

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM321150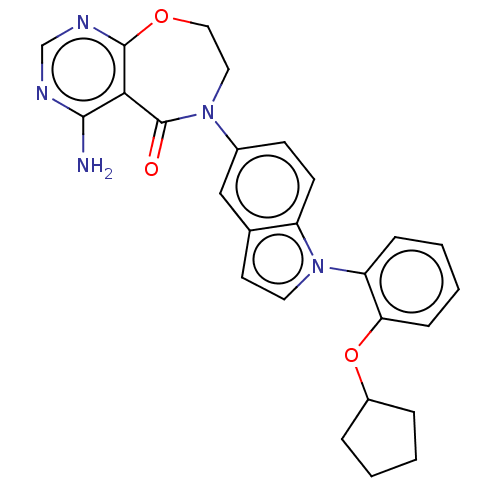 (US10174049, Example 17 | US9738658, Example 17 | U...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
GLAXOSMITHKLINE LLC US Patent | Assay Description For inhibition of triacylglycerol product formation, 11 uL reactions were run in white Polyplate-384 (PerkinElmer6007300) starting with a 30 minute p... | US Patent US9796729 (2017) BindingDB Entry DOI: 10.7270/Q2057J2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM321150 (US10174049, Example 17 | US9738658, Example 17 | U...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline LLC US Patent | Assay Description For inhibition of triacylglycerol product formation, 11 uL reactions were run in white Polyplate-384 (PerkinElmer6007300) starting with a 30 minute p... | US Patent US9738658 (2017) BindingDB Entry DOI: 10.7270/Q26Q20BZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM321150 (US10174049, Example 17 | US9738658, Example 17 | U...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxosmithkline LLC US Patent | Assay Description For inhibition of triacylglycerol product formation, 11 uL reactions were run in white Polyplate-384 (PerkinElmer6007300) starting with a 30 minute p... | US Patent US10174049 (2019) BindingDB Entry DOI: 10.7270/Q2X92DCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM321135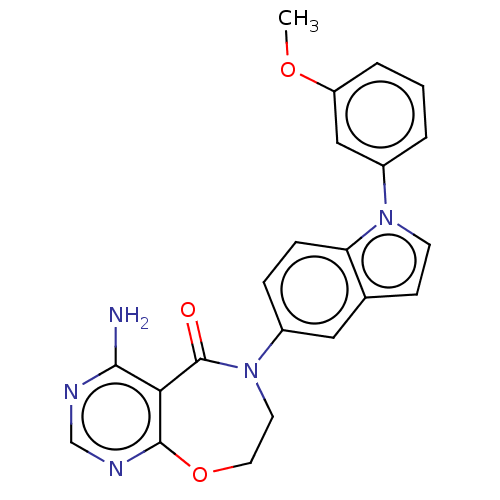 (US10174049, Example 2) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxosmithkline LLC US Patent | Assay Description For inhibition of triacylglycerol product formation, 11 uL reactions were run in white Polyplate-384 (PerkinElmer6007300) starting with a 30 minute p... | US Patent US10174049 (2019) BindingDB Entry DOI: 10.7270/Q2X92DCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM336120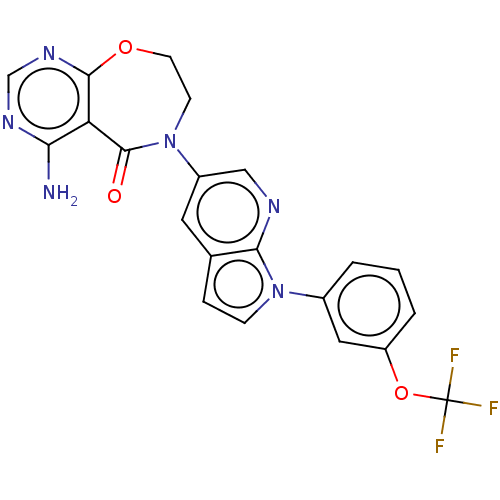 (US9738658, Example 2) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline LLC US Patent | Assay Description For inhibition of triacylglycerol product formation, 11 uL reactions were run in white Polyplate-384 (PerkinElmer6007300) starting with a 30 minute p... | US Patent US9738658 (2017) BindingDB Entry DOI: 10.7270/Q26Q20BZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM351918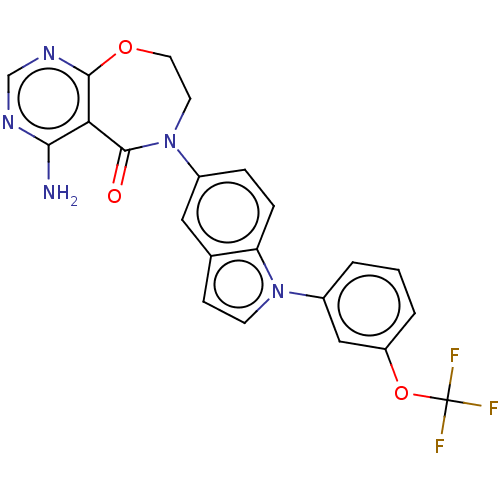 (4-Amino-6-(1-(3-(trifluoromethoxy)phenyl)-1H-indol...