

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50010495 (Acetyl-Ser-Leu-Asn-Phe-[CH(OH)CH2N]Pro-Ile-Val-OMe...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description In vitro binding affinity of the compound against HIV protease was measured | J Med Chem 34: 1222-5 (1991) BindingDB Entry DOI: 10.7270/Q2057DWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50010497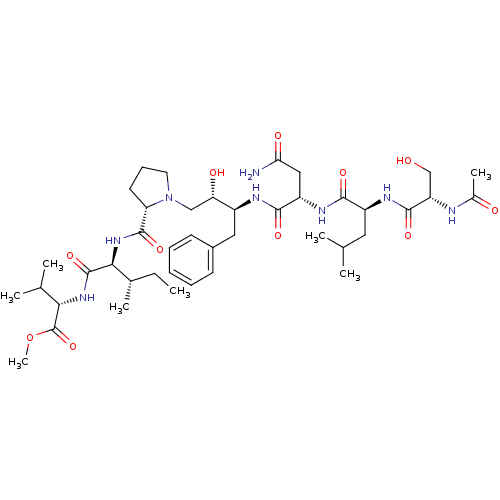 (Acetyl-Ser-Leu-Asn-Phe-[S]-[CH(OH)CH2N]Pro-Ile-Val...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description In vitro concentration of the compound required to inhibit 50% activity of HIV protease was measured | J Med Chem 34: 1222-5 (1991) BindingDB Entry DOI: 10.7270/Q2057DWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50010497 (Acetyl-Ser-Leu-Asn-Phe-[S]-[CH(OH)CH2N]Pro-Ile-Val...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description In vitro concentration of the compound required to inhibit 50% activity of HIV protease was measured (exp 2) | J Med Chem 34: 1222-5 (1991) BindingDB Entry DOI: 10.7270/Q2057DWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50023523 (2,2-Dimethyl-propionic acid 2-[2,4-bis-(2,2-dimeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 68 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human leukocyte elastase at 10e-8 M | J Med Chem 31: 1052-61 (1988) BindingDB Entry DOI: 10.7270/Q2PC31DJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50023518 (2,4-Bis-(2,2-dimethyl-propionyloxy)-benzoic acid 5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human leukocyte elastase at 10e-7 M | J Med Chem 31: 1052-61 (1988) BindingDB Entry DOI: 10.7270/Q2PC31DJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50023545 (2,2-Dimethyl-propionic acid 4-[4-(2,2-dimethyl-pro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human leukocyte elastase at 10e-8 M | J Med Chem 31: 1052-61 (1988) BindingDB Entry DOI: 10.7270/Q2PC31DJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50023542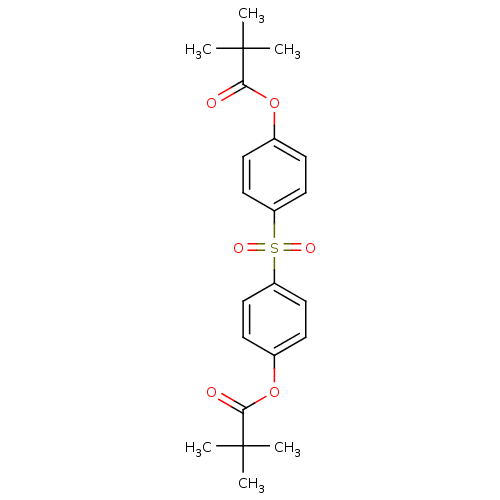 (2,2-Dimethyl-propionic acid 4-[4-(2,2-dimethyl-pro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against human leukocyte elastase at 10e-7 M | J Med Chem 31: 1052-61 (1988) BindingDB Entry DOI: 