

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50070476 ((1Z,4Z,9Z,15Z)-5,10,15,20-Tetrakis-(2,6-difluoro-p...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Inhibition constant (Ki) against electric eel Acetylcholinesterase as Km/Vmax versus inhibitor concentration replot | Bioorg Med Chem Lett 10: 1435-8 (2000) BindingDB Entry DOI: 10.7270/Q20K27SF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50070473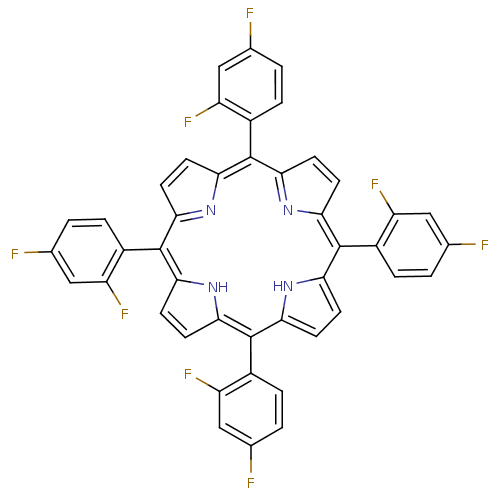 ((1Z,4Z,9Z,15Z)-5,10,15,20-Tetrakis-(2,4-difluoro-p...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Inhibition constant (Ki) against electric eel Acetylcholinesterase as Km/Vmax versus inhibitor concentration replot | Bioorg Med Chem Lett 10: 1435-8 (2000) BindingDB Entry DOI: 10.7270/Q20K27SF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50089503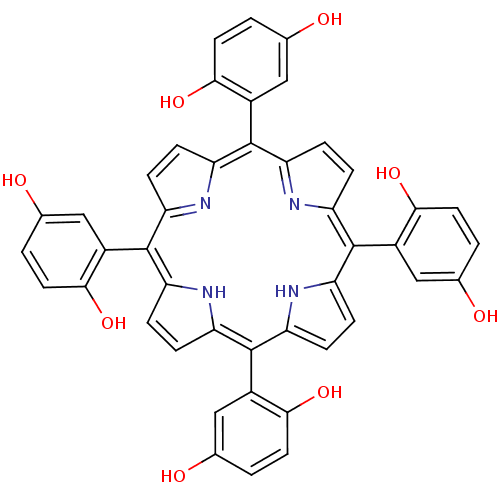 (2-[7,12,17-tri(2,5-hydroxyphenyl)-21,22,23,24-tetr...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Inhibition constant (Ki) against electric eel Acetylcholinesterase as Km/Vmax versus inhibitor concentration replot | Bioorg Med Chem Lett 10: 1435-8 (2000) BindingDB Entry DOI: 10.7270/Q20K27SF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50089504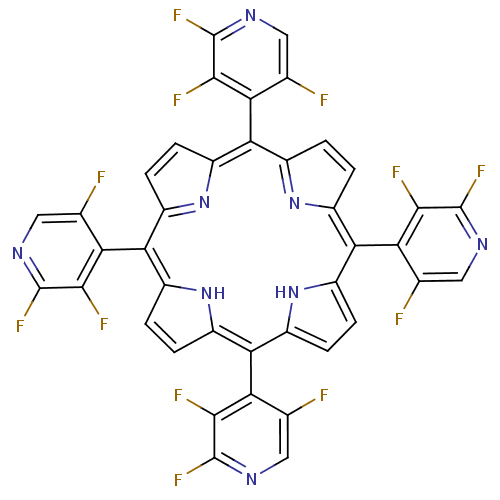 ((1Z,4Z,9Z,15Z)-5,10,15,20-Tetrakis-(2,3,5-trifluor...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Inhibition constant (Ki) against electric eel Acetylcholinesterase as Km/Vmax versus inhibitor concentration replot | Bioorg Med Chem Lett 10: 1435-8 (2000) BindingDB Entry DOI: 10.7270/Q20K27SF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50089505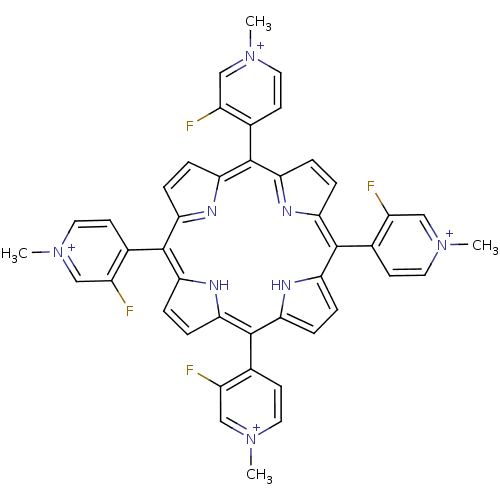 (2,7,12,17-tetra(3-fluoro-1-methyl-4-pyridiniumyl)-...