 Found 99 hits with Last Name = 'smith' and Initial = 'rj'
Found 99 hits with Last Name = 'smith' and Initial = 'rj' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.0460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong
Curated by ChEMBL
| Assay Description
Binding affinity against nicotinic acetylcholine receptor alpha4-beta2 using [3H]epibatidine as radioligand expressed in HEK293 cells or tsA cells |
Bioorg Med Chem Lett 14: 271-3 (2003)
BindingDB Entry DOI: 10.7270/Q2MC8ZD0 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-4
(Homo sapiens (Human)) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.0720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong
Curated by ChEMBL
| Assay Description
Binding affinity against nicotinic acetylcholine receptor using [3H]epibatidine as radioligand in rat brain tissue |
Bioorg Med Chem Lett 14: 271-3 (2003)
BindingDB Entry DOI: 10.7270/Q2MC8ZD0 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor; alpha2/beta4
(Homo sapiens (Human)) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 0.0910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong
Curated by ChEMBL
| Assay Description
Agonistic potency against nicotinic acetylcholine receptor alpha3-beta4 |
Bioorg Med Chem Lett 14: 271-3 (2003)
BindingDB Entry DOI: 10.7270/Q2MC8ZD0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neuronal acetylcholine receptor subunit alpha-3/beta-4
(Homo sapiens (Human)) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.457 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong
Curated by ChEMBL
| Assay Description
Binding affinity of the compound was determined against Nicotinic acetylcholine receptor using [3H]-(-)-nicotine radioligand |
Bioorg Med Chem Lett 14: 271-3 (2003)
BindingDB Entry DOI: 10.7270/Q2MC8ZD0 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50137787
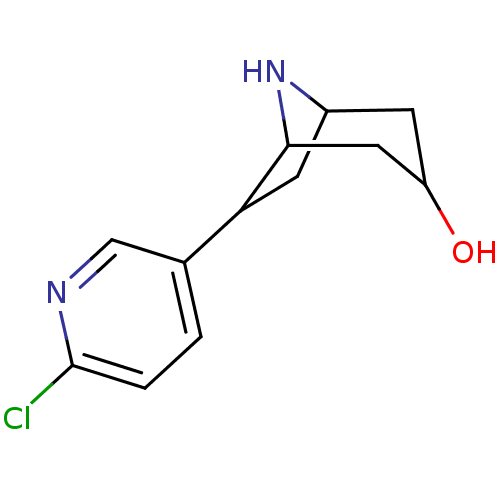
(6-(6-Chloro-pyridin-3-yl)-8-aza-bicyclo[3.2.1]octa...)Show SMILES OC1CC2CC(C(C1)N2)c1ccc(Cl)nc1 |TLB:0:1:8:5.4,THB:9:5:8:7.2.1| Show InChI InChI=1S/C12H15ClN2O/c13-12-2-1-7(6-14-12)10-4-8-3-9(16)5-11(10)15-8/h1-2,6,8-11,15-16H,3-5H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong
Curated by ChEMBL
| Assay Description
Binding affinity against nicotinic acetylcholine receptor alpha4-beta2 using [3H]epibatidine as radioligand expressed in HEK293 cells or tsA cells |
Bioorg Med Chem Lett 14: 271-3 (2003)
BindingDB Entry DOI: 10.7270/Q2MC8ZD0 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM82070

(CAS_29790-52-1 | NICOTINE-L (BASE) | Nicotine-D sa...)Show InChI InChI=1S/C10H14N2/c1-12-7-3-5-10(12)9-4-2-6-11-8-9/h2,4,6,8,10H,3,5,7H2,1H3/t10-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| 9.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong
Curated by ChEMBL
| Assay Description
Binding affinity against nicotinic acetylcholine receptor using [3H]epibatidine as radioligand in rat brain tissue |
Bioorg Med Chem Lett 14: 271-3 (2003)
BindingDB Entry DOI: 10.7270/Q2MC8ZD0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neuronal acetylcholine receptor; alpha2/beta4
(Homo sapiens (Human)) | BDBM50137787