10.7270/Q2PC31DJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50011680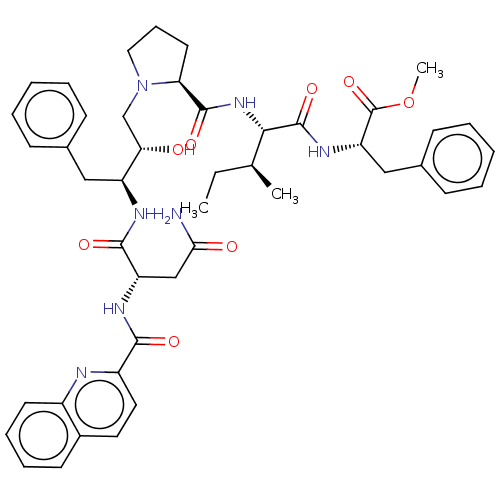 (CHEMBL3351098 | Qua-Asn-Phe-HEA(S)-Pro-Ile-Phe-OMe) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description In vitro concentration of the compound required to inhibit 50% activity of HIV protease was measured | J Med Chem 34: 1222-5 (1991) BindingDB Entry DOI: 10.7270/Q2057DWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM490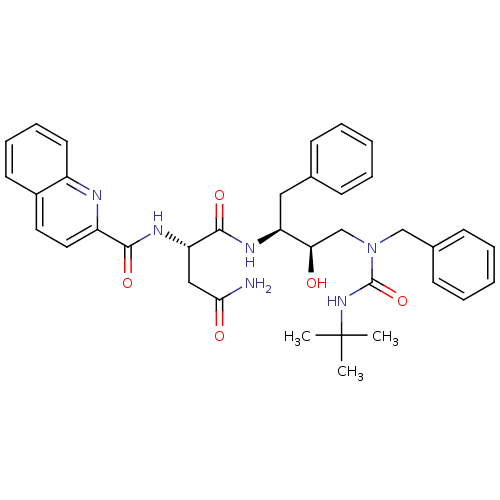 ((2S)-N-[(2S,3R)-4-[benzyl(tert-butylcarbamoyl)amin...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 6.4 | 25 |
Monsanto Corporate Research | Assay Description IC50 values for inhibition of recombinant HIV protease were determined using the spectrofluorometric assay, which utilized an intramolecularly quench... | J Med Chem 36: 288-91 (1993) Article DOI: 10.1021/jm00054a014 BindingDB Entry DOI: 10.7270/Q2WW7FTF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM486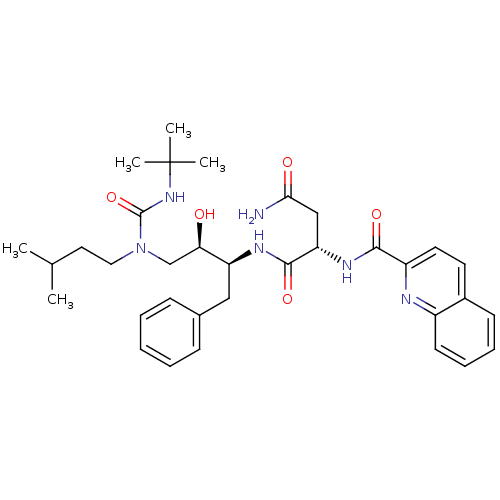 ((2S)-N-[(2S,3R)-4-[(tert-butylcarbamoyl)(3-methylb...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 6.4 | 25 |
Monsanto Corporate Research | Assay Description IC50 values for inhibition of recombinant HIV protease were determined using the spectrofluorometric assay, which utilized an intramolecularly quench... | J Med Chem 36: 288-91 (1993) Article DOI: 10.1021/jm00054a014 BindingDB Entry DOI: 10.7270/Q2WW7FTF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50011684 (CHEMBL3350878 | Cbz-Asn-Phe-HEA(S)-Pro-Ile-Phe-OMe) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description In vitro concentration of the compound required to inhibit 50% activity of HIV protease was measured | J Med Chem 34: 1222-5 (1991) BindingDB Entry DOI: 10.7270/Q2057DWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM488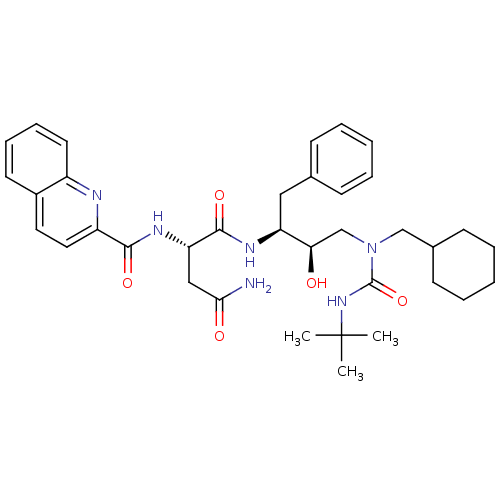 ((2S)-N-[(2S,3R)-4-[(tert-butylcarbamoyl)(cyclohexy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 6.4 | 25 |
Monsanto Corporate Research | Assay Description IC50 values for inhibition of recombinant HIV protease were determined using the spectrofluorometric assay, which utilized an intramolecularly quench... | J Med Chem 36: 288-91 (1993) Article DOI: 10.1021/jm00054a014 BindingDB Entry DOI: 10.7270/Q2WW7FTF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM483 ((2S)-N-[(2S,3R)-4-[(tert-butylcarbamoyl)(2-methylp...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 6.4 | 25 |
