

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50148285 (2-{(S)-3-[4-(2-Benzyl-thiazol-5-yl)-piperidin-1-yl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human C-C chemokine receptor type 5 assayed using [125I]-MIP-1 alpha as radioligand | Bioorg Med Chem Lett 14: 3419-24 (2004) Article DOI: 10.1016/j.bmcl.2004.04.078 BindingDB Entry DOI: 10.7270/Q2X63MD1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50148286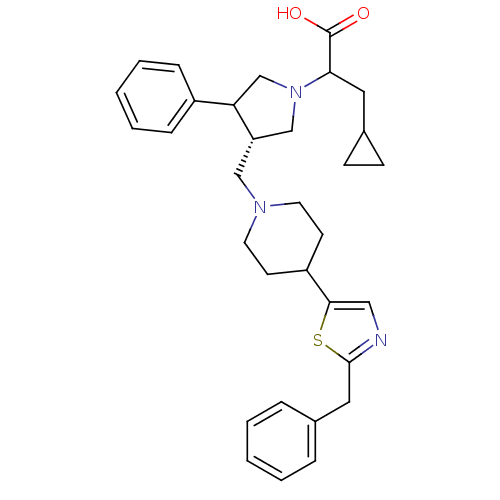 (2-{(S)-3-[4-(2-Benzyl-thiazol-5-yl)-piperidin-1-yl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human C-C chemokine receptor type 5 assayed using [125I]-MIP-1 alpha as radioligand | Bioorg Med Chem Lett 14: 3419-24 (2004) Article DOI: 10.1016/j.bmcl.2004.04.078 BindingDB Entry DOI: 10.7270/Q2X63MD1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50249465 ((R)-2-((3S,4S)-3-((4-(2-benzylthiazol-5-yl)piperid...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human C-C chemokine receptor type 5 assayed using [125I]-MIP-1 alpha as radioligand | Bioorg Med Chem Lett 14: 3419-24 (2004) Article DOI: 10.1016/j.bmcl.2004.04.078 BindingDB Entry DOI: 10.7270/Q2X63MD1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50249467 ((R)-2-((3S,4S)-3-((4-(2-benzyloxazol-5-yl)piperidi...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human C-C chemokine receptor type 5 assayed using [125I]-MIP-1 alpha as radioligand | Bioorg Med Chem Lett 14: 3419-24 (2004) Article DOI: 10.1016/j.bmcl.2004.04.078 BindingDB Entry DOI: 10.7270/Q2X63MD1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50404275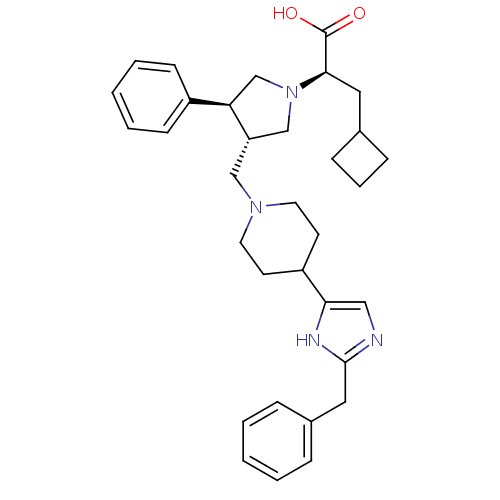 (CHEMBL2112559) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human C-C chemokine receptor type 5 assayed using [125I]-MIP-1 alpha as radioligand | Bioorg Med Chem Lett 14: 3419-24 (2004) Article DOI: 10.1016/j.bmcl.2004.04.078 BindingDB Entry DOI: 10.7270/Q2X63MD1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50249334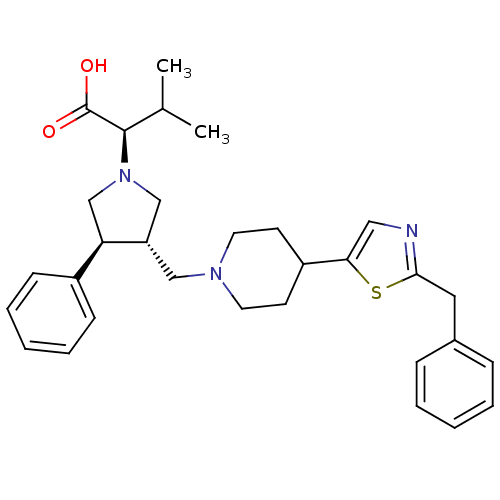 ((R)-2-((3S,4S)-3-((4-(2-benzylthiazol-5-yl)piperid...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human C-C chemokine receptor type 5 assayed using [125I]-MIP-1 alpha as radioligand | Bioorg Med Chem Lett 14: 3419-24 (2004) Article DOI: 10.1016/j.bmcl.2004.04.078 BindingDB Entry DOI: 10.7270/Q2X63MD1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50404276 (CHEMBL2112558) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human