 Found 2073 hits with Last Name = 'amin' and Initial = 's'
Found 2073 hits with Last Name = 'amin' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50082556

((S)-2,2-Dimethyl-4-[4-(pyridin-4-yloxy)-benzenesul...)Show SMILES CC1(C)SCCN([C@H]1C(=O)NO)S(=O)(=O)c1ccc(Oc2ccncc2)cc1 Show InChI InChI=1S/C18H21N3O5S2/c1-18(2)16(17(22)20-23)21(11-12-27-18)28(24,25)15-5-3-13(4-6-15)26-14-7-9-19-10-8-14/h3-10,16,23H,11-12H2,1-2H3,(H,20,22)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50082556

((S)-2,2-Dimethyl-4-[4-(pyridin-4-yloxy)-benzenesul...)Show SMILES CC1(C)SCCN([C@H]1C(=O)NO)S(=O)(=O)c1ccc(Oc2ccncc2)cc1 Show InChI InChI=1S/C18H21N3O5S2/c1-18(2)16(17(22)20-23)21(11-12-27-18)28(24,25)15-5-3-13(4-6-15)26-14-7-9-19-10-8-14/h3-10,16,23H,11-12H2,1-2H3,(H,20,22)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50062351

((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)CC(=O)NO |r| Show InChI InChI=1S/C20H28N4O4/c1-12(2)8-13(10-18(25)24-28)19(26)23-17(20(27)21-3)9-14-11-22-16-7-5-4-6-15(14)16/h4-7,11-13,17,22,28H,8-10H2,1-3H3,(H,21,27)(H,23,26)(H,24,25)/t13-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Sus scrofa (Pig)) | BDBM50457465

(CHEMBL4204736)Show SMILES CC(N)P(O)(=O)C[C@@H](Cc1ccccc1)C(=O)N[C@@](C)(Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C22H29N2O5P/c1-16(23)30(28,29)15-19(13-17-9-5-3-6-10-17)20(25)24-22(2,21(26)27)14-18-11-7-4-8-12-18/h3-12,16,19H,13-15,23H2,1-2H3,(H,24,25)(H,26,27)(H,28,29)/t16?,19-,22+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jadavpur University
Curated by ChEMBL
| Assay Description
Inhibition of APN in porcine kidney using Ala-p-NA as substrate |
J Med Chem 61: 6468-6490 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00782
BindingDB Entry DOI: 10.7270/Q28S4SHS |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Sus scrofa (Pig)) | BDBM50457475

(CHEMBL4216408)Show SMILES C[C@@](Cc1ccccc1)(NC(=O)[C@H](Cc1ccccc1)CP(O)(=O)C(N)Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C28H33N2O5P/c1-28(27(32)33,19-23-15-9-4-10-16-23)30-26(31)24(17-21-11-5-2-6-12-21)20-36(34,35)25(29)18-22-13-7-3-8-14-22/h2-16,24-25H,17-20,29H2,1H3,(H,30,31)(H,32,33)(H,34,35)/t24-,25?,28+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jadavpur University
Curated by ChEMBL
| Assay Description
Inhibition of APN in porcine kidney using Ala-p-NA as substrate |
J Med Chem 61: 6468-6490 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00782
BindingDB Entry DOI: 10.7270/Q28S4SHS |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50592900
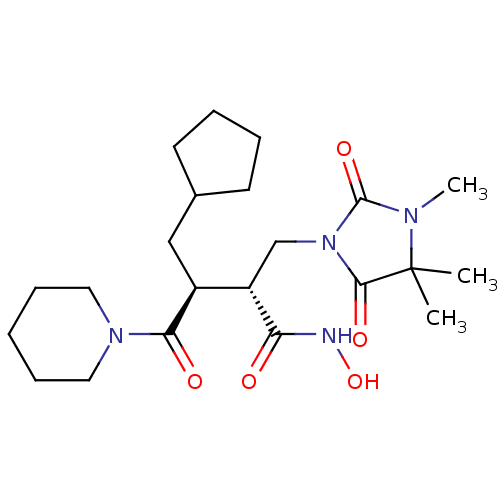
(CHEMBL5175770)Show SMILES CN1C(=O)N(C[C@@H]([C@H](CC2CCCC2)C(=O)N2CCCCC2)C(=O)NO)C(=O)C1(C)C |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM13126
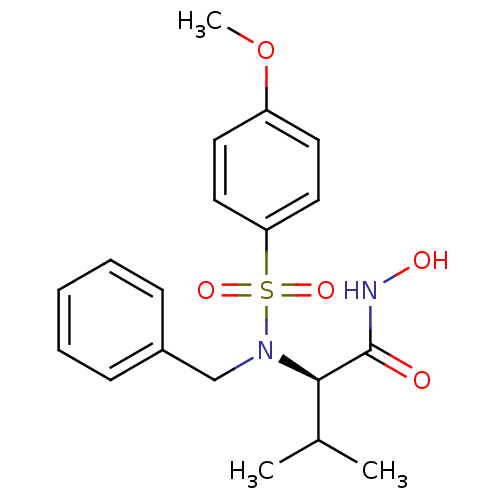
((2R)-2-[benzyl(4-methoxybenzene)sulfonamido]-N-hyd...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(Cc1ccccc1)[C@H](C(C)C)C(=O)NO |r| Show InChI InChI=1S/C19H24N2O5S/c1-14(2)18(19(22)20-23)21(13-15-7-5-4-6-8-15)27(24,25)17-11-9-16(26-3)10-12-17/h4-12,14,18,23H,13H2,1-3H3,(H,20,22)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Sphingosine kinase 1
(Homo sapiens (Human)) | BDBM50017018
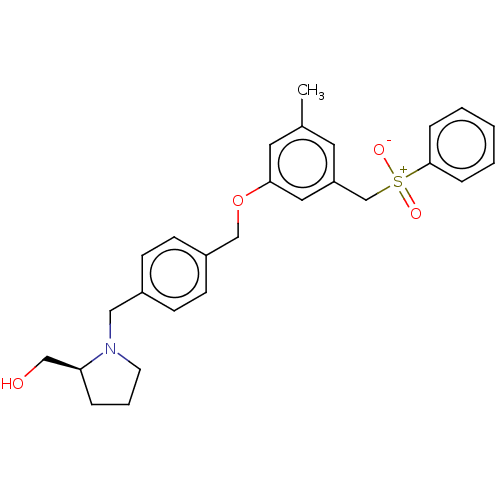
(CHEMBL3183090)Show SMILES Cc1cc(C[S+]([O-])(=O)c2ccccc2)cc(OCc2ccc(CN3CCC[C@H]3CO)cc2)c1 Show InChI InChI=1S/C27H31NO4S/c1-21-14-24(20-33(30,31)27-7-3-2-4-8-27)16-26(15-21)32-19-23-11-9-22(10-12-23)17-28-13-5-6-25(28)18-29/h2-4,7-12,14-16,25,29H,5-6,13,17-20H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
500 University Drive
Curated by ChEMBL
| Assay Description
Competitive inhibition of C-terminal His6-tagged human recombinant SphK1 expressed in baculovirus-infected Sf9 cells using sphingosine as substrate |
J Med Chem 57: 5509-24 (2014)
Article DOI: 10.1021/jm4011687
BindingDB Entry DOI: 10.7270/Q2474CF5 |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50592900