Monsanto Corporate Research | Assay Description IC50 values for inhibition of recombinant HIV protease were determined using the spectrofluorometric assay, which utilized an intramolecularly quench... | J Med Chem 36: 288-91 (1993) Article DOI: 10.1021/jm00054a014 BindingDB Entry DOI: 10.7270/Q2WW7FTF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50010495 (Acetyl-Ser-Leu-Asn-Phe-[CH(OH)CH2N]Pro-Ile-Val-OMe...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description In vitro binding affinity of the compound against HIV protease was measured | J Med Chem 34: 1222-5 (1991) BindingDB Entry DOI: 10.7270/Q2057DWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM485 ((Hydroxyethyl)urea Isostere deriv. 13 | benzyl N-[...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | 6.4 | 25 |
Monsanto Corporate Research | Assay Description IC50 values for inhibition of recombinant HIV protease were determined using the spectrofluorometric assay, which utilized an intramolecularly quench... | J Med Chem 36: 288-91 (1993) Article DOI: 10.1021/jm00054a014 BindingDB Entry DOI: 10.7270/Q2WW7FTF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50011682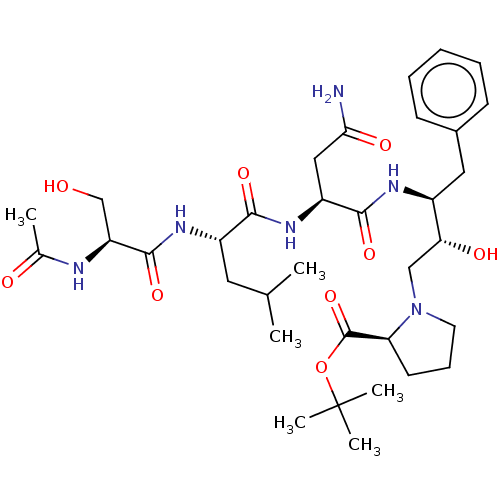 (Ac-Ser-Leu-Asn-Phe-HEA(S)-Pro-O-terbutyl | BDBM504...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description In vitro concentration of the compound required to inhibit 50% activity of HIV protease was measured | J Med Chem 34: 1222-5 (1991) BindingDB Entry DOI: 10.7270/Q2057DWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50011687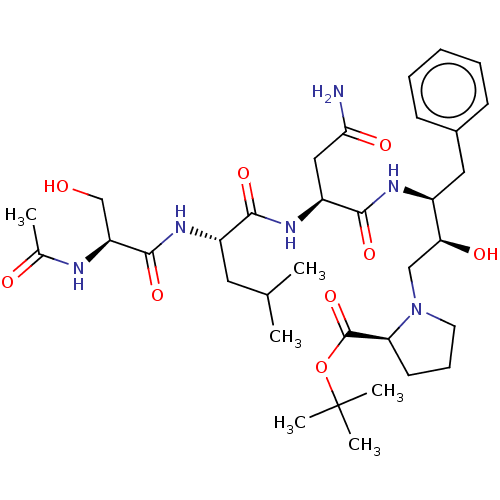 (Ac-Ser-Leu-Asn-Phe-HEA(R)-Pro-O-terbutyl | CHEMBL3...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description In vitro concentration of the compound required to inhibit 50% activity of HIV protease was measured | J Med Chem 34: 1222-5 (1991) BindingDB Entry DOI: 10.7270/Q2057DWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50011688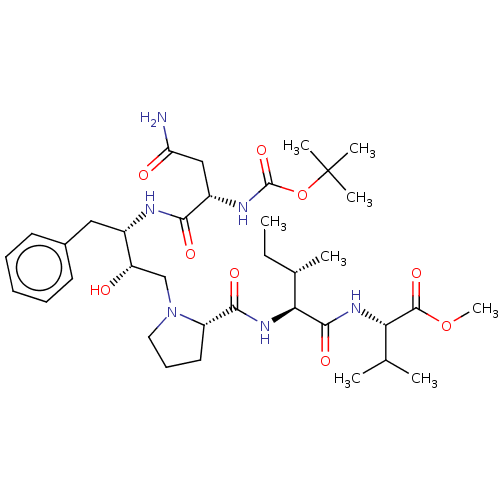 (Boc-Asn-Phe-HEA(R)-Pro-Ile-Val-OMe | CHEMBL3349554) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description In vitro concentration of the compound required to inhibit 50% activity of HIV protease was measured | J Med Chem 34: 1222-5 (1991) BindingDB Entry DOI: 10.7270/Q2057DWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM489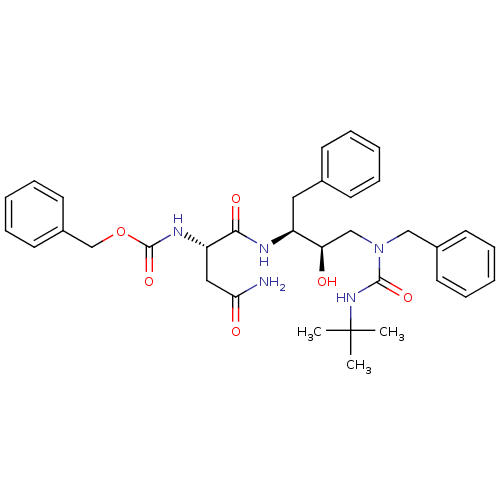 ((Hydroxyethyl)urea Isostere deriv. 17 | benzyl N-[...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | 6.4 | 25 |