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Inhibition constant (Ki) against electric eel Acetylcholinesterase as Km/Vmax versus inhibitor concentration replot | Bioorg Med Chem Lett 10: 1435-8 (2000) BindingDB Entry DOI: 10.7270/Q20K27SF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50089507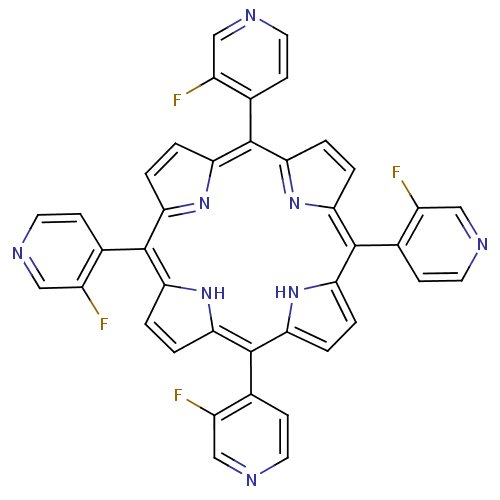 ((1Z,4Z,9Z,15Z)-5,10,15,20-Tetrakis-(3-fluoro-pyrid...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Inhibition constant (Ki) against electric eel Acetylcholinesterase as Km/Vmax versus inhibitor concentration replot | Bioorg Med Chem Lett 10: 1435-8 (2000) BindingDB Entry DOI: 10.7270/Q20K27SF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50089502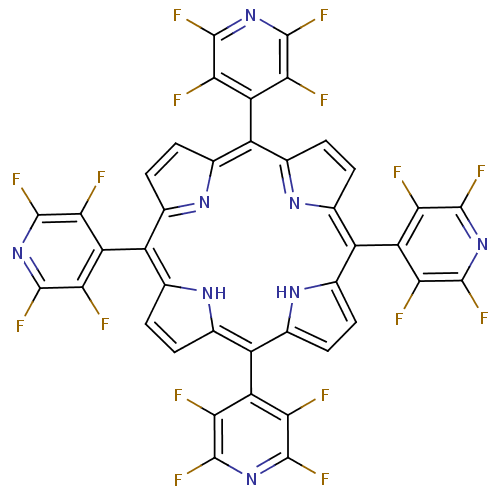 ((1Z,4Z,9Z,15Z)-5,10,15,20-Tetrakis-(2,3,5,6-tetraf...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Inhibition constant (Ki) against electric eel Acetylcholinesterase as Km/Vmax versus inhibitor concentration replot | Bioorg Med Chem Lett 10: 1435-8 (2000) BindingDB Entry DOI: 10.7270/Q20K27SF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50089510 ((1Z,4Z,9Z,15Z)-5,10,15,20-Tetrakis-(3-fluoro-pheny...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Inhibition constant (Ki) against electric eel Acetylcholinesterase as Km/Vmax versus inhibitor concentration replot | Bioorg Med Chem Lett 10: 1435-8 (2000) BindingDB Entry DOI: 10.7270/Q20K27SF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50070472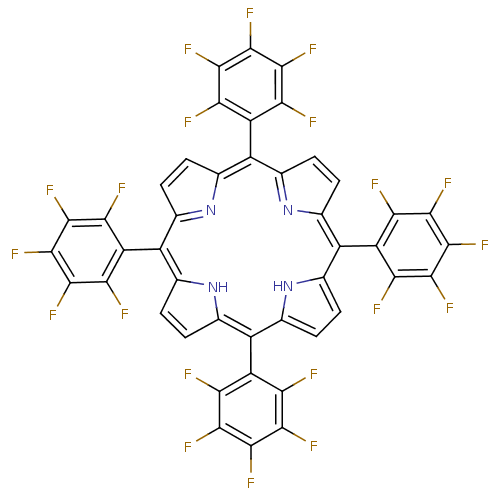 ((1Z,4Z,9Z,15Z)-5,10,15,20-Tetrakis-pentafluorophen...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.52E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Inhibition constant (Ki) against electric eel Acetylcholinesterase as Km/Vmax versus inhibitor concentration replot | Bioorg Med Chem Lett 10: 1435-8 (2000) BindingDB Entry DOI: 10.7270/Q20K27SF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50089509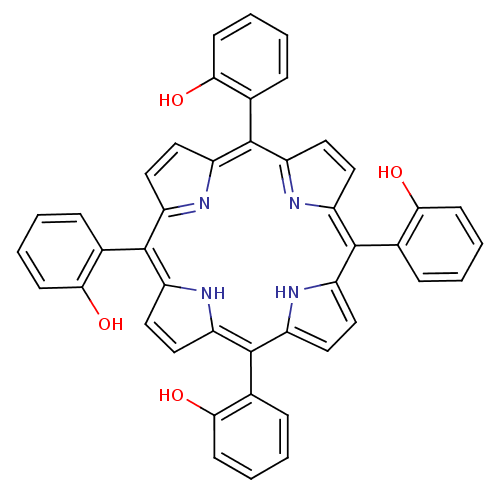 (2-[7,12,17-tri(2-hydroxyphenyl)-21,22,23,24-tetraa...