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GLAXOSMITHKLINE LLC US Patent | Assay Description For inhibition of triacylglycerol product formation, 11 uL reactions were run in white Polyplate-384 (PerkinElmer6007300) starting with a 30 minute p... | US Patent US9796729 (2017) BindingDB Entry DOI: 10.7270/Q2057J2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50203332 (CHEMBL3913908) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of recombinant CYP17 (unknown origin) overexpressed in human AD293 cells using [21-3H]17alpha-hydroxyl-pregenolone as substrate preincubat... | Bioorg Med Chem Lett 26: 5825-5829 (2016) Article DOI: 10.1016/j.bmcl.2016.10.016 BindingDB Entry DOI: 10.7270/Q2X63PW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50274157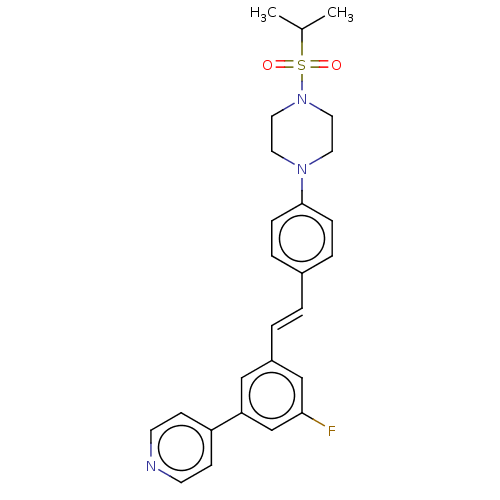 (CHEMBL4127656) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of recombinant CYP17 (unknown origin) expressed in human AD293 cells using [21-3H]17alpha-hydroxypregnenolone as substrate preincubated fo... | Bioorg Med Chem Lett 28: 2270-2274 (2018) Article DOI: 10.1016/j.bmcl.2018.05.040 BindingDB Entry DOI: 10.7270/Q2RJ4N0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50287311 (CHEMBL4176968) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of recombinant human DGAT1 expressed in baculovirus infected Sf9 insect microsomal membranes using C10-DAG and [3H]-labelled decanoyl-CoA ... | ACS Med Chem Lett 9: 103-108 (2018) Article DOI: 10.1021/acsmedchemlett.7b00450 BindingDB Entry DOI: 10.7270/Q2V127C9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50274165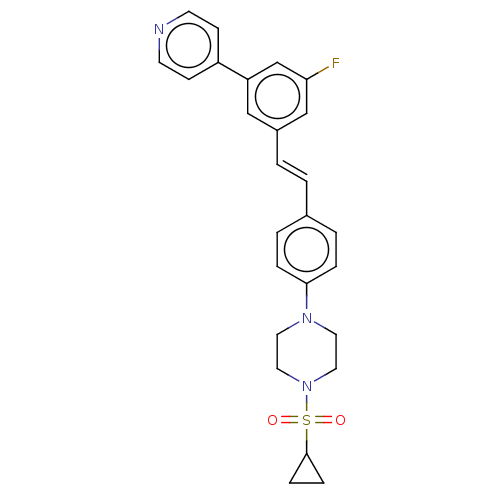 (CHEMBL4126996) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of recombinant CYP17 (unknown origin) expressed in human AD293 cells using [21-3H]17alpha-hydroxypregnenolone as substrate preincubated fo... | Bioorg Med Chem Lett 28: 2270-2274 (2018) Article DOI: 10.1016/j.bmcl.2018.05.040 BindingDB Entry DOI: 10.7270/Q2RJ4N0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM321161 (US10174049, Example 28 | US10385066, Example 28 | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxosmithkline LLC US Patent | Assay Description For inhibition of triacylglycerol product formation, 11 uL reactions were run in white Polyplate-384 (PerkinElmer6007300) starting with a 30 minute p... | US Patent US10174049 (2019) BindingDB Entry DOI: 10.7270/Q2X92DCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM321161 (US10174049, Example 28 | US10385066, Example 28 | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline LLC US Patent | Assay Description For inhibition of triacylglycerol product formation, 11 uL reactions were run in white Polyplate-384 (PerkinElmer6007300) starting with a 30 minute p... | US Patent US9738658 (2017) BindingDB Entry DOI: 10.7270/Q26Q20BZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM321161 (US10174049, Example 28 | US10385066, Example 28 | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