C-C chemokine receptor type 5 assayed using [125I]-MIP-1 alpha as radioligand | Bioorg Med Chem Lett 14: 3419-24 (2004) Article DOI: 10.1016/j.bmcl.2004.04.078 BindingDB Entry DOI: 10.7270/Q2X63MD1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50404274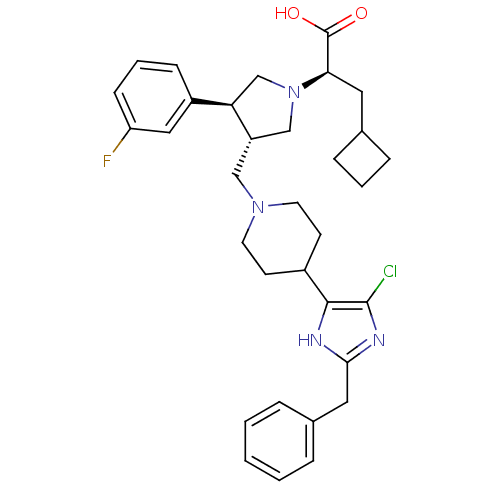 (CHEMBL2112557) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human C-C chemokine receptor type 5 assayed using [125I]-MIP-1 alpha as radioligand | Bioorg Med Chem Lett 14: 3419-24 (2004) Article DOI: 10.1016/j.bmcl.2004.04.078 BindingDB Entry DOI: 10.7270/Q2X63MD1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50249302 ((R)-2-((3S,4S)-3-((4-(2-benzyl-1H-imidazol-5-yl)pi...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human C-C chemokine receptor type 5 assayed using [125I]-MIP-1 alpha as radioligand | Bioorg Med Chem Lett 14: 3419-24 (2004) Article DOI: 10.1016/j.bmcl.2004.04.078 BindingDB Entry DOI: 10.7270/Q2X63MD1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50404272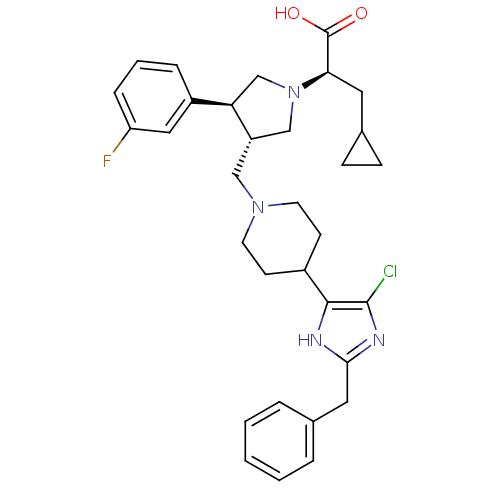 (CHEMBL2112552) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human C-C chemokine receptor type 5 assayed using [125I]-MIP-1 alpha as radioligand | Bioorg Med Chem Lett 14: 3419-24 (2004) Article DOI: 10.1016/j.bmcl.2004.04.078 BindingDB Entry DOI: 10.7270/Q2X63MD1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50404278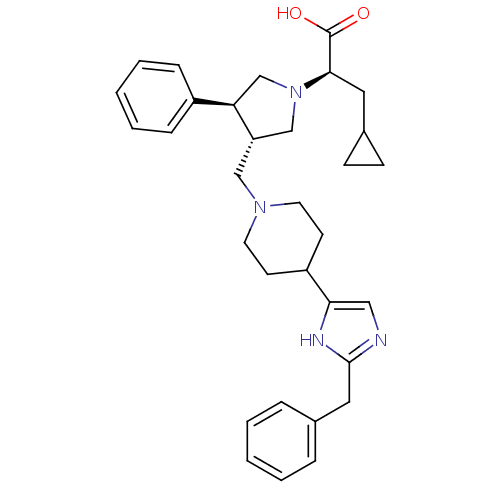 (CHEMBL2112560) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human C-C chemokine receptor type 5 assayed using [125I]-MIP-1 alpha as radioligand | Bioorg Med Chem Lett 14: 3419-24 (2004) Article DOI: 10.1016/j.bmcl.2004.04.078 BindingDB Entry DOI: 10.7270/Q2X63MD1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50404277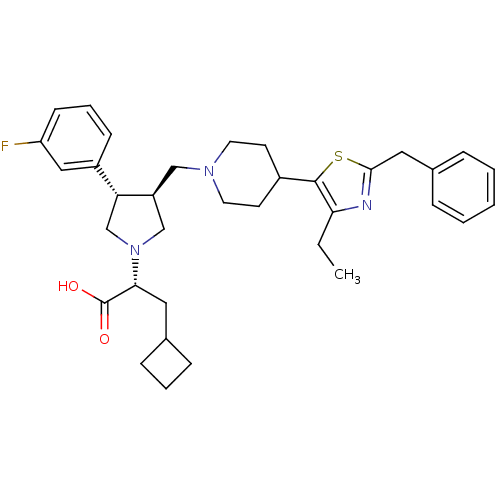 (CHEMBL2112553) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human C-C chemokine receptor type 5 assayed using [125I]-MIP-1 alpha as radioligand | Bioorg Med Chem Lett 14: 3419-24 (2004) Article DOI: 10.1016/j.bmcl.2004.04.078 BindingDB Entry DOI: 10.7270/Q2X63MD1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50404273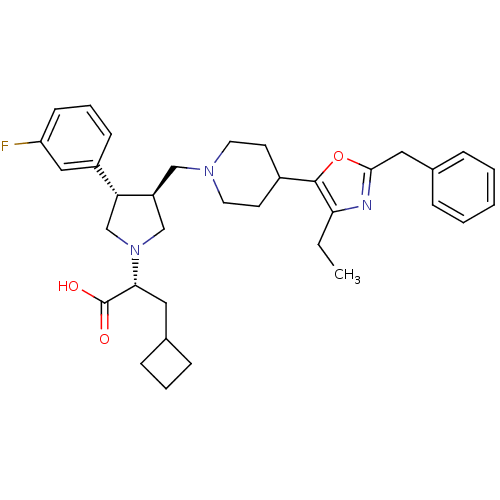 (CHEMBL2112556) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human C-C chemokine receptor type 5 assayed using [125I]-MIP-1 alpha as radioligand | Bioorg Med Chem Lett 14: 3419-24 (2004) Article DOI: 10.1016/j.bmcl.2004.04.078 BindingDB Entry DOI: 10.7270/Q2X63MD1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50105510 (Allyl-{1-[(S)-4-(benzenesulfonyl-methyl-amino)-3-m...