(6-(6-Chloro-pyridin-3-yl)-8-aza-bicyclo[3.2.1]octa...)Show SMILES OC1CC2CC(C(C1)N2)c1ccc(Cl)nc1 |TLB:0:1:8:5.4,THB:9:5:8:7.2.1| Show InChI InChI=1S/C12H15ClN2O/c13-12-2-1-7(6-14-12)10-4-8-3-9(16)5-11(10)15-8/h1-2,6,8-11,15-16H,3-5H2 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong
Curated by ChEMBL
| Assay Description
In vitro ability to displace [3H]-(-)-cytisine binding to whole rat brain membranes at Nicotinic acetylcholine receptor alpha4-beta2 |
Bioorg Med Chem Lett 14: 271-3 (2003)
BindingDB Entry DOI: 10.7270/Q2MC8ZD0 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-4
(Homo sapiens (Human)) | BDBM50137787

(6-(6-Chloro-pyridin-3-yl)-8-aza-bicyclo[3.2.1]octa...)Show SMILES OC1CC2CC(C(C1)N2)c1ccc(Cl)nc1 |TLB:0:1:8:5.4,THB:9:5:8:7.2.1| Show InChI InChI=1S/C12H15ClN2O/c13-12-2-1-7(6-14-12)10-4-8-3-9(16)5-11(10)15-8/h1-2,6,8-11,15-16H,3-5H2 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong
Curated by ChEMBL
| Assay Description
Binding affinity against nicotinic acetylcholine receptor alpha2-beta4 using [3H]epibatidine as radioligand expressed in HEK293 cells or tsA cells |
Bioorg Med Chem Lett 14: 271-3 (2003)
BindingDB Entry DOI: 10.7270/Q2MC8ZD0 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-3/beta-4
(Homo sapiens (Human)) | BDBM50137787

(6-(6-Chloro-pyridin-3-yl)-8-aza-bicyclo[3.2.1]octa...)Show SMILES OC1CC2CC(C(C1)N2)c1ccc(Cl)nc1 |TLB:0:1:8:5.4,THB:9:5:8:7.2.1| Show InChI InChI=1S/C12H15ClN2O/c13-12-2-1-7(6-14-12)10-4-8-3-9(16)5-11(10)15-8/h1-2,6,8-11,15-16H,3-5H2 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 88 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong
Curated by ChEMBL
| Assay Description
Binding affinity against nicotinic acetylcholine receptor alpha4-beta2 using [3H]epibatidine as radioligand expressed in HEK293 cells or tsA cells |
Bioorg Med Chem Lett 14: 271-3 (2003)
BindingDB Entry DOI: 10.7270/Q2MC8ZD0 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor; alpha2/beta4
(Homo sapiens (Human)) | BDBM82070

(CAS_29790-52-1 | NICOTINE-L (BASE) | Nicotine-D sa...)Show InChI InChI=1S/C10H14N2/c1-12-7-3-5-10(12)9-4-2-6-11-8-9/h2,4,6,8,10H,3,5,7H2,1H3/t10-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 103 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong
Curated by ChEMBL
| Assay Description
Binding affinity against nicotinic acetylcholine receptor alpha2-beta4 using [3H]epibatidine as radioligand expressed in HEK293 cells or tsA cells |
Bioorg Med Chem Lett 14: 271-3 (2003)
BindingDB Entry DOI: 10.7270/Q2MC8ZD0 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-4
(Homo sapiens (Human)) | BDBM82070

(CAS_29790-52-1 | NICOTINE-L (BASE) | Nicotine-D sa...)Show InChI InChI=1S/C10H14N2/c1-12-7-3-5-10(12)9-4-2-6-11-8-9/h2,4,6,8,10H,3,5,7H2,1H3/t10-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| 129 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong
Curated by ChEMBL
| Assay Description
Binding affinity against nicotinic acetylcholine receptor alpha4-beta4 using [3H]epibatidine as radioligand expressed in HEK293 cells or tsA cells |
Bioorg Med Chem Lett 14: 271-3 (2003)
BindingDB Entry DOI: 10.7270/Q2MC8ZD0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neuronal acetylcholine receptor subunit alpha-3/beta-4
(Homo sapiens (Human)) | BDBM82070

