

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM50187566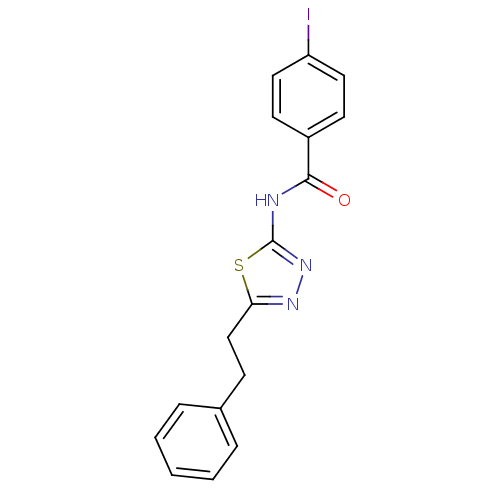 (4-iodo-N-(5-phenethyl-1,3,4-thiadiazol-2-yl)benzam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity to human recombinant Abl | J Med Chem 49: 3278-86 (2006) Article DOI: 10.1021/jm060236z BindingDB Entry DOI: 10.7270/Q2TH8M9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50103521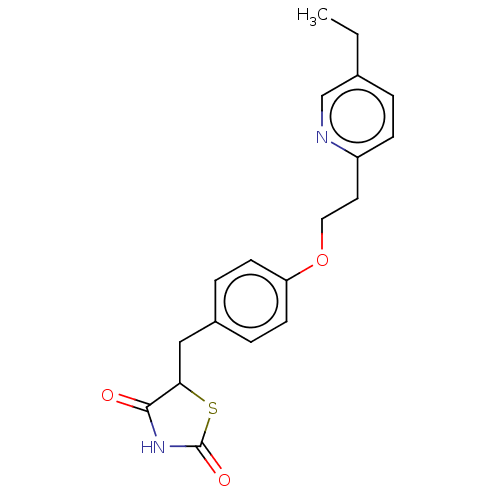 (Actos | CHEBI:8228 | Duetact | Pioglitazone | US10...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | DrugBank PDB Article PubMed | 420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Displacement of fluormone PanPPAR green tracer ligand from human 6His-tagged PPARgamma isoform 1 LBD (203 to 477 residues) expressed in Escherichia c... | J Med Chem 62: 2008-2023 (2019) Article DOI: 10.1021/acs.jmedchem.8b01573 BindingDB Entry DOI: 10.7270/Q2TH8R5M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50103521 (Actos | CHEBI:8228 | Duetact | Pioglitazone | US10...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | DrugBank PDB Article PubMed | 420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Displacement of fluormone PanPPAR green tracer ligand from human 6His-tagged PPARgamma isoform 1 LBD (203 to 477 residues) expressed in Escherichia c... | J Med Chem 62: 2008-2023 (2019) Article DOI: 10.1021/acs.jmedchem.8b01573 BindingDB Entry DOI: 10.7270/Q2TH8R5M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50187573 (3-(4-chlorophenyl)-1H-pyrazolo[3,4-d]pyrimidin-4-a...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity to human recombinant Src | J Med Chem 49: 3278-86 (2006) Article DOI: 10.1021/jm060236z BindingDB Entry DOI: 10.7270/Q2TH8M9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50187569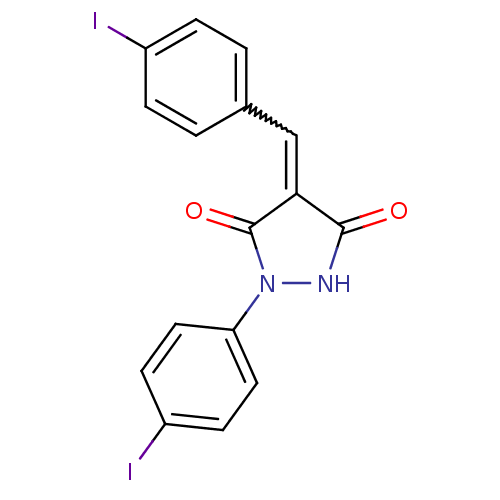 (4-(4-iodobenzylidene)-1-(4-iodophenyl)pyrazolidine...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity to human recombinant Src | J Med Chem 49: 3278-86 (2006) Article DOI: 10.1021/jm060236z BindingDB Entry DOI: 10.7270/Q2TH8M9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM50187573 (3-(4-chlorophenyl)-1H-pyrazolo[3,4-d]pyrimidin-4-a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity to human recombinant Abl | J Med Chem 49: 3278-86 (2006) Article DOI: 10.1021/jm060236z BindingDB Entry DOI: 10.7270/Q2TH8M9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50187579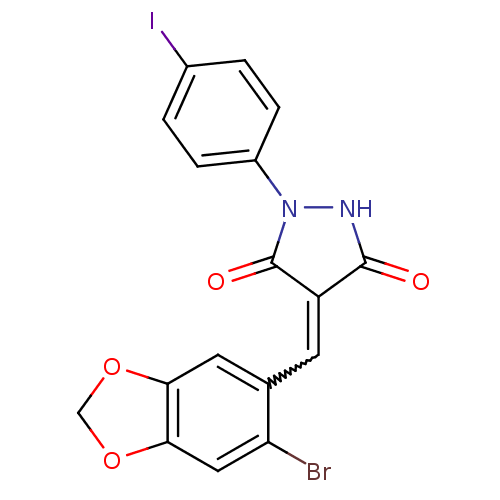 (4-((6-bromobenzo[d][1,3]dioxol-5-yl)methylene)-1-(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity to human recombinant Src | J Med Chem 49: 3278-86 (2006) Article DOI: 10.1021/jm060236z BindingDB Entry DOI: 10.7270/Q2TH8M9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM50187575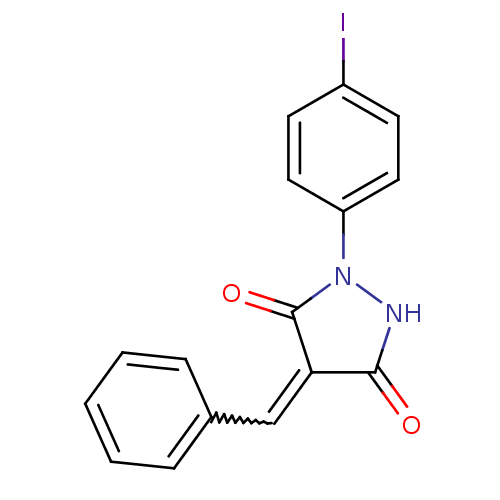 (4-benzylidene-1-(4-iodophenyl)pyrazolidine-3,5-dio...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity to human recombinant Abl | J Med Chem 49: 3278-86 (2006) Article DOI: 10.1021/jm060236z BindingDB Entry DOI: 10.7270/Q2TH8M9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM50187569 (4-(4-iodobenzylidene)-1-(4-iodophenyl)pyrazolidine...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity to human recombinant Abl | J Med Chem 49: 3278-86 (2006) Article DOI: 10.1021/jm060236z BindingDB Entry DOI: 10.7270/Q2TH8M9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50187570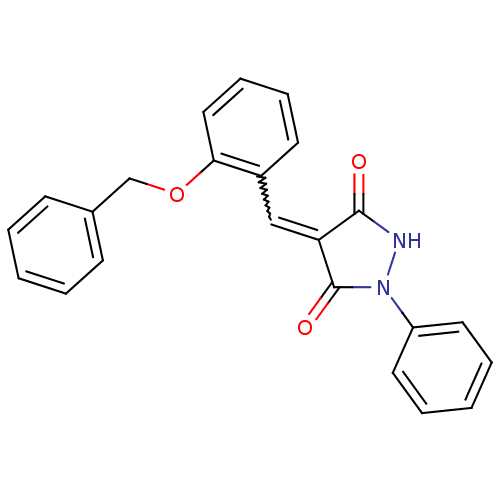 (4-(2-(benzyloxy)benzylidene)-1-phenylpyrazolidine-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity to human recombinant Src | J Med Chem 49: 3278-86 (2006) Article DOI: 10.1021/jm060236z BindingDB Entry DOI: 10.7270/Q2TH8M9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50187577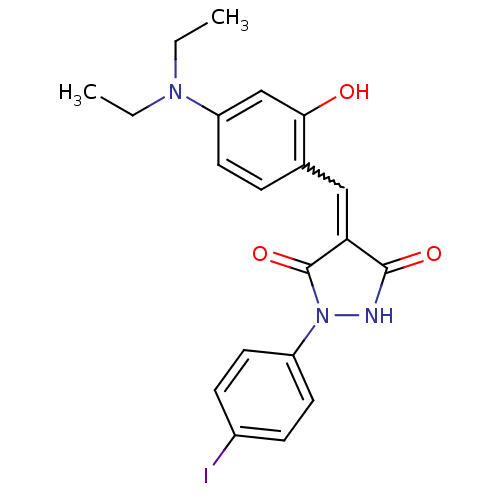 (4-(4-(diethylamino)-2-hydroxybenzylidene)-1-(4-iod...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity to human recombinant Src | J Med Chem 49: 3278-86 (2006) Article DOI: 10.1021/jm060236z BindingDB Entry DOI: 10.7270/Q2TH8M9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50187574 (4-((5-(3-chloro-4-methylphenyl)furan-2-yl)methylen...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity to human recombinant Src | J Med Chem 49: 3278-86 (2006) Article DOI: 10.1021/jm060236z BindingDB Entry DOI: 10.7270/Q2TH8M9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM50187572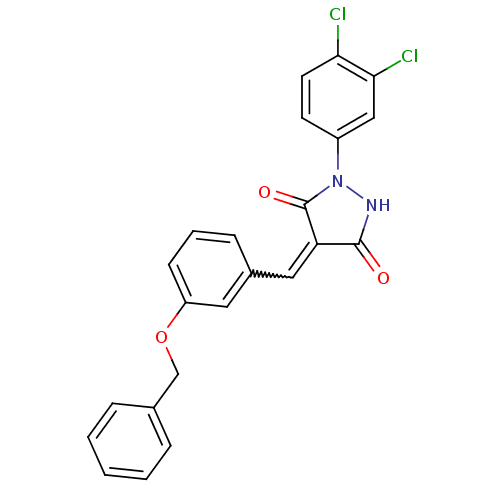 (4-(3-(benzyloxy)benzylidene)-1-(3,4-dichlorophenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity to human recombinant Abl | J Med Chem 49: 3278-86 (2006) Article DOI: 10.1021/jm060236z BindingDB Entry DOI: 10.7270/Q2TH8M9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50530214 (CHEBI:82937 | Leriglitazone | Min-102) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Displacement of