 Found 91 hits with Last Name = 'de alencastro' and Initial = 'rb'
Found 91 hits with Last Name = 'de alencastro' and Initial = 'rb' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50481036
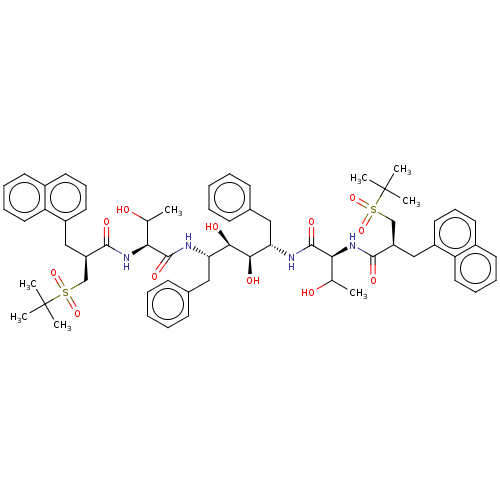
(CHEMBL568589)Show SMILES CC(O)[C@H](NC(=O)[C@H](Cc1cccc2ccccc12)CS(=O)(=O)C(C)(C)C)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)[C@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)[C@H](Cc1cccc2ccccc12)CS(=O)(=O)C(C)(C)C)C(C)O |r| Show InChI InChI=1S/C62H78N4O12S2/c1-39(67)53(65-57(71)47(37-79(75,76)61(3,4)5)35-45-29-19-27-43-25-15-17-31-49(43)45)59(73)63-51(33-41-21-11-9-12-22-41)55(69)56(70)52(34-42-23-13-10-14-24-42)64-60(74)54(40(2)68)66-58(72)48(38-80(77,78)62(6,7)8)36-46-30-20-28-44-26-16-18-32-50(44)46/h9-32,39-40,47-48,51-56,67-70H,33-38H2,1-8H3,(H,63,73)(H,64,74)(H,65,71)(H,66,72)/t39?,40?,47-,48-,51+,52+,53+,54+,55-,56-/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.302 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de Lavras
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease expressed in Escherichia coli K12 assessed as inhibition of enzyme activity |
Eur J Med Chem 44: 4344-52 (2009)
Article DOI: 10.1016/j.ejmech.2009.05.016
BindingDB Entry DOI: 10.7270/Q2JH3Q0K |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50481028
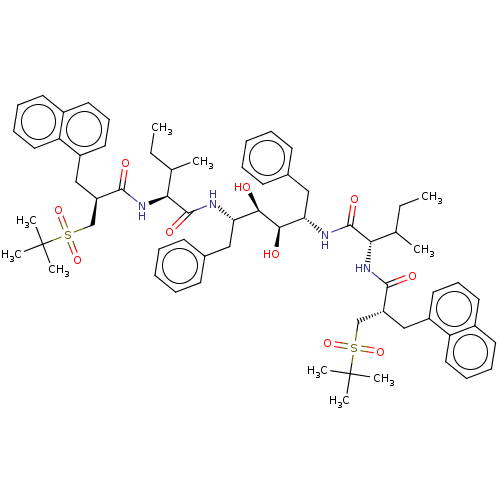
(CHEMBL566620)Show SMILES CCC(C)[C@H](NC(=O)[C@H](Cc1cccc2ccccc12)CS(=O)(=O)C(C)(C)C)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)[C@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)[C@H](Cc1cccc2ccccc12)CS(=O)(=O)C(C)(C)C)C(C)CC |r| Show InChI InChI=1S/C66H86N4O10S2/c1-11-43(3)57(69-61(73)51(41-81(77,78)65(5,6)7)39-49-33-23-31-47-29-19-21-35-53(47)49)63(75)67-55(37-45-25-15-13-16-26-45)59(71)60(72)56(38-46-27-17-14-18-28-46)68-64(76)58(44(4)12-2)70-62(74)52(42-82(79,80)66(8,9)10)40-50-34-24-32-48-30-20-22-36-54(48)50/h13-36,43-44,51-52,55-60,71-72H,11-12,37-42H2,1-10H3,(H,67,75)(H,68,76)(H,69,73)(H,70,74)/t43?,44?,51-,52-,55+,56+,57+,58+,59-,60-/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.302 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de Lavras
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease expressed in Escherichia coli K12 assessed as inhibition of enzyme activity |
Eur J Med Chem 44: 4344-52 (2009)
Article DOI: 10.1016/j.ejmech.2009.05.016
BindingDB Entry DOI: 10.7270/Q2JH3Q0K |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50481018
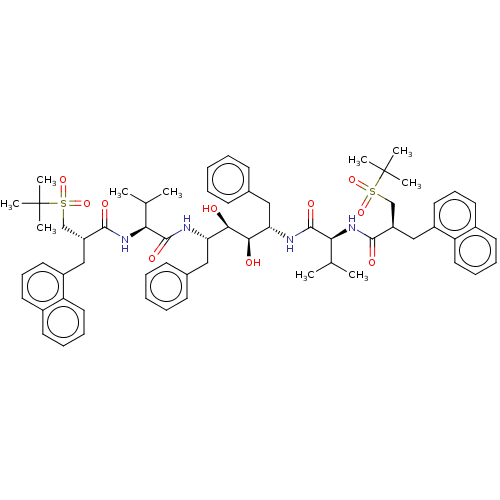
(CHEMBL568883)Show SMILES CC(C)[C@H](NC(=O)[C@H](Cc1cccc2ccccc12)CS(=O)(=O)C(C)(C)C)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)[C@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)[C@H](Cc1cccc2ccccc12)CS(=O)(=O)C(C)(C)C)C(C)C |r| Show InChI InChI=1S/C64H82N4O10S2/c1-41(2)55(67-59(71)49(39-79(75,76)63(5,6)7)37-47-31-21-29-45-27-17-19-33-51(45)47)61(73)65-53(35-43-23-13-11-14-24-43)57(69)58(70)54(36-44-25-15-12-16-26-44)66-62(74)56(42(3)4)68-60(72)50(40-80(77,78)64(8,9)10)38-48-32-22-30-46-28-18-20-34-52(46)48/h11-34,41-42,49-50,53-58,69-70H,35-40H2,1-10H3,(H,65,73)(H,66,74)(H,67,71)(H,68,72)/t49-,50-,53+,54+,55+,56+,57-,58-/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.302 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de Lavras
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease expressed in Escherichia coli K12 assessed as inhibition of enzyme activity |
Eur J Med Chem 44: 4344-52 (2009)
Article DOI: 10.1016/j.ejmech.2009.05.016
BindingDB Entry DOI: 10.7270/Q2JH3Q0K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50481029
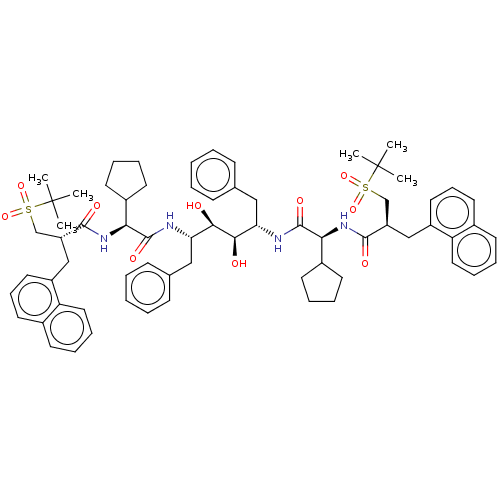
(CHEMBL569671)Show SMILES CC(C)(C)S(=O)(=O)C[C@@H](Cc1cccc2ccccc12)C(=O)N[C@@H](C1CCCC1)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)[C@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)[C@H](Cc1cccc2ccccc12)CS(=O)(=O)C(C)(C)C)C1CCCC1 |r| Show InChI InChI=1S/C68H86N4O10S2/c1-67(2,3)83(79,80)43-53(41-51-35-21-33-47-27-17-19-37-55(47)51)63(75)71-59(49-29-13-14-30-49)65(77)69-57(39-45-23-9-7-10-24-45)61(73)62(74)58(40-46-25-11-8-12-26-46)70-66(78)60(50-31-15-16-32-50)72-64(76)54(44-84(81,82)68(4,5)6)42-52-36-22-34-48-28-18-20-38-56(48)52/h7-12,17-28,33-38,49-50,53-54,57-62,73-74H,13-16,29-32,39-44H2,1-6H3,(H,69,77)(H,70,78)(H,71,75)(H,72,76)/t53-,54-,57+,58+,59+,60+,61-,62-/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.302 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de Lavras
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease expressed in Escherichia coli K12 assessed as inhibition of enzyme activity |
Eur J Med Chem 44: 4344-52 (2009)
Article DOI: 10.1016/j.ejmech.2009.05.016
BindingDB Entry DOI: 10.7270/Q2JH3Q0K |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50032162
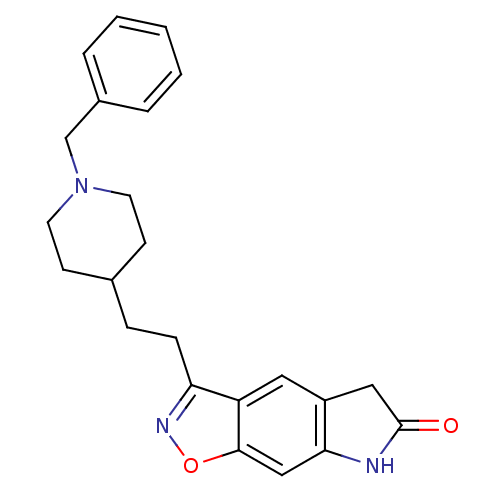
(3-[2-(1-Benzyl-piperidin-4-yl)-ethyl]-5,7-dihydro-...)Show SMILES O=C1Cc2cc3c(CCC4CCN(Cc5ccccc5)CC4)noc3cc2N1 Show InChI InChI=1S/C23H25N3O2/c27-23-13-18-12-19-20(25-28-22(19)14-21(18)24-23)7-6-16-8-10-26(11-9-16)15-17-4-2-1-3-5-17/h1-5,12,14,16H,6-11,13,15H2,(H,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.331 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