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against human C-C chemokine receptor type 5 in CHOcells with [125I]-MIP-1 alpha radioligand | Bioorg Med Chem Lett 15: 977-82 (2005) Article DOI: 10.1016/j.bmcl.2004.12.044 BindingDB Entry DOI: 10.7270/Q2G44PSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50404280 (CHEMBL2112555) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human C-C chemokine receptor type 5 assayed using [125I]-MIP-1 alpha as radioligand | Bioorg Med Chem Lett 14: 3419-24 (2004) Article DOI: 10.1016/j.bmcl.2004.04.078 BindingDB Entry DOI: 10.7270/Q2X63MD1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50404279 (CHEMBL2112554) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human C-C chemokine receptor type 5 assayed using [125I]-MIP-1 alpha as radioligand | Bioorg Med Chem Lett 14: 3419-24 (2004) Article DOI: 10.1016/j.bmcl.2004.04.078 BindingDB Entry DOI: 10.7270/Q2X63MD1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50249303 ((R)-2-((3S,4S)-3-((4-(2-benzylthiazol-4-yl)piperid...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human C-C chemokine receptor type 5 assayed using [125I]-MIP-1 alpha as radioligand | Bioorg Med Chem Lett 14: 3419-24 (2004) Article DOI: 10.1016/j.bmcl.2004.04.078 BindingDB Entry DOI: 10.7270/Q2X63MD1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50249304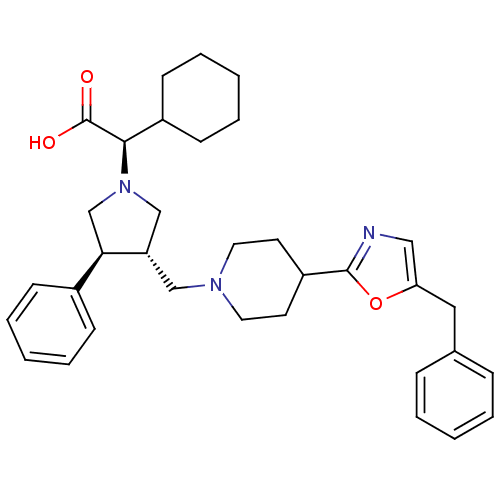 ((R)-2-((3S,4S)-3-((4-(5-benzyloxazol-2-yl)piperidi...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human C-C chemokine receptor type 5 assayed using [125I]-MIP-1 alpha as radioligand | Bioorg Med Chem Lett 14: 3419-24 (2004) Article DOI: 10.1016/j.bmcl.2004.04.078 BindingDB Entry DOI: 10.7270/Q2X63MD1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50249466 ((R)-2-((3S,4S)-3-((4-(2-benzyloxazol-5-yl)piperidi...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human C-C chemokine receptor type 5 assayed using [125I]-MIP-1 alpha as radioligand | Bioorg Med Chem Lett 14: 3419-24 (2004) Article DOI: 10.1016/j.bmcl.2004.04.078 BindingDB Entry DOI: 10.7270/Q2X63MD1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50160674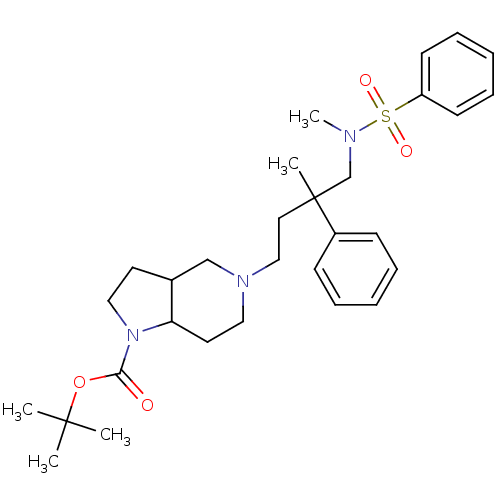 (5-[4-(Benzenesulfonyl-methyl-amino)-3-methyl-3-phe...