(CHEMBL5175770)Show SMILES CN1C(=O)N(C[C@@H]([C@H](CC2CCCC2)C(=O)N2CCCCC2)C(=O)NO)C(=O)C1(C)C |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50433864
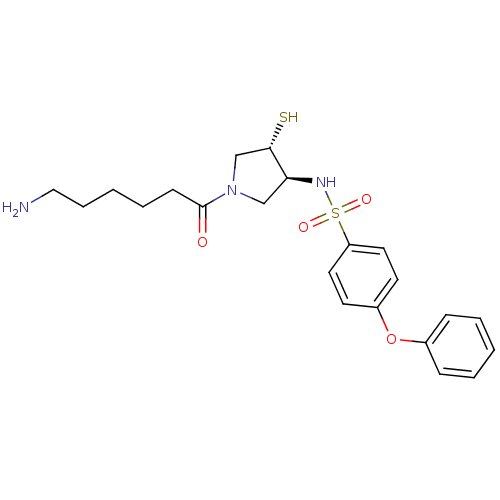
(CHEMBL2380403)Show SMILES NCCCCCC(=O)N1C[C@H](S)[C@H](C1)NS(=O)(=O)c1ccc(Oc2ccccc2)cc1 |r| Show InChI InChI=1S/C22H29N3O4S2/c23-14-6-2-5-9-22(26)25-15-20(21(30)16-25)24-31(27,28)19-12-10-18(11-13-19)29-17-7-3-1-4-8-17/h1,3-4,7-8,10-13,20-21,24,30H,2,5-6,9,14-16,23H2/t20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50335495
(4-(4-(thiiran-2-ylmethylsulfonyl)phenoxy)phenyl me...)Show SMILES CS(=O)(=O)Oc1ccc(Oc2ccc(cc2)S(=O)(=O)CC2CS2)cc1 Show InChI InChI=1S/C16H16O6S3/c1-24(17,18)22-14-4-2-12(3-5-14)21-13-6-8-16(9-7-13)25(19,20)11-15-10-23-15/h2-9,15H,10-11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50433864

(CHEMBL2380403)Show SMILES NCCCCCC(=O)N1C[C@H](S)[C@H](C1)NS(=O)(=O)c1ccc(Oc2ccccc2)cc1 |r| Show InChI InChI=1S/C22H29N3O4S2/c23-14-6-2-5-9-22(26)25-15-20(21(30)16-25)24-31(27,28)19-12-10-18(11-13-19)29-17-7-3-1-4-8-17/h1,3-4,7-8,10-13,20-21,24,30H,2,5-6,9,14-16,23H2/t20-,21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM207629