| Assay Description
Inhibition of human acetylcholinesterase |
Eur J Med Chem 46: 39-51 (2010)
Article DOI: 10.1016/j.ejmech.2010.10.009
BindingDB Entry DOI: 10.7270/Q2SX6FG0 |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50481014
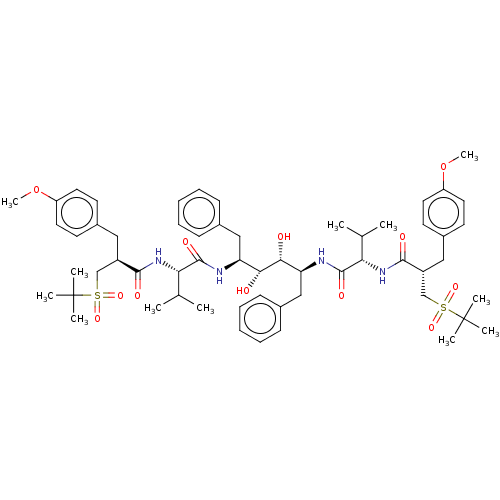
(CHEMBL568951)Show SMILES COc1ccc(C[C@H](CS(=O)(=O)C(C)(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc2ccccc2)[C@@H](O)[C@H](O)[C@H](Cc2ccccc2)NC(=O)[C@@H](NC(=O)[C@H](Cc2ccc(OC)cc2)CS(=O)(=O)C(C)(C)C)C(C)C)cc1 |r| Show InChI InChI=1S/C58H82N4O12S2/c1-37(2)49(61-53(65)43(35-75(69,70)57(5,6)7)31-41-23-27-45(73-11)28-24-41)55(67)59-47(33-39-19-15-13-16-20-39)51(63)52(64)48(34-40-21-17-14-18-22-40)60-56(68)50(38(3)4)62-54(66)44(36-76(71,72)58(8,9)10)32-42-25-29-46(74-12)30-26-42/h13-30,37-38,43-44,47-52,63-64H,31-36H2,1-12H3,(H,59,67)(H,60,68)(H,61,65)(H,62,66)/t43-,44-,47+,48+,49+,50+,51-,52-/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.339 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de Lavras
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease expressed in Escherichia coli K12 assessed as inhibition of enzyme activity |
Eur J Med Chem 44: 4344-52 (2009)
Article DOI: 10.1016/j.ejmech.2009.05.016
BindingDB Entry DOI: 10.7270/Q2JH3Q0K |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50481020
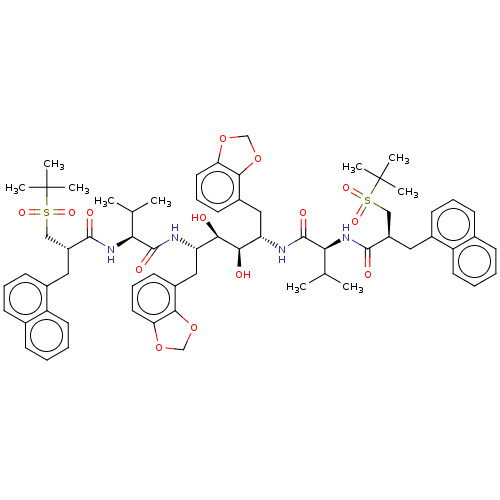
(CHEMBL565964)Show SMILES CC(C)[C@H](NC(=O)[C@H](Cc1cccc2ccccc12)CS(=O)(=O)C(C)(C)C)C(=O)N[C@@H](Cc1cccc2OCOc12)[C@@H](O)[C@H](O)[C@H](Cc1cccc2OCOc12)NC(=O)[C@@H](NC(=O)[C@H](Cc1cccc2ccccc12)CS(=O)(=O)C(C)(C)C)C(C)C |r| Show InChI InChI=1S/C66H82N4O14S2/c1-39(2)55(69-61(73)47(35-85(77,78)65(5,6)7)31-43-23-15-21-41-19-11-13-27-49(41)43)63(75)67-51(33-45-25-17-29-53-59(45)83-37-81-53)57(71)58(72)52(34-46-26-18-30-54-60(46)84-38-82-54)68-64(76)56(40(3)4)70-62(74)48(36-86(79,80)66(8,9)10)32-44-24-16-22-42-20-12-14-28-50(42)44/h11-30,39-40,47-48,51-52,55-58,71-72H,31-38H2,1-10H3,(H,67,75)(H,68,76)(H,69,73)(H,70,74)/t47-,48-,51+,52+,55+,56+,57-,58-/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de Lavras
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease expressed in Escherichia coli K12 assessed as inhibition of enzyme activity |
Eur J Med Chem 44: 4344-52 (2009)
Article DOI: 10.1016/j.ejmech.2009.05.016
BindingDB Entry DOI: 10.7270/Q2JH3Q0K |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50481023

(CHEMBL566849)Show SMILES CCC[C@H](NC(=O)[C@H](Cc1cccc2ccccc12)CS(=O)(=O)C(C)(C)C)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)[C@H](O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCC)NC(=O)[C@H](Cc1cccc2ccccc12)CS(=O)(=O)C(C)(C)C |r| Show InChI InChI=1S/C64H82N4O10S2/c1-9-23-53(65-59(71)49(41-79(75,76)63(3,4)5)39-47-33-21-31-45-29-17-19-35-51(45)47)61(73)67-55(37-43-25-13-11-14-26-43)57(69)58(70)56(38-44-27-15-12-16-28-44)68-62(74)54(24-10-2)66-60(72)50(42-80(77,78)64(6,7)8)40-48-34-22-32-46-30-18-20-36-52(46)48/h11-22,25-36,49-50,53-58,69-70H,9-10,23-24,37-42H2,1-8H3,(H,65,71)(H,66,72)(H,67,73)(H,68,74)/t49-,50-,53+,54+,55+,56+,57-,58-/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de Lavras
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease expressed in Escherichia coli K12 assessed as inhibition of enzyme activity |
Eur J Med Chem 44: 4344-52 (2009)
Article DOI: 10.1016/j.ejmech.2009.05.016
BindingDB Entry DOI: 10.7270/Q2JH3Q0K |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50032161
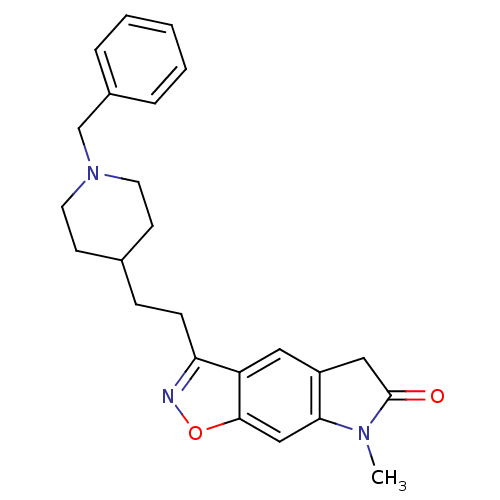
(3-[2-(1-Benzyl-piperidin-4-yl)-ethyl]-7-methyl-5,7...)Show SMILES CN1C(=O)Cc2cc3c(CCC4CCN(Cc5ccccc5)CC4)noc3cc12 Show InChI InChI=1S/C24H27N3O2/c1-26-22-15-23-20(13-19(22)14-24(26)28)21(25-29-23)8-7-17-9-11-27(12-10-17)16-18-5-3-2-4-6-18/h2-6,13,15,17H,7-12,14,16H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.479 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
| Assay Description
Inhibition of human acetylcholinesterase |
Eur J Med Chem 46: 39-51 (2010)
Article DOI: 10.1016/j.ejmech.2010.10.009
BindingDB Entry DOI: 10.7270/Q2SX6FG0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50417809
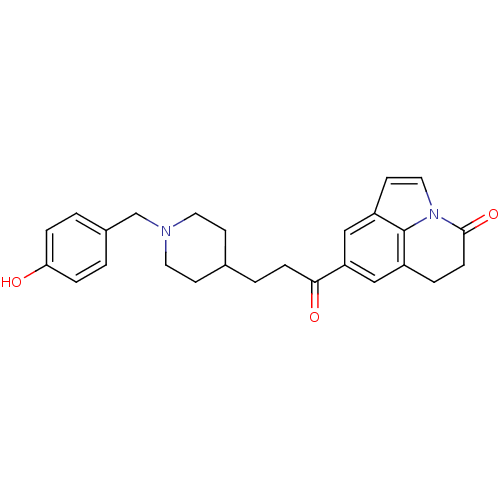
(CHEMBL1651249)Show SMILES Oc1ccc(CN2CCC(CCC(=O)c3cc4CCC(=O)n5ccc(c3)c45)CC2)cc1 Show InChI InChI=1S/C26H28N2O3/c29-23-5-1-19(2-6-23)17-27-12-9-18(10-13-27)3-7-24(30)22-15-20-4-8-25(31)28-14-11-21(16-22)26(20)28/h1-2,5-6,11,14-16,18,29H,3-4,7-10,12-13,17H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
| Assay Description
Inhibition of human acetylcholinesterase |
Eur J Med Chem 46: 39-51 (2010)
Article DOI: 10.1016/j.ejmech.2010.10.009
BindingDB Entry DOI: 10.7270/Q2SX6FG0 |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50481040
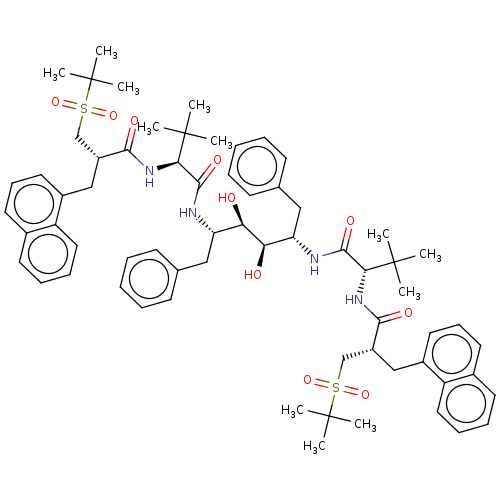