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against human C-C chemokine receptor type 5 in CHOcells with [125I]-MIP-1 alpha radioligand | Bioorg Med Chem Lett 15: 977-82 (2005) Article DOI: 10.1016/j.bmcl.2004.12.044 BindingDB Entry DOI: 10.7270/Q2G44PSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50249430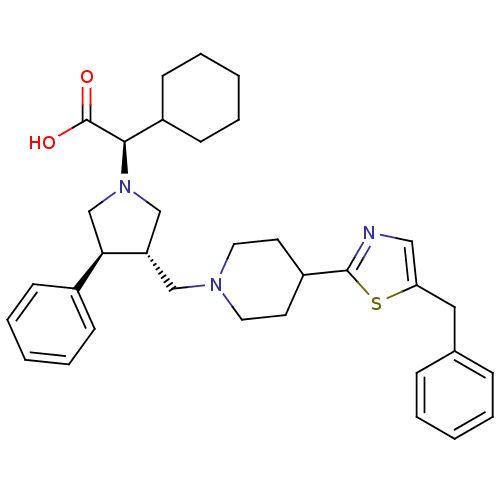 ((R)-2-((3S,4S)-3-((4-(5-benzylthiazol-2-yl)piperid...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human C-C chemokine receptor type 5 assayed using [125I]-MIP-1 alpha as radioligand | Bioorg Med Chem Lett 14: 3419-24 (2004) Article DOI: 10.1016/j.bmcl.2004.04.078 BindingDB Entry DOI: 10.7270/Q2X63MD1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50104235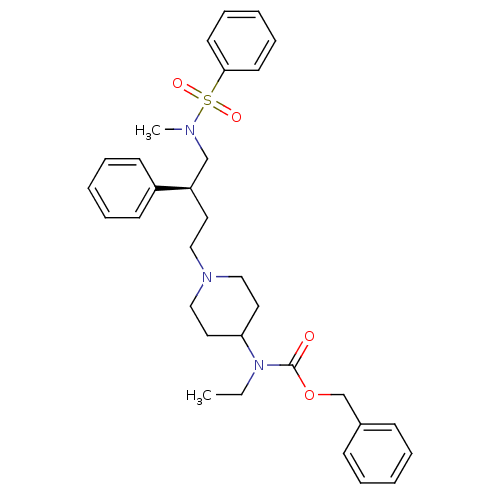 (CHEMBL85086 | {1-[(S)-4-(Benzenesulfonyl-methyl-am...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against human C-C chemokine receptor type 5 in CHOcells with [125I]-MIP-1 alpha radioligand | Bioorg Med Chem Lett 15: 977-82 (2005) Article DOI: 10.1016/j.bmcl.2004.12.044 BindingDB Entry DOI: 10.7270/Q2G44PSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50160678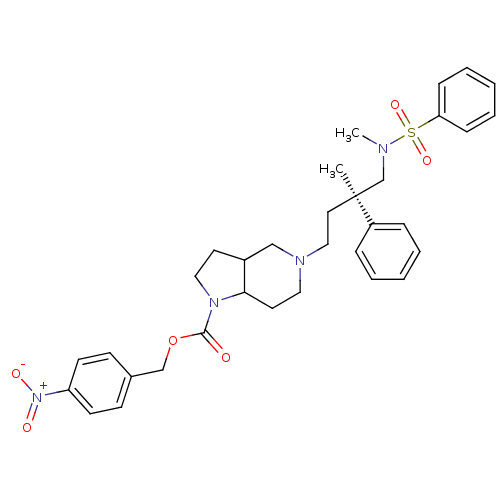 (5-[(R)-4-(Benzenesulfonyl-methyl-amino)-3-methyl-3...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against human C-C chemokine receptor type 5 in CHOcells with [125I]-MIP-1 alpha radioligand | Bioorg Med Chem Lett 15: 977-82 (2005) Article DOI: 10.1016/j.bmcl.2004.12.044 BindingDB Entry DOI: 10.7270/Q2G44PSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50249333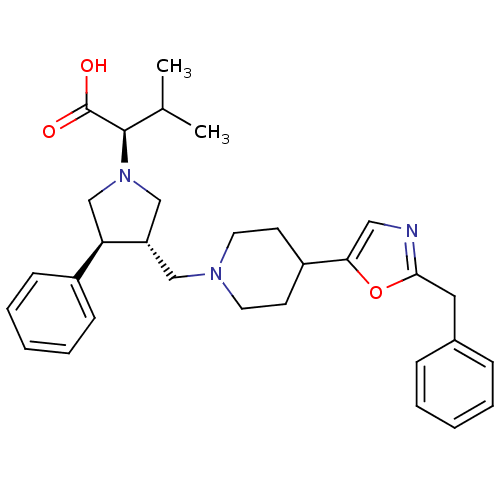 ((R)-2-((3S,4S)-3-((4-(2-benzyloxazol-5-yl)piperidi...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human C-C chemokine receptor type 5 assayed using [125I]-MIP-1 alpha as radioligand | Bioorg Med Chem Lett 14: 3419-24 (2004) Article DOI: 10.1016/j.bmcl.2004.04.078 BindingDB Entry DOI: 10.7270/Q2X63MD1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50160682 (CHEMBL180274 | N-Methyl-N-{(S)-2-methyl-4-[1-(4-ni...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against human C-C chemokine receptor type 5 in CHOcells with [125I]-MIP-1 alpha radioligand | Bioorg Med Chem Lett 15: 977-82 (2005) Article DOI: 10.1016/j.bmcl.2004.12.044 BindingDB Entry DOI: 10.7270/Q2G44PSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50249429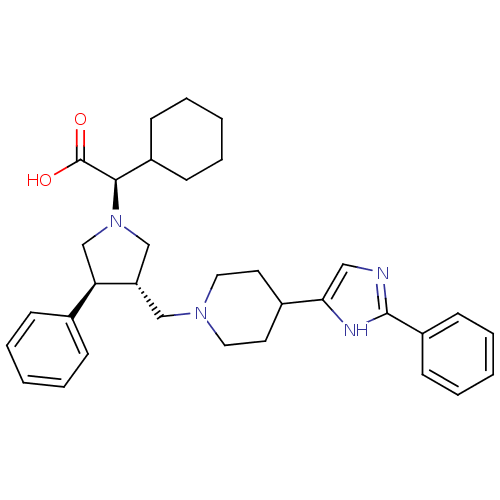 ((R)-2-cyclohexyl-2-((3S,4S)-3-phenyl-4-((4-(2-phen...