(US9265734, R01 | US9796664, Compound R01)Show InChI InChI=1S/C20H25N3O2/c1-15-10-12-16(13-11-15)20(25)22-14-6-2-3-9-19(24)23-18-8-5-4-7-17(18)21/h4-5,7-8,10-13H,2-3,6,9,14,21H2,1H3,(H,22,25)(H,23,24) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50388914
(CHEMBL2063274 | US10357546, p-OH SB-3CT)Show InChI InChI=1S/C15H14O4S2/c16-11-1-3-12(4-2-11)19-13-5-7-15(8-6-13)21(17,18)10-14-9-20-14/h1-8,14,16H,9-10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Homo sapiens (Human)) | BDBM50457479
(CHEMBL1159812)Show InChI InChI=1S/C5H13NS2/c1-8-3-2-5(6)4-7/h5,7H,2-4,6H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jadavpur University
Curated by ChEMBL
| Assay Description
Inhibition of APN in human placenta using AlabetaNA as substrate |
J Med Chem 61: 6468-6490 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00782
BindingDB Entry DOI: 10.7270/Q28S4SHS |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM13126

((2R)-2-[benzyl(4-methoxybenzene)sulfonamido]-N-hyd...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(Cc1ccccc1)[C@H](C(C)C)C(=O)NO |r| Show InChI InChI=1S/C19H24N2O5S/c1-14(2)18(19(22)20-23)21(13-15-7-5-4-6-8-15)27(24,25)17-11-9-16(26-3)10-12-17/h4-12,14,18,23H,13H2,1-3H3,(H,20,22)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50084661

(4-(4'-Chloro-biphenyl-4-yl)-4-oxo-2-phenylsulfanyl...)Show SMILES OC(=O)[C@@H](CSc1ccccc1)CC(=O)c1ccc(cc1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C23H19ClO3S/c24-20-12-10-17(11-13-20)16-6-8-18(9-7-16)22(25)14-19(23(26)27)15-28-21-4-2-1-3-5-21/h1-13,19H,14-15H2,(H,26,27)/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50129806
(CHEMBL3627987)Show SMILES [H][C@]1(NC(=O)[C@H](Cc2cc3ccccc3[nH]2)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](N)CCNC(=O)[C@@H](N)CSSC[C@H](NC(=O)[C@H](CCCCN)NC1=O)C(=O)N[C@@H](CC(C)C)C(N)=O)[C@H](C)O |r| Show InChI InChI=1S/C43H71N15O9S2/c1-22(2)17-31(35(47)60)55-41(66)33-21-69-68-20-27(46)36(61)50-16-13-26(45)37(62)53-30(12-8-15-51-43(48)49)38(63)56-32(19-25-18-24-9-4-5-10-28(24)52-25)40(65)58-34(23(3)59)42(67)54-29(39(64)57-33)11-6-7-14-44/h4-5,9-10,18,22-23,26-27,29-34,52,59H,6-8,11-17,19-21,44-46H2,1-3H3,(H2,47,60)(H,50,61)(H,53,62)(H,54,67)(H,55,66)(H,56,63)(H,57,64)(H,58,65)(H4,48,49,51)/t23-,26-,27-,29-,30-,31-,32-,33-,34-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brookhaven National Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum BoNT/A light chain 448 residue preincubated for 30 mins by FRET assay |
Bioorg Med Chem 23: 7264-73 (2015)
Article DOI: 10.1016/j.bmc.2015.10.024
BindingDB Entry DOI: 10.7270/Q2MG7RB8 |
More data for this
Ligand-Target Pair | |
Glutamyl aminopeptidase
(Homo sapiens (Human)) | BDBM50457482
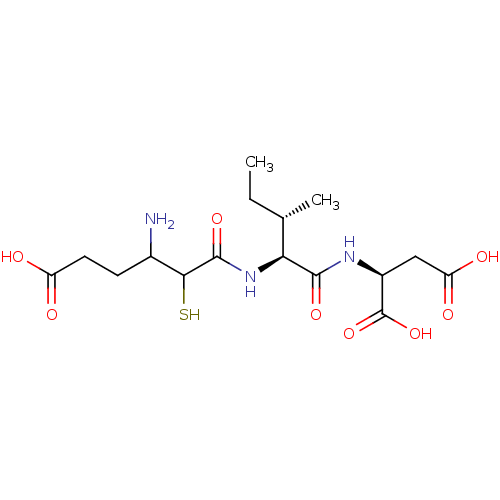
(CHEMBL4217064)Show SMILES CC[C@H](C)[C@H](NC(=O)C(S)C(N)CCC(O)=O)C(=O)N[C@@H](CC(O)=O)C(O)=O |r| Show InChI InChI=1S/C16H27N3O8S/c1-3-7(2)12(14(24)18-9(16(26)27)6-11(22)23)19-15(25)13(28)8(17)4-5-10(20)21/h7-9,12-13,28H,3-6,17H2,1-2H3,(H,18,24)(H,19,25)(H,20,21)(H,22,23)(H,26,27)/t7-,8?,9-,12-,13?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jadavpur University
Curated by ChEMBL
| Assay Description
Inhibition of APA (unknown origin) using GlubetaNA as substrate after 30 mins |
J Med Chem 61: 6468-6490 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00782
BindingDB Entry DOI: 10.7270/Q28S4SHS |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50264809