(CHEMBL566842)Show SMILES CC(C)(C)[C@H](NC(=O)[C@H](Cc1cccc2ccccc12)CS(=O)(=O)C(C)(C)C)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)[C@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)[C@H](Cc1cccc2ccccc12)CS(=O)(=O)C(C)(C)C)C(C)(C)C |r| Show InChI InChI=1S/C66H86N4O10S2/c1-63(2,3)57(69-59(73)49(41-81(77,78)65(7,8)9)39-47-33-23-31-45-29-19-21-35-51(45)47)61(75)67-53(37-43-25-15-13-16-26-43)55(71)56(72)54(38-44-27-17-14-18-28-44)68-62(76)58(64(4,5)6)70-60(74)50(42-82(79,80)66(10,11)12)40-48-34-24-32-46-30-20-22-36-52(46)48/h13-36,49-50,53-58,71-72H,37-42H2,1-12H3,(H,67,75)(H,68,76)(H,69,73)(H,70,74)/t49-,50-,53+,54+,55-,56-,57-,58-/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de Lavras
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease expressed in Escherichia coli K12 assessed as inhibition of enzyme activity |
Eur J Med Chem 44: 4344-52 (2009)
Article DOI: 10.1016/j.ejmech.2009.05.016
BindingDB Entry DOI: 10.7270/Q2JH3Q0K |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50032164
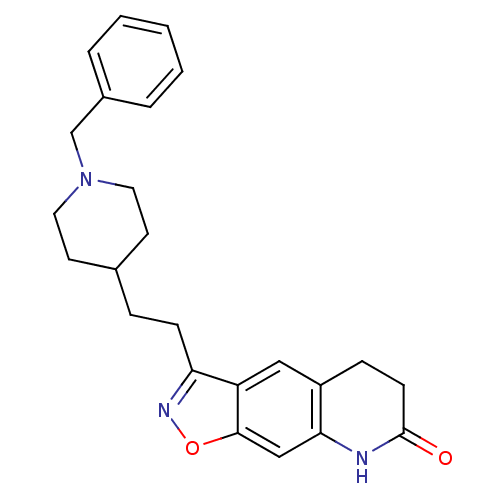
(3-(2-(1-benzylpiperidin-4-yl)ethyl)-5,6-dihydroiso...)Show SMILES O=C1CCc2cc3c(CCC4CCN(Cc5ccccc5)CC4)noc3cc2N1 Show InChI InChI=1S/C24H27N3O2/c28-24-9-7-19-14-20-21(26-29-23(20)15-22(19)25-24)8-6-17-10-12-27(13-11-17)16-18-4-2-1-3-5-18/h1-5,14-15,17H,6-13,16H2,(H,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.575 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
| Assay Description
Inhibition of human acetylcholinesterase |
Eur J Med Chem 46: 39-51 (2010)
Article DOI: 10.1016/j.ejmech.2010.10.009
BindingDB Entry DOI: 10.7270/Q2SX6FG0 |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50481024
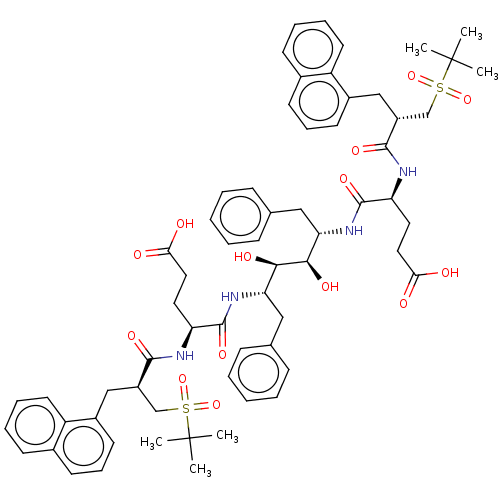
(CHEMBL566902)Show SMILES CC(C)(C)S(=O)(=O)C[C@@H](Cc1cccc2ccccc12)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)[C@H](O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1cccc2ccccc12)CS(=O)(=O)C(C)(C)C |r| Show InChI InChI=1S/C64H78N4O14S2/c1-63(2,3)83(79,80)39-47(37-45-27-17-25-43-23-13-15-29-49(43)45)59(75)65-51(31-33-55(69)70)61(77)67-53(35-41-19-9-7-10-20-41)57(73)58(74)54(36-42-21-11-8-12-22-42)68-62(78)52(32-34-56(71)72)66-60(76)48(40-84(81,82)64(4,5)6)38-46-28-18-26-44-24-14-16-30-50(44)46/h7-30,47-48,51-54,57-58,73-74H,31-40H2,1-6H3,(H,65,75)(H,66,76)(H,67,77)(H,68,78)(H,69,70)(H,71,72)/t47-,48-,51+,52+,53+,54+,57-,58-/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.741 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de Lavras
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease expressed in Escherichia coli K12 assessed as inhibition of enzyme activity |
Eur J Med Chem 44: 4344-52 (2009)
Article DOI: 10.1016/j.ejmech.2009.05.016
BindingDB Entry DOI: 10.7270/Q2JH3Q0K |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50417798
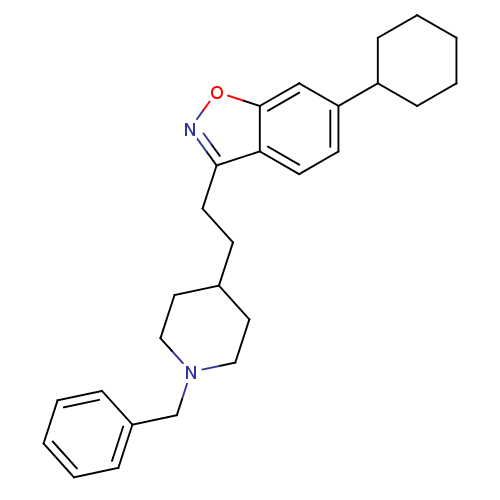
(CHEMBL1651247)Show SMILES C(Cc1noc2cc(ccc12)C1CCCCC1)C1CCN(Cc2ccccc2)CC1 Show InChI InChI=1S/C27H34N2O/c1-3-7-22(8-4-1)20-29-17-15-21(16-18-29)11-14-26-25-13-12-24(19-27(25)30-28-26)23-9-5-2-6-10-23/h1,3-4,7-8,12-13,19,21,23H,2,5-6,9-11,14-18,20H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
| Assay Description
Inhibition of human acetylcholinesterase |
Eur J Med Chem 46: 39-51 (2010)
Article DOI: 10.1016/j.ejmech.2010.10.009
BindingDB Entry DOI: 10.7270/Q2SX6FG0 |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50481015
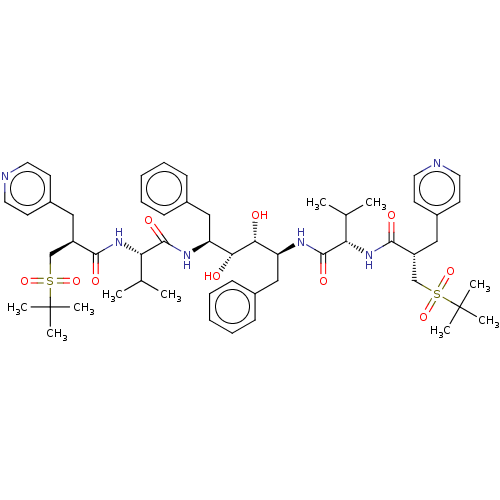
(CHEMBL568832)Show SMILES CC(C)[C@H](NC(=O)[C@H](Cc1ccncc1)CS(=O)(=O)C(C)(C)C)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)[C@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccncc1)CS(=O)(=O)C(C)(C)C)C(C)C |r| Show InChI InChI=1S/C54H76N6O10S2/c1-35(2)45(59-49(63)41(29-39-21-25-55-26-22-39)33-71(67,68)53(5,6)7)51(65)57-43(31-37-17-13-11-14-18-37)47(61)48(62)44(32-38-19-15-12-16-20-38)58-52(66)46(36(3)4)60-50(64)42(30-40-23-27-56-28-24-40)34-72(69,70)54(8,9)10/h11-28,35-36,41-48,61-62H,29-34H2,1-10H3,(H,57,65)(H,58,66)(H,59,63)(H,60,64)/t41-,42-,43+,44+,45+,46+,47-,48-/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de Lavras
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease expressed in Escherichia coli K12 assessed as inhibition of enzyme activity |
Eur J Med Chem 44: 4344-52 (2009)
Article DOI: 10.1016/j.ejmech.2009.05.016
BindingDB Entry DOI: 10.7270/Q2JH3Q0K |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50481017
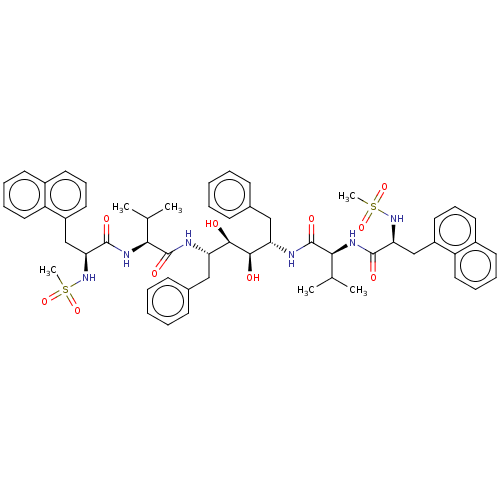
(CHEMBL568840)Show SMILES CC(C)[C@H](NC(=O)[C@H](Cc1cccc2ccccc12)NS(C)(=O)=O)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)[C@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)[C@H](Cc1cccc2ccccc12)NS(C)(=O)=O)C(C)C |r| Show InChI InChI=1S/C56H68N6O10S2/c1-35(2)49(59-53(65)47(61-73(5,69)70)33-41-27-17-25-39-23-13-15-29-43(39)41)55(67)57-45(31-37-19-9-7-10-20-37)51(63)52(64)46(32-38-21-11-8-12-22-38)58-56(68)50(36(3)4)60-54(66)48(62-74(6,71)72)34-42-28-18-26-40-24-14-16-30-44(40)42/h7-30,35-36,45-52,61-64H,31-34H2,1-6H3,(H,57,67)(H,58,68)(H,59,65)(H,60,66)/t45-,46-,47-,48-,49-,50-,51+,52+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de Lavras
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease expressed in Escherichia coli K12 assessed as inhibition of enzyme activity |