(CAS_29790-52-1 | NICOTINE-L (BASE) | Nicotine-D sa...)Show InChI InChI=1S/C10H14N2/c1-12-7-3-5-10(12)9-4-2-6-11-8-9/h2,4,6,8,10H,3,5,7H2,1H3/t10-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong
Curated by ChEMBL
| Assay Description
Binding affinity against nicotinic acetylcholine receptor alpha3-beta4 using [3H]epibatidine as radioligand expressed in HEK293 cells or tsA cells |
Bioorg Med Chem Lett 14: 271-3 (2003)
BindingDB Entry DOI: 10.7270/Q2MC8ZD0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50060425
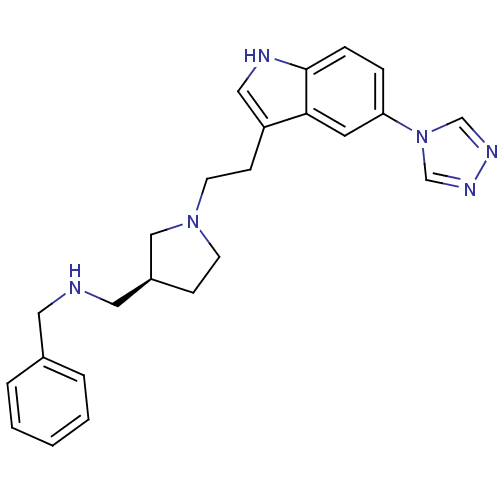
(Benzyl-{(S)-1-[2-(5-[1,2,4]triazol-4-yl-1H-indol-3...)Show SMILES C(Cc1c[nH]c2ccc(cc12)-n1cnnc1)N1CC[C@@H](CNCc2ccccc2)C1 Show InChI InChI=1S/C24H28N6/c1-2-4-19(5-3-1)13-25-14-20-8-10-29(16-20)11-9-21-15-26-24-7-6-22(12-23(21)24)30-17-27-28-18-30/h1-7,12,15,17-18,20,25-26H,8-11,13-14,16H2/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by ChEMBL
| Assay Description
Ability to displace [3H]- -5-HT binding to cloned 5-hydroxytryptamine 1D receptor stably expressed in chinese hamster cells (CHO cells) |
Bioorg Med Chem Lett 9: 2491-6 (1999)
BindingDB Entry DOI: 10.7270/Q27M074P |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50080889
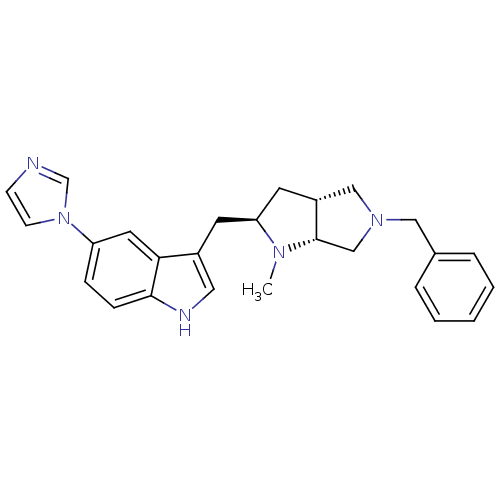
(3-((2R,3aR,6aR)-5-Benzyl-1-methyl-octahydro-pyrrol...)Show SMILES CN1[C@@H](Cc2c[nH]c3ccc(cc23)-n2ccnc2)C[C@@H]2CN(Cc3ccccc3)C[C@H]12 Show InChI InChI=1S/C26H29N5/c1-29-23(12-21-16-30(17-26(21)29)15-19-5-3-2-4-6-19)11-20-14-28-25-8-7-22(13-24(20)25)31-10-9-27-18-31/h2-10,13-14,18,21,23,26,28H,11-12,15-17H2,1H3/t21-,23+,26+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by ChEMBL
| Assay Description
Ability to displace [3H]- -5-HT binding to cloned 5-hydroxytryptamine 1D receptor stably expressed in chinese hamster cells (CHO cells) |
Bioorg Med Chem Lett 9: 2491-6 (1999)
BindingDB Entry DOI: 10.7270/Q27M074P |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50080894
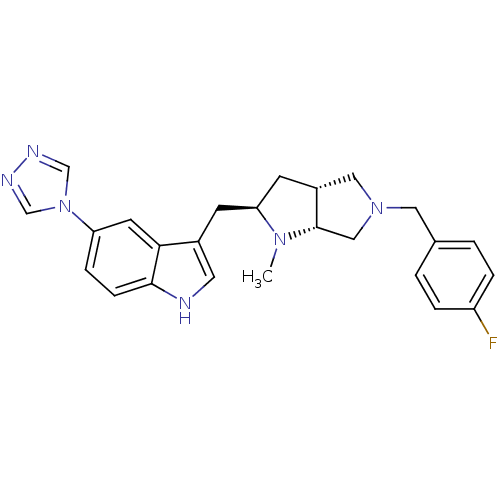