fluormone PanPPAR green tracer ligand from human 6His-tagged PPARgamma isoform 1 LBD (203 to 477 residues) expressed in Escherichia c... | J Med Chem 62: 2008-2023 (2019) Article DOI: 10.1021/acs.jmedchem.8b01573 BindingDB Entry DOI: 10.7270/Q2TH8R5M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50530214 (CHEBI:82937 | Leriglitazone | Min-102) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Displacement of fluormone PanPPAR green tracer ligand from human 6His-tagged PPARgamma isoform 1 LBD (203 to 477 residues) expressed in Escherichia c... | J Med Chem 62: 2008-2023 (2019) Article DOI: 10.1021/acs.jmedchem.8b01573 BindingDB Entry DOI: 10.7270/Q2TH8R5M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50187568 (2,4-dichloro-N-(5-phenethyl-1,3,4-thiadiazol-2-yl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity to human recombinant Src | J Med Chem 49: 3278-86 (2006) Article DOI: 10.1021/jm060236z BindingDB Entry DOI: 10.7270/Q2TH8M9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM50187565 (2-iodo-N-(5-phenethyl-1,3,4-thiadiazol-2-yl)benzam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity to human recombinant Abl | J Med Chem 49: 3278-86 (2006) Article DOI: 10.1021/jm060236z BindingDB Entry DOI: 10.7270/Q2TH8M9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50187576 (BAS-4844343 | CHEMBL207672 | N-(5-((1H-indol-3-yl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity to human recombinant Src | J Med Chem 49: 3278-86 (2006) Article DOI: 10.1021/jm060236z BindingDB Entry DOI: 10.7270/Q2TH8M9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM50187571 (2-methoxy-4-(methylthio)-N-(5-phenethyl-1,3,4-thia...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity to human recombinant Abl | J Med Chem 49: 3278-86 (2006) Article DOI: 10.1021/jm060236z BindingDB Entry DOI: 10.7270/Q2TH8M9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50187565 (2-iodo-N-(5-phenethyl-1,3,4-thiadiazol-2-yl)benzam...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity to human recombinant Src | J Med Chem 49: 3278-86 (2006) Article DOI: 10.1021/jm060236z BindingDB Entry DOI: 10.7270/Q2TH8M9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM50187579 (4-((6-bromobenzo[d][1,3]dioxol-5-yl)methylene)-1-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity to human recombinant Abl | J Med Chem 49: 3278-86 (2006) Article DOI: 10.1021/jm060236z