Monsanto Corporate Research | Assay Description IC50 values for inhibition of recombinant HIV protease were determined using the spectrofluorometric assay, which utilized an intramolecularly quench... | J Med Chem 36: 288-91 (1993) Article DOI: 10.1021/jm00054a014 BindingDB Entry DOI: 10.7270/Q2WW7FTF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM494 ((2S)-N-[(2S,3R)-4-[(tert-butylcarbamoyl)(pyridin-4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | 6.4 | 25 |
Monsanto Corporate Research | Assay Description IC50 values for inhibition of recombinant HIV protease were determined using the spectrofluorometric assay, which utilized an intramolecularly quench... | J Med Chem 36: 288-91 (1993) Article DOI: 10.1021/jm00054a014 BindingDB Entry DOI: 10.7270/Q2WW7FTF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50023523 (2,2-Dimethyl-propionic acid 2-[2,4-bis-(2,2-dimeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human leukocyte elastase at 10e-7 M | J Med Chem 31: 1052-61 (1988) BindingDB Entry DOI: 10.7270/Q2PC31DJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50023518 (2,4-Bis-(2,2-dimethyl-propionyloxy)-benzoic acid 5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against human leukocyte elastase at 10e-8 M | J Med Chem 31: 1052-61 (1988) BindingDB Entry DOI: 10.7270/Q2PC31DJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM487 ((Hydroxyethyl)urea Isostere deriv. 15 | benzyl N-[...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | 6.4 | 25 |
Monsanto Corporate Research | Assay Description IC50 values for inhibition of recombinant HIV protease were determined using the spectrofluorometric assay, which utilized an intramolecularly quench... | J Med Chem 36: 288-91 (1993) Article DOI: 10.1021/jm00054a014 BindingDB Entry DOI: 10.7270/Q2WW7FTF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM481 ((Hydroxyethyl)urea Isostere deriv. 9 | benzyl N-[(...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | 6.4 | 25 |