Eur J Med Chem 44: 4344-52 (2009)
Article DOI: 10.1016/j.ejmech.2009.05.016
BindingDB Entry DOI: 10.7270/Q2JH3Q0K |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50481025
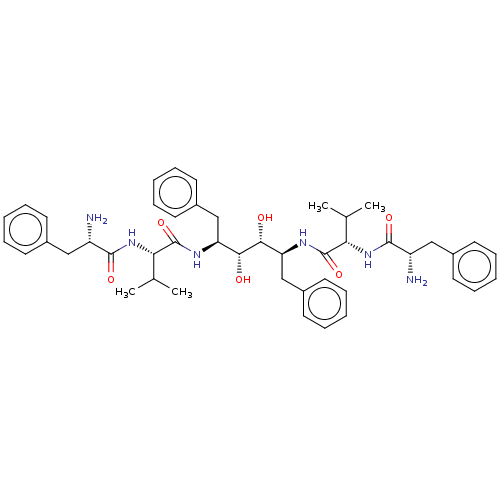
(CHEMBL567444)Show SMILES CC(C)[C@H](NC(=O)[C@@H](N)Cc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)[C@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)[C@@H](N)Cc1ccccc1)C(C)C |r| Show InChI InChI=1S/C46H60N6O6/c1-29(2)39(51-43(55)35(47)25-31-17-9-5-10-18-31)45(57)49-37(27-33-21-13-7-14-22-33)41(53)42(54)38(28-34-23-15-8-16-24-34)50-46(58)40(30(3)4)52-44(56)36(48)26-32-19-11-6-12-20-32/h5-24,29-30,35-42,53-54H,25-28,47-48H2,1-4H3,(H,49,57)(H,50,58)(H,51,55)(H,52,56)/t35-,36-,37-,38-,39-,40-,41+,42+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.851 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de Lavras
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease expressed in Escherichia coli K12 assessed as inhibition of enzyme activity |
Eur J Med Chem 44: 4344-52 (2009)
Article DOI: 10.1016/j.ejmech.2009.05.016
BindingDB Entry DOI: 10.7270/Q2JH3Q0K |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50481033
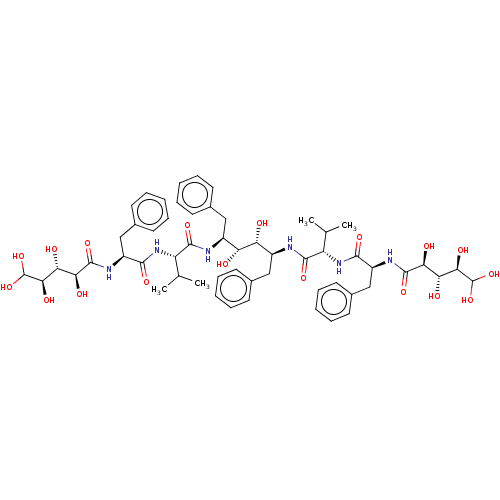
(CHEMBL565528)Show SMILES CC(C)[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](O)[C@@H](O)[C@@H](O)C(O)O)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)[C@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](O)[C@@H](O)[C@@H](O)C(O)O)C(C)C |r| Show InChI InChI=1S/C56H76N6O18/c1-29(2)39(61-49(71)37(27-33-21-13-7-14-22-33)59-53(75)45(67)43(65)47(69)55(77)78)51(73)57-35(25-31-17-9-5-10-18-31)41(63)42(64)36(26-32-19-11-6-12-20-32)58-52(74)40(30(3)4)62-50(72)38(28-34-23-15-8-16-24-34)60-54(76)46(68)44(66)48(70)56(79)80/h5-24,29-30,35-48,55-56,63-70,77-80H,25-28H2,1-4H3,(H,57,73)(H,58,74)(H,59,75)(H,60,76)(H,61,71)(H,62,72)/t35-,36-,37-,38-,39-,40-,41+,42+,43+,44+,45-,46-,47+,48+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.891 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de Lavras
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease expressed in Escherichia coli K12 assessed as inhibition of enzyme activity |
Eur J Med Chem 44: 4344-52 (2009)
Article DOI: 10.1016/j.ejmech.2009.05.016
BindingDB Entry DOI: 10.7270/Q2JH3Q0K |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50032163
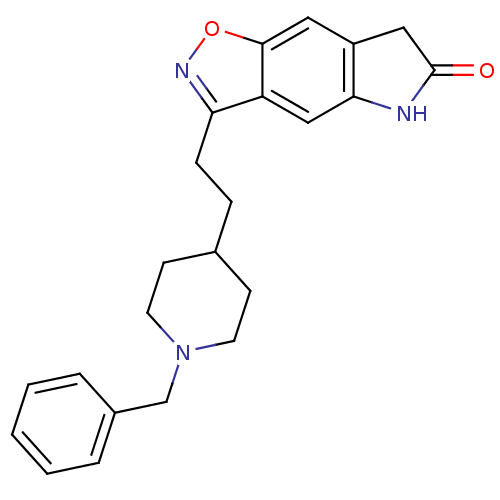
(3-(2-(1-benzylpiperidin-4-yl)ethyl)-5H-isoxazolo[5...)Show SMILES O=C1Cc2cc3onc(CCC4CCN(Cc5ccccc5)CC4)c3cc2N1 Show InChI InChI=1S/C23H25N3O2/c27-23-13-18-12-22-19(14-21(18)24-23)20(25-28-22)7-6-16-8-10-26(11-9-16)15-17-4-2-1-3-5-17/h1-5,12,14,16H,6-11,13,15H2,(H,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.955 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
| Assay Description
Inhibition of human acetylcholinesterase |
Eur J Med Chem 46: 39-51 (2010)
Article DOI: 10.1016/j.ejmech.2010.10.009
BindingDB Entry DOI: 10.7270/Q2SX6FG0 |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50481035
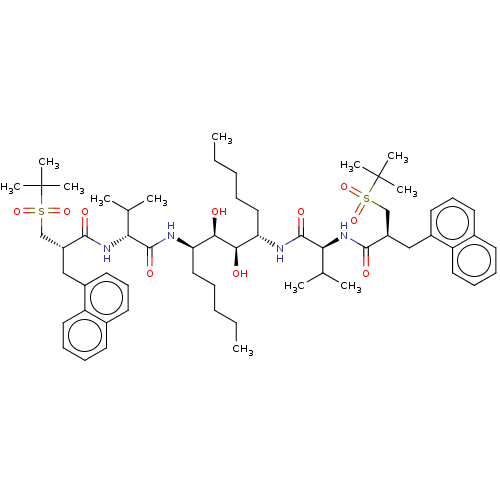
(CHEMBL569670)Show SMILES CCCCC[C@@H](NC(=O)[C@H](NC(=O)[C@H](Cc1cccc2ccccc12)CS(=O)(=O)C(C)(C)C)C(C)C)[C@@H](O)[C@H](O)[C@H](CCCCC)NC(=O)[C@@H](NC(=O)[C@H](Cc1cccc2ccccc12)CS(=O)(=O)C(C)(C)C)C(C)C |r| Show InChI InChI=1S/C60H90N4O10S2/c1-13-15-17-33-49(61-57(69)51(39(3)4)63-55(67)45(37-75(71,72)59(7,8)9)35-43-29-23-27-41-25-19-21-31-47(41)43)53(65)54(66)50(34-18-16-14-2)62-58(70)52(40(5)6)64-56(68)46(38-76(73,74)60(10,11)12)36-44-30-24-28-42-26-20-22-32-48(42)44/h19-32,39-40,45-46,49-54,65-66H,13-18,33-38H2,1-12H3,(H,61,69)(H,62,70)(H,63,67)(H,64,68)/t45-,46-,49-,50+,51-,52+,53-,54-/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de Lavras
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease expressed in Escherichia coli K12 assessed as inhibition of enzyme activity |
Eur J Med Chem 44: 4344-52 (2009)
Article DOI: 10.1016/j.ejmech.2009.05.016
BindingDB Entry DOI: 10.7270/Q2JH3Q0K |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50417791
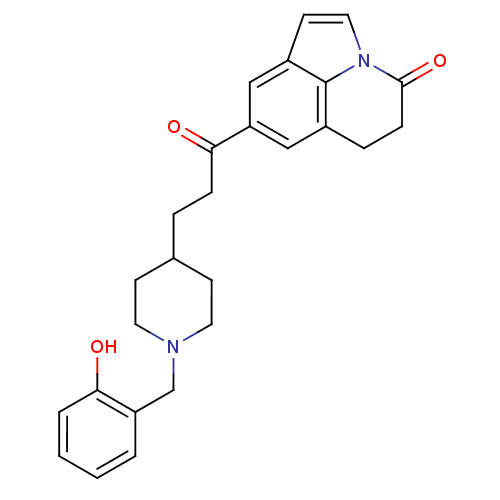
(CHEMBL1651139)Show SMILES Oc1ccccc1CN1CCC(CCC(=O)c2cc3CCC(=O)n4ccc(c2)c34)CC1 Show InChI InChI=1S/C26H28N2O3/c29-23-4-2-1-3-21(23)17-27-12-9-18(10-13-27)5-7-24(30)22-15-19-6-8-25(31)28-14-11-20(16-22)26(19)28/h1-4,11,14-16,18,29H,5-10,12-13,17H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
| Assay Description
Inhibition of human acetylcholinesterase |
Eur J Med Chem 46: 39-51 (2010)
Article DOI: 10.1016/j.ejmech.2010.10.009
BindingDB Entry DOI: 10.7270/Q2SX6FG0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50417787
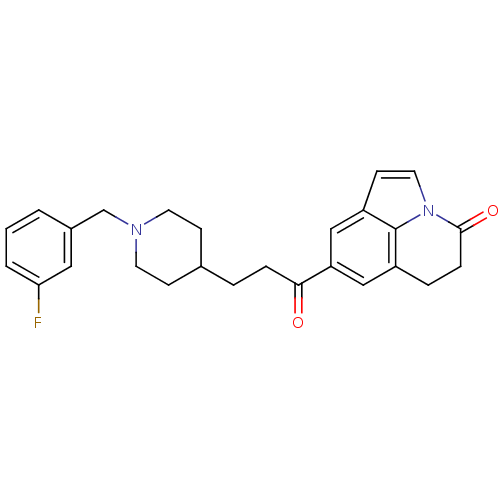
(CHEMBL1651132)Show SMILES Fc1cccc(CN2CCC(CCC(=O)c3cc4CCC(=O)n5ccc(c3)c45)CC2)c1 Show InChI InChI=1S/C26H27FN2O2/c27-23-3-1-2-19(14-23)17-28-11-8-18(9-12-28)4-6-24(30)22-15-20-5-7-25(31)29-13-10-21(16-22)26(20)29/h1-3,10,13-16,18H,4-9,11-12,17H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.29 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
| Assay Description
