cont... | Bioorg Med Chem 17: 3072-9 (2009) Article DOI: 10.1016/j.bmc.2009.03.013 BindingDB Entry DOI: 10.7270/Q2G44NMS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A2 [1-425] (Clostridium botulinum) | BDBM28910 (2-mercaptoacetamide, 12h | N-[3-(4-fluorophenyl)-1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.35E+4 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Absolute Science, Inc. | Assay Description Reactions between recombinant BoNTA-LC and fluorescent peptide substrate were carried out in 96-well microplates. Reaction progress was measured cont... | Bioorg Med Chem 17: 3072-9 (2009) Article DOI: 10.1016/j.bmc.2009.03.013 BindingDB Entry DOI: 10.7270/Q2G44NMS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A2 [1-425] (Clostridium botulinum) | BDBM28909 (2-mercaptoacetamide, 12e | N-(3-phenyl-1H-pyrazol-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.45E+4 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Absolute Science, Inc. | Assay Description Reactions between recombinant BoNTA-LC and fluorescent peptide substrate were carried out in 96-well microplates. Reaction progress was measured cont... | Bioorg Med Chem 17: 3072-9 (2009) Article DOI: 10.1016/j.bmc.2009.03.013 BindingDB Entry DOI: 10.7270/Q2G44NMS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A2 [1-425] (Clostridium botulinum) | BDBM28914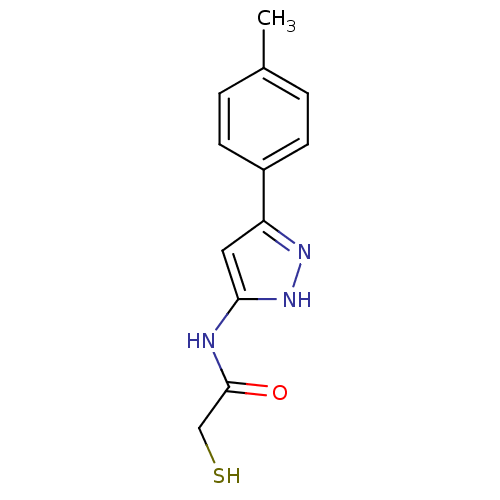 (2-mercaptoacetamide, 12j | N-[3-(4-methylphenyl)-1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Absolute Science, Inc. | Assay Description Reactions between recombinant BoNTA-LC and fluorescent peptide substrate were carried out in 96-well microplates. Reaction progress was measured cont... | Bioorg Med Chem 17: 3072-9 (2009) Article DOI: 10.1016/j.bmc.2009.03.013 BindingDB Entry DOI: 10.7270/Q2G44NMS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A2 [1-425] (Clostridium botulinum) | BDBM28916 (2-mercaptoacetamide, 12b | N-[3-(2-chlorophenyl)-1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60E+4 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Absolute Science, Inc. | Assay Description Reactions between recombinant BoNTA-LC and fluorescent peptide substrate were carried out in 96-well microplates. Reaction progress was measured cont... | Bioorg Med Chem 17: 3072-9 (2009) Article DOI: 10.1016/j.bmc.2009.03.013 BindingDB Entry DOI: 10.7270/Q2G44NMS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A2 [1-425] (Clostridium botulinum) | BDBM28915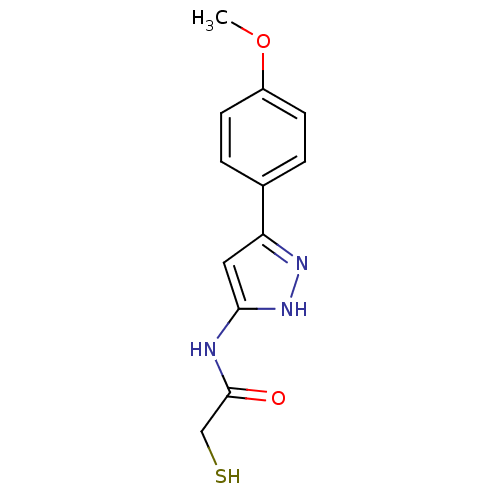 (2-mercaptoacetamide, 12k | N-[3-(4-methoxyphenyl)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.45E+4 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Absolute