(3-[(2R,3aR,6aR)-5-(4-Fluoro-benzyl)-1-methyl-octah...)Show SMILES CN1[C@@H](Cc2c[nH]c3ccc(cc23)-n2cnnc2)C[C@@H]2CN(Cc3ccc(F)cc3)C[C@H]12 Show InChI InChI=1S/C25H27FN6/c1-30-22(9-19-13-31(14-25(19)30)12-17-2-4-20(26)5-3-17)8-18-11-27-24-7-6-21(10-23(18)24)32-15-28-29-16-32/h2-7,10-11,15-16,19,22,25,27H,8-9,12-14H2,1H3/t19-,22+,25+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by ChEMBL
| Assay Description
Ability to displace [3H]- -5-HT binding to cloned 5-hydroxytryptamine 1D receptor stably expressed in chinese hamster cells (CHO cells) |
Bioorg Med Chem Lett 9: 2491-6 (1999)
BindingDB Entry DOI: 10.7270/Q27M074P |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50080892
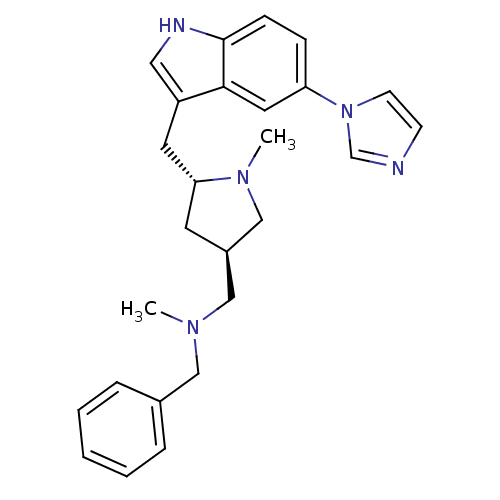
(Benzyl-[(3R,5R)-5-(5-imidazol-1-yl-1H-indol-3-ylme...)Show SMILES CN(C[C@H]1C[C@H](Cc2c[nH]c3ccc(cc23)-n2ccnc2)N(C)C1)Cc1ccccc1 Show InChI InChI=1S/C26H31N5/c1-29(16-20-6-4-3-5-7-20)17-21-12-24(30(2)18-21)13-22-15-28-26-9-8-23(14-25(22)26)31-11-10-27-19-31/h3-11,14-15,19,21,24,28H,12-13,16-18H2,1-2H3/t21-,24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by ChEMBL
| Assay Description
Ability to displace [3H]- -5-HT binding to cloned 5-hydroxytryptamine 1D receptor stably expressed in chinese hamster cells (CHO cells) |
Bioorg Med Chem Lett 9: 2491-6 (1999)
BindingDB Entry DOI: 10.7270/Q27M074P |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50080890
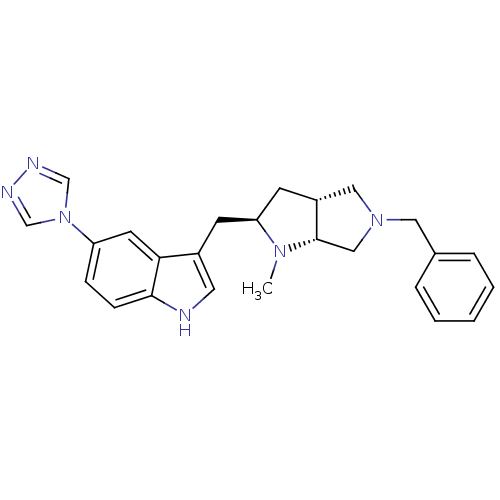
(3-((2R,3aR,6aR)-5-Benzyl-1-methyl-octahydro-pyrrol...)Show SMILES CN1[C@@H](Cc2c[nH]c3ccc(cc23)-n2cnnc2)C[C@@H]2CN(Cc3ccccc3)C[C@H]12 Show InChI InChI=1S/C25H28N6/c1-29-22(10-20-14-30(15-25(20)29)13-18-5-3-2-4-6-18)9-19-12-26-24-8-7-21(11-23(19)24)31-16-27-28-17-31/h2-8,11-12,16-17,20,22,25-26H,9-10,13-15H2,1H3/t20-,22+,25+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by ChEMBL
| Assay Description
Measurement of agonist induced [35S]-GTP-gammaS, binding in CHO cells stably transfected with 5-hydroxytryptamine 1D receptor |
Bioorg Med Chem Lett 9: 2491-6 (1999)
BindingDB Entry DOI: 10.7270/Q27M074P |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50080891