BindingDB Entry DOI: 10.7270/Q2TH8M9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50187578 (BAS-0338868 | CHEMBL378903 | N-(5-phenethyl-1,3,4-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity to human recombinant Src | J Med Chem 49: 3278-86 (2006) Article DOI: 10.1021/jm060236z BindingDB Entry DOI: 10.7270/Q2TH8M9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50187571 (2-methoxy-4-(methylthio)-N-(5-phenethyl-1,3,4-thia...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity to human recombinant Src | J Med Chem 49: 3278-86 (2006) Article DOI: 10.1021/jm060236z BindingDB Entry DOI: 10.7270/Q2TH8M9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50187575 (4-benzylidene-1-(4-iodophenyl)pyrazolidine-3,5-dio...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity to human recombinant Src | J Med Chem 49: 3278-86 (2006) Article DOI: 10.1021/jm060236z BindingDB Entry DOI: 10.7270/Q2TH8M9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM50187574 (4-((5-(3-chloro-4-methylphenyl)furan-2-yl)methylen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity to human recombinant Abl | J Med Chem 49: 3278-86 (2006) Article DOI: 10.1021/jm060236z BindingDB Entry DOI: 10.7270/Q2TH8M9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50187572 (4-(3-(benzyloxy)benzylidene)-1-(3,4-dichlorophenyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity to human recombinant Src | J Med Chem 49: 3278-86 (2006) Article DOI: 10.1021/jm060236z BindingDB Entry DOI: 10.7270/Q2TH8M9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM50187580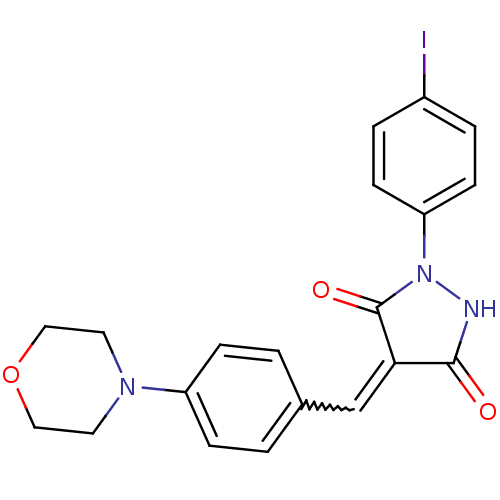 (4-(4-morpholinobenzylidene)-1-(4-iodophenyl)pyrazo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity to human recombinant Abl | J Med Chem 49: 3278-86 (2006) Article DOI: 10.1021/jm060236z BindingDB Entry DOI: 10.7270/Q2TH8M9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM50187567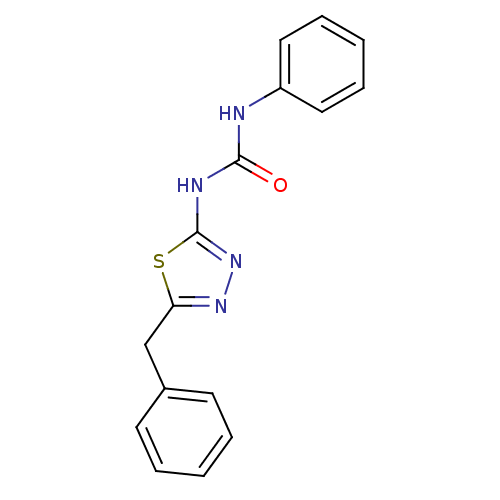 (1-(5-benzyl-1,3,4-thiadiazol-2-yl)-3-phenylurea | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity to human recombinant Abl | J Med Chem 49: 3278-86 (2006) Article DOI: 10.1021/jm060236z BindingDB Entry DOI: 10.7270/Q2TH8M9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50308732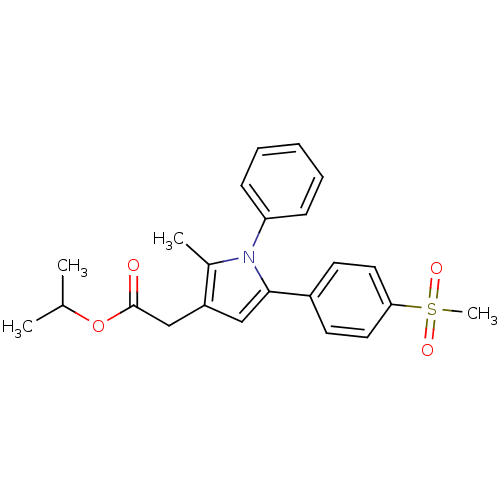 (CHEMBL605820 | Isopropyl-2-methyl-5-[4-(methylsulf...