Science, Inc. | Assay Description Reactions between recombinant BoNTA-LC and fluorescent peptide substrate were carried out in 96-well microplates. Reaction progress was measured cont... | Bioorg Med Chem 17: 3072-9 (2009) Article DOI: 10.1016/j.bmc.2009.03.013 BindingDB Entry DOI: 10.7270/Q2G44NMS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A2 [1-425] (Clostridium botulinum) | BDBM28905 (3-mercaptopropionamide analog, 5 | N-[3-(4-chlorop...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.95E+5 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Absolute Science, Inc. | Assay Description Reactions between recombinant BoNTA-LC and fluorescent peptide substrate were carried out in 96-well microplates. Reaction progress was measured cont... | Bioorg Med Chem 17: 3072-9 (2009) Article DOI: 10.1016/j.bmc.2009.03.013 BindingDB Entry DOI: 10.7270/Q2G44NMS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A2 [1-425] (Clostridium botulinum) | BDBM28907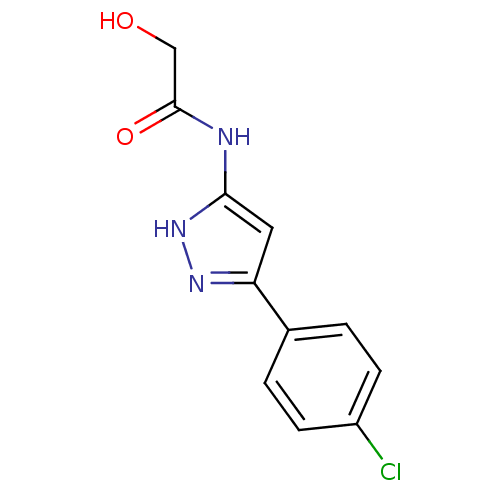 (2-hydroxyacetamide, 6 | N-[3-(4-chlorophenyl)-1H-p...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Absolute Science, Inc. | Assay Description Reactions between recombinant BoNTA-LC and fluorescent peptide substrate were carried out in 96-well microplates. Reaction progress was measured cont... | Bioorg Med Chem 17: 3072-9 (2009) Article DOI: 10.1016/j.bmc.2009.03.013 BindingDB Entry DOI: 10.7270/Q2G44NMS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A2 [1-425] (Clostridium botulinum) | BDBM28908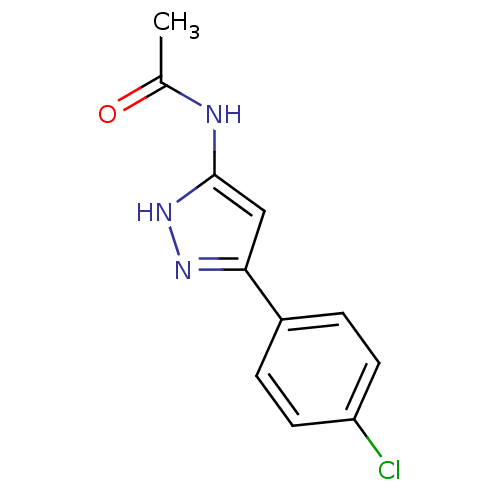 (N-[3-(4-chlorophenyl)-1H-pyrazol-5-yl]acetamide | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Absolute Science, Inc. | Assay Description Reactions between recombinant BoNTA-LC and fluorescent peptide substrate were carried out in 96-well microplates. Reaction progress was measured cont... | Bioorg Med Chem 17: 3072-9 (2009) Article DOI: 10.1016/j.bmc.2009.03.013 BindingDB Entry DOI: 10.7270/Q2G44NMS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM323617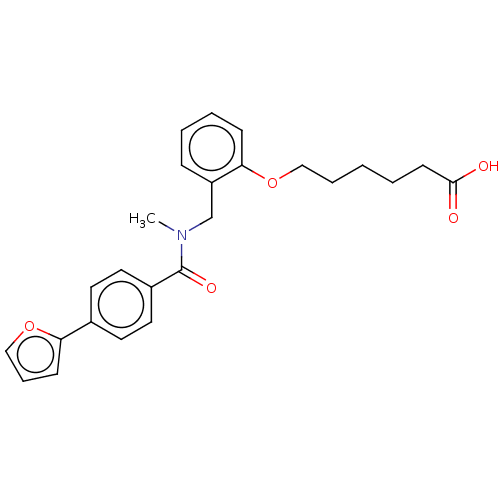 (US10188627, Comparator Cmpd. 1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 13 | n/a | n/a | n/a | n/a |
Mitobridge, Inc. Curated by ChEMBL | Assay Description Activation of human PPARdelta LBD expressed in CHO-K1 cells by beta galactosidase enzyme fragment complement based fluorescence assay | Bioorg Med Chem Lett 27: 5230-5234 (2017) Article DOI: 10.1016/j.bmcl.2017.10.037 BindingDB Entry DOI: 10.7270/Q21V5HJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM323617 (US10188627, Comparator Cmpd. 