(3-{(2R,3aR,6aR)-5-[2-(3-Fluoro-phenyl)-ethyl]-1-me...)Show SMILES CN1[C@@H](Cc2c[nH]c3ccc(cc23)-n2cnnc2)C[C@@H]2CN(CCc3cccc(F)c3)C[C@H]12 Show InChI InChI=1S/C26H29FN6/c1-31-23(10-19-13-28-25-6-5-22(12-24(19)25)33-16-29-30-17-33)11-20-14-32(15-26(20)31)8-7-18-3-2-4-21(27)9-18/h2-6,9,12-13,16-17,20,23,26,28H,7-8,10-11,14-15H2,1H3/t20-,23+,26+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by ChEMBL
| Assay Description
Ability to displace [3H]- -5-HT binding to cloned 5-hydroxytryptamine 1D receptor stably expressed in chinese hamster cells (CHO cells) |
Bioorg Med Chem Lett 9: 2491-6 (1999)
BindingDB Entry DOI: 10.7270/Q27M074P |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50080893
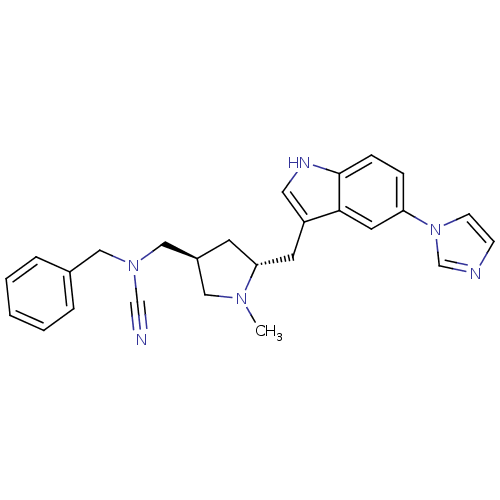
(Benzyl-[(3S,5R)-5-(5-imidazol-1-yl-1H-indol-3-ylme...)Show SMILES CN1C[C@@H](CN(Cc2ccccc2)C#N)C[C@@H]1Cc1c[nH]c2ccc(cc12)-n1ccnc1 Show InChI InChI=1S/C26H28N6/c1-30-15-21(17-31(18-27)16-20-5-3-2-4-6-20)11-24(30)12-22-14-29-26-8-7-23(13-25(22)26)32-10-9-28-19-32/h2-10,13-14,19,21,24,29H,11-12,15-17H2,1H3/t21-,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by ChEMBL
| Assay Description
Ability to displace [3H]- -5-HT binding to cloned 5-hydroxytryptamine 1D receptor stably expressed in chinese hamster cells (CHO cells) |
Bioorg Med Chem Lett 9: 2491-6 (1999)
BindingDB Entry DOI: 10.7270/Q27M074P |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50604564
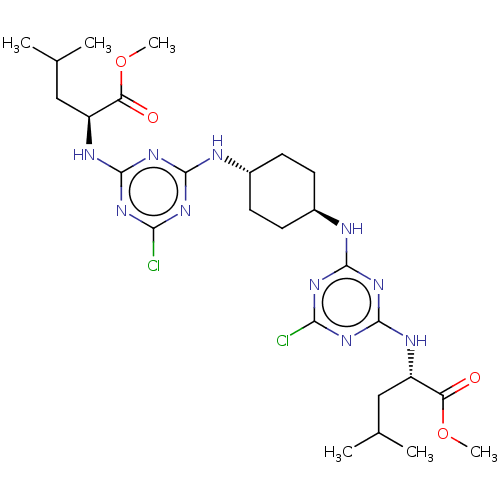
(CHEMBL5191531)Show SMILES COC(=O)[C@H](CC(C)C)Nc1nc(Cl)nc(N[C@H]2CC[C@@H](CC2)Nc2nc(Cl)nc(N[C@@H](CC(C)C)C(=O)OC)n2)n1 |r,wU:31.31,17.16,wD:20.23,4.8,(7.32,6.16,;5.99,5.39,;6,3.85,;4.66,3.08,;7.32,3.08,;8.66,3.86,;9.99,3.08,;11.33,3.86,;9.99,1.54,;7.33,1.54,;6,.77,;6,-.78,;4.66,-1.55,;4.66,-3.09,;3.33,-.77,;3.34,.77,;2.01,1.53,;.67,.77,;.67,-.77,;-.66,-1.54,;-1.99,-.76,;-2,.78,;-.66,1.54,;-3.31,-1.54,;-4.65,-.76,;-4.65,.79,;-5.99,1.55,;-5.99,3.09,;-7.32,.78,;-7.32,-.76,;-8.66,-1.54,;-8.66,-3.08,;-7.32,-3.84,;-7.32,-5.38,;-5.99,-6.16,;-8.66,-6.16,;-9.99,-3.84,;-9.99,-5.38,;-11.33,-3.08,;-11.33,-1.54,;-5.99,-1.54,;4.67,1.53,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.96E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01974
BindingDB Entry DOI: 10.7270/Q2V98D48 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50604573
(CHEMBL5174834)Show SMILES CC(C)C[C@H](Nc1ncnc(Nc2ccc(NC(C)=O)cc2)n1)C(=O)OC(C)(C)C |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01974
BindingDB Entry DOI: 10.7270/Q2V98D48 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50604566