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human C-C chemokine receptor type 5 assayed using [125I]-MIP-1 alpha as radioligand | Bioorg Med Chem Lett 14: 3419-24 (2004) Article DOI: 10.1016/j.bmcl.2004.04.078 BindingDB Entry DOI: 10.7270/Q2X63MD1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50160679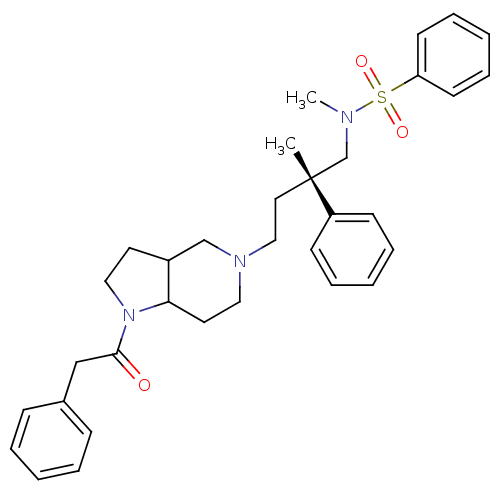 (CHEMBL426048 | N-Methyl-N-[(S)-2-methyl-2-phenyl-4...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against human C-C chemokine receptor type 5 in CHOcells with [125I]-MIP-1 alpha radioligand | Bioorg Med Chem Lett 15: 977-82 (2005) Article DOI: 10.1016/j.bmcl.2004.12.044 BindingDB Entry DOI: 10.7270/Q2G44PSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50249332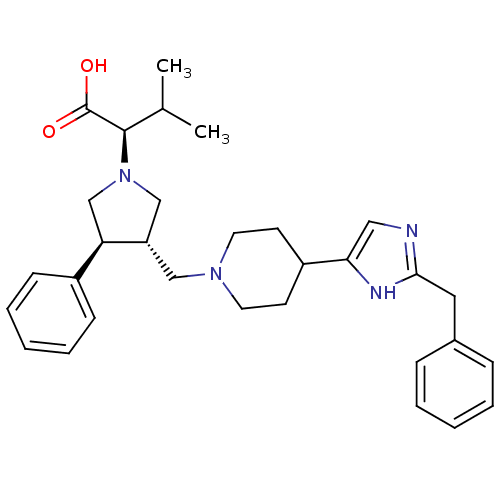 ((R)-2-((3S,4S)-3-((4-(2-benzyl-1H-imidazol-5-yl)pi...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human C-C chemokine receptor type 5 assayed using [125I]-MIP-1 alpha as radioligand | Bioorg Med Chem Lett 14: 3419-24 (2004) Article DOI: 10.1016/j.bmcl.2004.04.078 BindingDB Entry DOI: 10.7270/Q2X63MD1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50160683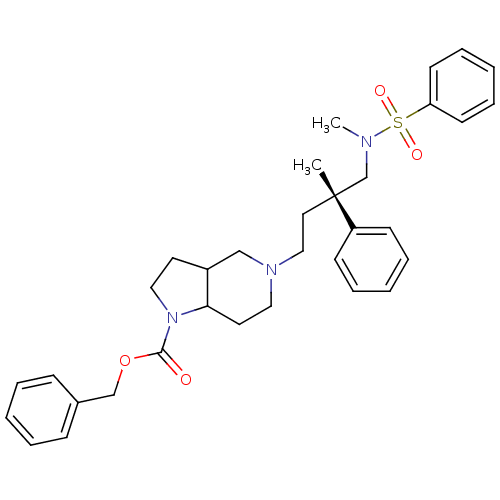 (5-[(S)-4-(Benzenesulfonyl-methyl-amino)-3-methyl-3...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against human C-C chemokine receptor type 5 in CHOcells with [125I]-MIP-1 alpha radioligand | Bioorg Med Chem Lett 15: 977-82 (2005) Article DOI: 10.1016/j.bmcl.2004.12.044 BindingDB Entry DOI: 10.7270/Q2G44PSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50160675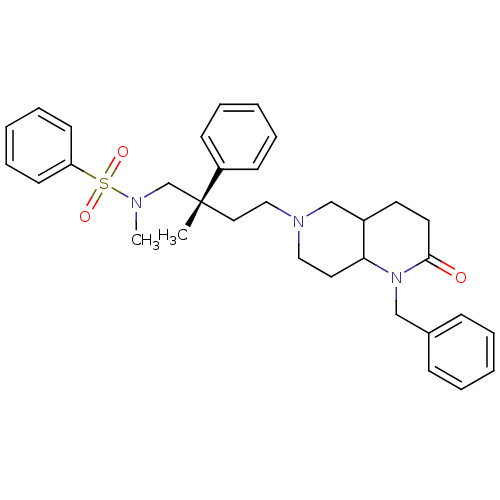 (CHEMBL361585 | N-[(S)-4-(1-Benzyl-2-oxo-octahydro-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against human C-C chemokine receptor type 5 in CHOcells with [125I]-MIP-1 alpha radioligand | Bioorg Med Chem Lett 15: 977-82 (2005) Article DOI: 10.1016/j.bmcl.2004.12.044 BindingDB Entry DOI: 10.7270/Q2G44PSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50160677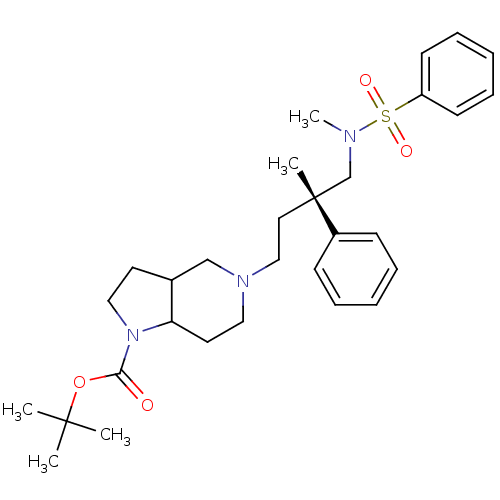 (5-[(S)-4-(Benzenesulfonyl-methyl-amino)-3-methyl-3...