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Mitobridge, Inc. Curated by ChEMBL | Assay Description Activation of human PPARgamma LBD expressed in CHO-K1 cells by beta galactosidase enzyme fragment complement based fluorescence assay | Bioorg Med Chem Lett 27: 5230-5234 (2017) Article DOI: 10.1016/j.bmcl.2017.10.037 BindingDB Entry DOI: 10.7270/Q21V5HJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM323597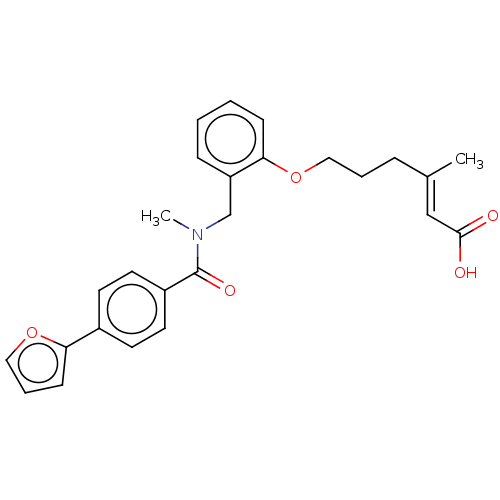 ((E)-6-(2-((4-(furan-2-yl)-N-methylbenzamido)methyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Mitobridge, Inc. Curated by ChEMBL | Assay Description Transactivation of yeast GAL4 DNA binding domain-fused PPARdelta LBD (unknown origin) expressed in CV-1 cells after 24 hrs by luciferase reporter gen... | Bioorg Med Chem Lett 27: 5230-5234 (2017) Article DOI: 10.1016/j.bmcl.2017.10.037 BindingDB Entry DOI: 10.7270/Q21V5HJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50192829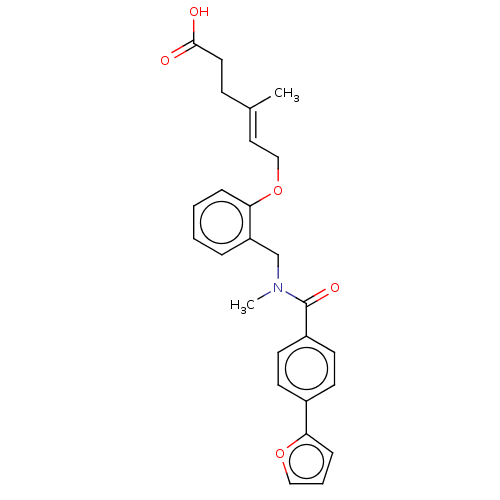 (CHEMBL3958704 | US10188627, Compound 8a) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Mitobridge, Inc. Curated by ChEMBL | Assay Description Transactivation of yeast GAL4 DNA binding domain-fused PPARgamma LBD (unknown origin) expressed in CV-1 cells after 24 hrs by luciferase reporter gen... | Bioorg Med Chem Lett 27: 5230-5234 (2017) Article DOI: 10.1016/j.bmcl.2017.10.037 BindingDB Entry DOI: 10.7270/Q21V5HJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50192829 (CHEMBL3958704 | US10188627, Compound 8a) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a |
Mitobridge, Inc. Curated by ChEMBL | Assay Description Binding affinity to His-tagged PPARdelta (unknown origin) by SPR assay | Bioorg Med Chem Lett 27: 5230-5234 (2017) Article DOI: 10.1016/j.bmcl.2017.10.037 BindingDB Entry DOI: 10.7270/Q21V5HJT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50192831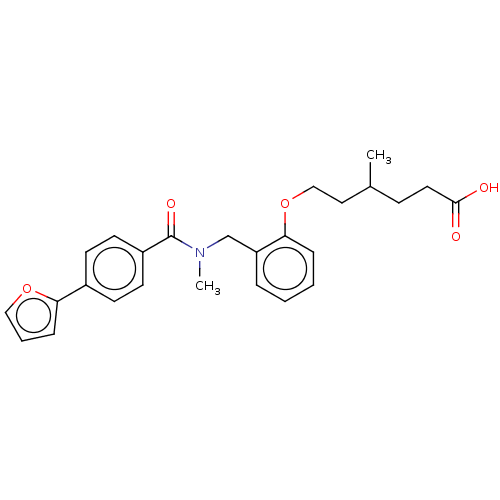 (CHEMBL3895104 | US10188627, Compound 8h) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 990 | n/a | n/a | n/a | n/a |
Mitobridge, Inc. Curated by ChEMBL | Assay Description Transactivation of yeast GAL4 DNA binding domain-fused PPARdelta LBD (unknown origin) expressed in CV-1 cells after 24 hrs by luciferase reporter gen... | Bioorg Med Chem Lett 27: 5230-5234 (2017) Article DOI: 10.1016/j.bmcl.2017.10.037 BindingDB Entry DOI: 10.7270/Q21V5HJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50452128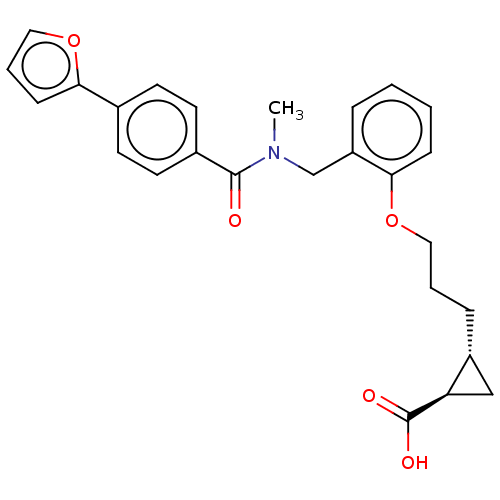 (CHEMBL4214179) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.63E+3 | n/a | n/a | n/a | n/a |