(2-((4-phenoxyphenylsulfonyl)methyl)thiirane | CHEM...)Show InChI InChI=1S/C15H14O3S2/c16-20(17,11-14-10-19-14)15-8-6-13(7-9-15)18-12-4-2-1-3-5-12/h1-9,14H,10-11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50264810

(CHEMBL445971 | N-(4-(4-(thiiran-2-ylmethylsulfonyl...)Show SMILES CS(=O)(=O)Nc1ccc(Oc2ccc(cc2)S(=O)(=O)CC2CS2)cc1 Show InChI InChI=1S/C16H17NO5S3/c1-24(18,19)17-12-2-4-13(5-3-12)22-14-6-8-16(9-7-14)25(20,21)11-15-10-23-15/h2-9,15,17H,10-11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Homo sapiens (Human)) | BDBM50457467
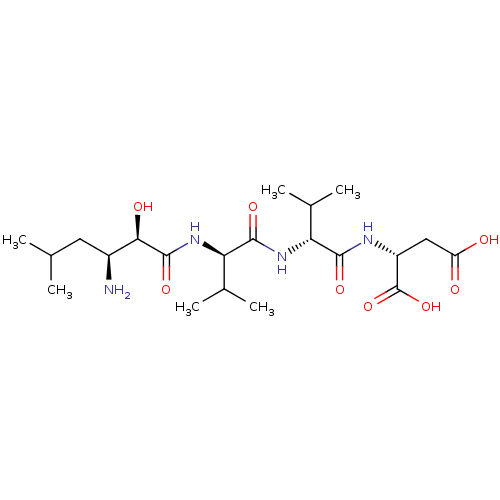
(CHEMBL4217873)Show SMILES CC(C)C[C@H](N)[C@@H](O)C(=O)N[C@H](C(C)C)C(=O)N[C@H](C(C)C)C(=O)N[C@H](CC(O)=O)C(O)=O |r| Show InChI InChI=1S/C21H38N4O8/c1-9(2)7-12(22)17(28)20(31)25-16(11(5)6)19(30)24-15(10(3)4)18(29)23-13(21(32)33)8-14(26)27/h9-13,15-17,28H,7-8,22H2,1-6H3,(H,23,29)(H,24,30)(H,25,31)(H,26,27)(H,32,33)/t12-,13+,15+,16+,17+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jadavpur University
Curated by ChEMBL
| Assay Description
Competitive inhibition of aminopeptidase-M (unknown origin) |
J Med Chem 61: 6468-6490 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00782
BindingDB Entry DOI: 10.7270/Q28S4SHS |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Homo sapiens (Human)) | BDBM50457485
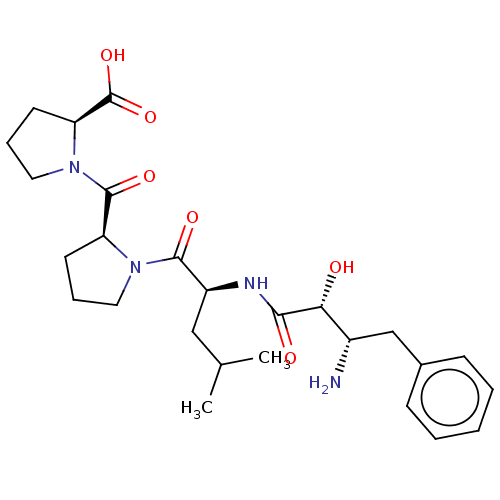
(CHEMBL4205440)Show SMILES CC(C)C[C@H](NC(=O)[C@H](O)[C@@H](N)Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(O)=O |r| Show InChI InChI=1S/C26H38N4O6/c1-16(2)14-19(28-23(32)22(31)18(27)15-17-8-4-3-5-9-17)24(33)29-12-6-10-20(29)25(34)30-13-7-11-21(30)26(35)36/h3-5,8-9,16,18-22,31H,6-7,10-15,27H2,1-2H3,(H,28,32)(H,35,36)/t18-,19-,20-,21-,22+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jadavpur University
Curated by ChEMBL
| Assay Description
Competitive inhibition of aminopeptidase-M (unknown origin) |
J Med Chem 61: 6468-6490 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00782
BindingDB Entry DOI: 10.7270/Q28S4SHS |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM13126

((2R)-2-[benzyl(4-methoxybenzene)sulfonamido]-N-hyd...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(Cc1ccccc1)[C@H](C(C)C)C(=O)NO |r| Show InChI InChI=1S/C19H24N2O5S/c1-14(2)18(19(22)20-23)21(13-15-7-5-4-6-8-15)27(24,25)17-11-9-16(26-3)10-12-17/h4-12,14,18,23H,13H2,1-3H3,(H,20,22)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Homo sapiens (Human)) | BDBM50457477