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against human C-C chemokine receptor type 5 in CHOcells with [125I]-MIP-1 alpha radioligand | Bioorg Med Chem Lett 15: 977-82 (2005) Article DOI: 10.1016/j.bmcl.2004.12.044 BindingDB Entry DOI: 10.7270/Q2G44PSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50164777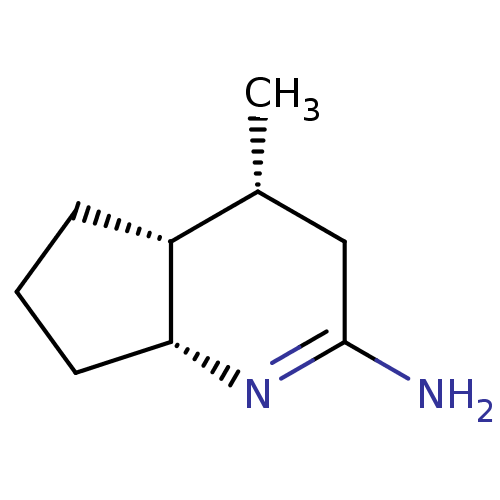 ((4R,4aR,7aR)-4-Methyl-octahydro-[1]pyrindin-(2E)-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human Inducible nitric oxide synthase (iNOS) | Bioorg Med Chem Lett 15: 1997-2001 (2005) Article DOI: 10.1016/j.bmcl.2005.02.067 BindingDB Entry DOI: 10.7270/Q2XK8F3M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50164777 ((4R,4aR,7aR)-4-Methyl-octahydro-[1]pyrindin-(2E)-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human Inducible nitric oxide synthase (iNOS) | Bioorg Med Chem Lett 15: 1997-2001 (2005) Article DOI: 10.1016/j.bmcl.2005.02.067 BindingDB Entry DOI: 10.7270/Q2XK8F3M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50062133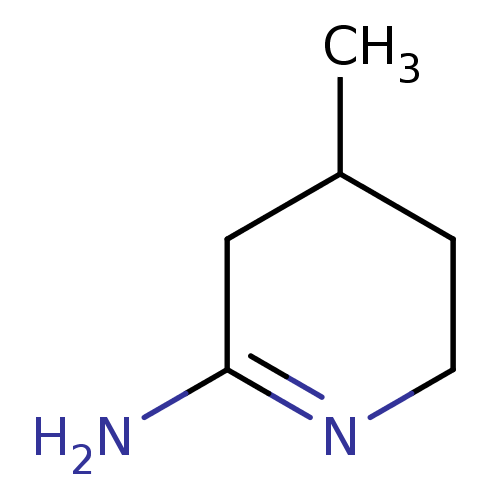 (4-Methyl-piperidin-(2E)-ylideneamine | 4-Methyl-pi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human Neuronal nitric oxide synthase (nNOS) | Bioorg Med Chem Lett 15: 1997-2001 (2005) Article DOI: 10.1016/j.bmcl.2005.02.067 BindingDB Entry DOI: 10.7270/Q2XK8F3M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50160684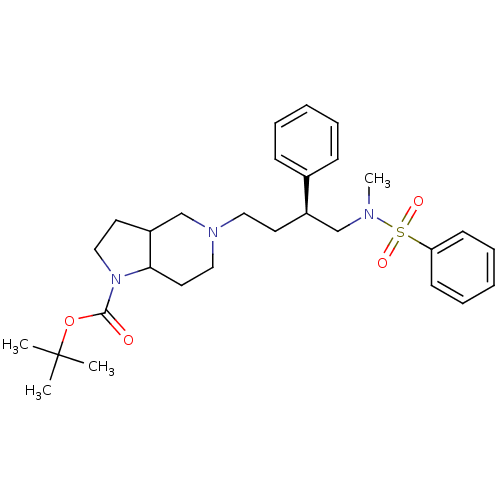 (5-[(S)-4-(Benzenesulfonyl-methyl-amino)-3-phenyl-b...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against human C-C chemokine receptor type 5 in CHOcells with [125I]-MIP-1 alpha radioligand | Bioorg Med Chem Lett 15: 977-82 (2005) Article DOI: 10.1016/j.bmcl.2004.12.044 BindingDB Entry DOI: 10.7270/Q2G44PSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50249395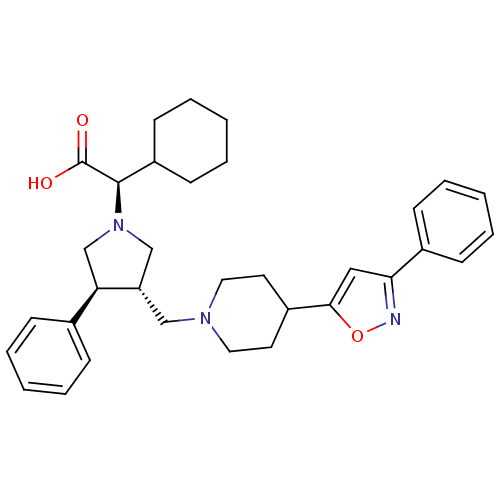 ((R)-2-cyclohexyl-2-((3S,4S)-3-phenyl-4-((4-(3-phen...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration of the compound against C-C chemokine receptor type 5 using [125I]-MIP-1 alpha as radioligand | Bioorg Med Chem Lett 14: 3419-24 (2004) Article DOI: 10.1016/j.bmcl.2004.04.078 BindingDB Entry DOI: 10.7270/Q2X63MD1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50066778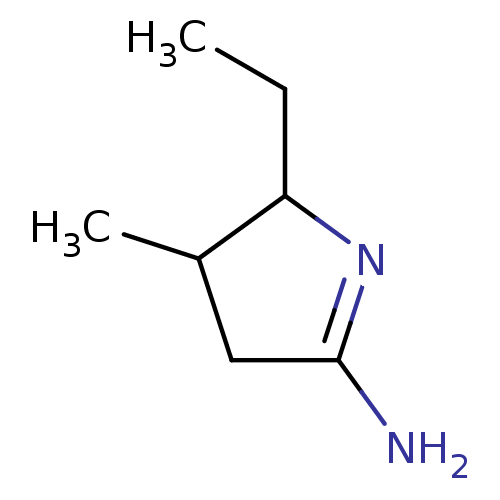 (5-Ethyl-4-methyl-pyrrolidin-(2E)-ylideneamine; hyd...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibitory activity against Inducible nitric oxide synthase | Bioorg Med Chem Lett 14: 4539-44 (2004) Article DOI: 10.1016/j.bmcl.2004.06.033 BindingDB Entry DOI: 10.7270/Q218377V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50160681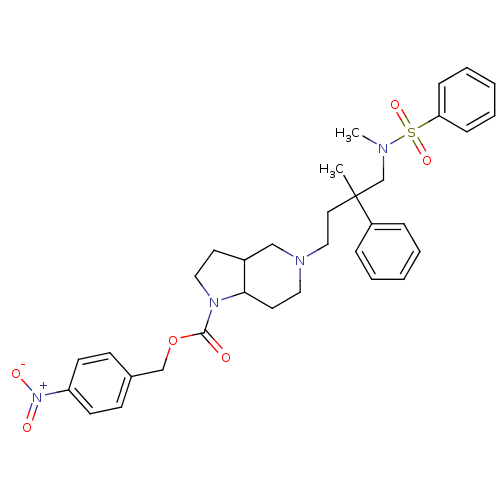 (5-[4-(Benzenesulfonyl-methyl-amino)-3-methyl-3-phe...