Mitobridge, Inc. Curated by ChEMBL | Assay Description Transactivation of yeast GAL4 DNA binding domain-fused PPARdelta LBD (unknown origin) expressed in CV-1 cells after 24 hrs by luciferase reporter gen... | Bioorg Med Chem Lett 27: 5230-5234 (2017) Article DOI: 10.1016/j.bmcl.2017.10.037 BindingDB Entry DOI: 10.7270/Q21V5HJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50192835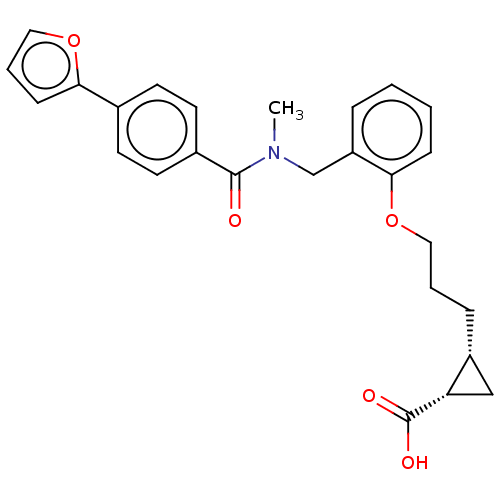 (CHEMBL3981654 | US10188627, Compound 8x) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 330 | n/a | n/a | n/a | n/a |
Mitobridge, Inc. Curated by ChEMBL | Assay Description Transactivation of yeast GAL4 DNA binding domain-fused PPARdelta LBD (unknown origin) expressed in CV-1 cells after 24 hrs by luciferase reporter gen... | Bioorg Med Chem Lett 27: 5230-5234 (2017) Article DOI: 10.1016/j.bmcl.2017.10.037 BindingDB Entry DOI: 10.7270/Q21V5HJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50452129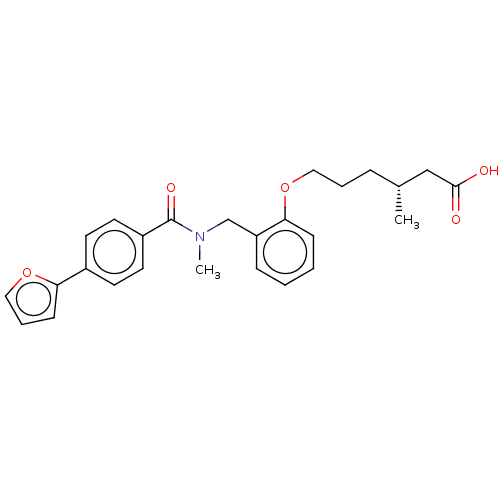 (CHEMBL4206950) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 417 | n/a | n/a | n/a | n/a |
Mitobridge, Inc. Curated by ChEMBL | Assay Description Transactivation of yeast GAL4 DNA binding domain-fused PPARdelta LBD (unknown origin) expressed in CV-1 cells after 24 hrs by luciferase reporter gen... | Bioorg Med Chem Lett 27: 5230-5234 (2017) Article DOI: 10.1016/j.bmcl.2017.10.037 BindingDB Entry DOI: 10.7270/Q21V5HJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM323603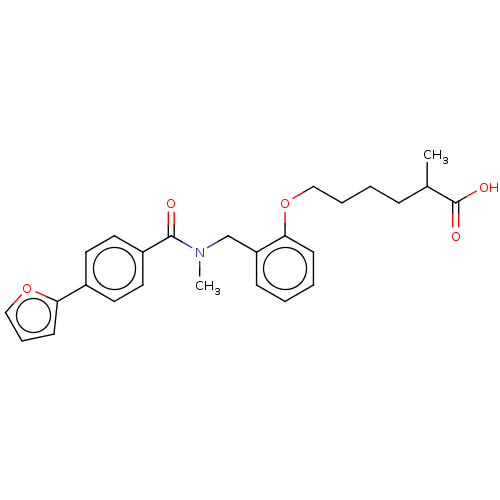 (6-(2-((4-(furan-2-yl)-N-methylbenzamido)methyl)phe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 640 | n/a | n/a | n/a | n/a |
Mitobridge, Inc. Curated by ChEMBL | Assay Description Transactivation of yeast GAL4 DNA binding domain-fused PPARdelta LBD (unknown origin) expressed in CV-1 cells after 24 hrs by luciferase reporter gen... | Bioorg Med Chem Lett 27: 5230-5234 (2017) Article DOI: 10.1016/j.bmcl.2017.10.037 BindingDB Entry DOI: 10.7270/Q21V5HJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50452130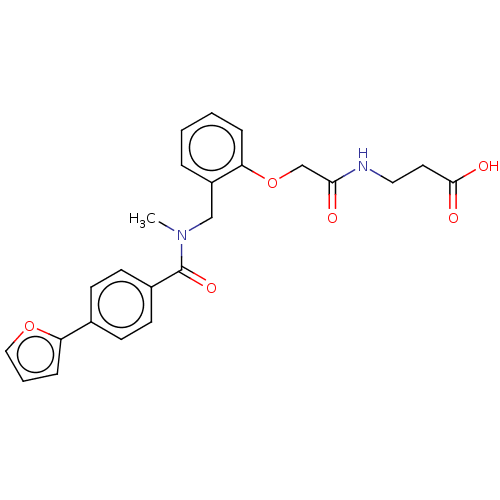 (CHEMBL4203252) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
Mitobridge, Inc. Curated by ChEMBL | Assay Description Activation of human PPARdelta LBD expressed in CHO-K1 cells by beta galactosidase enzyme fragment complement based fluorescence assay | Bioorg Med Chem Lett 27: 5230-5234 (2017) Article DOI: 10.1016/j.bmcl.2017.10.037 BindingDB Entry DOI: 10.7270/Q21V5HJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM323599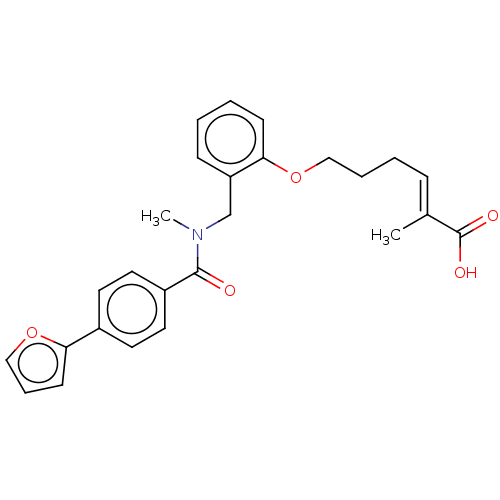 ((E)-6-(2-((4-(furan-2-yl)-N-methylbenzamido)methyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Mitobridge, Inc. Curated by ChEMBL | Assay Description Transactivation of yeast GAL4 DNA binding domain-fused PPARdelta LBD (unknown origin) expressed in CV-1 cells after 24 hrs by luciferase reporter gen... | Bioorg Med Chem Lett 27: 5230-5234 (2017) Article DOI: 10.1016/j.bmcl.2017.10.037 BindingDB Entry DOI: 10.7270/Q21V5HJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM323617 (US10188627, Comparator Cmpd. 1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 47 | n/a | n/a | n/a | n/a |