Inhibition of human acetylcholinesterase |
Eur J Med Chem 46: 39-51 (2010)
Article DOI: 10.1016/j.ejmech.2010.10.009
BindingDB Entry DOI: 10.7270/Q2SX6FG0 |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50481037
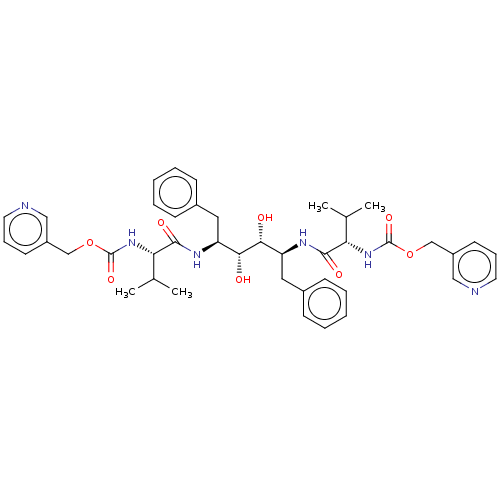
(CHEMBL569236)Show SMILES CC(C)[C@H](NC(=O)OCc1cccnc1)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)[C@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)OCc1cccnc1)C(C)C |r| Show InChI InChI=1S/C42H52N6O8/c1-27(2)35(47-41(53)55-25-31-17-11-19-43-23-31)39(51)45-33(21-29-13-7-5-8-14-29)37(49)38(50)34(22-30-15-9-6-10-16-30)46-40(52)36(28(3)4)48-42(54)56-26-32-18-12-20-44-24-32/h5-20,23-24,27-28,33-38,49-50H,21-22,25-26H2,1-4H3,(H,45,51)(H,46,52)(H,47,53)(H,48,54)/t33-,34-,35-,36-,37+,38+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de Lavras
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease expressed in Escherichia coli K12 assessed as inhibition of enzyme activity |
Eur J Med Chem 44: 4344-52 (2009)
Article DOI: 10.1016/j.ejmech.2009.05.016
BindingDB Entry DOI: 10.7270/Q2JH3Q0K |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50481032
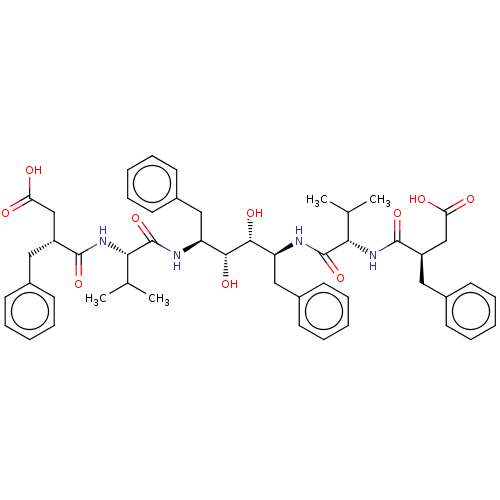
(CHEMBL569569)Show SMILES CC(C)[C@H](NC(=O)[C@@H](CC(O)=O)Cc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)[C@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)[C@@H](CC(O)=O)Cc1ccccc1)C(C)C |r| Show InChI InChI=1S/C50H62N4O10/c1-31(2)43(53-47(61)37(29-41(55)56)25-33-17-9-5-10-18-33)49(63)51-39(27-35-21-13-7-14-22-35)45(59)46(60)40(28-36-23-15-8-16-24-36)52-50(64)44(32(3)4)54-48(62)38(30-42(57)58)26-34-19-11-6-12-20-34/h5-24,31-32,37-40,43-46,59-60H,25-30H2,1-4H3,(H,51,63)(H,52,64)(H,53,61)(H,54,62)(H,55,56)(H,57,58)/t37-,38-,39+,40+,43+,44+,45-,46-/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de Lavras
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease expressed in Escherichia coli K12 assessed as inhibition of enzyme activity |
Eur J Med Chem 44: 4344-52 (2009)
Article DOI: 10.1016/j.ejmech.2009.05.016
BindingDB Entry DOI: 10.7270/Q2JH3Q0K |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50481022
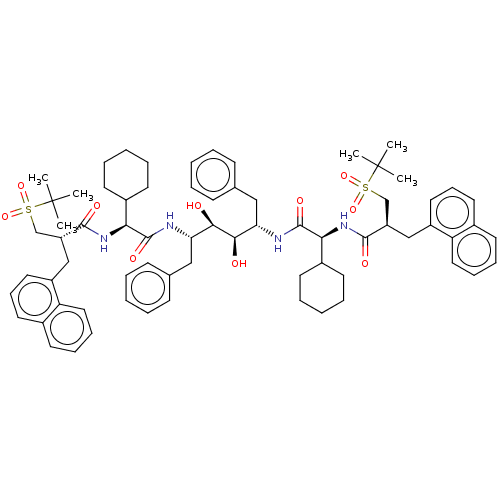
(CHEMBL568365)Show SMILES CC(C)(C)S(=O)(=O)C[C@@H](Cc1cccc2ccccc12)C(=O)N[C@@H](C1CCCCC1)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)[C@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)[C@H](Cc1cccc2ccccc12)CS(=O)(=O)C(C)(C)C)C1CCCCC1 |r| Show InChI InChI=1S/C70H90N4O10S2/c1-69(2,3)85(81,82)45-55(43-53-37-23-35-49-29-19-21-39-57(49)53)65(77)73-61(51-31-15-9-16-32-51)67(79)71-59(41-47-25-11-7-12-26-47)63(75)64(76)60(42-48-27-13-8-14-28-48)72-68(80)62(52-33-17-10-18-34-52)74-66(78)56(46-86(83,84)70(4,5)6)44-54-38-24-36-50-30-20-22-40-58(50)54/h7-8,11-14,19-30,35-40,51-52,55-56,59-64,75-76H,9-10,15-18,31-34,41-46H2,1-6H3,(H,71,79)(H,72,80)(H,73,77)(H,74,78)/t55-,56-,59+,60+,61+,62+,63-,64-/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de Lavras
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease expressed in Escherichia coli K12 assessed as inhibition of enzyme activity |
Eur J Med Chem 44: 4344-52 (2009)
Article DOI: 10.1016/j.ejmech.2009.05.016
BindingDB Entry DOI: 10.7270/Q2JH3Q0K |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM586099
(BDBM50064200 | TL-3)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)[C@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)OCc1ccccc1)C(C)C | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de Lavras
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease expressed in Escherichia coli K12 assessed as inhibition of enzyme activity |
Eur J Med Chem 44: 4344-52 (2009)
Article DOI: 10.1016/j.ejmech.2009.05.016
BindingDB Entry DOI: 10.7270/Q2JH3Q0K |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50481019
(CHEMBL571731)Show SMILES CC(C)[C@H](NC(=O)[C@H](Cc1cccc2ccccc12)CS(=O)(=O)C(C)(C)C)C(=O)NC[C@@H](O)[C@H](O)CNC(=O)[C@@H](NC(=O)[C@H](Cc1cccc2ccccc12)CS(=O)(=O)C(C)(C)C)C(C)C |r| Show InChI InChI=1S/C50H70N4O10S2/c1-31(2)43(53-45(57)37(29-65(61,62)49(5,6)7)25-35-21-15-19-33-17-11-13-23-39(33)35)47(59)51-27-41(55)42(56)28-52-48(60)44(32(3)4)54-46(58)38(30-66(63,64)50(8,9)10)26-36-22-16-20-34-18-12-14-24-40(34)36/h11-24,31-32,37-38,41-44,55-56H,25-30H2,1-10H3,(H,51,59)(H,52,60)(H,53,57)(H,54,58)/t37-,38-,41-,42-,43+,44+/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de Lavras
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease expressed in Escherichia coli K12 assessed as inhibition of enzyme activity |
Eur J Med Chem 44: 4344-52 (2009)
Article DOI: 10.1016/j.ejmech.2009.05.016
BindingDB Entry DOI: 10.7270/Q2JH3Q0K |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50417786
(CHEMBL1651131)Show SMILES Fc1ccccc1CN1CCC(CCC(=O)c2cc3CCC(=O)n4ccc(c2)c34)CC1 Show InChI InChI=1S/C26H27FN2O2/c27-23-4-2-1-3-21(23)17-28-12-9-18(10-13-28)5-7-24(30)22-15-19-6-8-25(31)29-14-11-20(16-22)26(19)29/h1-4,11,14-16,18H,5-10,12-13,17H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.51 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
| Assay Description