(CHEMBL4210855)Show InChI InChI=1S/C14H13NO/c15-13-7-10-6-5-9-3-1-2-4-11(9)12(10)8-14(13)16/h1-6,13H,7-8,15H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jadavpur University
Curated by ChEMBL
| Assay Description
Inhibition of aminopeptidase-M (unknown origin) |
J Med Chem 61: 6468-6490 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00782
BindingDB Entry DOI: 10.7270/Q28S4SHS |
More data for this
Ligand-Target Pair | |
Cytosol aminopeptidase [435-519]
(Sus scrofa) | BDBM50022748
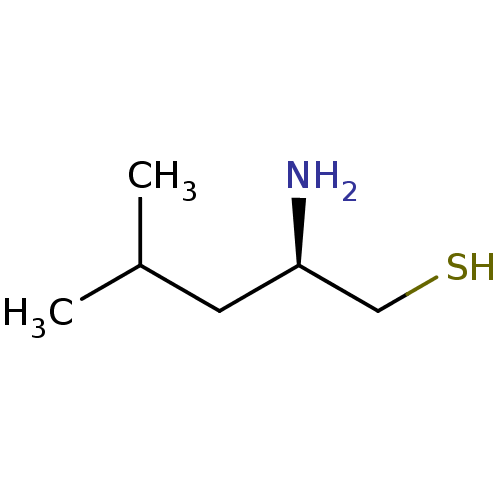
(2-Amino-4-methyl-pentane-1-thiol | CHEMBL69349)Show InChI InChI=1S/C6H15NS/c1-5(2)3-6(7)4-8/h5-6,8H,3-4,7H2,1-2H3/t6-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jadavpur University
Curated by ChEMBL
| Assay Description
Competitive inhibition of LAP in porcine kidney microsomes |
J Med Chem 61: 6468-6490 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00782
BindingDB Entry DOI: 10.7270/Q28S4SHS |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50335495

(4-(4-(thiiran-2-ylmethylsulfonyl)phenoxy)phenyl me...)Show SMILES CS(=O)(=O)Oc1ccc(Oc2ccc(cc2)S(=O)(=O)CC2CS2)cc1 Show InChI InChI=1S/C16H16O6S3/c1-24(17,18)22-14-4-2-12(3-5-14)21-13-6-8-16(9-7-13)25(19,20)11-15-10-23-15/h2-9,15H,10-11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50354624
(CHEMBL1834422 | US10357546, ND-322 | US9951035, ND...)Show InChI InChI=1S/C15H15NO3S2/c16-11-1-3-12(4-2-11)19-13-5-7-15(8-6-13)21(17,18)10-14-9-20-14/h1-8,14H,9-10,16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50354624

(CHEMBL1834422 | US10357546, ND-322 | US9951035, ND...)Show InChI InChI=1S/C15H15NO3S2/c16-11-1-3-12(4-2-11)19-13-5-7-15(8-6-13)21(17,18)10-14-9-20-14/h1-8,14H,9-10,16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50129806

(CHEMBL3627987)Show SMILES [H][C@]1(NC(=O)[C@H](Cc2cc3ccccc3[nH]2)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](N)CCNC(=O)[C@@H](N)CSSC[C@H](NC(=O)[C@H](CCCCN)NC1=O)C(=O)N[C@@H](CC(C)C)C(N)=O)[C@H](C)O |r| Show InChI InChI=1S/C43H71N15O9S2/c1-22(2)17-31(35(47)60)55-41(66)33-21-69-68-20-27(46)36(61)50-16-13-26(45)37(62)53-30(12-8-15-51-43(48)49)38(63)56-32(19-25-18-24-9-4-5-10-28(24)52-25)40(65)58-34(23(3)59)42(67)54-29(39(64)57-33)11-6-7-14-44/h4-5,9-10,18,22-23,26-27,29-34,52,59H,6-8,11-17,19-21,44-46H2,1-3H3,(H2,47,60)(H,50,61)(H,53,62)(H,54,67)(H,55,66)(H,56,63)(H,57,64)(H,58,65)(H4,48,49,51)/t23-,26-,27-,29-,30-,31-,32-,33-,34-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brookhaven National Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum BoNT/A light chain 448 residue coincubated with TCEP for 30 mins and TCEP absent during reaction by FRET assay |
Bioorg Med Chem 23: 7264-73 (2015)
Article DOI: 10.1016/j.bmc.2015.10.024
BindingDB Entry DOI: 10.7270/Q2MG7RB8 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50082556

((S)-2,2-Dimethyl-4-[4-(pyridin-4-yloxy)-benzenesul...)Show SMILES CC1(C)SCCN([C@H]1C(=O)NO)S(=O)(=O)c1ccc(Oc2ccncc2)cc1 Show InChI InChI=1S/C18H21N3O5S2/c1-18(2)16(17(22)20-23)21(11-12-27-18)28(24,25)15-5-3-13(4-6-15)26-14-7-9-19-10-8-14/h3-10,16,23H,11-12H2,1-2H3,(H,20,22)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50082556