(CHEMBL5193490)Show SMILES CC(=O)Nc1ccc(Nc2ncnc(N[C@@H](Cc3ccccc3)C(=O)OC(C)(C)C)n2)cc1 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.72E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01974
BindingDB Entry DOI: 10.7270/Q2V98D48 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50604566

(CHEMBL5193490)Show SMILES CC(=O)Nc1ccc(Nc2ncnc(N[C@@H](Cc3ccccc3)C(=O)OC(C)(C)C)n2)cc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01974
BindingDB Entry DOI: 10.7270/Q2V98D48 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50604566

(CHEMBL5193490)Show SMILES CC(=O)Nc1ccc(Nc2ncnc(N[C@@H](Cc3ccccc3)C(=O)OC(C)(C)C)n2)cc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.47E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01974
BindingDB Entry DOI: 10.7270/Q2V98D48 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50604573

(CHEMBL5174834)Show SMILES CC(C)C[C@H](Nc1ncnc(Nc2ccc(NC(C)=O)cc2)n1)C(=O)OC(C)(C)C |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.64E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01974
BindingDB Entry DOI: 10.7270/Q2V98D48 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50604573

(CHEMBL5174834)Show SMILES CC(C)C[C@H](Nc1ncnc(Nc2ccc(NC(C)=O)cc2)n1)C(=O)OC(C)(C)C |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.67E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01974
BindingDB Entry DOI: 10.7270/Q2V98D48 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50604566

(CHEMBL5193490)Show SMILES CC(=O)Nc1ccc(Nc2ncnc(N[C@@H](Cc3ccccc3)C(=O)OC(C)(C)C)n2)cc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.77E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01974
BindingDB Entry DOI: 10.7270/Q2V98D48 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50604566

(CHEMBL5193490)Show SMILES CC(=O)Nc1ccc(Nc2ncnc(N[C@@H](Cc3ccccc3)C(=O)OC(C)(C)C)n2)cc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.98E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01974
BindingDB Entry DOI: 10.7270/Q2V98D48 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50604573

(CHEMBL5174834)Show SMILES CC(C)C[C@H](Nc1ncnc(Nc2ccc(NC(C)=O)cc2)n1)C(=O)OC(C)(C)C |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.68E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01974
BindingDB Entry DOI: 10.7270/Q2V98D48 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50604573

(CHEMBL5174834)Show SMILES CC(C)C[C@H](Nc1ncnc(Nc2ccc(NC(C)=O)cc2)n1)C(=O)OC(C)(C)C |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.99E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01974
BindingDB Entry DOI: 10.7270/Q2V98D48 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50604573

(CHEMBL5174834)Show SMILES CC(C)C[C@H](Nc1ncnc(Nc2ccc(NC(C)=O)cc2)n1)C(=O)OC(C)(C)C |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.06E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01974
BindingDB Entry DOI: 10.7270/Q2V98D48 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50604564