Monsanto Corporate Research | Assay Description IC50 values for inhibition of recombinant HIV protease were determined using the spectrofluorometric assay, which utilized an intramolecularly quench... | J Med Chem 36: 288-91 (1993) Article DOI: 10.1021/jm00054a014 BindingDB Entry DOI: 10.7270/Q2WW7FTF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50005687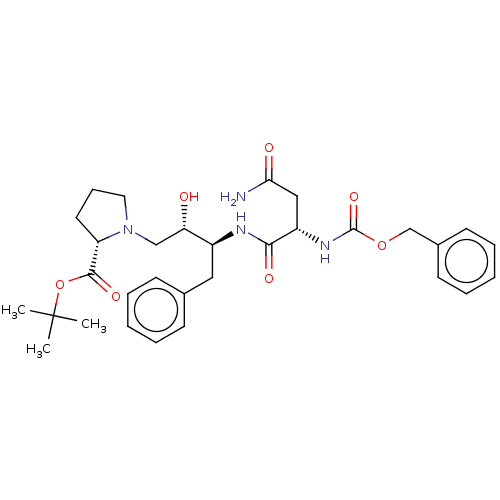 (1-[3-(2-Benzyloxycarbonylamino-3-carbamoyl-propion...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description In vitro concentration of the compound required to inhibit 50% activity of HIV protease was measured (exp 2) | J Med Chem 34: 1222-5 (1991) BindingDB Entry DOI: 10.7270/Q2057DWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50023517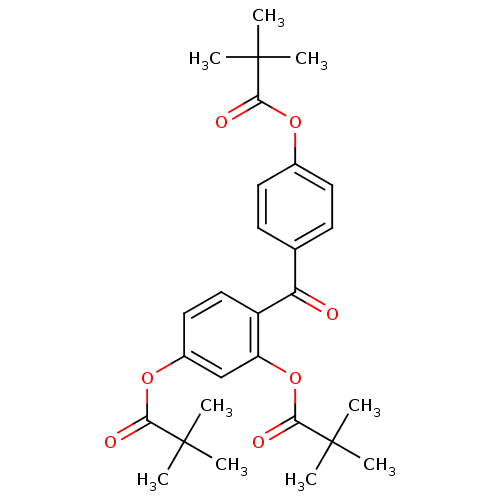 (2,2-Dimethyl-propionic acid 5-(2,2-dimethyl-propio...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against human leukocyte elastase at 10e-5 M | J Med Chem 31: 1052-61 (1988) BindingDB Entry DOI: 10.7270/Q2PC31DJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50010497 (Acetyl-Ser-Leu-Asn-Phe-[S]-[CH(OH)CH2N]Pro-Ile-Val...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description In vitro concentration of the compound required to inhibit 50% activity of HIV protease was measured | J Med Chem 34: 1222-5 (1991) BindingDB Entry DOI: 10.7270/Q2057DWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50010497 (Acetyl-Ser-Leu-Asn-Phe-[S]-[CH(OH)CH2N]Pro-Ile-Val...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description In vitro concentration of the compound required to inhibit 50% activity of HIV protease was measured | J Med Chem 34: 1222-5 (1991) BindingDB Entry DOI: 10.7270/Q2057DWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50023522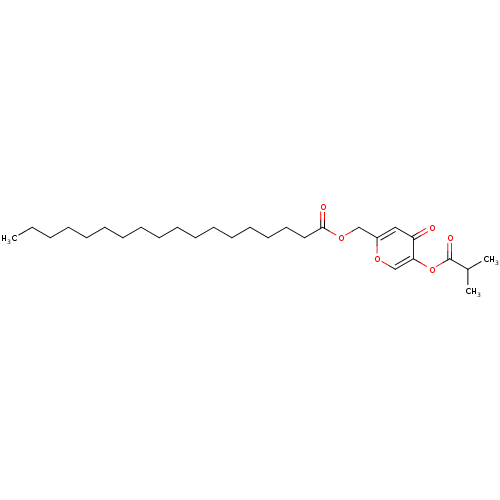 (CHEMBL9969 | Octadecanoic acid 5-isobutyryloxy-4-o...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against human leukocyte elastase at 10e-8 M | J Med Chem 31: 1052-61 (1988) BindingDB Entry DOI: 10.7270/Q2PC31DJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50023542 (2,2-Dimethyl-propionic acid 4-[4-(2,2-dimethyl-pro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against human leukocyte elastase at 10e-8 M | J Med Chem 31: 1052-61 (1988) BindingDB Entry