Mitobridge, Inc. Curated by ChEMBL | Assay Description Transactivation of yeast GAL4 DNA binding domain-fused PPARdelta LBD (unknown origin) expressed in CV-1 cells after 24 hrs by luciferase reporter gen... | Bioorg Med Chem Lett 27: 5230-5234 (2017) Article DOI: 10.1016/j.bmcl.2017.10.037 BindingDB Entry DOI: 10.7270/Q21V5HJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50192829 (CHEMBL3958704 | US10188627, Compound 8a) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 5 | n/a | n/a | n/a | n/a |
Mitobridge, Inc. Curated by ChEMBL | Assay Description Activation of human PPARdelta LBD expressed in CHO-K1 cells by beta galactosidase enzyme fragment complement based fluorescence assay | Bioorg Med Chem Lett 27: 5230-5234 (2017) Article DOI: 10.1016/j.bmcl.2017.10.037 BindingDB Entry DOI: 10.7270/Q21V5HJT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50192831 (CHEMBL3895104 | US10188627, Compound 8h) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 40 | n/a | n/a | n/a | n/a |
Mitobridge, Inc. Curated by ChEMBL | Assay Description Activation of human PPARdelta LBD expressed in CHO-K1 cells by beta galactosidase enzyme fragment complement based fluorescence assay | Bioorg Med Chem Lett 27: 5230-5234 (2017) Article DOI: 10.1016/j.bmcl.2017.10.037 BindingDB Entry DOI: 10.7270/Q21V5HJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM323599 ((E)-6-(2-((4-(furan-2-yl)-N-methylbenzamido)methyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.85E+3 | n/a | n/a | n/a | n/a |
Mitobridge, Inc. Curated by ChEMBL | Assay Description Activation of human PPARdelta LBD expressed in CHO-K1 cells by beta galactosidase enzyme fragment complement based fluorescence assay | Bioorg Med Chem Lett 27: 5230-5234 (2017) Article DOI: 10.1016/j.bmcl.2017.10.037 BindingDB Entry DOI: 10.7270/Q21V5HJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM323616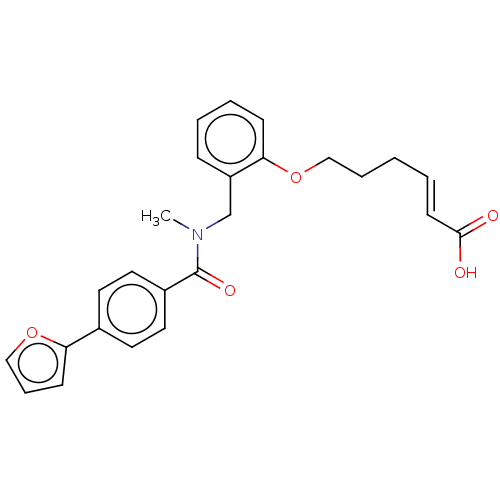 ((E)-6-(2-((4-(furan-2-yl)-N-methylbenzamido)methyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 80 | n/a | n/a | n/a | n/a |
Mitobridge, Inc. Curated by ChEMBL | Assay Description Activation of human PPARdelta LBD expressed in CHO-K1 cells by beta galactosidase enzyme fragment complement based fluorescence assay | Bioorg Med Chem Lett 27: 5230-5234 (2017) Article DOI: 10.1016/j.bmcl.2017.10.037 BindingDB Entry DOI: 10.7270/Q21V5HJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50452128 (CHEMBL4214179) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 210 | n/a | n/a | n/a | n/a |
