

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P2Y purinoceptor 14 (Homo sapiens (Human)) | BDBM50512416 (CHEMBL4447162) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of human P2Y14R | J Med Chem 63: 9563-9589 (2020) Article DOI: 10.1021/acs.jmedchem.0c00745 BindingDB Entry DOI: 10.7270/Q20R9SZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Rattus norvegicus) | BDBM50456168 (CHEMBL1800685) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Antagonist activity at P2Y14R in rat C6 cells assessed as suppression of UDP-glucose-mediated inhibition of forskolin-stimulated [3H]cyclic-AMP accum... | J Med Chem 61: 4860-4882 (2018) Article DOI: 10.1021/acs.jmedchem.8b00168 BindingDB Entry DOI: 10.7270/Q2X069NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM50523511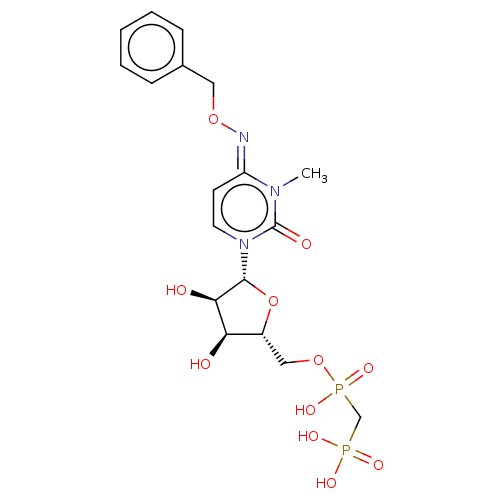 (CHEMBL4443977) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of recombinant rat C-terminal His-tagged soluble form of CD73 expressed in baculovirus infected Sf9 insect cells using [2,8-3H]AMP as subs... | J Med Chem 62: 3677-3695 (2019) Article DOI: 10.1021/acs.jmedchem.9b00164 BindingDB Entry DOI: 10.7270/Q2M61PNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM50523526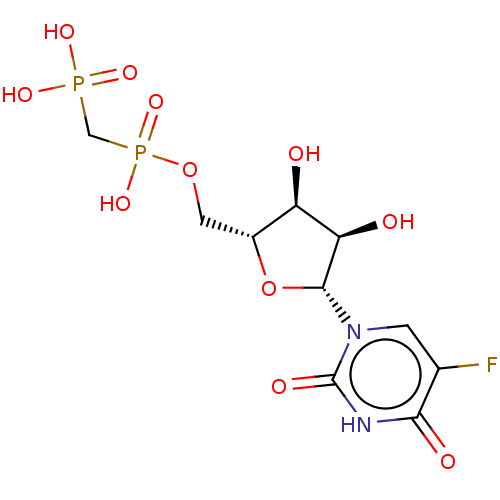 (CHEMBL3606064) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of CD73 in human MDA-MB-231 cell membranes using [2,8-3H]AMP as substrate measured after 25 mins by scintillation counting method | J Med Chem 62: 3677-3695 (2019) Article DOI: 10.1021/acs.jmedchem.9b00164 BindingDB Entry DOI: 10.7270/Q2M61PNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM50523510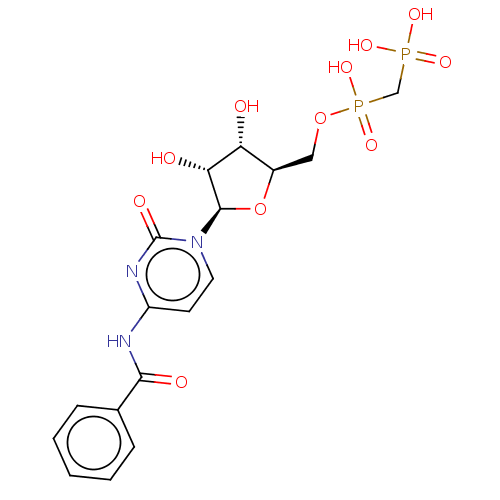 (CHEMBL4483379) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of recombinant human C-terminal His-tagged soluble form of CD73 expressed in baculovirus infected Sf9 insect cells using [2,8-3H]AMP as su... | J Med Chem 62: 3677-3695 (2019) Article DOI: 10.1021/acs.jmedchem.9b00164 BindingDB Entry DOI: 10.7270/Q2M61PNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM50523526 (CHEMBL3606064) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of recombinant human C-terminal His-tagged soluble form of CD73 expressed in baculovirus infected Sf9 insect cells using [2,8-3H]AMP as su... | J Med Chem 62: 3677-3695 (2019) Article DOI: 10.1021/acs.jmedchem.9b00164 BindingDB Entry DOI: 10.7270/Q2M61PNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM50523510 (CHEMBL4483379) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of CD73 in human MDA-MB-231 cell membranes using [2,8-3H]AMP as substrate