DOI: 10.7270/Q2PC31DJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50023550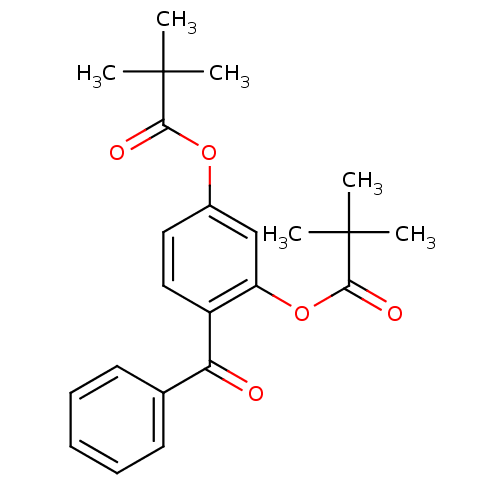 (2,2-Dimethyl-propionic acid 2-benzoyl-5-(2,2-dimet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against human leukocyte elastase at 10e-8 M | J Med Chem 31: 1052-61 (1988) BindingDB Entry DOI: 10.7270/Q2PC31DJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50023555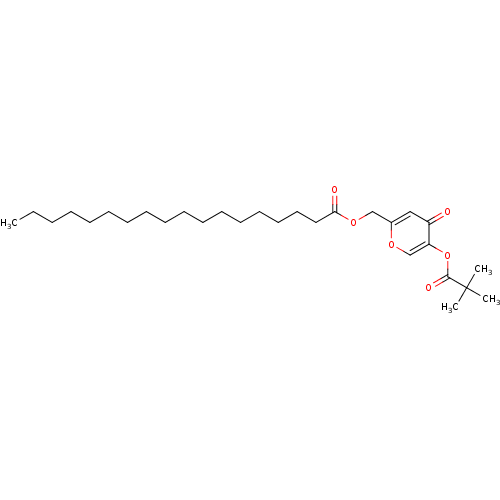 (CHEMBL9809 | Octadecanoic acid 5-(2,2-dimethyl-pro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against human leukocyte elastase at 10e-7 M | J Med Chem 31: 1052-61 (1988) BindingDB Entry DOI: 10.7270/Q2PC31DJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50023569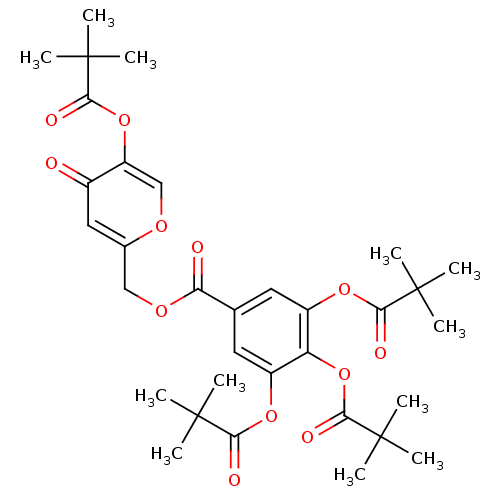 (3,4,5-Tris-(2,2-dimethyl-propionyloxy)-benzoic aci...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against human leukocyte elastase at 10e-7 M | J Med Chem 31: 1052-61 (1988) BindingDB Entry DOI: 10.7270/Q2PC31DJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM493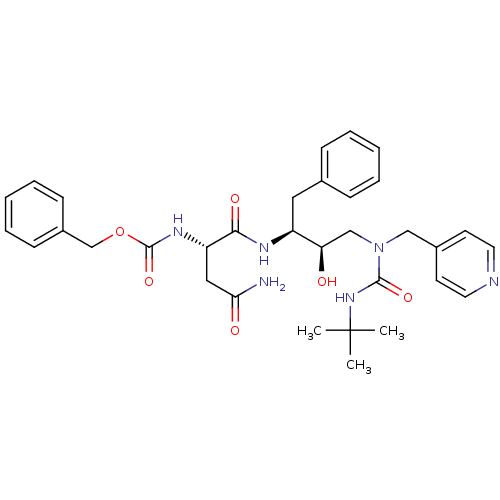 ((Hydroxyethyl)urea Isostere deriv. 21 | benzyl N-[...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 105 | n/a | n/a | n/a | n/a | 6.4 | 25 |
Monsanto Corporate Research | Assay Description IC50 values for inhibition of recombinant HIV protease were determined using the spectrofluorometric assay, which utilized an intramolecularly quench... | J Med Chem 36: 288-91 (1993) Article DOI: 10.1021/jm00054a014 BindingDB Entry DOI: 10.7270/Q2WW7FTF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50023570 (CHEMBL9895 | Hexadecanoic acid 5-(2,2-dimethyl-pro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against human leukocyte elastase at 10e-7 M | J Med Chem 31: 1052-61 (1988) BindingDB Entry DOI: 10.7270/Q2PC31DJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50023545 (2,2-Dimethyl-propionic acid 4-[4-(2,2-dimethyl-pro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against human leukocyte elastase at 10e-8 M | J Med Chem 31: 1052-61 (1988) BindingDB Entry DOI: 10.7270/Q2PC31DJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM477 ((2S)-N-[(2S,3R)-4-[(butylcarbamoyl)(2-methylpropyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 126 | n/a | n/a | n/a | n/a | 6.4 | 25 |