Mitobridge, Inc. Curated by ChEMBL | Assay Description Activation of human PPARdelta LBD expressed in CHO-K1 cells by beta galactosidase enzyme fragment complement based fluorescence assay | Bioorg Med Chem Lett 27: 5230-5234 (2017) Article DOI: 10.1016/j.bmcl.2017.10.037 BindingDB Entry DOI: 10.7270/Q21V5HJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50452131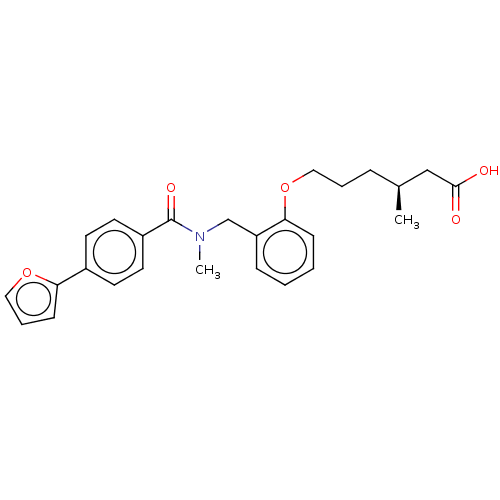 (CHEMBL4210212) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Mitobridge, Inc. Curated by ChEMBL | Assay Description Activation of human PPARdelta LBD expressed in CHO-K1 cells by beta galactosidase enzyme fragment complement based fluorescence assay | Bioorg Med Chem Lett 27: 5230-5234 (2017) Article DOI: 10.1016/j.bmcl.2017.10.037 BindingDB Entry DOI: 10.7270/Q21V5HJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50452129 (CHEMBL4206950) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 270 | n/a | n/a | n/a | n/a |
Mitobridge, Inc. Curated by ChEMBL | Assay Description Activation of human PPARdelta LBD expressed in CHO-K1 cells by beta galactosidase enzyme fragment complement based fluorescence assay | Bioorg Med Chem Lett 27: 5230-5234 (2017) Article DOI: 10.1016/j.bmcl.2017.10.037 BindingDB Entry DOI: 10.7270/Q21V5HJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM323617 (US10188627, Comparator Cmpd. 1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Mitobridge, Inc. Curated by ChEMBL | Assay Description Activation of human PPARalpha LBD expressed in CHO-K1 cells by beta galactosidase enzyme fragment complement based fluorescence assay | Bioorg Med Chem Lett 27: 5230-5234 (2017) Article DOI: 10.1016/j.bmcl.2017.10.037 BindingDB Entry DOI: 10.7270/Q21V5HJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM323603 (6-(2-((4-(furan-2-yl)-N-methylbenzamido)methyl)phe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Mitobridge, Inc. Curated by ChEMBL | Assay Description Activation of human PPARalpha LBD expressed in CHO-K1 cells by beta galactosidase enzyme fragment complement based fluorescence assay | Bioorg Med Chem Lett 27: 5230-5234 (2017) Article DOI: 10.1016/j.bmcl.2017.10.037 BindingDB Entry DOI: 10.7270/Q21V5HJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM323607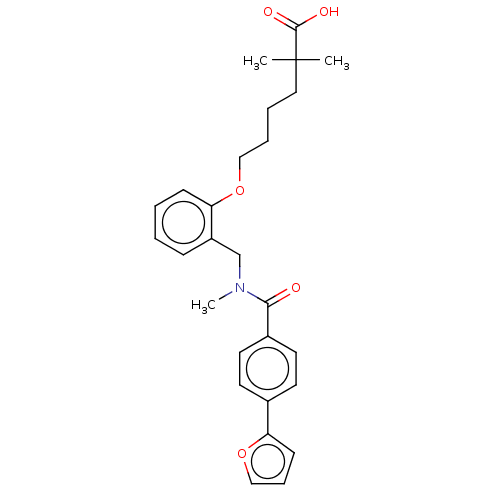 (6-(2-((4-(furan-2-yl)-N-methylbenzamido)methyl)phe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Mitobridge, Inc. Curated by ChEMBL | Assay Description Activation of human PPARalpha LBD expressed in CHO-K1 cells by beta galactosidase