(CHEMBL5191531)Show SMILES COC(=O)[C@H](CC(C)C)Nc1nc(Cl)nc(N[C@H]2CC[C@@H](CC2)Nc2nc(Cl)nc(N[C@@H](CC(C)C)C(=O)OC)n2)n1 |r,wU:31.31,17.16,wD:20.23,4.8,(7.32,6.16,;5.99,5.39,;6,3.85,;4.66,3.08,;7.32,3.08,;8.66,3.86,;9.99,3.08,;11.33,3.86,;9.99,1.54,;7.33,1.54,;6,.77,;6,-.78,;4.66,-1.55,;4.66,-3.09,;3.33,-.77,;3.34,.77,;2.01,1.53,;.67,.77,;.67,-.77,;-.66,-1.54,;-1.99,-.76,;-2,.78,;-.66,1.54,;-3.31,-1.54,;-4.65,-.76,;-4.65,.79,;-5.99,1.55,;-5.99,3.09,;-7.32,.78,;-7.32,-.76,;-8.66,-1.54,;-8.66,-3.08,;-7.32,-3.84,;-7.32,-5.38,;-5.99,-6.16,;-8.66,-6.16,;-9.99,-3.84,;-9.99,-5.38,;-11.33,-3.08,;-11.33,-1.54,;-5.99,-1.54,;4.67,1.53,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.78E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01974
BindingDB Entry DOI: 10.7270/Q2V98D48 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50604565

(CHEMBL5180826)Show SMILES CC(=O)Nc1ccc(Nc2ncnc(NCCC(=O)OC(C)(C)C)n2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01974
BindingDB Entry DOI: 10.7270/Q2V98D48 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50604569

(CHEMBL5170051)Show SMILES CC(C)C[C@H](Nc1ncnc(Nc2ccc(NC(C)=O)cc2)n1)C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01974
BindingDB Entry DOI: 10.7270/Q2V98D48 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50604565

(CHEMBL5180826)Show SMILES CC(=O)Nc1ccc(Nc2ncnc(NCCC(=O)OC(C)(C)C)n2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01974
BindingDB Entry DOI: 10.7270/Q2V98D48 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50604569

(CHEMBL5170051)Show SMILES CC(C)C[C@H](Nc1ncnc(Nc2ccc(NC(C)=O)cc2)n1)C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01974
BindingDB Entry DOI: 10.7270/Q2V98D48 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50604565

(CHEMBL5180826)Show SMILES CC(=O)Nc1ccc(Nc2ncnc(NCCC(=O)OC(C)(C)C)n2)cc1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01974
BindingDB Entry DOI: 10.7270/Q2V98D48 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50604569

(CHEMBL5170051)Show SMILES CC(C)C[C@H](Nc1ncnc(Nc2ccc(NC(C)=O)cc2)n1)C(O)=O |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01974
BindingDB Entry DOI: 10.7270/Q2V98D48 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50604565

(CHEMBL5180826)Show SMILES CC(=O)Nc1ccc(Nc2ncnc(NCCC(=O)OC(C)(C)C)n2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01974
BindingDB Entry DOI: 10.7270/Q2V98D48 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50604569

(CHEMBL5170051)Show SMILES CC(C)C[C@H](Nc1ncnc(Nc2ccc(NC(C)=O)cc2)n1)C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01974
BindingDB Entry DOI: 10.7270/Q2V98D48 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50604565

(CHEMBL5180826)Show SMILES CC(=O)Nc1ccc(Nc2ncnc(NCCC(=O)OC(C)(C)C)n2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01974
BindingDB Entry DOI: 10.7270/Q2V98D48 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50604569

(CHEMBL5170051)Show SMILES CC(C)C[C@H](Nc1ncnc(Nc2ccc(NC(C)=O)cc2)n1)C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01974
BindingDB Entry DOI: 10.7270/Q2V98D48 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50604565

(CHEMBL5180826)Show SMILES CC(=O)Nc1ccc(Nc2ncnc(NCCC(=O)OC(C)(C)C)n2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01974
BindingDB Entry DOI: 10.7270/Q2V98D48 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50604569

(CHEMBL5170051)Show SMILES CC(C)C[C@H](Nc1ncnc(Nc2ccc(NC(C)=O)cc2)n1)C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01974
BindingDB Entry DOI: 10.7270/Q2V98D48 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50080894

(3-[(2R,3aR,6aR)-5-(4-Fluoro-benzyl)-1-methyl-octah...)Show SMILES CN1[C@@H](Cc2c[nH]c3ccc(cc23)-n2cnnc2)C[C@@H]2CN(Cc3ccc(F)cc3)C[C@H]12 Show InChI InChI=1S/C25H27FN6/c1-30-22(9-19-13-31(14-25(19)30)12-17-2-4-20(26)5-3-17)8-18-11-27-24-7-6-21(10-23(18)24)32-15-28-29-16-32/h2-7,10-11,15-16,19,22,25,27H,8-9,12-14H2,1H3/t19-,22+,25+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by ChEMBL
| Assay Description
Measurement of agonist induced [35S]-GTP-gammaS, binding in CHO cells stably transfected with 5-hydroxytryptamine 1D receptor |
Bioorg Med Chem Lett 9: 2491-6 (1999)
BindingDB Entry DOI: 10.7270/Q27M074P |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50080889