Monsanto Corporate Research | Assay Description IC50 values for inhibition of recombinant HIV protease were determined using the spectrofluorometric assay, which utilized an intramolecularly quench... | J Med Chem 36: 288-91 (1993) Article DOI: 10.1021/jm00054a014 BindingDB Entry DOI: 10.7270/Q2WW7FTF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50023551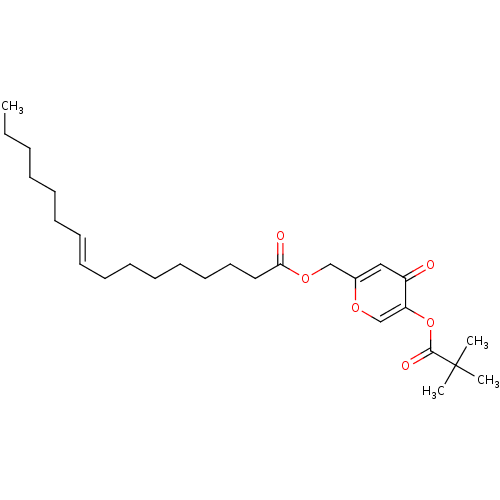 (CHEMBL9865 | Hexadec-9-enoic acid 5-(2,2-dimethyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against human leukocyte elastase at 10e-7 M | J Med Chem 31: 1052-61 (1988) BindingDB Entry DOI: 10.7270/Q2PC31DJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50023540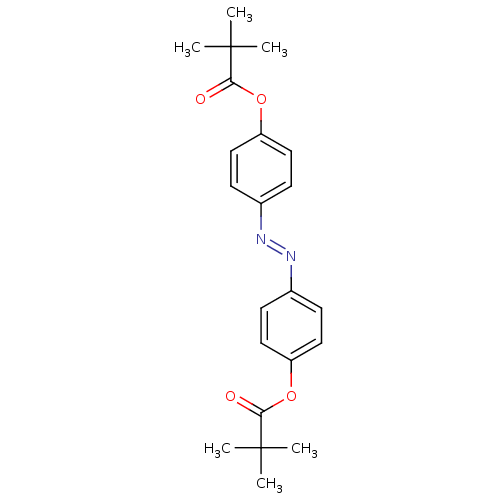 (2,2-Dimethyl-propionic acid 4-[4-(2,2-dimethyl-pro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against human leukocyte elastase at 10e-7 M | J Med Chem 31: 1052-61 (1988) BindingDB Entry DOI: 10.7270/Q2PC31DJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50023558 (CHEMBL9978 | Icosanoic acid 5-(2,2-dimethyl-propio...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against human leukocyte elastase at 10e-7 M | J Med Chem 31: 1052-61 (1988) BindingDB Entry DOI: 10.7270/Q2PC31DJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50005687 (1-[3-(2-Benzyloxycarbonylamino-3-carbamoyl-propion...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description In vitro concentration of the compound required to inhibit 50% activity of HIV protease was measured | J Med Chem 34: 1222-5 (1991) BindingDB Entry DOI: 10.7270/Q2057DWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50023547 (2,2-Dimethyl-propionic acid 4-(4-chloro-benzoyl)-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against human leukocyte elastase at 10e-7 M | J Med Chem 31: 1052-61 (1988) BindingDB Entry DOI: 10.7270/Q2PC31DJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50023566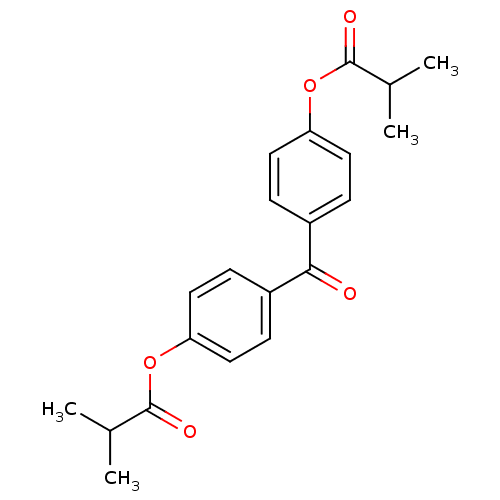 (CHEMBL269669 | Isobutyric acid 4-(4-isobutyryloxy-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against human leukocyte elastase at 10e-7 M | J Med Chem 31: 1052-61 (1988) BindingDB Entry DOI: 10.7270/Q2PC31DJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50023529 (CHEMBL9644 | Docos-13-enoic acid 5-(2,2-dimethyl-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against