Inhibition of human acetylcholinesterase |
Eur J Med Chem 46: 39-51 (2010)
Article DOI: 10.1016/j.ejmech.2010.10.009
BindingDB Entry DOI: 10.7270/Q2SX6FG0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50032165
(CHEMBL92736 | CHEMBL94217 | N-{3-[2-(1-Benzyl-pipe...)Show SMILES CC(=O)Nc1ccc2c(CCC3CCN(Cc4ccccc4)CC3)noc2c1 Show InChI InChI=1S/C23H27N3O2/c1-17(27)24-20-8-9-21-22(25-28-23(21)15-20)10-7-18-11-13-26(14-12-18)16-19-5-3-2-4-6-19/h2-6,8-9,15,18H,7,10-14,16H2,1H3,(H,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.82 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
| Assay Description
Inhibition of human acetylcholinesterase |
Eur J Med Chem 46: 39-51 (2010)
Article DOI: 10.1016/j.ejmech.2010.10.009
BindingDB Entry DOI: 10.7270/Q2SX6FG0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50417793
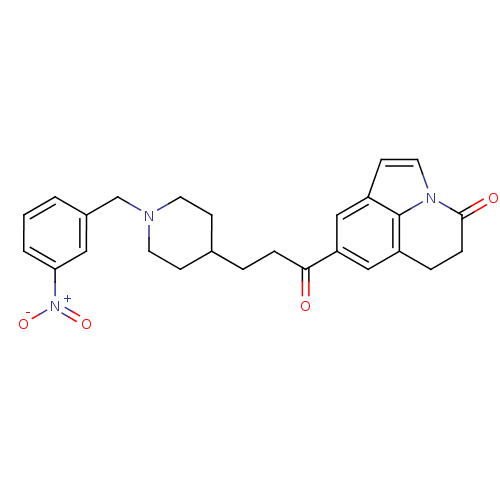
(CHEMBL1651243)Show SMILES [O-][N+](=O)c1cccc(CN2CCC(CCC(=O)c3cc4CCC(=O)n5ccc(c3)c45)CC2)c1 Show InChI InChI=1S/C26H27N3O4/c30-24(22-15-20-5-7-25(31)28-13-10-21(16-22)26(20)28)6-4-18-8-11-27(12-9-18)17-19-2-1-3-23(14-19)29(32)33/h1-3,10,13-16,18H,4-9,11-12,17H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.88 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
| Assay Description
Inhibition of human acetylcholinesterase |
Eur J Med Chem 46: 39-51 (2010)
Article DOI: 10.1016/j.ejmech.2010.10.009
BindingDB Entry DOI: 10.7270/Q2SX6FG0 |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50481031
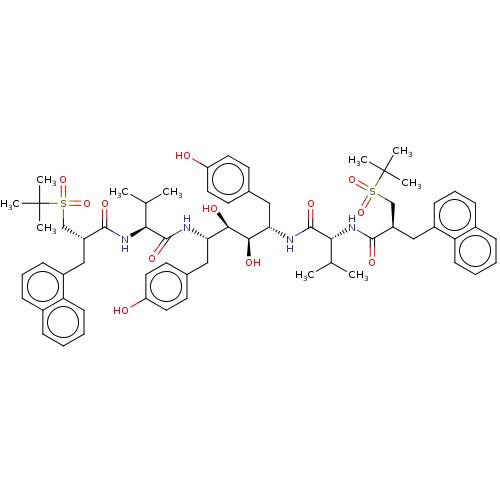
(CHEMBL567043)Show SMILES CC(C)[C@H](NC(=O)[C@H](Cc1cccc2ccccc12)CS(=O)(=O)C(C)(C)C)C(=O)N[C@@H](Cc1ccc(O)cc1)[C@@H](O)[C@H](O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](NC(=O)[C@H](Cc1cccc2ccccc12)CS(=O)(=O)C(C)(C)C)C(C)C |r| Show InChI InChI=1S/C64H82N4O12S2/c1-39(2)55(67-59(73)47(37-81(77,78)63(5,6)7)35-45-21-15-19-43-17-11-13-23-51(43)45)61(75)65-53(33-41-25-29-49(69)30-26-41)57(71)58(72)54(34-42-27-31-50(70)32-28-42)66-62(76)56(40(3)4)68-60(74)48(38-82(79,80)64(8,9)10)36-46-22-16-20-44-18-12-14-24-52(44)46/h11-32,39-40,47-48,53-58,69-72H,33-38H2,1-10H3,(H,65,75)(H,66,76)(H,67,73)(H,68,74)/t47-,48-,53+,54+,55-,56+,57-,58-/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de Lavras
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease expressed in Escherichia coli K12 assessed as inhibition of enzyme activity |
Eur J Med Chem 44: 4344-52 (2009)
Article DOI: 10.1016/j.ejmech.2009.05.016
BindingDB Entry DOI: 10.7270/Q2JH3Q0K |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50481041
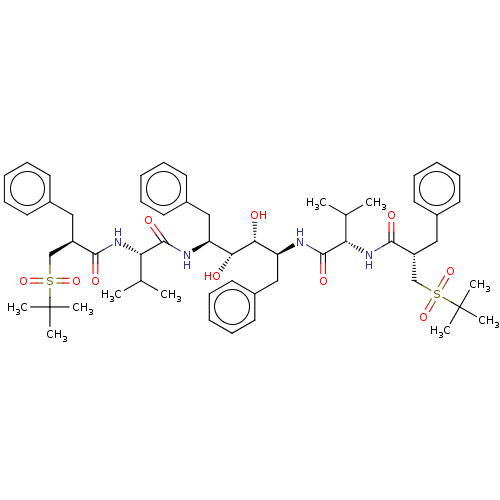
(CHEMBL569673)Show SMILES CC(C)[C@H](NC(=O)[C@H](Cc1ccccc1)CS(=O)(=O)C(C)(C)C)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)[C@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)CS(=O)(=O)C(C)(C)C)C(C)C |r| Show InChI InChI=1S/C56H78N4O10S2/c1-37(2)47(59-51(63)43(31-39-23-15-11-16-24-39)35-71(67,68)55(5,6)7)53(65)57-45(33-41-27-19-13-20-28-41)49(61)50(62)46(34-42-29-21-14-22-30-42)58-54(66)48(38(3)4)60-52(64)44(32-40-25-17-12-18-26-40)36-72(69,70)56(8,9)10/h11-30,37-38,43-50,61-62H,31-36H2,1-10H3,(H,57,65)(H,58,66)(H,59,63)(H,60,64)/t43-,44-,45+,46+,47+,48+,49-,50-/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de Lavras
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease expressed in Escherichia coli K12 assessed as inhibition of enzyme activity |
Eur J Med Chem 44: 4344-52 (2009)
Article DOI: 10.1016/j.ejmech.2009.05.016
BindingDB Entry DOI: 10.7270/Q2JH3Q0K |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50417785
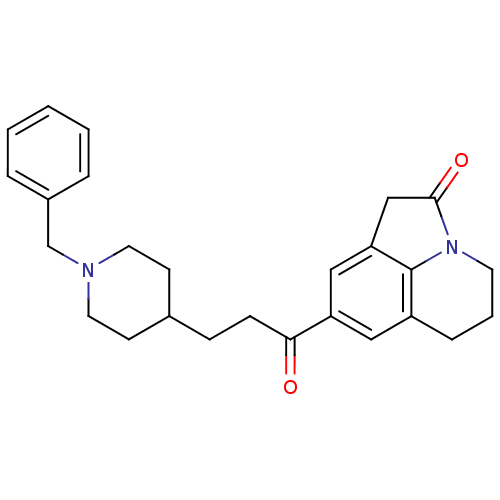
(CHEMBL1651129)Show SMILES O=C(CCC1CCN(Cc2ccccc2)CC1)c1cc2CC(=O)N3CCCc(c1)c23 Show InChI InChI=1S/C26H30N2O2/c29-24(22-15-21-7-4-12-28-25(30)17-23(16-22)26(21)28)9-8-19-10-13-27(14-11-19)18-20-5-2-1-3-6-20/h1-3,5-6,15-16,19H,4,7-14,17-18H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.63 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
| Assay Description
Inhibition of human acetylcholinesterase |
Eur J Med Chem 46: 39-51 (2010)
Article DOI: 10.1016/j.ejmech.2010.10.009
BindingDB Entry DOI: 10.7270/Q2SX6FG0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50032160
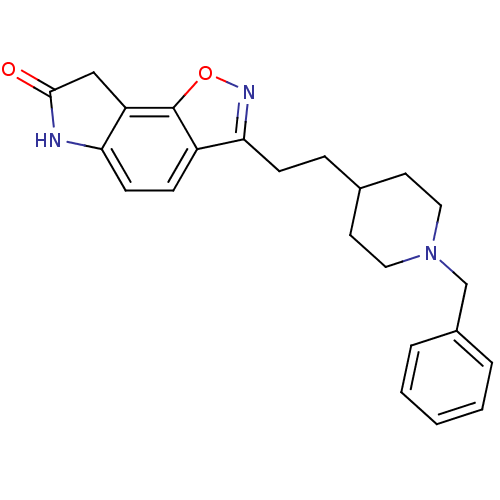
(3-(2-(1-benzylpiperidin-4-yl)ethyl)-6H-isoxazolo[5...)Show SMILES O=C1Cc2c(N1)ccc1c(CCC3CCN(Cc4ccccc4)CC3)noc21 Show InChI InChI=1S/C23H25N3O2/c27-22-14-19-20(24-22)9-7-18-21(25-28-23(18)19)8-6-16-10-12-26(13-11-16)15-17-4-2-1-3-5-17/h1-5,7,9,16H,6,8,10-15H2,(H,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.63 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
| Assay Description
Inhibition of human acetylcholinesterase |
Eur J Med Chem 46: 39-51 (2010)
Article DOI: 10.1016/j.ejmech.2010.10.009
BindingDB Entry DOI: 10.7270/Q2SX6FG0 |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50481016
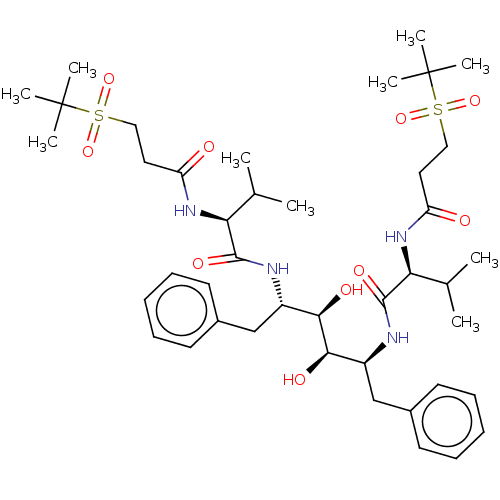