measured after 25 mins by scintillation counting method | J Med Chem 62: 3677-3695 (2019) Article DOI: 10.1021/acs.jmedchem.9b00164 BindingDB Entry DOI: 10.7270/Q2M61PNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM50523527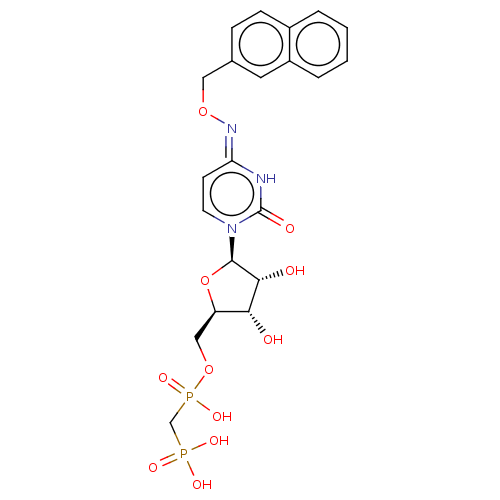 (CHEMBL4443094) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of CD73 in human MDA-MB-231 cell membranes using [2,8-3H]AMP as substrate measured after 25 mins by scintillation counting method | J Med Chem 62: 3677-3695 (2019) Article DOI: 10.1021/acs.jmedchem.9b00164 BindingDB Entry DOI: 10.7270/Q2M61PNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM50523527 (CHEMBL4443094) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of recombinant human C-terminal His-tagged soluble form of CD73 expressed in baculovirus infected Sf9 insect cells using [2,8-3H]AMP as su... | J Med Chem 62: 3677-3695 (2019) Article DOI: 10.1021/acs.jmedchem.9b00164 BindingDB Entry DOI: 10.7270/Q2M61PNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM50523511 (CHEMBL4443977) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of CD73 in human MDA-MB-231 cell membranes using [2,8-3H]AMP as substrate measured after 25 mins by scintillation counting method | J Med Chem 62: 3677-3695 (2019) Article DOI: 10.1021/acs.jmedchem.9b00164 BindingDB Entry DOI: 10.7270/Q2M61PNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM50523529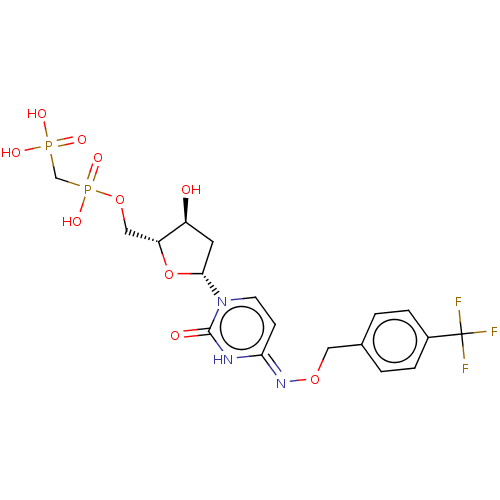 (CHEMBL4551648) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of CD73 in human MDA-MB-231 cell membranes using [2,8-3H]AMP as substrate measured after 25 mins by scintillation counting method | J Med Chem 62: 3677-3695 (2019) Article DOI: 10.1021/acs.jmedchem.9b00164 BindingDB Entry DOI: 10.7270/Q2M61PNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM50523511 (CHEMBL4443977) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of recombinant human C-terminal His-tagged soluble form of CD73 expressed in baculovirus infected Sf9 insect cells using [2,8-3H]AMP as su... | J Med Chem 62: 3677-3695 (2019) Article DOI: 10.1021/acs.jmedchem.9b00164 BindingDB Entry DOI: 10.7270/Q2M61PNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM50523529 (CHEMBL4551648) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of recombinant human C-terminal His-tagged soluble form of CD73 expressed in baculovirus infected Sf9 insect cells using [2,8-3H]AMP as su... | J Med Chem 62: 3677-3695 (2019) Article DOI: 10.1021/acs.jmedchem.9b00164 BindingDB Entry DOI: 10.7270/Q2M61PNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM50523510 (CHEMBL4483379) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of recombinant rat C-terminal His-tagged soluble form of CD73 expressed in baculovirus infected Sf9 insect cells using [2,8-3H]AMP as subs... | J Med Chem 62: 3677-3695 (2019) Article DOI: 10.1021/acs.jmedchem.9b00164 BindingDB Entry DOI: 10.7270/Q2M61PNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM50523526 (CHEMBL3606064) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of recombinant rat C-terminal His-tagged soluble form of CD73 expressed in baculovirus infected Sf9 insect cells using [2,8-3H]AMP as subs... | J Med Chem 62: 3677-3695 (2019) Article DOI: 10.1021/acs.jmedchem.9b00164 BindingDB Entry DOI: 10.7270/Q2M61PNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM50523528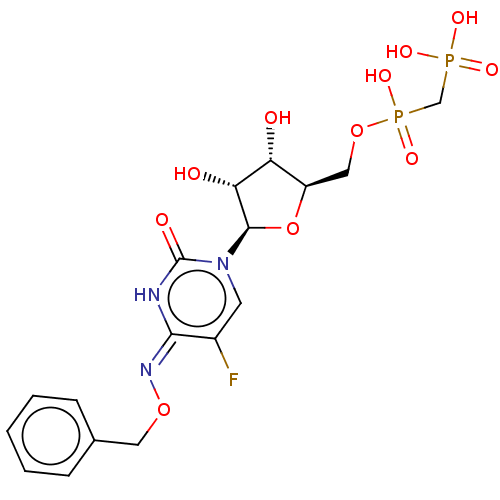 (CHEMBL4470616) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of recombinant human C-terminal His-tagged soluble form of CD73 expressed in baculovirus infected Sf9 insect cells using [2,8-3H]AMP as su... | J Med Chem 62: 3677-3695 (2019) Article DOI: 10.1021/acs.jmedchem.9b00164 BindingDB Entry DOI: 10.7270/Q2M61PNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM50523528 (CHEMBL4470616) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of CD73 in human MDA-MB-231 cell membranes using [2,8-3H]AMP as substrate measured after 25 mins by scintillation counting method | J Med Chem 62: 3677-3695 (2019) Article DOI: 10.1021/acs.jmedchem.9b00164 BindingDB Entry DOI: 10.7270/Q2M61PNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM50523527 (CHEMBL4443094) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of recombinant rat C-terminal His-tagged soluble form of CD73 expressed in baculovirus infected Sf9 insect cells using [2,8-3H]AMP as subs... | J Med Chem 62: 3677-3695 (2019) Article DOI: 10.1021/acs.jmedchem.9b00164 BindingDB Entry DOI: 10.7270/Q2M61PNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (Homo sapiens (Human)) | BDBM50456166 (CHEMBL4216870) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]Rauwolscine from human adrenergic alpha2A receptor expressed in MDCK cell membranes after 90 mins by scintillation counting metho... | J Med Chem 61: 4860-4882 (2018) Article DOI: 10.1021/acs.jmedchem.8b00168 BindingDB Entry DOI: 10.7270/Q2X069NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM50523529 (CHEMBL4551648) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of recombinant rat C-terminal His-tagged soluble form of CD73 expressed in baculovirus infected Sf9 insect cells using [2,8-3H]AMP as subs... | J Med Chem 