GLAXOSMITHKLINE LLC US Patent | Assay Description For inhibition of triacylglycerol product formation, 11 uL reactions were run in white Polyplate-384 (PerkinElmer6007300) starting with a 30 minute p... | US Patent US9796729 (2017) BindingDB Entry DOI: 10.7270/Q2057J2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM321169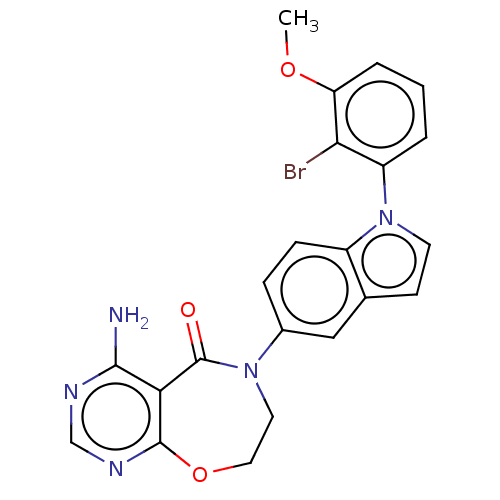 (US10174049, Example 36 | US9738658, Example 36 | U...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxosmithkline LLC US Patent | Assay Description For inhibition of triacylglycerol product formation, 11 uL reactions were run in white Polyplate-384 (PerkinElmer6007300) starting with a 30 minute p... | US Patent US10174049 (2019) BindingDB Entry DOI: 10.7270/Q2X92DCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM321145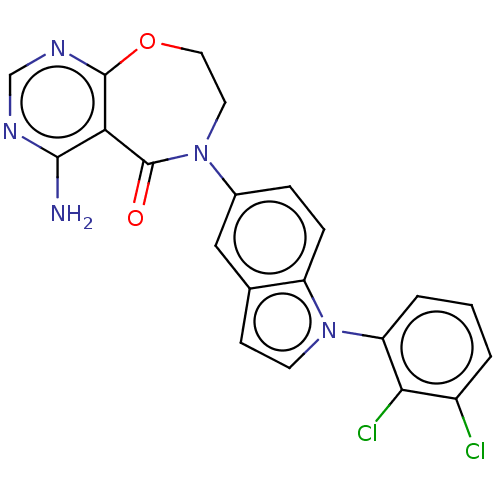 (US10174049, Example 12 | US9738658, Example 12 | U...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxosmithkline LLC US Patent | Assay Description For inhibition of triacylglycerol product formation, 11 uL reactions were run in white Polyplate-384 (PerkinElmer6007300) starting with a 30 minute p... | US Patent US10174049 (2019) BindingDB Entry DOI: 10.7270/Q2X92DCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM321169 (US10174049, Example 36 | US9738658, Example 36 | U...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
GLAXOSMITHKLINE LLC US Patent | Assay Description For inhibition of triacylglycerol product formation, 11 uL reactions were run in white Polyplate-384 (PerkinElmer6007300) starting with a 30 minute p... | US Patent US9796729 (2017) BindingDB Entry DOI: 10.7270/Q2057J2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM321155 (US10174049, Example 22 | US10385066, Example 22 | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
GLAXOSMITHKLINE LLC US Patent | Assay Description For inhibition of triacylglycerol product formation, 11 uL reactions were run in white Polyplate-384 (PerkinElmer6007300) starting with a 30 minute p... | US Patent US9796729 (2017) BindingDB Entry DOI: 10.7270/Q2057J2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM321155 (US10174049, Example 22 | US10385066, Example 22 | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxosmithkline LLC US Patent | Assay Description For inhibition of triacylglycerol product formation, 11 uL reactions were run in white Polyplate-384 (PerkinElmer6007300) starting with a 30 minute p... | US Patent US10174049 (2019) BindingDB Entry DOI: 10.7270/Q2X92DCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM321169 (US10174049, Example 36 | US9738658, Example 36 | U...