(3-((2R,3aR,6aR)-5-Benzyl-1-methyl-octahydro-pyrrol...)Show SMILES CN1[C@@H](Cc2c[nH]c3ccc(cc23)-n2ccnc2)C[C@@H]2CN(Cc3ccccc3)C[C@H]12 Show InChI InChI=1S/C26H29N5/c1-29-23(12-21-16-30(17-26(21)29)15-19-5-3-2-4-6-19)11-20-14-28-25-8-7-22(13-24(20)25)31-10-9-27-18-31/h2-10,13-14,18,21,23,26,28H,11-12,15-17H2,1H3/t21-,23+,26+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by ChEMBL
| Assay Description
Ability to displace [3H]- -5-HT binding to cloned 5-hydroxytryptamine 1D receptor stably expressed in chinese hamster cells (CHO cells) |
Bioorg Med Chem Lett 9: 2491-6 (1999)
BindingDB Entry DOI: 10.7270/Q27M074P |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50060425

(Benzyl-{(S)-1-[2-(5-[1,2,4]triazol-4-yl-1H-indol-3...)Show SMILES C(Cc1c[nH]c2ccc(cc12)-n1cnnc1)N1CC[C@@H](CNCc2ccccc2)C1 Show InChI InChI=1S/C24H28N6/c1-2-4-19(5-3-1)13-25-14-20-8-10-29(16-20)11-9-21-15-26-24-7-6-22(12-23(21)24)30-17-27-28-18-30/h1-7,12,15,17-18,20,25-26H,8-11,13-14,16H2/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by ChEMBL
| Assay Description
Measurement of agonist induced [35S]-GTP-gammaS, binding in CHO cells stably transfected with 5-hydroxytryptamine 1D receptor |
Bioorg Med Chem Lett 9: 2491-6 (1999)
BindingDB Entry DOI: 10.7270/Q27M074P |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50080891

(3-{(2R,3aR,6aR)-5-[2-(3-Fluoro-phenyl)-ethyl]-1-me...)Show SMILES CN1[C@@H](Cc2c[nH]c3ccc(cc23)-n2cnnc2)C[C@@H]2CN(CCc3cccc(F)c3)C[C@H]12 Show InChI InChI=1S/C26H29FN6/c1-31-23(10-19-13-28-25-6-5-22(12-24(19)25)33-16-29-30-17-33)11-20-14-32(15-26(20)31)8-7-18-3-2-4-21(27)9-18/h2-6,9,12-13,16-17,20,23,26,28H,7-8,10-11,14-15H2,1H3/t20-,23+,26+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by ChEMBL
| Assay Description
Measurement of agonist induced [35S]-GTP-gammaS, binding in CHO cells stably transfected with 5-hydroxytryptamine 1D receptor |
Bioorg Med Chem Lett 9: 2491-6 (1999)
BindingDB Entry DOI: 10.7270/Q27M074P |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-3/beta-4
(Homo sapiens (Human)) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 19 | n/a | n/a | n/a | n/a |
University of Wollongong
Curated by ChEMBL
| Assay Description
Binding affinity against nicotinic acetylcholine receptor alpha3-beta4 using [3H]epibatidine as radioligand expressed in HEK293 cells or tsA cells |
Bioorg Med Chem Lett 14: 271-3 (2003)
BindingDB Entry DOI: 10.7270/Q2MC8ZD0 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-3/beta-4
(Homo sapiens (Human)) | BDBM82070

(CAS_29790-52-1 | NICOTINE-L (BASE) | Nicotine-D sa...)Show InChI InChI=1S/C10H14N2/c1-12-7-3-5-10(12)9-4-2-6-11-8-9/h2,4,6,8,10H,3,5,7H2,1H3/t10-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a |
University of Wollongong
Curated by ChEMBL
| Assay Description
Agonistic potency against nicotinic acetylcholine receptor alpha3-beta4 |
Bioorg Med Chem Lett 14: 271-3 (2003)
BindingDB Entry DOI: 10.7270/Q2MC8ZD0 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data