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.83E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Inhibition constant (Ki) against electric eel Acetylcholinesterase as Km/Vmax versus inhibitor concentration replot | Bioorg Med Chem Lett 10: 1435-8 (2000) BindingDB Entry DOI: 10.7270/Q20K27SF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50089508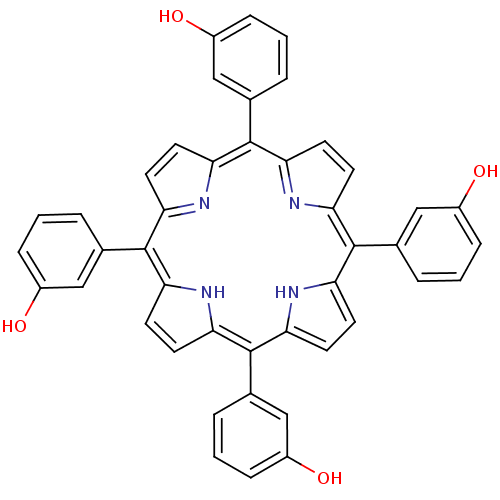 (2-[7,12,17-tri(3-hydroxyphenyl)-21,22,23,24-tetraa...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.96E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Inhibition constant (Ki) against electric eel Acetylcholinesterase as Km/Vmax versus inhibitor concentration replot | Bioorg Med Chem Lett 10: 1435-8 (2000) BindingDB Entry DOI: 10.7270/Q20K27SF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50089506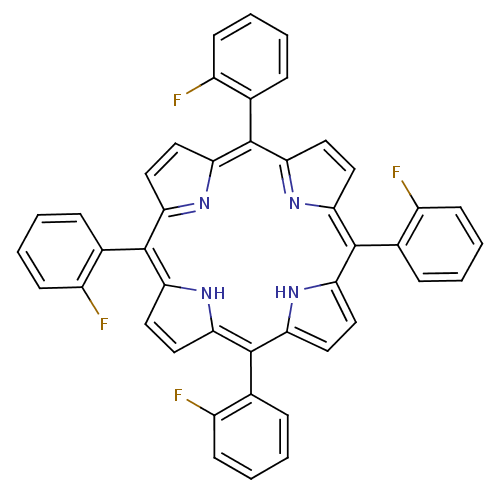 ((1Z,4Z,9Z,15Z)-5,10,15,20-Tetrakis-(2-fluoro-pheny...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 5.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Inhibition constant (Ki) against electric eel Acetylcholinesterase as Km/Vmax versus inhibitor concentration replot | Bioorg Med Chem Lett 10: 1435-8 (2000) BindingDB Entry DOI: 10.7270/Q20K27SF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk1 (Homo sapiens (Human)) | BDBM50185219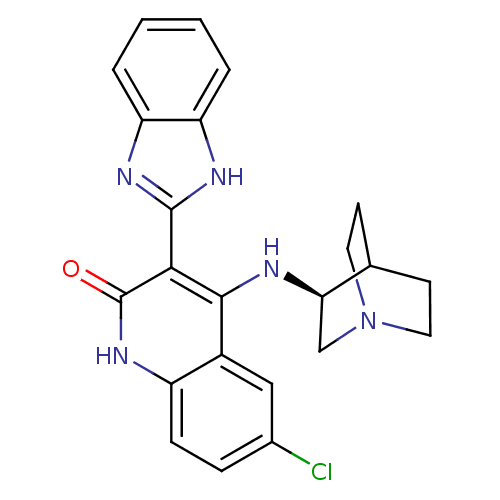 ((S)-3-(1H-benzo[d]imidazol-2-yl)-6-chloro-4-(quinu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2022.114304 BindingDB Entry DOI: 10.7270/Q25B06GC | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetolactate synthase 2, chloroplastic (Nicotiana tabacum) | BDBM50004707 (CHEMBL2269035) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul Women's University Curated