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ La Sapienza Curated by ChEMBL | Assay Description Inhibition of COX2-dependent PGE2 production in LPS-stimulated mouse J774 cells by RIA | J Med Chem 53: 723-33 (2010) Article DOI: 10.1021/jm901269y BindingDB Entry DOI: 10.7270/Q25X291D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50224118 (CHEMBL237592 | ethyl-2-methyl-5-[4-(methylsulfonyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Università La Sapienza Curated by ChEMBL | Assay Description Inhibition of COX2 in LPS-stimulated J774 cells assessed as inhibition of PGE2 levels by radioimmunoassay | J Med Chem 50: 5403-11 (2007) Article DOI: 10.1021/jm0707525 BindingDB Entry DOI: 10.7270/Q2PK0FV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50224129 (CHEMBL237626 | ethyl-2-methyl-5-[4-(methylsulfonyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Università La Sapienza Curated by ChEMBL | Assay Description Inhibition of COX2 in LPS-stimulated J774 cells assessed as inhibition of PGE2 levels by radioimmunoassay | J Med Chem 50: 5403-11 (2007) Article DOI: 10.1021/jm0707525 BindingDB Entry DOI: 10.7270/Q2PK0FV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50308736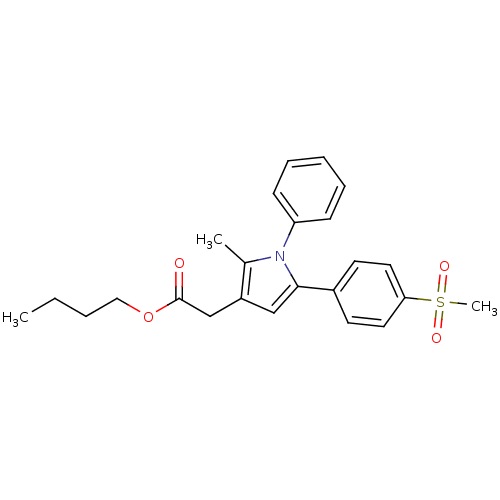 (CHEMBL602237 | n-Butyl-2-Methyl-5-[4-(methylsulfon...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ La Sapienza Curated by ChEMBL | Assay Description Inhibition of COX2-dependent PGE2 production in LPS-stimulated mouse J774 cells by RIA | J Med Chem 53: 723-33 (2010) Article DOI: 10.1021/jm901269y BindingDB Entry DOI: 10.7270/Q25X291D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50224128 (CHEMBL238027 | ethyl-2-methyl-5-[4-(methylsulfonyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Università La Sapienza Curated by ChEMBL | Assay Description Inhibition of COX2 in LPS-stimulated J774 cells assessed as inhibition of PGE2 levels by radioimmunoassay | J Med Chem 50: 5403-11 (2007) Article DOI: 10.1021/jm0707525 BindingDB Entry DOI: 10.7270/Q2PK0FV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50308738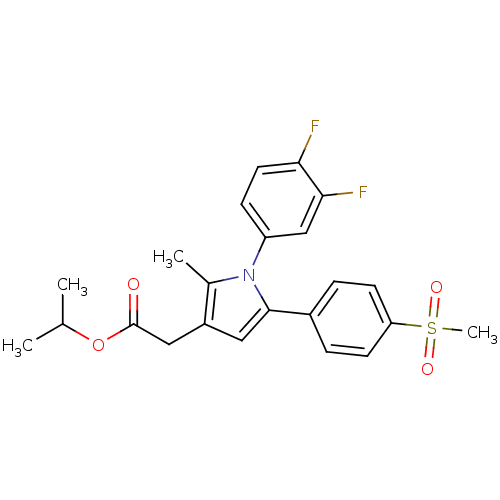 (CHEMBL589813 | Isopropyl-2-methyl-5-[4-(methylsulf...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ La Sapienza Curated by ChEMBL | Assay Description Inhibition of COX2-dependent PGE2 production in LPS-stimulated mouse J774 cells by RIA | J Med Chem 53: 723-33 (2010) Article DOI: 10.1021/jm901269y BindingDB Entry DOI: 10.7270/Q25X291D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50308740 (CHEMBL600167 | Isopropyl-2-methyl-5-[4-(methylsulf...