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline LLC US Patent | Assay Description For inhibition of triacylglycerol product formation, 11 uL reactions were run in white Polyplate-384 (PerkinElmer6007300) starting with a 30 minute p... | US Patent US9738658 (2017) BindingDB Entry DOI: 10.7270/Q26Q20BZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM336140 (US9738658, Example 22) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline LLC US Patent | Assay Description For inhibition of triacylglycerol product formation, 11 uL reactions were run in white Polyplate-384 (PerkinElmer6007300) starting with a 30 minute p... | US Patent US9738658 (2017) BindingDB Entry DOI: 10.7270/Q26Q20BZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM321145 (US10174049, Example 12 | US9738658, Example 12 | U...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline LLC US Patent | Assay Description For inhibition of triacylglycerol product formation, 11 uL reactions were run in white Polyplate-384 (PerkinElmer6007300) starting with a 30 minute p... | US Patent US9738658 (2017) BindingDB Entry DOI: 10.7270/Q26Q20BZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM321145 (US10174049, Example 12 | US9738658, Example 12 | U...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
GLAXOSMITHKLINE LLC US Patent | Assay Description For inhibition of triacylglycerol product formation, 11 uL reactions were run in white Polyplate-384 (PerkinElmer6007300) starting with a 30 minute p... | US Patent US9796729 (2017) BindingDB Entry DOI: 10.7270/Q2057J2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50274156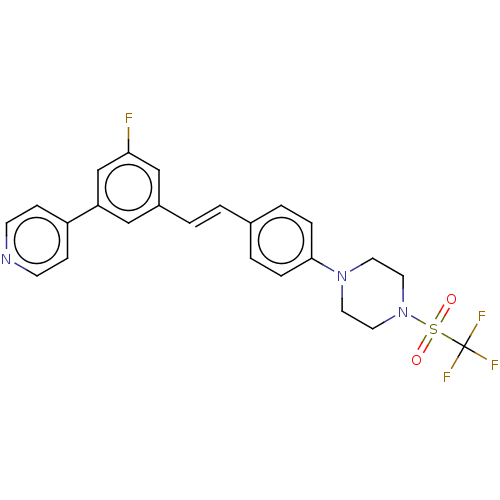 (CHEMBL4128368) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of recombinant CYP17 (unknown origin) expressed in human AD293 cells using [21-3H]17alpha-hydroxypregnenolone as substrate preincubated fo... | Bioorg Med Chem Lett 28: 2270-2274 (2018) Article DOI: 10.1016/j.bmcl.2018.05.040 BindingDB Entry DOI: 10.7270/Q2RJ4N0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50287453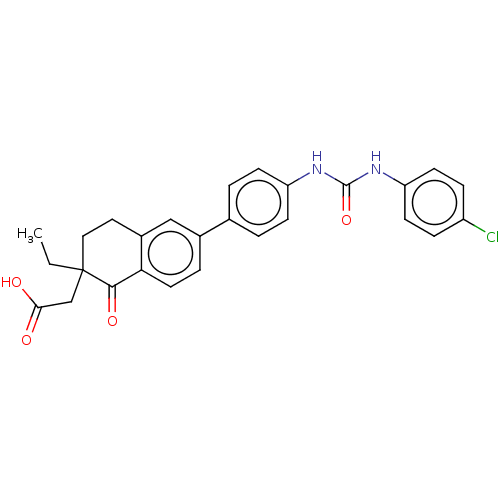 (CHEMBL4163300) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of recombinant human DGAT1 expressed in baculovirus infected Sf9 insect microsomal membranes using C10-DAG and C10-CoA as substrate pretre... | ACS Med Chem Lett 9: 103-108 (2018) Article DOI: 10.1021/acsmedchemlett.7b00450 BindingDB Entry DOI: 10.7270/Q2V127C9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50287389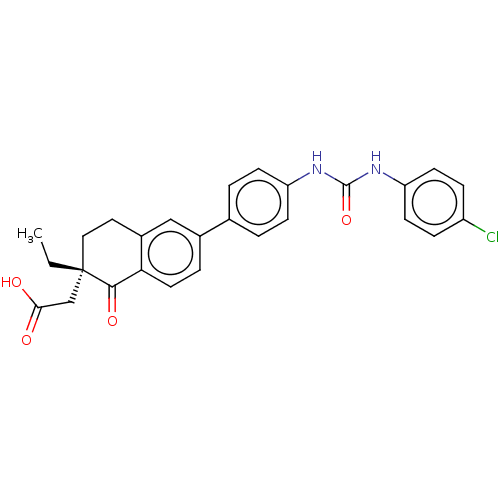 (CHEMBL4174767) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of recombinant human DGAT1 expressed in baculovirus infected Sf9 insect microsomal