62: 3677-3695 (2019) Article DOI: 10.1021/acs.jmedchem.9b00164 BindingDB Entry DOI: 10.7270/Q2M61PNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM50523528 (CHEMBL4470616) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of recombinant rat C-terminal His-tagged soluble form of CD73 expressed in baculovirus infected Sf9 insect cells using [2,8-3H]AMP as subs... | J Med Chem 62: 3677-3695 (2019) Article DOI: 10.1021/acs.jmedchem.9b00164 BindingDB Entry DOI: 10.7270/Q2M61PNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM50523545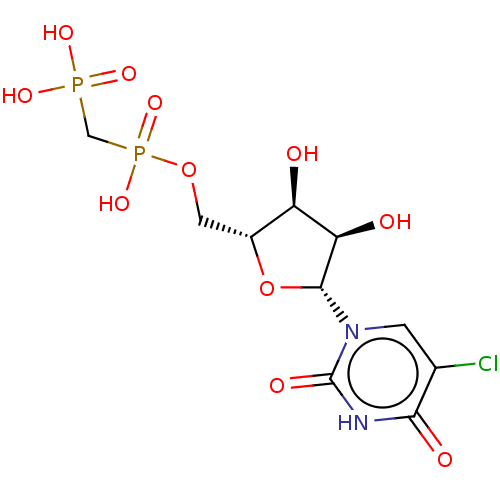 (CHEMBL4538320) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 87 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of recombinant rat C-terminal His-tagged soluble form of CD73 expressed in baculovirus infected Sf9 insect cells using [2,8-3H]AMP as subs... | J Med Chem 62: 3677-3695 (2019) Article DOI: 10.1021/acs.jmedchem.9b00164 BindingDB Entry DOI: 10.7270/Q2M61PNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM50523552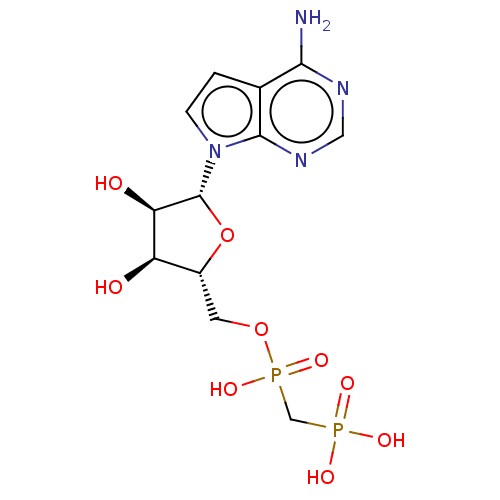 (CHEMBL4561816) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of recombinant rat C-terminal His-tagged soluble form of CD73 expressed in baculovirus infected Sf9 insect cells using [2,8-3H]AMP as subs... | J Med Chem 62: 3677-3695 (2019) Article DOI: 10.1021/acs.jmedchem.9b00164 BindingDB Entry DOI: 10.7270/Q2M61PNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM50523532 (CHEMBL4457058) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of recombinant rat C-terminal His-tagged soluble form of CD73 expressed in baculovirus infected Sf9 insect cells using [2,8-3H]AMP as subs... | J Med Chem 62: 3677-3695 (2019) Article DOI: 10.1021/acs.jmedchem.9b00164 BindingDB Entry DOI: 10.7270/Q2M61PNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50444918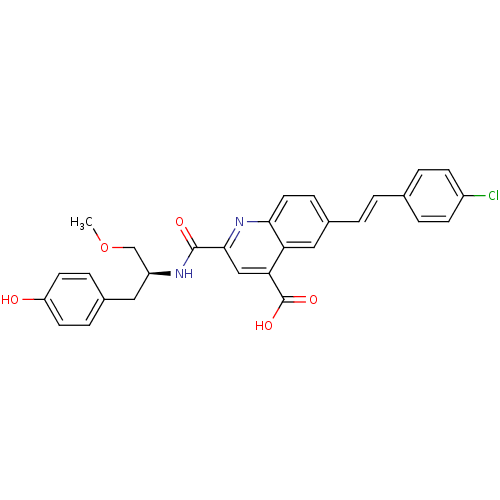 (CHEMBL3099753) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | <100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of B-Alexa-Fluor647 from CDK2 (unknown origin) by fluorescence polarization assay | Bioorg Med Chem Lett 24: 199-203 (2013) Article DOI: 10.1016/j.bmcl.2013.11.041 BindingDB Entry DOI: 10.7270/Q2SN0BF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50444921 (CHEMBL3099750) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of B-Alexa-Fluor647 from CDK2 (unknown origin) by fluorescence polarization assay | Bioorg Med Chem Lett 24: 199-203 (2013) Article DOI: 10.1016/j.bmcl.2013.11.041 BindingDB Entry DOI: 10.7270/Q2SN0BF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50444933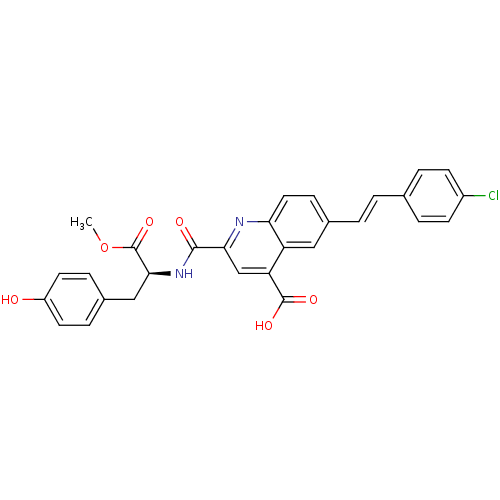 (CHEMBL3099763) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | <100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of B-Alexa-Fluor647 from CDK2 (unknown origin) by fluorescence polarization assay | Bioorg Med Chem Lett 24: 199-203 (2013) Article DOI: 10.1016/j.bmcl.2013.11.041 BindingDB Entry DOI: 10.7270/Q2SN0BF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50444932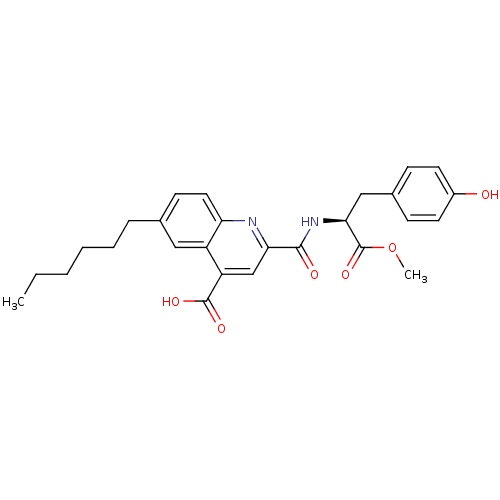 (CHEMBL3099764) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | <100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of B-Alexa-Fluor647 from CDK2 (unknown origin) by fluorescence polarization assay | Bioorg Med Chem Lett 24: 199-203 (2013) Article DOI: 10.1016/j.bmcl.2013.11.041 BindingDB Entry DOI: 10.7270/Q2SN0BF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50444934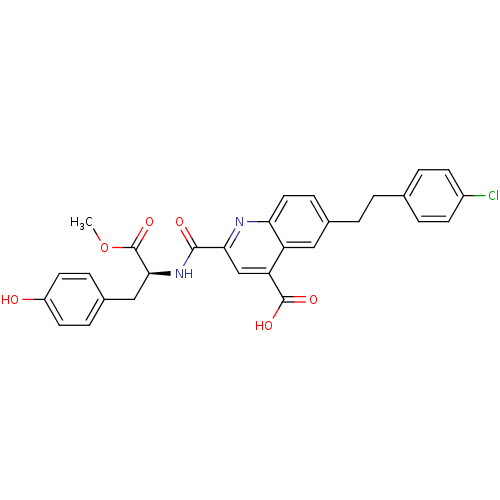 (CHEMBL3099762) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | <100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of B-Alexa-Fluor647 from CDK2 (unknown origin) by fluorescence polarization assay | Bioorg Med Chem Lett 24: 199-203 (2013) Article DOI: 10.1016/j.bmcl.2013.11.041 BindingDB Entry DOI: 10.7270/Q2SN0BF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50444935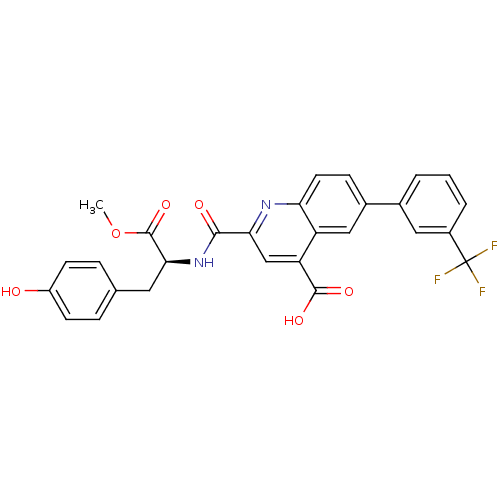 (CHEMBL3099761) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | <100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of B-Alexa-Fluor647 from CDK2 (unknown origin) by fluorescence polarization assay | Bioorg Med Chem Lett 24: 199-203 (2013) Article DOI: 10.1016/j.bmcl.2013.11.041 BindingDB Entry DOI: 10.7270/Q2SN0BF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50444919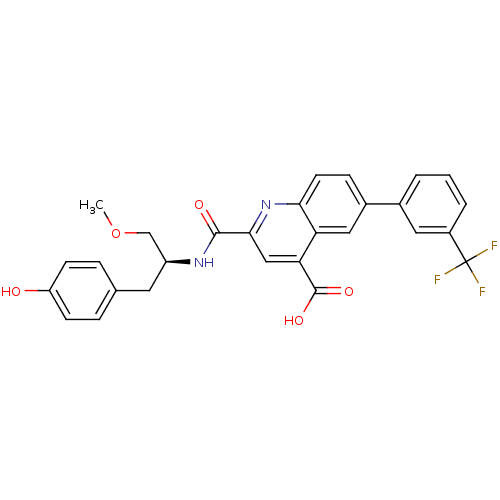 (CHEMBL3099752) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of B-Alexa-Fluor647 from CDK2 (unknown origin) by fluorescence polarization assay | Bioorg Med Chem Lett 24: 199-203 (2013) Article DOI: 10.1016/j.bmcl.2013.11.041 BindingDB Entry DOI: 10.7270/Q2SN0BF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM50523521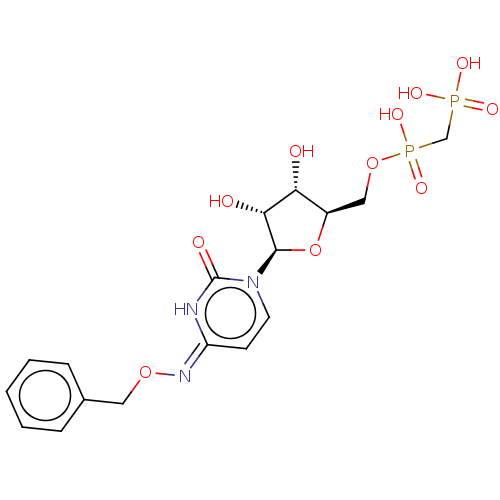 (CHEMBL4299847) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 112 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of recombinant rat C-terminal His-tagged soluble form of CD73 expressed in baculovirus infected Sf9 insect cells using [2,8-3H]AMP as subs... | J Med Chem 62: 3677-3695 (2019) Article DOI: 10.1021/acs.jmedchem.9b00164 BindingDB Entry DOI: 10.7270/Q2M61PNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50444941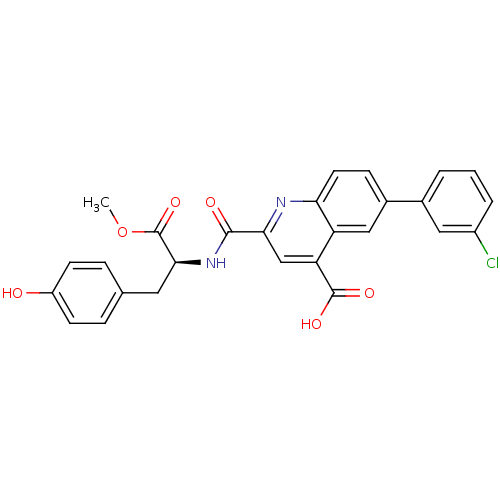 (CHEMBL3099755) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of B-Alexa-Fluor647 from CDK2 (unknown origin) by fluorescence polarization assay | Bioorg Med Chem Lett 24: 199-203 (2013) Article DOI: 10.1016/j.bmcl.2013.11.041 BindingDB Entry DOI: 10.7270/Q2SN0BF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50444920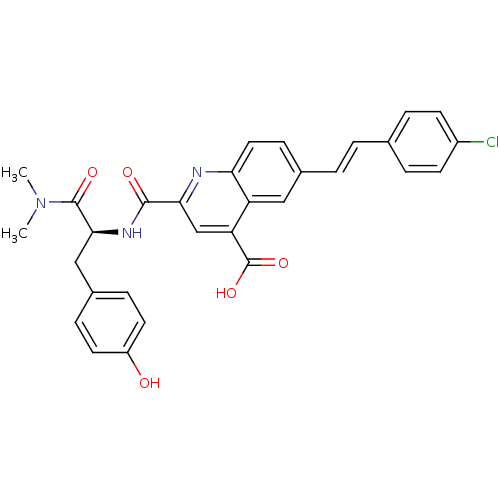 (CHEMBL3099751) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of B-Alexa-Fluor647 from CDK2 (unknown origin) by fluorescence polarization assay | Bioorg Med Chem Lett 24: 199-203 (2013) Article DOI: 10.1016/j.bmcl.2013.11.041 BindingDB Entry DOI: 10.7270/Q2SN0BF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM50319142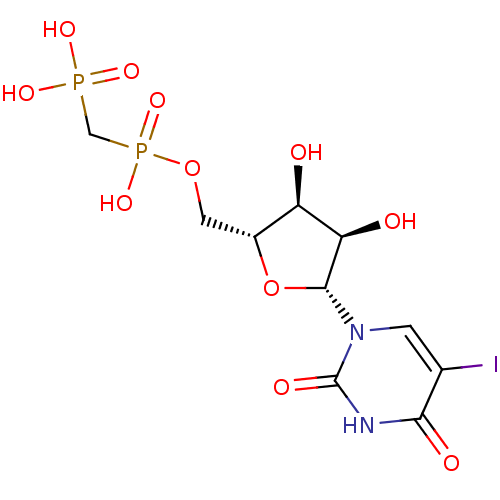 (5-Iodouridine5'-Methylenediphosphate Triethylammon...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 162 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of recombinant rat C-terminal His-tagged soluble form of CD73 expressed in baculovirus infected Sf9 insect cells using [2,8-3H]AMP as subs... | J Med Chem 62: 3677-3695 (2019) Article DOI: 10.1021/acs.jmedchem.9b00164 BindingDB Entry DOI: 10.7270/Q2M61PNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM18136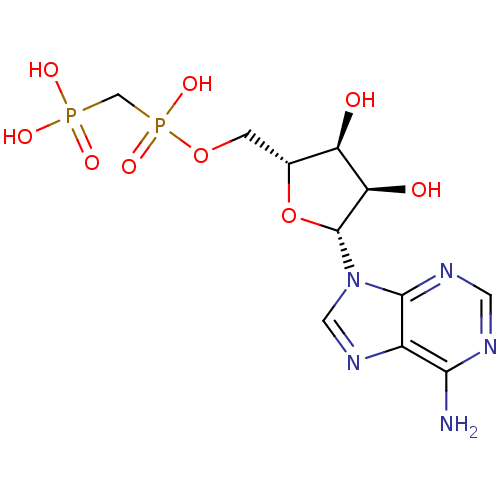 (ADP, alpha beta-me | AMPCPP | [({[(2R,3S,4R,5R)-5-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 167 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of recombinant rat C-terminal His-tagged soluble form of CD73 expressed in baculovirus infected Sf9 insect cells using [2,8-3H]AMP as subs... | J Med Chem 62: 3677-3695 (2019) Article DOI: 10.1021/acs.jmedchem.9b00164 BindingDB Entry DOI: 10.7270/Q2M61PNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50444938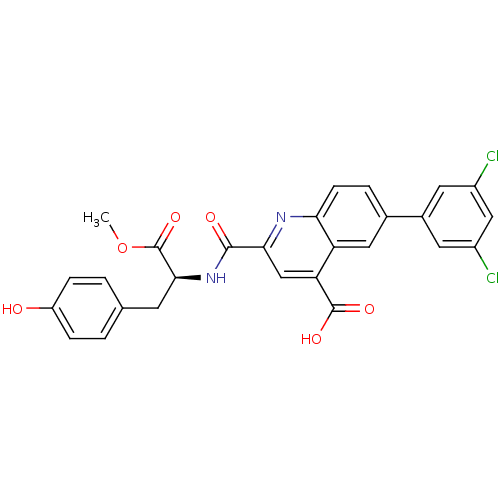 (CHEMBL3099758) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of B-Alexa-Fluor647 from CDK2 (unknown origin) by fluorescence polarization assay | Bioorg Med Chem Lett 24: 199-203 (2013) Article DOI: 10.1016/j.bmcl.2013.11.041 BindingDB Entry DOI: 10.7270/Q2SN0BF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM50523547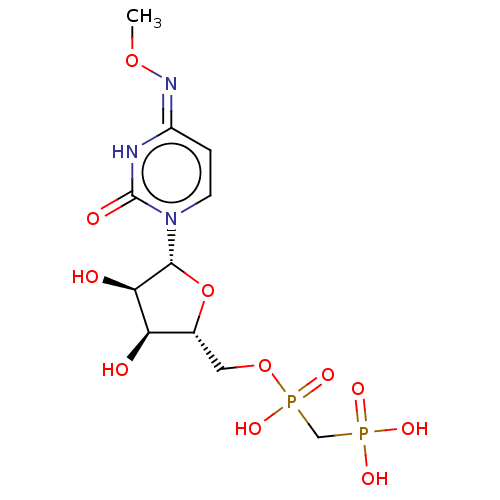 (CHEMBL1198873) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 257 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of recombinant rat C-terminal His-tagged soluble form of CD73 expressed in baculovirus infected Sf9 insect cells using [2,8-3H]AMP as subs... | J Med Chem 62: 3677-3695 (2019) Article DOI: 10.1021/acs.jmedchem.9b00164 BindingDB Entry DOI: 