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ La Sapienza Curated by ChEMBL | Assay Description Inhibition of COX2-dependent PGE2 production in LPS-stimulated mouse J774 cells by RIA | J Med Chem 53: 723-33 (2010) Article DOI: 10.1021/jm901269y BindingDB Entry DOI: 10.7270/Q25X291D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50308737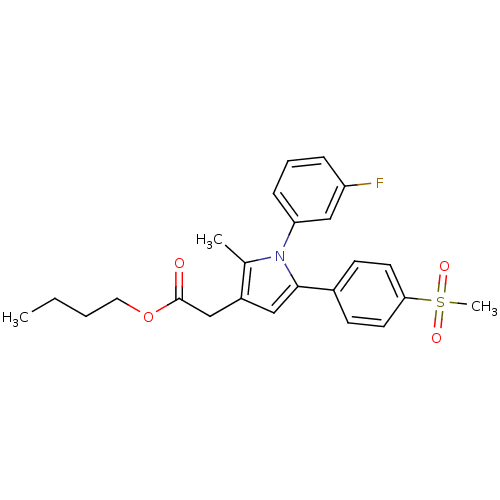 (CHEMBL600844 | n-Butyl-2-methyl-5-[4-(methylsulfon...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ La Sapienza Curated by ChEMBL | Assay Description Inhibition of COX2-dependent PGE2 production in LPS-stimulated mouse J774 cells by RIA | J Med Chem 53: 723-33 (2010) Article DOI: 10.1021/jm901269y BindingDB Entry DOI: 10.7270/Q25X291D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50224122 (CHEMBL398382 | ethyl-2-methyl-5-[4-(methylsulfonyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Università La Sapienza Curated by ChEMBL | Assay Description Inhibition of COX2 in LPS-stimulated J774 cells assessed as inhibition of PGE2 levels by radioimmunoassay | J Med Chem 50: 5403-11 (2007) Article DOI: 10.1021/jm0707525 BindingDB Entry DOI: 10.7270/Q2PK0FV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50308735 (2-Methyl-5-[4-(methylsulfonyl)phenyl]-1-[3-(fluoro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ La Sapienza Curated by ChEMBL | Assay Description Inhibition of COX2-dependent PGE2 production in LPS-stimulated mouse J774 cells by RIA | J Med Chem 53: 723-33 (2010) Article DOI: 10.1021/jm901269y BindingDB Entry DOI: 10.7270/Q25X291D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50308739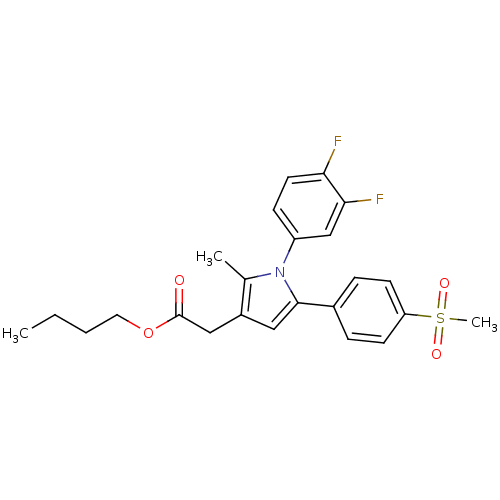 (CHEMBL589087 | n-Butyl-2-methyl-5-[4-(methylsulfon...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ La Sapienza Curated by ChEMBL | Assay Description Inhibition of COX2-dependent PGE2 production in LPS-stimulated mouse J774 cells by RIA | J Med Chem 53: 723-33 (2010) Article DOI: 10.1021/jm901269y BindingDB Entry DOI: 10.7270/Q25X291D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50308741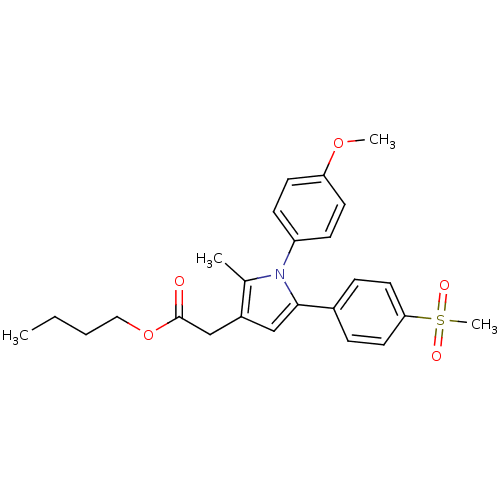 (CHEMBL589166 | n-Butyl-2-methyl-5-[4-(methylsulfon...