membranes using C10-DAG and C10-CoA as substrate pretre... | ACS Med Chem Lett 9: 103-108 (2018) Article DOI: 10.1021/acsmedchemlett.7b00450 BindingDB Entry DOI: 10.7270/Q2V127C9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50007606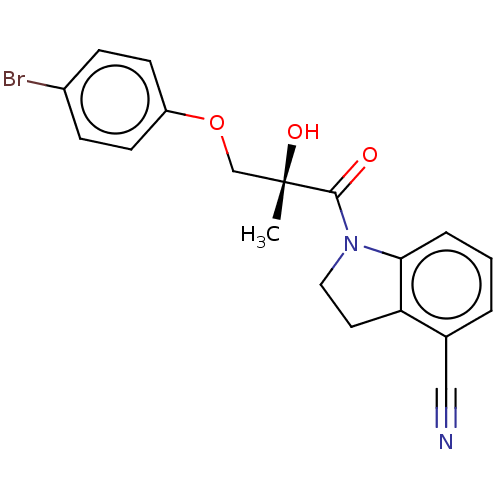 (CHEMBL3238280) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]mibolerone from full length human AR expressed in COS cells after 3.5 hrs by liquid scintillation counting | J Med Chem 57: 2462-71 (2014) Article DOI: 10.1021/jm401625b BindingDB Entry DOI: 10.7270/Q2RB763C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50287311 (CHEMBL4176968) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of recombinant human DGAT1 expressed in baculovirus infected Sf9 insect microsomal membranes using C10-DAG and C10-CoA as substrate pretre... | ACS Med Chem Lett 9: 103-108 (2018) Article DOI: 10.1021/acsmedchemlett.7b00450 BindingDB Entry DOI: 10.7270/Q2V127C9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM321166 (US10174049, Example 33 | US10385066, Example 33 | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline LLC US Patent | Assay Description For inhibition of triacylglycerol product formation, 11 uL reactions were run in white Polyplate-384 (PerkinElmer6007300) starting with a 30 minute p... | US Patent US9738658 (2017) BindingDB Entry DOI: 10.7270/Q26Q20BZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM321166 (US10174049, Example 33 | US10385066, Example 33 | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxosmithkline LLC US Patent | Assay Description For inhibition of triacylglycerol product formation, 11 uL reactions were run in white Polyplate-384 (PerkinElmer6007300) starting with a 30 minute p... | US Patent US10174049 (2019) BindingDB Entry DOI: 10.7270/Q2X92DCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM321166 (US10174049, Example 33 | US10385066, Example 33 | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GLAXOSMITHKLINE LLC US Patent | Assay Description For inhibition of triacylglycerol product formation, 11 uL reactions were run in white Polyplate-384 (PerkinElmer6007300) starting with a 30 minute p... | US Patent US9796729 (2017) BindingDB Entry DOI: 10.7270/Q2057J2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM321141 (US10174049, Example 8 | US10385066, Example 8 | US...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline LLC US Patent | Assay Description For inhibition of triacylglycerol product formation, 11 uL reactions were run in white Polyplate-384 (PerkinElmer6007300) starting with a 30 minute p... | US Patent US9738658 (2017) BindingDB Entry DOI: 10.7270/Q26Q20BZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM351925 (US9796729, 8) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