enzyme fragment complement based fluorescence assay | Bioorg Med Chem Lett 27: 5230-5234 (2017) Article DOI: 10.1016/j.bmcl.2017.10.037 BindingDB Entry DOI: 10.7270/Q21V5HJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50452131 (CHEMBL4210212) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Mitobridge, Inc. Curated by ChEMBL | Assay Description Activation of human PPARalpha LBD expressed in CHO-K1 cells by beta galactosidase enzyme fragment complement based fluorescence assay | Bioorg Med Chem Lett 27: 5230-5234 (2017) Article DOI: 10.1016/j.bmcl.2017.10.037 BindingDB Entry DOI: 10.7270/Q21V5HJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50192835 (CHEMBL3981654 | US10188627, Compound 8x) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Mitobridge, Inc. Curated by ChEMBL | Assay Description Activation of human PPARalpha LBD expressed in CHO-K1 cells by beta galactosidase enzyme fragment complement based fluorescence assay | Bioorg Med Chem Lett 27: 5230-5234 (2017) Article DOI: 10.1016/j.bmcl.2017.10.037 BindingDB Entry DOI: 10.7270/Q21V5HJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM323616 ((E)-6-(2-((4-(furan-2-yl)-N-methylbenzamido)methyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Mitobridge, Inc. Curated by ChEMBL | Assay Description Activation of human PPARalpha LBD expressed in CHO-K1 cells by beta galactosidase enzyme fragment complement based fluorescence assay | Bioorg Med Chem Lett 27: 5230-5234 (2017) Article DOI: 10.1016/j.bmcl.2017.10.037 BindingDB Entry DOI: 10.7270/Q21V5HJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM323597 ((E)-6-(2-((4-(furan-2-yl)-N-methylbenzamido)methyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Mitobridge, Inc. Curated by ChEMBL | Assay Description Activation of human PPARalpha LBD expressed in CHO-K1 cells by beta galactosidase enzyme fragment complement based fluorescence assay | Bioorg Med Chem Lett 27: 5230-5234 (2017) Article DOI: 10.1016/j.bmcl.2017.10.037 BindingDB Entry DOI: 10.7270/Q21V5HJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50192831 (CHEMBL3895104 | US10188627, Compound 8h) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Mitobridge, Inc. Curated by ChEMBL | Assay Description Activation of human PPARalpha LBD expressed in CHO-K1 cells by beta galactosidase enzyme fragment complement based fluorescence assay | Bioorg Med Chem Lett 27: 5230-5234 (2017) Article DOI: 10.1016/j.bmcl.2017.10.037 BindingDB Entry DOI: 10.7270/Q21V5HJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM323607 (6-(2-((4-(furan-2-yl)-N-methylbenzamido)methyl)phe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Mitobridge, Inc. Curated