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ La Sapienza Curated by ChEMBL | Assay Description Inhibition of COX2-dependent PGE2 production in LPS-stimulated mouse J774 cells by RIA | J Med Chem 53: 723-33 (2010) Article DOI: 10.1021/jm901269y BindingDB Entry DOI: 10.7270/Q25X291D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50166278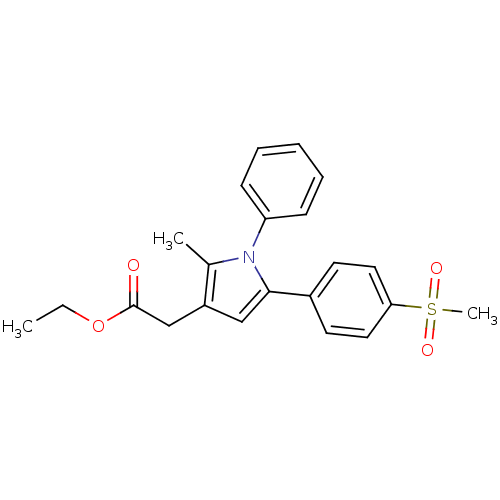 (CHEMBL191941 | [5-(4-Methanesulfonyl-phenyl)-2-met...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ La Sapienza Curated by ChEMBL | Assay Description Inhibition of COX2-dependent PGE2 production in LPS-stimulated mouse J774 cells by RIA | J Med Chem 53: 723-33 (2010) Article DOI: 10.1021/jm901269y BindingDB Entry DOI: 10.7270/Q25X291D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50308734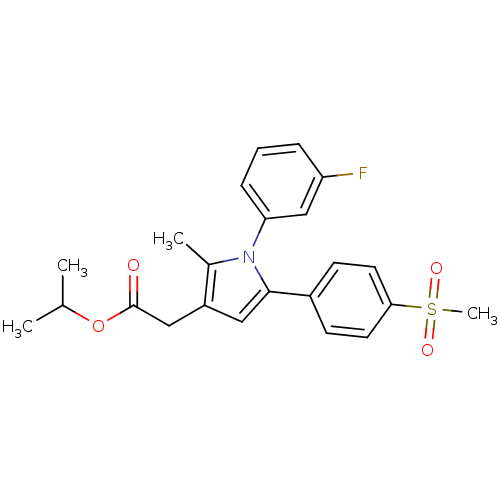 (CHEMBL589812 | Isopropyl-2-methyl-5-[4-(methylsulf...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ La Sapienza Curated by ChEMBL | Assay Description Inhibition of COX2-dependent PGE2 production in LPS-stimulated mouse J774 cells by RIA | J Med Chem 53: 723-33 (2010) Article DOI: 10.1021/jm901269y BindingDB Entry DOI: 10.7270/Q25X291D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM11639 (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Università La Sapienza Curated by ChEMBL | Assay Description Inhibition of COX2 in LPS-stimulated mouse J774 cells assessed as inhibition of PGE2 production after 15 mins by radioimmunoassay | Bioorg Med Chem 16: 8072-81 (2008) Article DOI: 10.1016/j.bmc.2008.07.058 BindingDB Entry DOI: 10.7270/Q24J0DZW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM11639 (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Università La Sapienza Curated by ChEMBL | Assay Description Inhibition of COX2 in LPS-stimulated J774 cells assessed as inhibition of PGE2 levels by radioimmunoassay | J Med Chem 50: 5403-11 (2007) Article DOI: 10.1021/jm0707525 BindingDB Entry DOI: 10.7270/Q2PK0FV8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50253541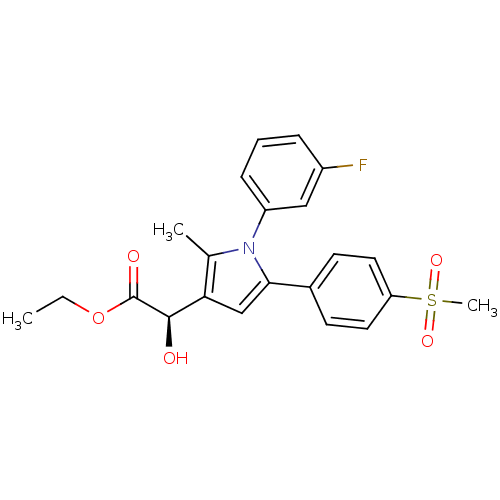 ((-)-(R)-Ethyl-[2-hydroxy-2-[1-(3-fluoro)phenyl-2-m...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
Università La Sapienza Curated by ChEMBL | Assay Description Inhibition of COX2 in LPS-stimulated mouse J774 cells assessed as inhibition of PGE2 production after 15 mins by radioimmunoassay | Bioorg Med Chem 16: 8072-81 (2008) Article DOI: 10.1016/j.bmc.2008.07.058 BindingDB Entry DOI: 10.7270/Q24J0DZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50253543 ((+)-(S)-Ethyl-[2-hydroxy-2-[1-(4-methoxy)phenyl-2-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