GLAXOSMITHKLINE LLC US Patent | Assay Description For inhibition of triacylglycerol product formation, 11 uL reactions were run in white Polyplate-384 (PerkinElmer6007300) starting with a 30 minute p... | US Patent US9796729 (2017) BindingDB Entry DOI: 10.7270/Q2057J2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM321141 (US10174049, Example 8 | US10385066, Example 8 | US...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxosmithkline LLC US Patent | Assay Description For inhibition of triacylglycerol product formation, 11 uL reactions were run in white Polyplate-384 (PerkinElmer6007300) starting with a 30 minute p... | US Patent US10174049 (2019) BindingDB Entry DOI: 10.7270/Q2X92DCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50287329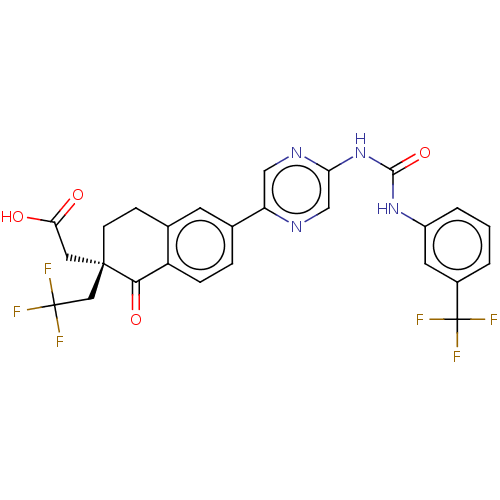 (CHEMBL4163009) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of recombinant human DGAT1 expressed in baculovirus infected Sf9 insect microsomal membranes using C10-DAG and [3H]-labelled decanoyl-CoA ... | ACS Med Chem Lett 9: 103-108 (2018) Article DOI: 10.1021/acsmedchemlett.7b00450 BindingDB Entry DOI: 10.7270/Q2V127C9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50287449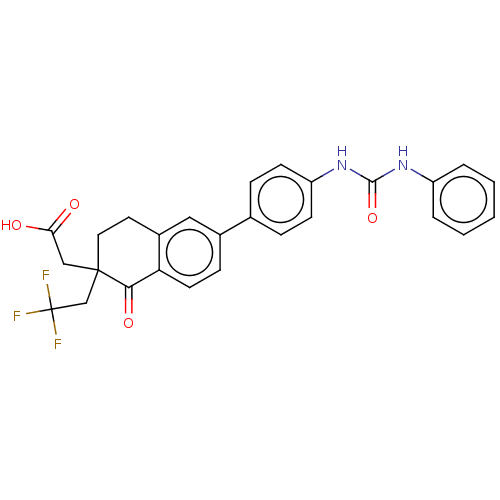 (CHEMBL4176181) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of recombinant human DGAT1 expressed in baculovirus infected Sf9 insect microsomal membranes using C10-DAG and C10-CoA as substrate pretre... | ACS Med Chem Lett 9: 103-108 (2018) Article DOI: 10.1021/acsmedchemlett.7b00450 BindingDB Entry DOI: 10.7270/Q2V127C9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50287310 (CHEMBL4173715) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of recombinant human DGAT1 expressed in baculovirus infected Sf9 insect microsomal membranes using C10-DAG and [3H]-labelled decanoyl-CoA ... | ACS Med Chem Lett 9: 103-108 (2018) Article DOI: 10.1021/acsmedchemlett.7b00450 BindingDB Entry DOI: 10.7270/Q2V127C9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50287313 (CHEMBL4168991) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of recombinant human DGAT1 expressed in baculovirus infected Sf9 insect microsomal membranes using C10-DAG and C10-CoA as substrate pretre... | ACS Med Chem Lett 9: 103-108 (2018) Article DOI: 10.1021/acsmedchemlett.7b00450 BindingDB Entry DOI: 10.7270/Q2V127C9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50007591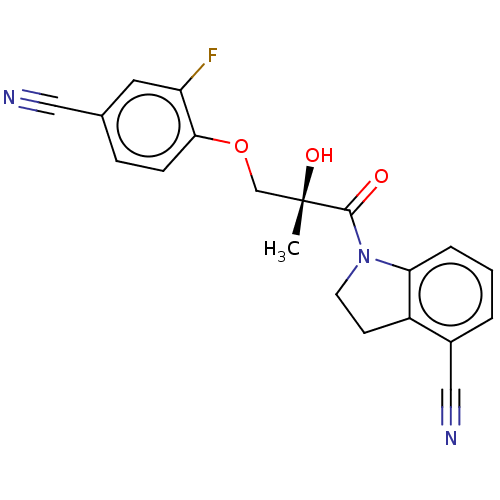 (CHEMBL3238292) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]mibolerone from full length human AR expressed in COS cells after 3.5 hrs by liquid scintillation counting | J Med Chem 57: 2462-71 (2014) Article DOI: 10.1021/jm401625b BindingDB Entry DOI: 10.7270/Q2RB763C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM321140 (US10174049, Example 7 | US10385066, Example 7 | US...