((S)-2,2-Dimethyl-4-[4-(pyridin-4-yloxy)-benzenesul...)Show SMILES CC1(C)SCCN([C@H]1C(=O)NO)S(=O)(=O)c1ccc(Oc2ccncc2)cc1 Show InChI InChI=1S/C18H21N3O5S2/c1-18(2)16(17(22)20-23)21(11-12-27-18)28(24,25)15-5-3-13(4-6-15)26-14-7-9-19-10-8-14/h3-10,16,23H,11-12H2,1-2H3,(H,20,22)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50082556

((S)-2,2-Dimethyl-4-[4-(pyridin-4-yloxy)-benzenesul...)Show SMILES CC1(C)SCCN([C@H]1C(=O)NO)S(=O)(=O)c1ccc(Oc2ccncc2)cc1 Show InChI InChI=1S/C18H21N3O5S2/c1-18(2)16(17(22)20-23)21(11-12-27-18)28(24,25)15-5-3-13(4-6-15)26-14-7-9-19-10-8-14/h3-10,16,23H,11-12H2,1-2H3,(H,20,22)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50082556

((S)-2,2-Dimethyl-4-[4-(pyridin-4-yloxy)-benzenesul...)Show SMILES CC1(C)SCCN([C@H]1C(=O)NO)S(=O)(=O)c1ccc(Oc2ccncc2)cc1 Show InChI InChI=1S/C18H21N3O5S2/c1-18(2)16(17(22)20-23)21(11-12-27-18)28(24,25)15-5-3-13(4-6-15)26-14-7-9-19-10-8-14/h3-10,16,23H,11-12H2,1-2H3,(H,20,22)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Sus scrofa (Pig)) | BDBM50036969

((S)-2-Amino-pentane-1,5-dithiol | CHEMBL101771)Show InChI InChI=1S/C5H13NS2/c6-5(4-8)2-1-3-7/h5,7-8H,1-4,6H2/t5-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jadavpur University
Curated by ChEMBL
| Assay Description
Inhibition of hog kidney APN using [3H]Tyr1-Leu5-enkephalin as substrate preincubated for 15 mins followed by substrate addition measured after 15 mi... |
J Med Chem 61: 6468-6490 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00782
BindingDB Entry DOI: 10.7270/Q28S4SHS |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM13126

((2R)-2-[benzyl(4-methoxybenzene)sulfonamido]-N-hyd...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(Cc1ccccc1)[C@H](C(C)C)C(=O)NO |r| Show InChI InChI=1S/C19H24N2O5S/c1-14(2)18(19(22)20-23)21(13-15-7-5-4-6-8-15)27(24,25)17-11-9-16(26-3)10-12-17/h4-12,14,18,23H,13H2,1-3H3,(H,20,22)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Sus scrofa (Pig)) | BDBM50036827

(CHEMBL27040 | [4-(2-Amino-3-mercapto-propyl)-cyclo...)Show SMILES NC(CS)CC1CCC(CC(O)=O)CC1 |(.78,-9.51,;.77,-7.97,;-.58,-7.2,;-1.91,-7.99,;2.1,-7.19,;3.43,-7.94,;3.43,-9.48,;4.76,-10.25,;6.11,-9.46,;7.44,-10.23,;8.77,-9.44,;10.11,-10.21,;8.76,-7.9,;6.1,-7.92,;4.75,-7.15,)| Show InChI InChI=1S/C11H21NO2S/c12-10(7-15)5-8-1-3-9(4-2-8)6-11(13)14/h8-10,15H,1-7,12H2,(H,13,14) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jadavpur University
Curated by ChEMBL
| Assay Description
Inhibition of hog kidney APN using [3H]Tyr1-Leu5-enkephalin as substrate preincubated for 15 mins followed by substrate addition measured after 15 mi... |
J Med Chem 61: 6468-6490 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00782
BindingDB Entry DOI: 10.7270/Q28S4SHS |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Sus scrofa (Pig)) | BDBM50190112
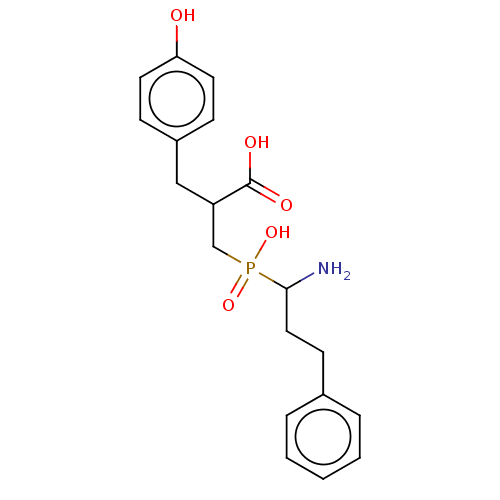
(CHEMBL88808)Show SMILES NC(CCc1ccccc1)P(O)(=O)CC(Cc1ccc(O)cc1)C(O)=O Show InChI InChI=1S/C19H24NO5P/c20-18(11-8-14-4-2-1-3-5-14)26(24,25)13-16(19(22)23)12-15-6-9-17(21)10-7-15/h1-7,9-10,16,18,21H,8,11-13,20H2,(H,22,23)(H,24,25) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jadavpur University
Curated by ChEMBL
| Assay Description
Inhibition of APN in porcine kidney using L-leucine p-nitroanilide as substrate preincubated for 60 mins followed by substrate addition measured afte... |
J Med Chem 61: 6468-6490 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00782
BindingDB Entry DOI: 10.7270/Q28S4SHS |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Sus scrofa (Pig)) | BDBM50036968