Università La Sapienza Curated by ChEMBL | Assay Description Inhibition of COX2 in LPS-stimulated mouse J774 cells assessed as inhibition of PGE2 production after 15 mins by radioimmunoassay | Bioorg Med Chem 16: 8072-81 (2008) Article DOI: 10.1016/j.bmc.2008.07.058 BindingDB Entry DOI: 10.7270/Q24J0DZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM11639 (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ La Sapienza Curated by ChEMBL | Assay Description Inhibition of COX2-dependent PGE2 production in LPS-stimulated mouse J774 cells by RIA | J Med Chem 53: 723-33 (2010) Article DOI: 10.1021/jm901269y BindingDB Entry DOI: 10.7270/Q25X291D | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50253627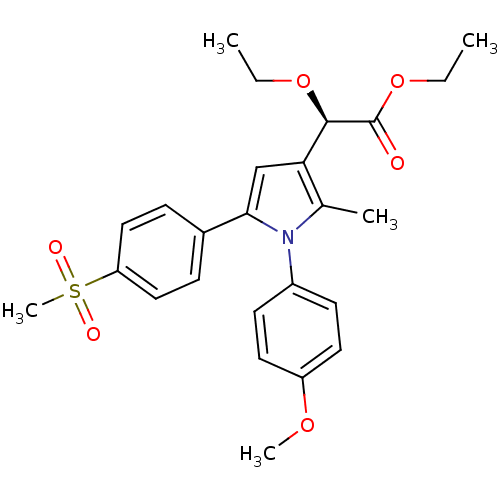 ((-)-(R)-Ethyl-[2-ethoxy-2-[1-(4-methoxy)phenyl-2-m...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Università La Sapienza Curated by ChEMBL | Assay Description Inhibition of COX2 in LPS-stimulated mouse J774 cells assessed as inhibition of PGE2 production after 15 mins by radioimmunoassay | Bioorg Med Chem 16: 8072-81 (2008) Article DOI: 10.1016/j.bmc.2008.07.058 BindingDB Entry DOI: 10.7270/Q24J0DZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50253569 ((+/-)-Ethyl-[2-ethoxy-2-[1-(3-fluoro)phenyl-2-meth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Università La Sapienza Curated by ChEMBL | Assay Description Inhibition of COX2 in LPS-stimulated mouse J774 cells assessed as inhibition of PGE2 production after 15 mins by radioimmunoassay | Bioorg Med Chem 16: 8072-81 (2008) Article DOI: 10.1016/j.bmc.2008.07.058 BindingDB Entry DOI: 10.7270/Q24J0DZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50253567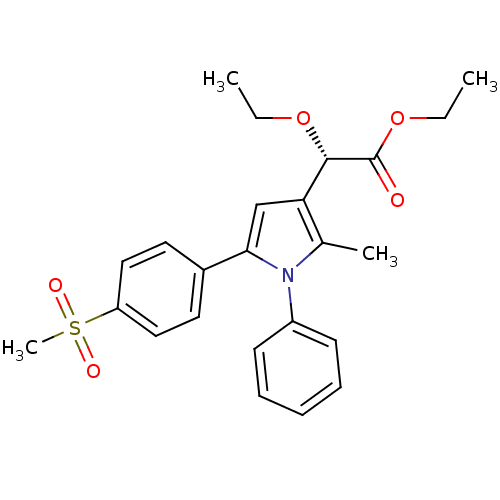 ((+)-(S)-Ethyl-[2-ethoxy-2-[-2-methyl-5-(4-methylsu...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Università La Sapienza Curated by ChEMBL | Assay Description Inhibition of COX2 in LPS-stimulated mouse J774 cells assessed as inhibition of PGE2 production after 15 mins by radioimmunoassay | Bioorg Med Chem 16: 8072-81 (2008) Article DOI: 10.1016/j.bmc.2008.07.058 BindingDB Entry DOI: 10.7270/Q24J0DZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 283 total ) | Next | Last >> |