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline LLC US Patent | Assay Description For inhibition of triacylglycerol product formation, 11 uL reactions were run in white Polyplate-384 (PerkinElmer6007300) starting with a 30 minute p... | US Patent US9738658 (2017) BindingDB Entry DOI: 10.7270/Q26Q20BZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM321140 (US10174049, Example 7 | US10385066, Example 7 | US...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxosmithkline LLC US Patent | Assay Description For inhibition of triacylglycerol product formation, 11 uL reactions were run in white Polyplate-384 (PerkinElmer6007300) starting with a 30 minute p... | US Patent US10174049 (2019) BindingDB Entry DOI: 10.7270/Q2X92DCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM351924 (US9796729, 7) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
GLAXOSMITHKLINE LLC US Patent | Assay Description For inhibition of triacylglycerol product formation, 11 uL reactions were run in white Polyplate-384 (PerkinElmer6007300) starting with a 30 minute p... | US Patent US9796729 (2017) BindingDB Entry DOI: 10.7270/Q2057J2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM351950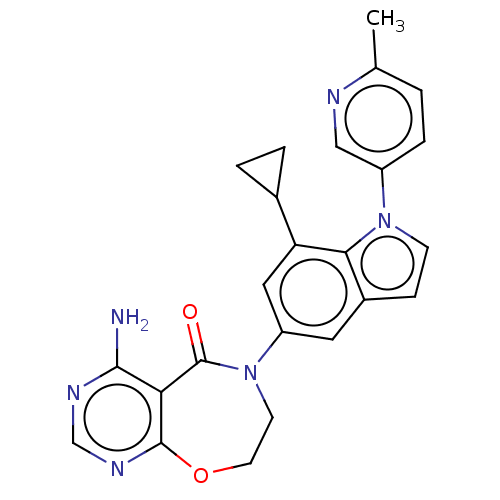 (US10385066, Example 26 | US9796729, 26) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
GLAXOSMITHKLINE LLC US Patent | Assay Description For inhibition of triacylglycerol product formation, 11 uL reactions were run in white Polyplate-384 (PerkinElmer6007300) starting with a 30 minute p... | US Patent US9796729 (2017) BindingDB Entry DOI: 10.7270/Q2057J2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM321159 (US10174049, Example 26) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxosmithkline LLC US Patent | Assay Description For inhibition of triacylglycerol product formation, 11 uL reactions were run in white Polyplate-384 (PerkinElmer6007300) starting with a 30 minute p... | US Patent US10174049 (2019) BindingDB Entry DOI: 10.7270/Q2X92DCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM336144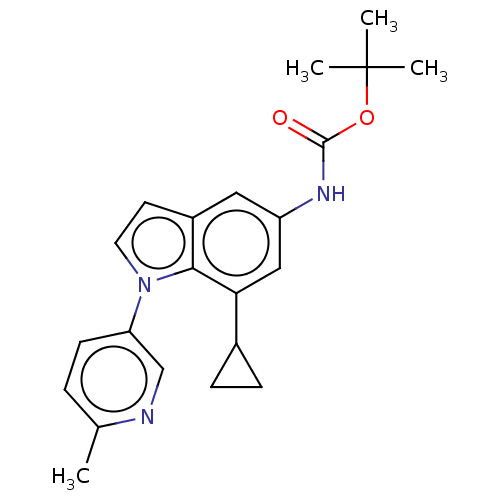 (US9738658, Example 26) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline LLC US Patent | Assay Description For inhibition of triacylglycerol product formation, 11 uL reactions were run in white Polyplate-384 (PerkinElmer6007300) starting with a 30 minute p... | US