10.7270/Q2M61PNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM50523523 (CHEMBL4440236) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 262 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of recombinant rat C-terminal His-tagged soluble form of CD73 expressed in baculovirus infected Sf9 insect cells using [2,8-3H]AMP as subs... | J Med Chem 62: 3677-3695 (2019) Article DOI: 10.1021/acs.jmedchem.9b00164 BindingDB Entry DOI: 10.7270/Q2M61PNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM50523550 (CHEMBL4462889) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 276 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of recombinant rat C-terminal His-tagged soluble form of CD73 expressed in baculovirus infected Sf9 insect cells using [2,8-3H]AMP as subs... | J Med Chem 62: 3677-3695 (2019) Article DOI: 10.1021/acs.jmedchem.9b00164 BindingDB Entry DOI: 10.7270/Q2M61PNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50444922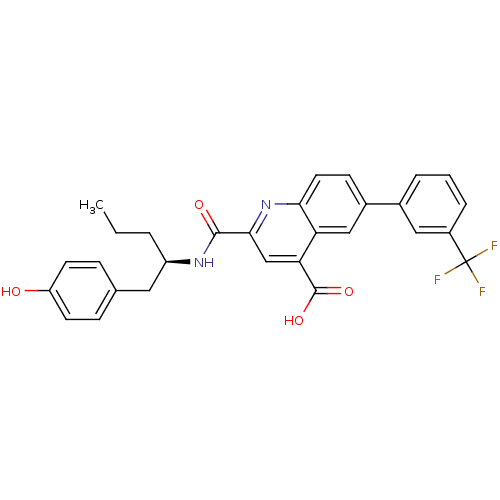 (CHEMBL3099749) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of B-Alexa-Fluor647 from CDK2 (unknown origin) by fluorescence polarization assay | Bioorg Med Chem Lett 24: 199-203 (2013) Article DOI: 10.1016/j.bmcl.2013.11.041 BindingDB Entry DOI: 10.7270/Q2SN0BF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM50523514 (CHEMBL4474742) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 321 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of recombinant rat C-terminal His-tagged soluble form of CD73 expressed in baculovirus infected Sf9 insect cells using [2,8-3H]AMP as subs... | J Med Chem 62: 3677-3695 (2019) Article DOI: 10.1021/acs.jmedchem.9b00164 BindingDB Entry DOI: 10.7270/Q2M61PNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM50523541 (CHEMBL3606109) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 338 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of recombinant rat C-terminal His-tagged soluble form of CD73 expressed in baculovirus infected Sf9 insect cells using [2,8-3H]AMP as subs... | J Med Chem 62: 3677-3695 (2019) Article DOI: 10.1021/acs.jmedchem.9b00164 BindingDB Entry DOI: 10.7270/Q2M61PNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM50523519 (CHEMBL4441328) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 349 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of recombinant rat C-terminal His-tagged soluble form of CD73 expressed in baculovirus infected Sf9 insect cells using [2,8-3H]AMP as subs... | J Med Chem 62: 3677-3695 (2019) Article DOI: 10.1021/acs.jmedchem.9b00164 BindingDB Entry DOI: 