by ChEMBL | Assay Description Activation of human PPARgamma LBD expressed in CHO-K1 cells by beta galactosidase enzyme fragment complement based fluorescence assay | Bioorg Med Chem Lett 27: 5230-5234 (2017) Article DOI: 10.1016/j.bmcl.2017.10.037 BindingDB Entry DOI: 10.7270/Q21V5HJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50452131 (CHEMBL4210212) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Mitobridge, Inc. Curated by ChEMBL | Assay Description Activation of human PPARgamma LBD expressed in CHO-K1 cells by beta galactosidase enzyme fragment complement based fluorescence assay | Bioorg Med Chem Lett 27: 5230-5234 (2017) Article DOI: 10.1016/j.bmcl.2017.10.037 BindingDB Entry DOI: 10.7270/Q21V5HJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50192835 (CHEMBL3981654 | US10188627, Compound 8x) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Mitobridge, Inc. Curated by ChEMBL | Assay Description Activation of human PPARgamma LBD expressed in CHO-K1 cells by beta galactosidase enzyme fragment complement based fluorescence assay | Bioorg Med Chem Lett 27: 5230-5234 (2017) Article DOI: 10.1016/j.bmcl.2017.10.037 BindingDB Entry DOI: 10.7270/Q21V5HJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM323616 ((E)-6-(2-((4-(furan-2-yl)-N-methylbenzamido)methyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Mitobridge, Inc. Curated by ChEMBL | Assay Description Activation of human PPARgamma LBD expressed in CHO-K1 cells by beta galactosidase enzyme fragment complement based fluorescence assay | Bioorg Med Chem Lett 27: 5230-5234 (2017) Article DOI: 10.1016/j.bmcl.2017.10.037 BindingDB Entry DOI: 10.7270/Q21V5HJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM323599 ((E)-6-(2-((4-(furan-2-yl)-N-methylbenzamido)methyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Mitobridge, Inc. Curated by ChEMBL | Assay Description Activation of human PPARgamma LBD expressed in CHO-K1 cells by beta galactosidase enzyme fragment complement based fluorescence assay | Bioorg Med Chem Lett 27: 5230-5234 (2017) Article DOI: 10.1016/j.bmcl.2017.10.037 BindingDB Entry DOI: 10.7270/Q21V5HJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50192831 (CHEMBL3895104 | US10188627, Compound 8h) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Mitobridge, Inc. Curated by ChEMBL | Assay Description Activation of human PPARgamma LBD expressed in CHO-K1 cells by beta galactosidase enzyme fragment complement based fluorescence assay | Bioorg Med Chem Lett 27: 5230-5234 (2017) Article DOI: 10.1016/j.bmcl.2017.10.037 BindingDB Entry DOI: 10.7270/Q21V5HJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 69 total ) | Next | Last >> |