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against human C-C chemokine receptor type 5 in CHOcells with [125I]-MIP-1 alpha radioligand | Bioorg Med Chem Lett 15: 977-82 (2005) Article DOI: 10.1016/j.bmcl.2004.12.044 BindingDB Entry DOI: 10.7270/Q2G44PSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50160681 (5-[4-(Benzenesulfonyl-methyl-amino)-3-methyl-3-phe...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against human C-C chemokine receptor type 5 in CHOcells with [125I]-MIP-1 alpha radioligand | Bioorg Med Chem Lett 15: 977-82 (2005) Article DOI: 10.1016/j.bmcl.2004.12.044 BindingDB Entry DOI: 10.7270/Q2G44PSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50164782 ((4R,4aR,5R)-4-Methyl-octahydro-quinolin-(2E)-ylide...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human Inducible nitric oxide synthase (iNOS) | Bioorg Med Chem Lett 15: 1997-2001 (2005) Article DOI: 10.1016/j.bmcl.2005.02.067 BindingDB Entry DOI: 10.7270/Q2XK8F3M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50249428 ((R)-2-cyclohexyl-2-((3S,4S)-3-phenyl-4-((4-(4-phen...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human C-C chemokine receptor type 5 assayed using [125I]-MIP-1 alpha as radioligand | Bioorg Med Chem Lett 14: 3419-24 (2004) Article DOI: 10.1016/j.bmcl.2004.04.078 BindingDB Entry DOI: 10.7270/Q2X63MD1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50249396 ((R)-2-cyclohexyl-2-((3S,4S)-3-phenyl-4-((4-(4-phen...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human C-C chemokine receptor type 5 assayed using [125I]-MIP-1 alpha as radioligand | Bioorg Med Chem Lett 14: 3419-24 (2004) Article DOI: 10.1016/j.bmcl.2004.04.078 BindingDB Entry DOI: 10.7270/Q2X63MD1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50160676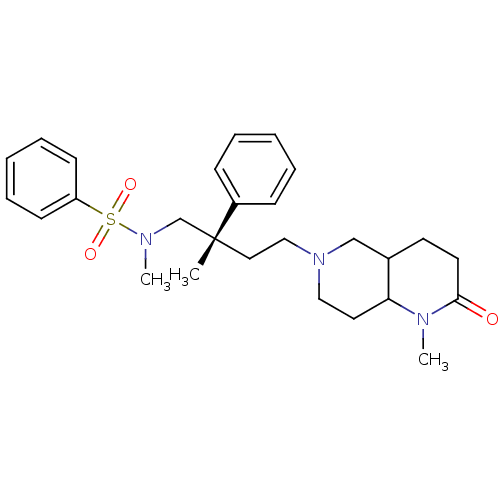 (CHEMBL182188 | N-Methyl-N-[(S)-2-methyl-4-(1-methy...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against human C-C chemokine receptor type 5 in CHOcells with [125I]-MIP-1 alpha radioligand | Bioorg Med Chem Lett 15: 977-82 (2005) Article DOI: 10.1016/j.bmcl.2004.12.044 BindingDB Entry DOI: 10.7270/Q2G44PSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50164777 ((4R,4aR,7aR)-4-Methyl-octahydro-[1]pyrindin-(2E)-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human Neuronal nitric oxide synthase (nNOS) | Bioorg Med Chem Lett 15: 1997-2001 (2005) Article DOI: 10.1016/j.bmcl.2005.02.067 BindingDB Entry DOI: 