10.7270/Q2M61PNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Homo sapiens (Human)) | BDBM50456166 (CHEMBL4216870) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 365 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [125I]-Iodoaminopotentidine from human histamine H2 receptor expressed in HEK293T cell membranes after 90 mins by scintillation count... | J Med Chem 61: 4860-4882 (2018) Article DOI: 10.1021/acs.jmedchem.8b00168 BindingDB Entry DOI: 10.7270/Q2X069NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM50523533 (CHEMBL4574475) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 424 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of recombinant rat C-terminal His-tagged soluble form of CD73 expressed in baculovirus infected Sf9 insect cells using [2,8-3H]AMP as subs... | J Med Chem 62: 3677-3695 (2019) Article DOI: 10.1021/acs.jmedchem.9b00164 BindingDB Entry DOI: 10.7270/Q2M61PNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50543252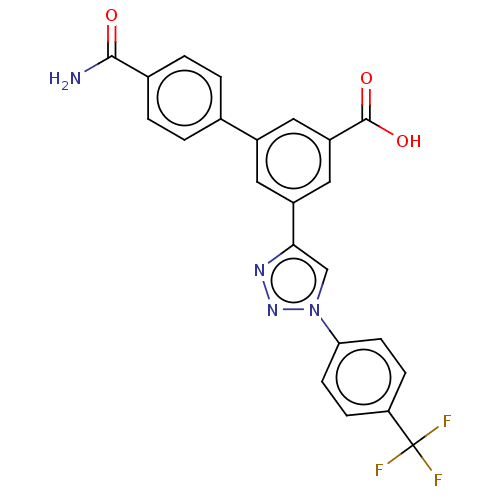 (CHEMBL4642215) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity to sigma 1 receptor (unknown origin) | J Med Chem 63: 9563-9589 (2020) Article DOI: 10.1021/acs.jmedchem.0c00745 BindingDB Entry DOI: 10.7270/Q20R9SZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM50523543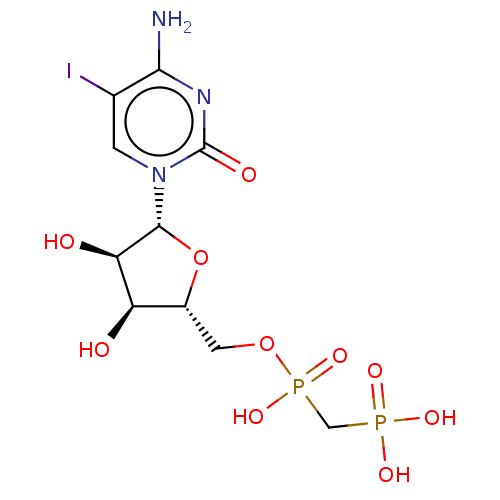 (CHEMBL4436640) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 502 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of recombinant rat C-terminal His-tagged soluble form of CD73 expressed in baculovirus infected Sf9 insect cells using [2,8-3H]AMP as subs... | J Med Chem 62: 3677-3695 (2019) Article DOI: 10.1021/acs.jmedchem.9b00164 BindingDB Entry DOI: 10.7270/Q2M61PNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Homo sapiens (Human)) | BDBM50541998 (CHEMBL4642592) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity to TSPO receptor (unknown origin) | J Med Chem 63: 9563-9589 (2020) Article DOI: 10.1021/acs.jmedchem.0c00745 BindingDB Entry DOI: 10.7270/Q20R9SZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM50523508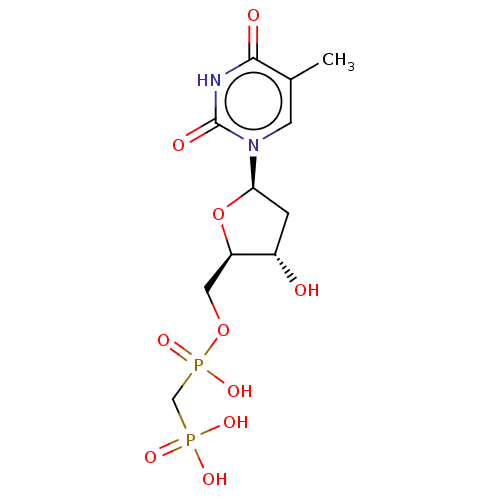 (CHEMBL4543740) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 639 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of recombinant rat C-terminal His-tagged soluble form of CD73 expressed in baculovirus infected Sf9 insect cells using [2,8-3H]AMP as subs... | J Med Chem 62: 3677-3695 (2019) Article DOI: 10.1021/acs.jmedchem.9b00164 BindingDB Entry DOI: 10.7270/Q2M61PNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 640 total ) | Next | Last >> |