by ChEMBL | Assay Description Inhibition of wild type Nicotiana tabacum (tobacco) GST-tagged acetolactate synthase expressed in Escherichia coli by modified Westerfeld method | Biochem Biophys Res Commun 258: 797-801 (1999) Article DOI: 10.1006/bbrc.1999.0708 BindingDB Entry DOI: 10.7270/Q2CR5VV2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetolactate synthase 2, chloroplastic (Nicotiana tabacum) | BDBM50004746 (CHEMBL2269037) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul Women's University Curated by ChEMBL | Assay Description Inhibition of wild type Nicotiana tabacum (tobacco) GST-tagged acetolactate synthase expressed in Escherichia coli by modified Westerfeld method | Biochem Biophys Res Commun 258: 797-801 (1999) Article DOI: 10.1006/bbrc.1999.0708 BindingDB Entry DOI: 10.7270/Q2CR5VV2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetolactate synthase 2, chloroplastic (Nicotiana tabacum) | BDBM50004747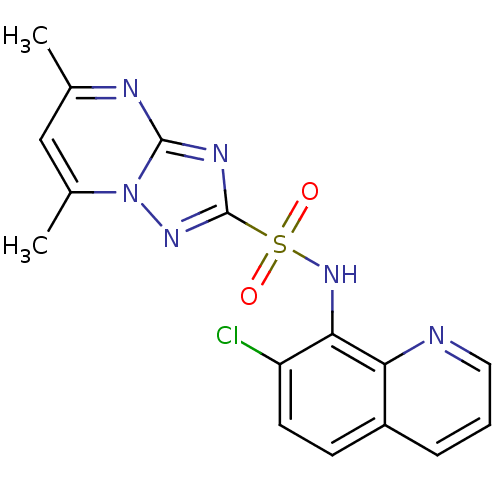 (CHEMBL2269036) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul Women's University Curated by ChEMBL | Assay Description Inhibition of wild type Nicotiana tabacum (tobacco) GST-tagged acetolactate synthase expressed in Escherichia coli by modified Westerfeld method | Biochem Biophys Res Commun 258: 797-801 (1999) Article DOI: 10.1006/bbrc.1999.0708 BindingDB Entry DOI: 10.7270/Q2CR5VV2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetolactate synthase 2, chloroplastic (Nicotiana tabacum) | BDBM50004706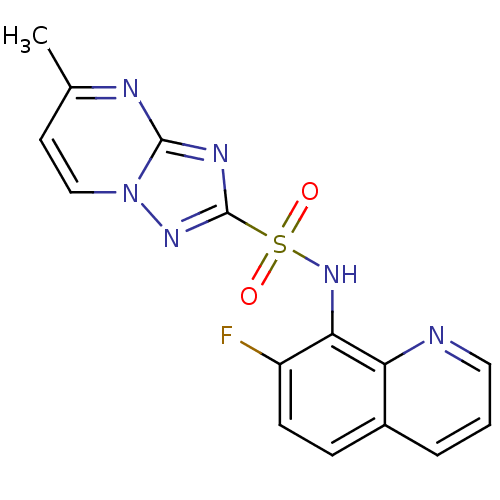 (CHEMBL2269029) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul Women's University Curated by ChEMBL | Assay Description Inhibition of wild type Nicotiana tabacum (tobacco) GST-tagged acetolactate synthase expressed in Escherichia coli by modified Westerfeld method | Biochem Biophys