((S)-5-Amino-6-mercapto-hexanoic acid hydroxyamide ...)Show InChI InChI=1S/C6H14N2O2S/c7-5(4-11)2-1-3-6(9)8-10/h5,10-11H,1-4,7H2,(H,8,9)/t5-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jadavpur University
Curated by ChEMBL
| Assay Description
Inhibition of hog kidney APN using [3H]Tyr1-Leu5-enkephalin as substrate preincubated for 15 mins followed by substrate addition measured after 15 mi... |
J Med Chem 61: 6468-6490 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00782
BindingDB Entry DOI: 10.7270/Q28S4SHS |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50129806

(CHEMBL3627987)Show SMILES [H][C@]1(NC(=O)[C@H](Cc2cc3ccccc3[nH]2)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](N)CCNC(=O)[C@@H](N)CSSC[C@H](NC(=O)[C@H](CCCCN)NC1=O)C(=O)N[C@@H](CC(C)C)C(N)=O)[C@H](C)O |r| Show InChI InChI=1S/C43H71N15O9S2/c1-22(2)17-31(35(47)60)55-41(66)33-21-69-68-20-27(46)36(61)50-16-13-26(45)37(62)53-30(12-8-15-51-43(48)49)38(63)56-32(19-25-18-24-9-4-5-10-28(24)52-25)40(65)58-34(23(3)59)42(67)54-29(39(64)57-33)11-6-7-14-44/h4-5,9-10,18,22-23,26-27,29-34,52,59H,6-8,11-17,19-21,44-46H2,1-3H3,(H2,47,60)(H,50,61)(H,53,62)(H,54,67)(H,55,66)(H,56,63)(H,57,64)(H,58,65)(H4,48,49,51)/t23-,26-,27-,29-,30-,31-,32-,33-,34-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brookhaven National Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum truncated-BoNT/A light chain 424 residue preincubated for 30 mins by FRET assay |
Bioorg Med Chem 23: 7264-73 (2015)
Article DOI: 10.1016/j.bmc.2015.10.024
BindingDB Entry DOI: 10.7270/Q2MG7RB8 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM408486
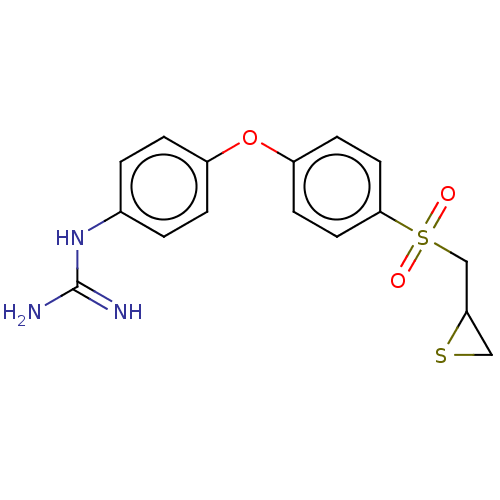
(US10357546, ND-3640)Show SMILES NC(=N)Nc1ccc(Oc2ccc(cc2)S(=O)(=O)CC2CS2)cc1 Show InChI InChI=1S/C16H17N3O3S2/c17-16(18)19-11-1-3-12(4-2-11)22-13-5-7-15(8-6-13)24(20,21)10-14-9-23-14/h1-8,14H,9-10H2,(H4,17,18,19) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50534918
(CHEMBL4474592)Show SMILES CSCC[C@H](NC(=O)C(CCN)NC(=O)[C@@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCCNC(N)=N)NC(=O)C(N)CCNc1ccc(cc1[N+]([O-])=O)[N+]([O-])=O)[C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(O)=O |r| Show InChI InChI=1S/C46H69N15O13S/c1-24(2)20-36(45(69)70)58-42(66)34(15-19-75-4)55-41(65)33(13-16-47)56-44(68)38(25(3)62)59-43(67)35(21-26-23-53-30-9-6-5-8-28(26)30)57-40(64)32(10-7-17-52-46(49)50)54-39(63)29(48)14-18-51-31-12-11-27(60(71)72)22-37(31)61(73)74/h5-6,8-9,11-12,22-25,29,32-36,38,51,53,62H,7,10,13-21,47-48H2,1-4H3,(H,54,63)(H,55,65)(H,56,68)(H,57,64)(H,58,66)(H,59,67)(H,69,70)(H4,49,50,52)/t25-,29?,32+,33?,34+,35+,36+,38+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stony Brook University
Curated by ChEMBL
| Assay Description
Inhibition of protease activity of Clostridium botulinum BoNT/A using N(K)-acetyl)-SNKTRIDEANQRATKML-carboxamide as substrate |
Bioorg Med Chem 24: 4875-4889 (2016)
Article DOI: 10.1016/j.bmc.2016.07.031
BindingDB Entry DOI: 10.7270/Q2JH3QPJ |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM13126