human leukocyte elastase at 10e-7 M | J Med Chem 31: 1052-61 (1988) BindingDB Entry DOI: 10.7270/Q2PC31DJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50023511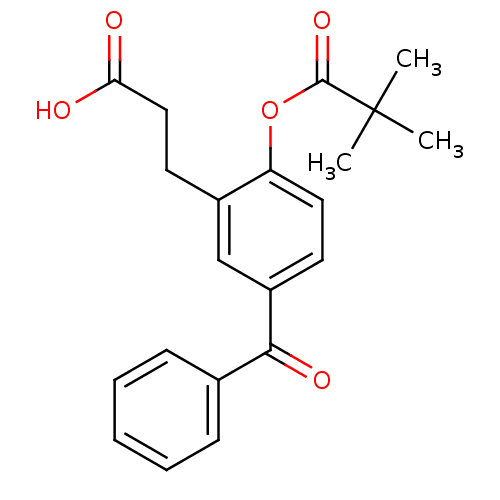 (2,2-Dimethyl-propionic acid 4-benzoyl-2-(2-carboxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against human leukocyte elastase at 10e-6 M | J Med Chem 31: 1052-61 (1988) BindingDB Entry DOI: 10.7270/Q2PC31DJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50023549 (4-(2,2-Dimethyl-propionyloxy)-benzoic acid 5-(2,2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against human leukocyte elastase at 10e-7 M | J Med Chem 31: 1052-61 (1988) BindingDB Entry DOI: 10.7270/Q2PC31DJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50023562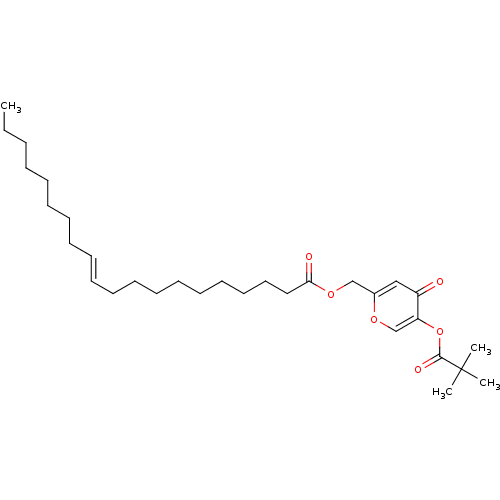 (CHEMBL9792 | Icos-11-enoic acid 5-(2,2-dimethyl-pr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against human leukocyte elastase at 10e-7 M | J Med Chem 31: 1052-61 (1988) BindingDB Entry DOI: 10.7270/Q2PC31DJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50023525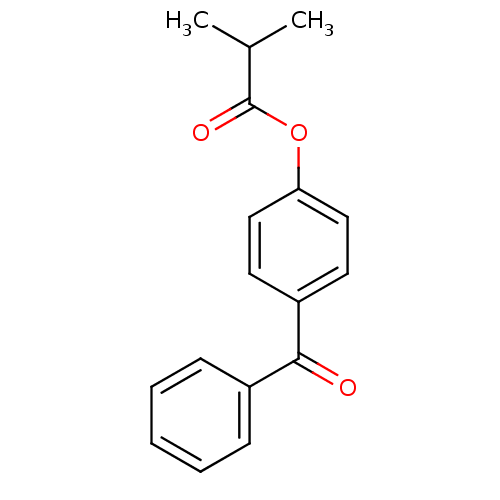 (CHEMBL10348 | Isobutyric acid 4-benzoyl-phenyl est...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against human leukocyte elastase at 10e-7 M | J Med Chem 31: 1052-61 (1988) BindingDB Entry DOI: 10.7270/Q2PC31DJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM480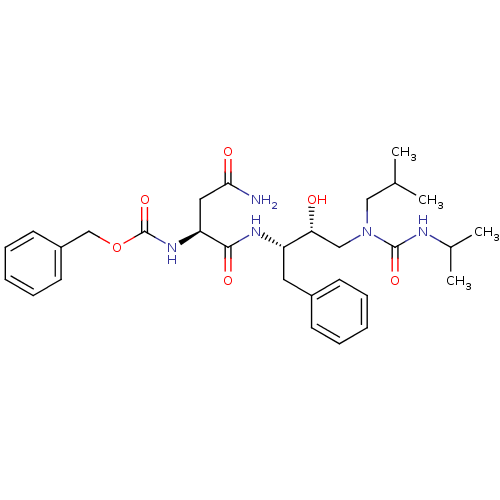 ((Hydroxyethyl)urea Isostere deriv. 8 | benzyl N-[(...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | 6.4 | 25 |
Monsanto Corporate Research | Assay Description IC50 values for inhibition of recombinant HIV protease were determined using the spectrofluorometric assay, which utilized an intramolecularly quench... | J Med Chem 36: 288-91 (1993) Article DOI: 10.1021/jm00054a014 BindingDB Entry DOI: 10.7270/Q2WW7FTF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50010500 (1-[3-(2-Benzyloxycarbonylamino-3-carbamoyl-propion...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description In vitro binding affinity of the compound against HIV protease was measured | J Med Chem 34: 1222-5 (1991) BindingDB Entry DOI: 10.7270/Q2057DWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 107 total ) | Next | Last >> |