Patent US9738658 (2017) BindingDB Entry DOI: 10.7270/Q26Q20BZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50007595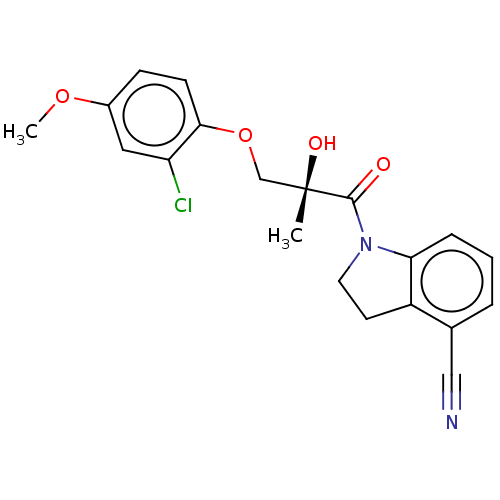 (CHEMBL3233069) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]mibolerone from full length human AR expressed in COS cells after 3.5 hrs by liquid scintillation counting | J Med Chem 57: 2462-71 (2014) Article DOI: 10.1021/jm401625b BindingDB Entry DOI: 10.7270/Q2RB763C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50203339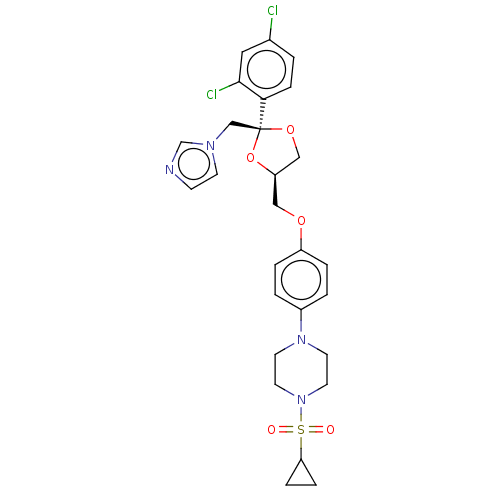 (CHEMBL3922888) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of recombinant CYP17 (unknown origin) overexpressed in human AD293 cells using [21-3H]17alpha-hydroxyl-pregenolone as substrate preincubat... | Bioorg Med Chem Lett 26: 5825-5829 (2016) Article DOI: 10.1016/j.bmcl.2016.10.016 BindingDB Entry DOI: 10.7270/Q2X63PW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM321153 (US10174049, Example 20 | US10385066, Example 20 | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline LLC US Patent | Assay Description For inhibition of triacylglycerol product formation, 11 uL reactions were run in white Polyplate-384 (PerkinElmer6007300) starting with a 30 minute p... | US Patent US9738658 (2017) BindingDB Entry DOI: 10.7270/Q26Q20BZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM321153 (US10174049, Example 20 | US10385066, Example 20 | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
GLAXOSMITHKLINE LLC US Patent | Assay Description For inhibition of triacylglycerol product formation, 11 uL reactions were run in white Polyplate-384 (PerkinElmer6007300) starting with a 30 minute p... | US Patent US9796729 (2017) BindingDB Entry DOI: 10.7270/Q2057J2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM321153 (US10174049, Example 20 | US10385066, Example 20 | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxosmithkline LLC US Patent | Assay Description For inhibition of triacylglycerol product formation, 11 uL reactions were run in white Polyplate-384 (PerkinElmer6007300) starting with a 30 minute p... | US Patent US10174049 (2019) BindingDB Entry DOI: 10.7270/Q2X92DCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50274164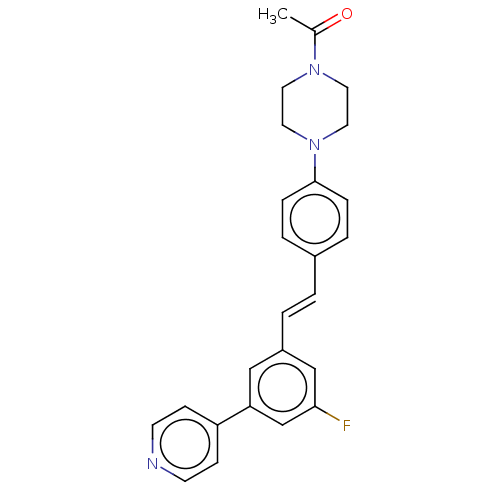 (CHEMBL4129721) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of recombinant CYP17 (unknown origin) expressed in human AD293 cells using [21-3H]17alpha-hydroxypregnenolone as substrate preincubated fo... | Bioorg Med Chem Lett 28: 2270-2274 (2018) Article DOI: 10.1016/j.bmcl.2018.05.040 BindingDB Entry DOI: 10.7270/Q2RJ4N0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 352 total ) | Next | Last >> |