10.7270/Q2XK8F3M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50164777 ((4R,4aR,7aR)-4-Methyl-octahydro-[1]pyrindin-(2E)-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human Neuronal nitric oxide synthase (nNOS) | Bioorg Med Chem Lett 15: 1997-2001 (2005) Article DOI: 10.1016/j.bmcl.2005.02.067 BindingDB Entry DOI: 10.7270/Q2XK8F3M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50160688 (CHEMBL359752 | N-Methyl-N-{(S)-4-[4-((R)-2-oxo-5-p...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 31.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against human C-C chemokine receptor type 5 in CHOcells with [125I]-MIP-1 alpha radioligand | Bioorg Med Chem Lett 15: 977-82 (2005) Article DOI: 10.1016/j.bmcl.2004.12.044 BindingDB Entry DOI: 10.7270/Q2G44PSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50164784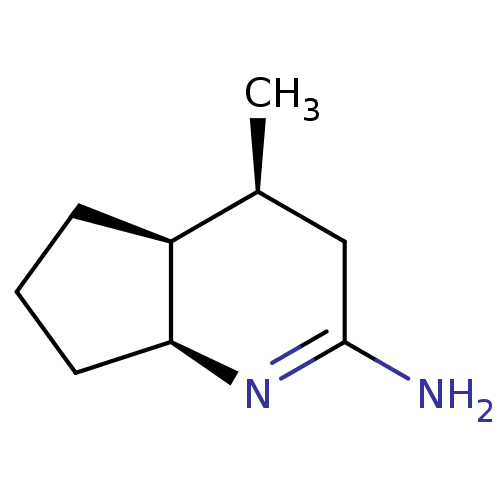 ((4S,7S)-4-Methyl-octahydro-[1]pyrindin-(2E)-yliden...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human Neuronal nitric oxide synthase (nNOS) | Bioorg Med Chem Lett 15: 1997-2001 (2005) Article DOI: 10.1016/j.bmcl.2005.02.067 BindingDB Entry DOI: 10.7270/Q2XK8F3M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50160685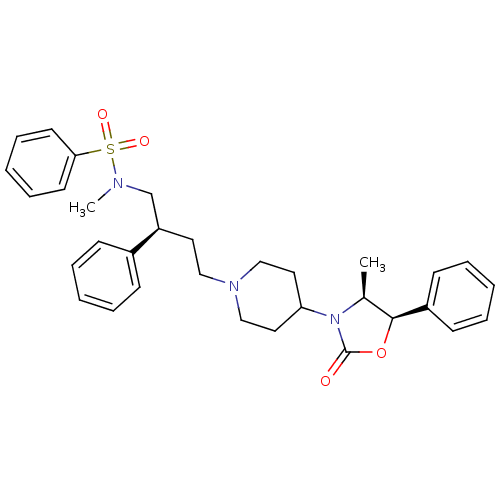 (CHEMBL178337 | N-Methyl-N-{(S)-4-[4-((R)-4-methyl-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 36.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against human C-C chemokine receptor type 5 in CHOcells with [125I]-MIP-1 alpha radioligand | Bioorg Med Chem Lett 15: 977-82 (2005) Article DOI: 10.1016/j.bmcl.2004.12.044 BindingDB Entry DOI: 10.7270/Q2G44PSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50062133 (4-Methyl-piperidin-(2E)-ylideneamine | 4-Methyl-pi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human Inducible nitric oxide synthase (iNOS) | Bioorg Med Chem Lett 15: 1997-2001 (2005) Article DOI: 10.1016/j.bmcl.2005.02.067 BindingDB Entry DOI: 10.7270/Q2XK8F3M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50164779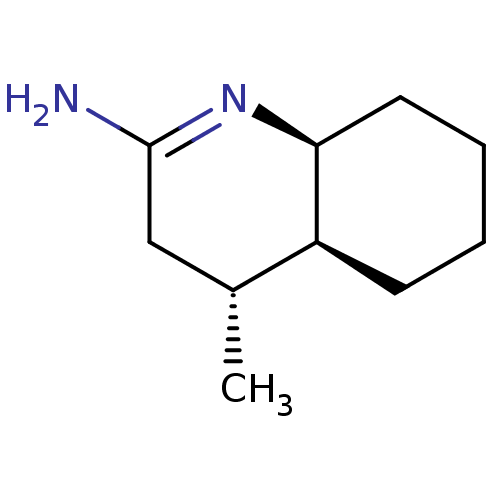 ((4R,4aS,5S)-4-Methyl-octahydro-quinolin-(2E)-ylide...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human Inducible nitric oxide synthase (iNOS) | Bioorg Med Chem Lett 15: 1997-2001 (2005) Article DOI: 10.1016/j.bmcl.2005.02.067 BindingDB Entry DOI: 10.7270/Q2XK8F3M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 170 total ) | Next | Last >> |