(CHEMBL568817)Show SMILES CC(C)[C@H](NC(=O)CCS(=O)(=O)C(C)(C)C)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)[C@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)CCS(=O)(=O)C(C)(C)C)C(C)C |r| Show InChI InChI=1S/C42H66N4O10S2/c1-27(2)35(45-33(47)21-23-57(53,54)41(5,6)7)39(51)43-31(25-29-17-13-11-14-18-29)37(49)38(50)32(26-30-19-15-12-16-20-30)44-40(52)36(28(3)4)46-34(48)22-24-58(55,56)42(8,9)10/h11-20,27-28,31-32,35-38,49-50H,21-26H2,1-10H3,(H,43,51)(H,44,52)(H,45,47)(H,46,48)/t31-,32-,35-,36-,37+,38+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de Lavras
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease expressed in Escherichia coli K12 assessed as inhibition of enzyme activity |
Eur J Med Chem 44: 4344-52 (2009)
Article DOI: 10.1016/j.ejmech.2009.05.016
BindingDB Entry DOI: 10.7270/Q2JH3Q0K |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50481038
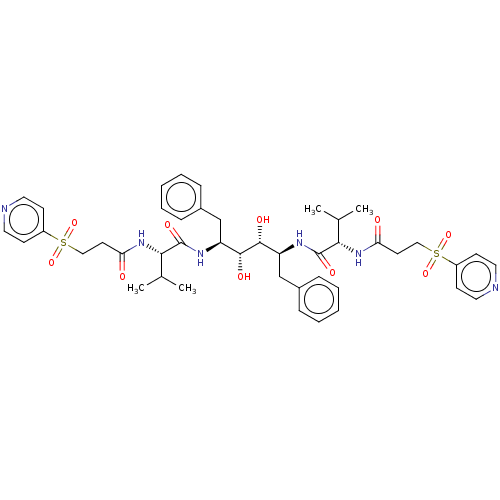
(CHEMBL568977)Show SMILES CC(C)[C@H](NC(=O)CCS(=O)(=O)c1ccncc1)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)[C@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)CCS(=O)(=O)c1ccncc1)C(C)C |r| Show InChI InChI=1S/C44H56N6O10S2/c1-29(2)39(49-37(51)19-25-61(57,58)33-15-21-45-22-16-33)43(55)47-35(27-31-11-7-5-8-12-31)41(53)42(54)36(28-32-13-9-6-10-14-32)48-44(56)40(30(3)4)50-38(52)20-26-62(59,60)34-17-23-46-24-18-34/h5-18,21-24,29-30,35-36,39-42,53-54H,19-20,25-28H2,1-4H3,(H,47,55)(H,48,56)(H,49,51)(H,50,52)/t35-,36-,39-,40-,41+,42+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de Lavras
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease expressed in Escherichia coli K12 assessed as inhibition of enzyme activity |
Eur J Med Chem 44: 4344-52 (2009)
Article DOI: 10.1016/j.ejmech.2009.05.016
BindingDB Entry DOI: 10.7270/Q2JH3Q0K |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50034001
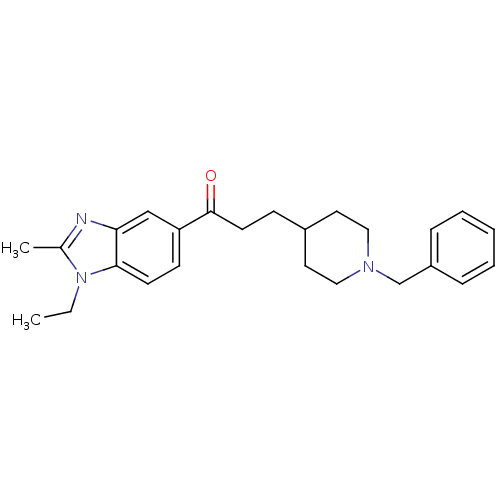
(3-(1-Benzyl-piperidin-4-yl)-1-(1-ethyl-2-methyl-1H...)Show SMILES CCn1c(C)nc2cc(ccc12)C(=O)CCC1CCN(Cc2ccccc2)CC1 Show InChI InChI=1S/C25H31N3O/c1-3-28-19(2)26-23-17-22(10-11-24(23)28)25(29)12-9-20-13-15-27(16-14-20)18-21-7-5-4-6-8-21/h4-8,10-11,17,20H,3,9,12-16,18H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.27 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
| Assay Description
Inhibition of human acetylcholinesterase |
Eur J Med Chem 46: 39-51 (2010)
Article DOI: 10.1016/j.ejmech.2010.10.009
BindingDB Entry DOI: 10.7270/Q2SX6FG0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50417799
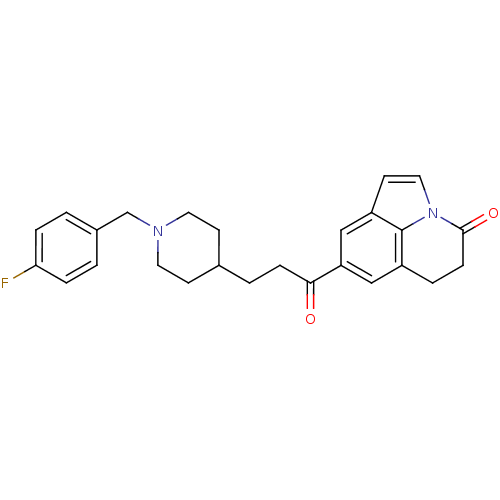
(CHEMBL1651248)Show SMILES Fc1ccc(CN2CCC(CCC(=O)c3cc4CCC(=O)n5ccc(c3)c45)CC2)cc1 Show InChI InChI=1S/C26H27FN2O2/c27-23-5-1-19(2-6-23)17-28-12-9-18(10-13-28)3-7-24(30)22-15-20-4-8-25(31)29-14-11-21(16-22)26(20)29/h1-2,5-6,11,14-16,18H,3-4,7-10,12-13,17H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.57 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
| Assay Description
Inhibition of human acetylcholinesterase |
Eur J Med Chem 46: 39-51 (2010)
Article DOI: 10.1016/j.ejmech.2010.10.009
BindingDB Entry DOI: 10.7270/Q2SX6FG0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50417788
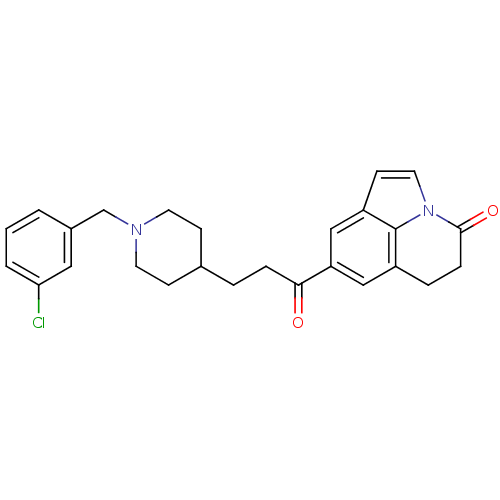
(CHEMBL1651134)Show SMILES Clc1cccc(CN2CCC(CCC(=O)c3cc4CCC(=O)n5ccc(c3)c45)CC2)c1 Show InChI InChI=1S/C26H27ClN2O2/c27-23-3-1-2-19(14-23)17-28-11-8-18(9-12-28)4-6-24(30)22-15-20-5-7-25(31)29-13-10-21(16-22)26(20)29/h1-3,10,13-16,18H,4-9,11-12,17H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
| Assay Description
Inhibition of human acetylcholinesterase |
Eur J Med Chem 46: 39-51 (2010)
Article DOI: 10.1016/j.ejmech.2010.10.009
BindingDB Entry DOI: 10.7270/Q2SX6FG0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50417803
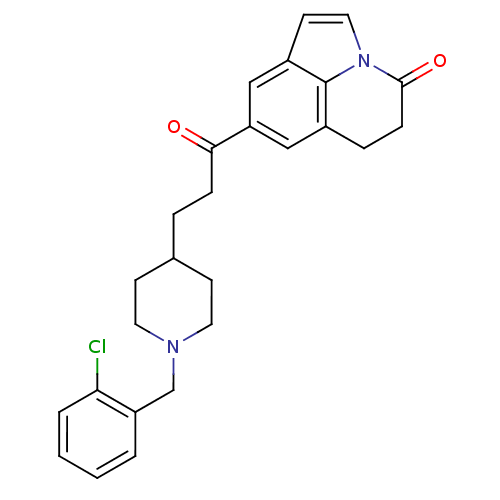
(CHEMBL1651133)Show SMILES Clc1ccccc1CN1CCC(CCC(=O)c2cc3CCC(=O)n4ccc(c2)c34)CC1 Show InChI InChI=1S/C26H27ClN2O2/c27-23-4-2-1-3-21(23)17-28-12-9-18(10-13-28)5-7-24(30)22-15-19-6-8-25(31)29-14-11-20(16-22)26(19)29/h1-4,11,14-16,18H,5-10,12-13,17H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.13 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
| Assay Description
Inhibition of human acetylcholinesterase |
Eur J Med Chem 46: 39-51 (2010)
Article DOI: 10.1016/j.ejmech.2010.10.009
BindingDB Entry DOI: 10.7270/Q2SX6FG0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50039729
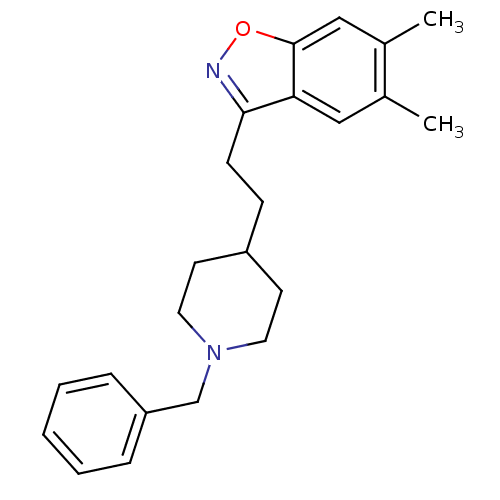
(3-(2-(1-benzylpiperidin-4-yl)ethyl)-5,6-dimethylbe...)Show InChI InChI=1S/C23H28N2O/c1-17-14-21-22(24-26-23(21)15-18(17)2)9-8-19-10-12-25(13-11-19)16-20-6-4-3-5-7-20/h3-7,14-15,19H,8-13,16H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.75 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
| Assay Description
Inhibition of human acetylcholinesterase |
Eur J Med Chem 46: 39-51 (2010)
Article DOI: 10.1016/j.ejmech.2010.10.009
BindingDB Entry DOI: 10.7270/Q2SX6FG0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50034002

(3-(1-Benzyl-piperidin-4-yl)-1-(2-methyl-benzothiaz...)Show InChI InChI=1S/C23H26N2OS/c1-17-24-21-9-8-20(15-23(21)27-17)22(26)10-7-18-11-13-25(14-12-18)16-19-5-3-2-4-6-19/h2-6,8-9,15,18H,7,10-14,16H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.76 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