((2R)-2-[benzyl(4-methoxybenzene)sulfonamido]-N-hyd...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(Cc1ccccc1)[C@H](C(C)C)C(=O)NO |r| Show InChI InChI=1S/C19H24N2O5S/c1-14(2)18(19(22)20-23)21(13-15-7-5-4-6-8-15)27(24,25)17-11-9-16(26-3)10-12-17/h4-12,14,18,23H,13H2,1-3H3,(H,20,22)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50129806

(CHEMBL3627987)Show SMILES [H][C@]1(NC(=O)[C@H](Cc2cc3ccccc3[nH]2)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](N)CCNC(=O)[C@@H](N)CSSC[C@H](NC(=O)[C@H](CCCCN)NC1=O)C(=O)N[C@@H](CC(C)C)C(N)=O)[C@H](C)O |r| Show InChI InChI=1S/C43H71N15O9S2/c1-22(2)17-31(35(47)60)55-41(66)33-21-69-68-20-27(46)36(61)50-16-13-26(45)37(62)53-30(12-8-15-51-43(48)49)38(63)56-32(19-25-18-24-9-4-5-10-28(24)52-25)40(65)58-34(23(3)59)42(67)54-29(39(64)57-33)11-6-7-14-44/h4-5,9-10,18,22-23,26-27,29-34,52,59H,6-8,11-17,19-21,44-46H2,1-3H3,(H2,47,60)(H,50,61)(H,53,62)(H,54,67)(H,55,66)(H,56,63)(H,57,64)(H,58,65)(H4,48,49,51)/t23-,26-,27-,29-,30-,31-,32-,33-,34-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brookhaven National Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum BoNT/A light chain 448 residue coincubated with TCEP for 60 mins and TCEP absent during reaction by FRET assay |
Bioorg Med Chem 23: 7264-73 (2015)
Article DOI: 10.1016/j.bmc.2015.10.024
BindingDB Entry DOI: 10.7270/Q2MG7RB8 |
More data for this
Ligand-Target Pair | |
Cytosol aminopeptidase
(Homo sapiens (Human)) | BDBM50457478
(CHEMBL4208547)Show InChI InChI=1S/C14H22BNO2/c1-13(2)14(3,4)18-15(17-13)12(16)10-11-8-6-5-7-9-11/h5-9,12H,10,16H2,1-4H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jadavpur University
Curated by ChEMBL
| Assay Description
Inhibition of LAP (unknown origin) |
J Med Chem 61: 6468-6490 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00782
BindingDB Entry DOI: 10.7270/Q28S4SHS |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50592900

(CHEMBL5175770)Show SMILES CN1C(=O)N(C[C@@H]([C@H](CC2CCCC2)C(=O)N2CCCCC2)C(=O)NO)C(=O)C1(C)C |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50433867
(CHEMBL2380401)Show SMILES CN([C@H]1CN(C[C@@H]1S)C(N)=O)S(=O)(=O)c1ccc(Oc2ccccc2)cc1 |r| Show InChI InChI=1S/C18H21N3O4S2/c1-20(16-11-21(18(19)22)12-17(16)26)27(23,24)15-9-7-14(8-10-15)25-13-5-3-2-4-6-13/h2-10,16-17,26H,11-12H2,1H3,(H2,19,22)/t16-,17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Glutamyl aminopeptidase
(Homo sapiens (Human)) | BDBM50190112

(CHEMBL88808)Show SMILES NC(CCc1ccccc1)P(O)(=O)CC(Cc1ccc(O)cc1)C(O)=O Show InChI InChI=1S/C19H24NO5P/c20-18(11-8-14-4-2-1-3-5-14)26(24,25)13-16(19(22)23)12-15-6-9-17(21)10-7-15/h1-7,9-10,16,18,21H,8,11-13,20H2,(H,22,23)(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 67 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jadavpur University
Curated by ChEMBL
| Assay Description
Inhibition of APA (unknown origin) |
J Med Chem 61: 6468-6490 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00782
BindingDB Entry DOI: 10.7270/Q28S4SHS |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50354625
(CHEMBL1834423)Show InChI InChI=1S/C15H15NO4S2/c16-14-6-3-11(7-15(14)17)20-10-1-4-13(5-2-10)22(18,19)9-12-8-21-12/h1-7,12,17H,8-9,16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 78 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Homo sapiens (Human)) | BDBM50457476
(CHEMBL4215801)Show InChI InChI=1S/C22H21NO/c23-20-14-17-12-11-16-8-4-5-9-18(16)21(17)19(22(20)24)13-10-15-6-2-1-3-7-15/h1-9,11-12,19-20H,10,13-14,23H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jadavpur University
Curated by ChEMBL
| Assay Description
Inhibition of aminopeptidase-M (unknown origin) |
J Med Chem 61: 6468-6490 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00782
BindingDB Entry DOI: 10.7270/Q28S4SHS |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data