| Assay Description
Inhibition of human acetylcholinesterase |
Eur J Med Chem 46: 39-51 (2010)
Article DOI: 10.1016/j.ejmech.2010.10.009
BindingDB Entry DOI: 10.7270/Q2SX6FG0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50039713
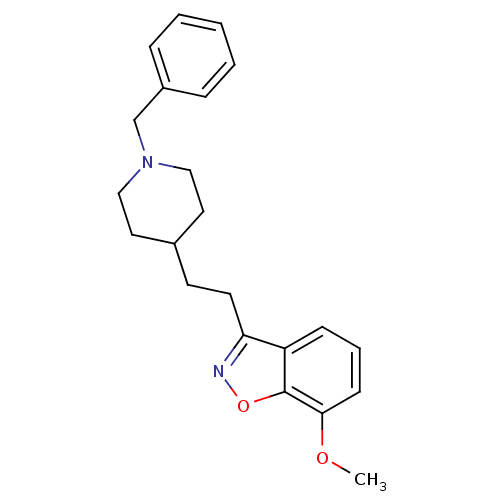
(3-(2-(1-benzylpiperidin-4-yl)ethyl)-7-methoxybenzo...)Show InChI InChI=1S/C22H26N2O2/c1-25-21-9-5-8-19-20(23-26-22(19)21)11-10-17-12-14-24(15-13-17)16-18-6-3-2-4-7-18/h2-9,17H,10-16H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.08 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
| Assay Description
Inhibition of human acetylcholinesterase |
Eur J Med Chem 46: 39-51 (2010)
Article DOI: 10.1016/j.ejmech.2010.10.009
BindingDB Entry DOI: 10.7270/Q2SX6FG0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50039712
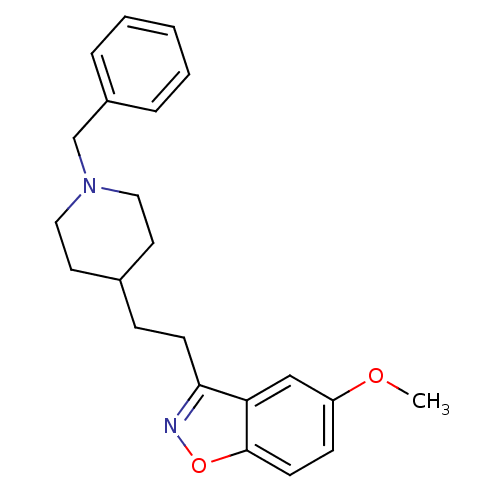
(3-(2-(1-benzylpiperidin-4-yl)ethyl)-5-methoxybenzo...)Show InChI InChI=1S/C22H26N2O2/c1-25-19-8-10-22-20(15-19)21(23-26-22)9-7-17-11-13-24(14-12-17)16-18-5-3-2-4-6-18/h2-6,8,10,15,17H,7,9,11-14,16H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.24 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
| Assay Description
Inhibition of human acetylcholinesterase |
Eur J Med Chem 46: 39-51 (2010)
Article DOI: 10.1016/j.ejmech.2010.10.009
BindingDB Entry DOI: 10.7270/Q2SX6FG0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50039728
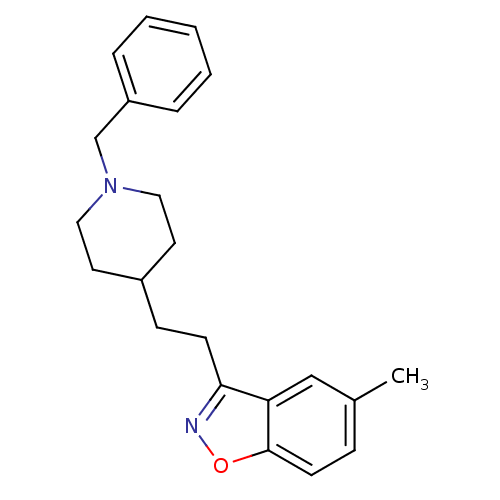
(3-(2-(1-benzylpiperidin-4-yl)ethyl)-5-methylbenzo[...)Show InChI InChI=1S/C22H26N2O/c1-17-7-10-22-20(15-17)21(23-25-22)9-8-18-11-13-24(14-12-18)16-19-5-3-2-4-6-19/h2-7,10,15,18H,8-9,11-14,16H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.76 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
| Assay Description
Inhibition of human acetylcholinesterase |
Eur J Med Chem 46: 39-51 (2010)
Article DOI: 10.1016/j.ejmech.2010.10.009
BindingDB Entry DOI: 10.7270/Q2SX6FG0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50039717
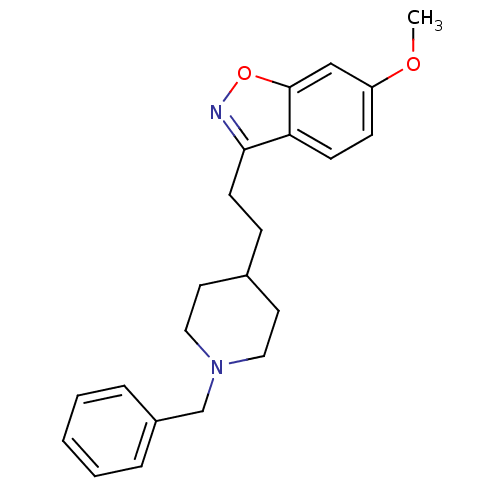
(3-(2-(1-benzylpiperidin-4-yl)ethyl)-6-methoxybenzo...)Show InChI InChI=1S/C22H26N2O2/c1-25-19-8-9-20-21(23-26-22(20)15-19)10-7-17-11-13-24(14-12-17)16-18-5-3-2-4-6-18/h2-6,8-9,15,17H,7,10-14,16H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.32 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
| Assay Description
Inhibition of human acetylcholinesterase |
Eur J Med Chem 46: 39-51 (2010)
Article DOI: 10.1016/j.ejmech.2010.10.009
BindingDB Entry DOI: 10.7270/Q2SX6FG0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50039723
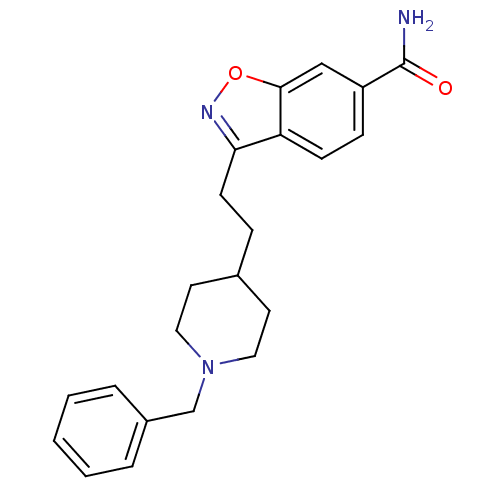
(3-(2-(1-benzylpiperidin-4-yl)ethyl)benzo[d]isoxazo...)Show InChI InChI=1S/C22H25N3O2/c23-22(26)18-7-8-19-20(24-27-21(19)14-18)9-6-16-10-12-25(13-11-16)15-17-4-2-1-3-5-17/h1-5,7-8,14,16H,6,9-13,15H2,(H2,23,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.71 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
| Assay Description
Inhibition of human acetylcholinesterase |
Eur J Med Chem 46: 39-51 (2010)
Article DOI: 10.1016/j.ejmech.2010.10.009
BindingDB Entry DOI: 10.7270/Q2SX6FG0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50417792

(CHEMBL1651140)Show SMILES Oc1cccc(CN2CCC(CCC(=O)c3cc4CCC(=O)n5ccc(c3)c45)CC2)c1 Show InChI InChI=1S/C26H28N2O3/c29-23-3-1-2-19(14-23)17-27-11-8-18(9-12-27)4-6-24(30)22-15-20-5-7-25(31)28-13-10-21(16-22)26(20)28/h1-3,10,13-16,18,29H,4-9,11-12,17H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.71 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
| Assay Description
Inhibition of human acetylcholinesterase |
Eur J Med Chem 46: 39-51 (2010)
Article DOI: 10.1016/j.ejmech.2010.10.009
BindingDB Entry DOI: 10.7270/Q2SX6FG0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50039727
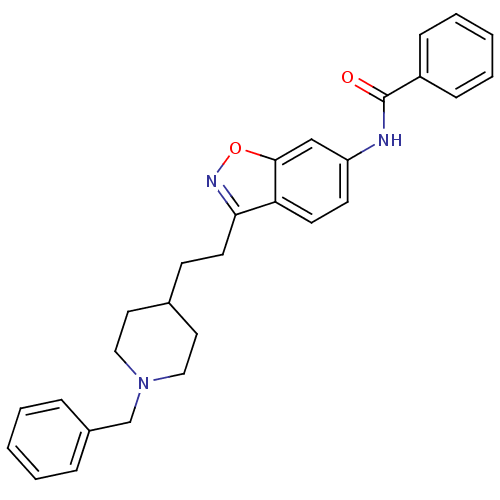
(CHEMBL93123 | N-(3-(2-(1-benzylpiperidin-4-yl)ethy...)Show SMILES O=C(Nc1ccc2c(CCC3CCN(Cc4ccccc4)CC3)noc2c1)c1ccccc1 Show InChI InChI=1S/C28H29N3O2/c32-28(23-9-5-2-6-10-23)29-24-12-13-25-26(30-33-27(25)19-24)14-11-21-15-17-31(18-16-21)20-22-7-3-1-4-8-22/h1-10,12-13,19,21H,11,14-18,20H2,(H,29,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.33 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
| Assay Description
Inhibition of human acetylcholinesterase |
Eur J Med Chem 46: 39-51 (2010)
Article DOI: 10.1016/j.ejmech.2010.10.009
BindingDB Entry DOI: 10.7270/Q2SX6FG0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50417802

(CHEMBL1651130)Show SMILES O=C(CCC1CCN(Cc2ccccc2)CC1)c1cc2CCN3c2c(CCC3=O)c1 Show InChI InChI=1S/C26H30N2O2/c29-24(23-16-21-7-9-25(30)28-15-12-22(17-23)26(21)28)8-6-19-10-13-27(14-11-19)18-20-4-2-1-3-5-20/h1-5,16-17,19H,6-15,18H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.77 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
| Assay Description
Inhibition of human acetylcholinesterase |
Eur J Med Chem 46: 39-51 (2010)
Article DOI: 10.1016/j.ejmech.2010.10.009
BindingDB Entry DOI: 10.7270/Q2SX6FG0 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data