Res Commun 258: 797-801 (1999) Article DOI: 10.1006/bbrc.1999.0708 BindingDB Entry DOI: 10.7270/Q2CR5VV2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetolactate synthase 2, chloroplastic (Nicotiana tabacum) | BDBM50004709 (CHEMBL2269031) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul Women's University Curated by ChEMBL | Assay Description Inhibition of wild type Nicotiana tabacum (tobacco) GST-tagged acetolactate synthase expressed in Escherichia coli by modified Westerfeld method | Biochem Biophys Res Commun 258: 797-801 (1999) Article DOI: 10.1006/bbrc.1999.0708 BindingDB Entry DOI: 10.7270/Q2CR5VV2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toll-like receptor 9 (Homo sapiens (Human)) | BDBM50467770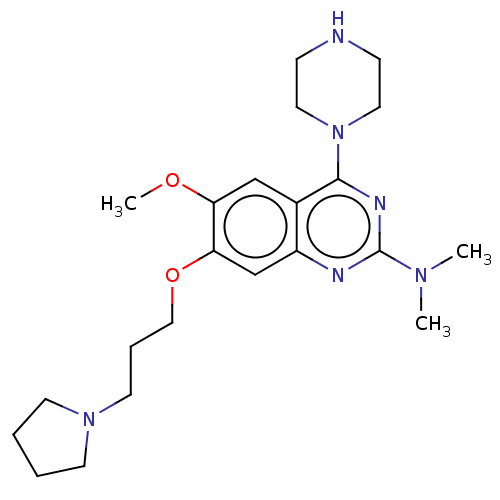 (CHEMBL4279467) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Biology Curated by ChEMBL | Assay Description Antagonist activity at human TLR9 expressed in HEK-Blue cells assessed as reduction in CpGB-induced NF-kappaB levels after 24 hrs by spectrophotometr... | Eur J Med Chem 159: 187-205 (2018) Article DOI: 10.1016/j.ejmech.2018.09.058 BindingDB Entry DOI: 10.7270/Q2C2504D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toll-like receptor 9 (Homo sapiens (Human)) | BDBM50467784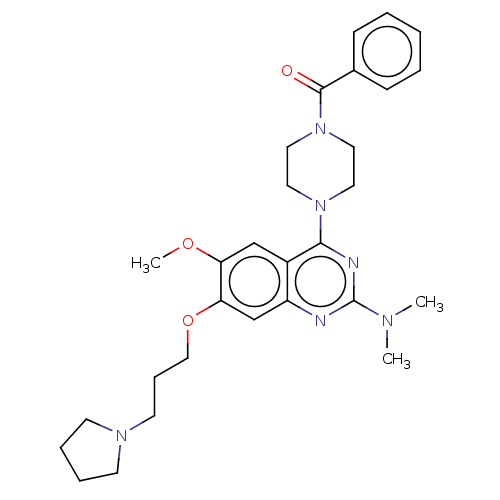 (CHEMBL4278635) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Biology Curated by ChEMBL | Assay Description Antagonist activity at human TLR9 expressed in HEK-Blue cells assessed as reduction in CpGB-induced NF-kappaB levels after 24 hrs by spectrophotometr... | Eur J Med Chem 159: 187-205 (2018) Article DOI: 10.1016/j.ejmech.2018.09.058 BindingDB Entry DOI: 10.7270/Q2C2504D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toll-like receptor 9 (Homo sapiens (Human)) | BDBM50467766 (CHEMBL4276673) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Biology Curated by ChEMBL | Assay Description Antagonist activity at human TLR9 expressed in HEK-Blue cells assessed as reduction in CpGB-induced NF-kappaB levels after 24 hrs by spectrophotometr... | Eur J Med Chem 159: 187-205 (2018) Article DOI: 10.1016/j.ejmech.2018.09.058 BindingDB Entry DOI: 10.7270/Q2C2504D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toll-like receptor 9 (Homo sapiens (Human)) | BDBM50467773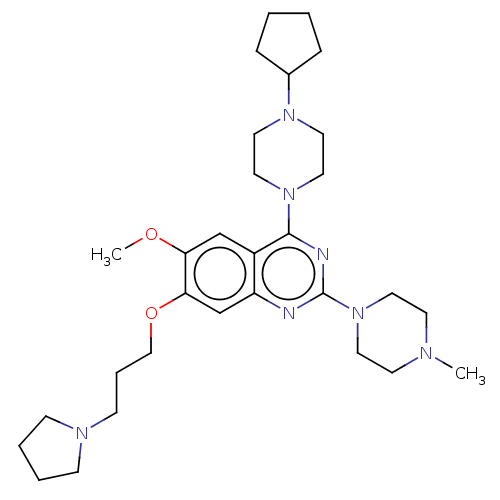 (CHEMBL4282131) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Biology Curated by ChEMBL | Assay Description Antagonist activity at human TLR9 expressed in HEK-Blue cells assessed as reduction in CpGB-induced NF-kappaB levels after 24 hrs by spectrophotometr... | Eur J Med Chem 159: 187-205 (2018) Article DOI: 10.1016/j.ejmech.2018.09.058 BindingDB Entry DOI: 10.7270/Q2C2504D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50510239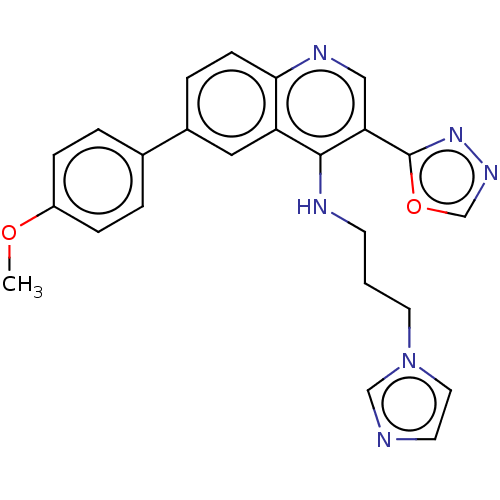 (CHEMBL4454135) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2022.114304 BindingDB Entry DOI: 10.7270/Q25B06GC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toll-like receptor 9 (Homo sapiens (Human)) | BDBM50467774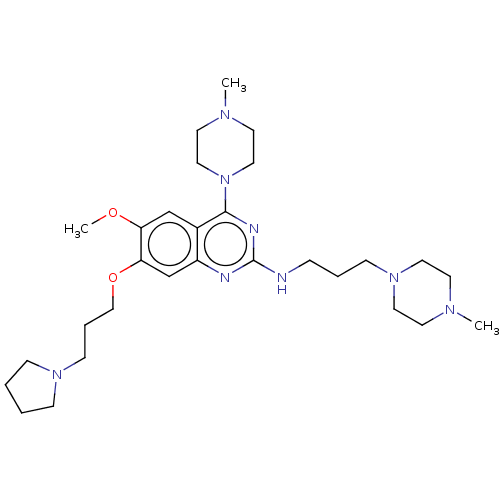 (CHEMBL4286933) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Biology Curated by ChEMBL | Assay Description Antagonist activity at human TLR9 expressed in HEK-Blue cells assessed as reduction in CpGB-induced NF-kappaB levels after 24 hrs by spectrophotometr... | Eur J Med Chem 159: 187-205 (2018) Article DOI: 10.1016/j.ejmech.2018.09.058 BindingDB Entry DOI: 10.7270/Q2C2504D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toll-like receptor 9 (Homo sapiens (Human)) | BDBM50467782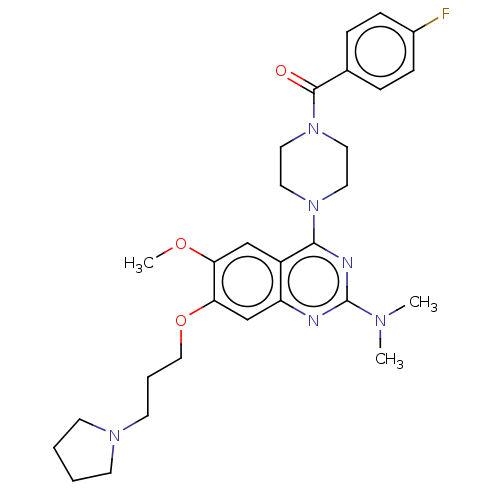 (CHEMBL4279648) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Biology Curated by ChEMBL | Assay Description Antagonist activity at human TLR9 expressed in HEK-Blue cells assessed as reduction in CpGB-induced NF-kappaB levels after 24 hrs by spectrophotometr... | Eur J Med Chem 159: 187-205 (2018) Article DOI: 10.1016/j.ejmech.2018.09.058 BindingDB Entry DOI: 10.7270/Q2C2504D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toll-like receptor 9 (Homo sapiens (Human)) | BDBM50467786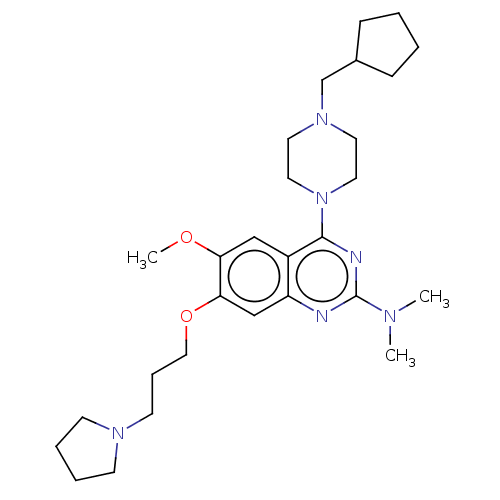 (CHEMBL4281194) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Biology Curated by ChEMBL | Assay Description Antagonist activity at human TLR9 expressed in HEK-Blue cells assessed as reduction in CpGB-induced NF-kappaB levels after 24 hrs by spectrophotometr... | Eur J Med Chem 159: 187-205 (2018) Article DOI: 10.1016/j.ejmech.2018.09.058 BindingDB Entry DOI: 10.7270/Q2C2504D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toll-like receptor 9 (Homo sapiens (Human)) | BDBM50467775 (CHEMBL4278682) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Biology Curated by ChEMBL | Assay Description Antagonist activity at human TLR9 expressed in HEK-Blue cells assessed as reduction in CpGB-induced NF-kappaB levels after 24 hrs by spectrophotometr... | Eur J Med Chem 159: 187-205 (2018) Article DOI: 10.1016/j.ejmech.2018.09.058 BindingDB Entry DOI: 10.7270/Q2C2504D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toll-like receptor 9 (Homo sapiens (Human)) | BDBM50467794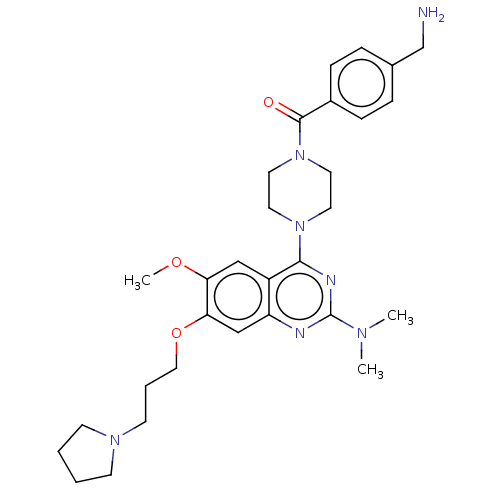 (CHEMBL4290314) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Biology Curated by ChEMBL | Assay Description Antagonist activity at human TLR9 expressed in HEK-Blue cells assessed as reduction in CpGB-induced NF-kappaB levels after 24 hrs by spectrophotometr... | Eur J Med Chem 159: 187-205 (2018) Article DOI: 10.1016/j.ejmech.2018.09.058 BindingDB Entry DOI: 10.7270/Q2C2504D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetolactate synthase 2, chloroplastic (Nicotiana tabacum) | BDBM50004719 (CHEMBL2269024) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul Women's University Curated by ChEMBL | Assay Description Inhibition of wild type Nicotiana tabacum (tobacco) GST-tagged acetolactate synthase expressed in Escherichia coli by modified Westerfeld method | Biochem Biophys Res Commun 258: 797-801 (1999) Article DOI: 10.1006/bbrc.1999.0708 BindingDB Entry DOI: 10.7270/Q2CR5VV2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetolactate synthase 2, chloroplastic (Nicotiana tabacum) | BDBM50004708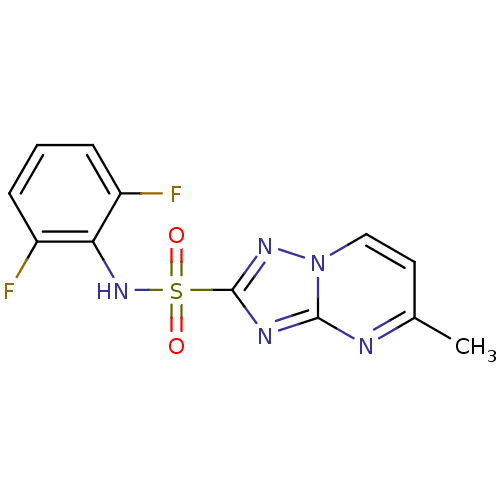 (FLUMETSULAM) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul Women's University Curated by ChEMBL | Assay Description Inhibition of wild type Nicotiana tabacum (tobacco) GST-tagged acetolactate synthase expressed in Escherichia coli by modified Westerfeld method | Biochem Biophys Res Commun 258: 797-801 (1999) Article DOI: 10.1006/bbrc.1999.0708 BindingDB Entry DOI: 10.7270/Q2CR5VV2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetolactate synthase 2, chloroplastic (Nicotiana tabacum) | BDBM50004712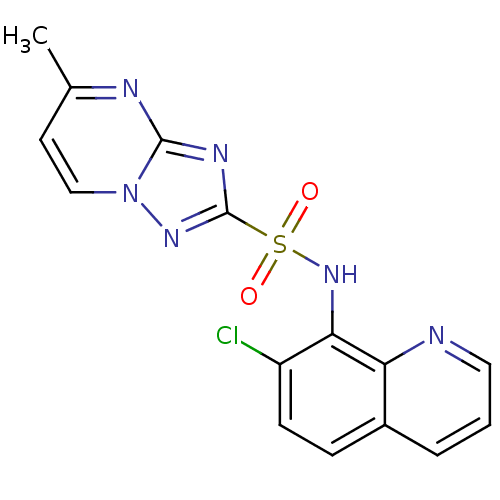 (CHEMBL2269030) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul Women's University Curated by ChEMBL | Assay Description Inhibition of wild type Nicotiana tabacum (tobacco) GST-tagged acetolactate synthase expressed in Escherichia coli by modified Westerfeld method | Biochem Biophys Res Commun 258: 797-801 (1999) Article DOI: 10.1006/bbrc.1999.0708 BindingDB Entry DOI: 10.7270/Q2CR5VV2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toll-like receptor 9 (Homo sapiens (Human)) | BDBM50467787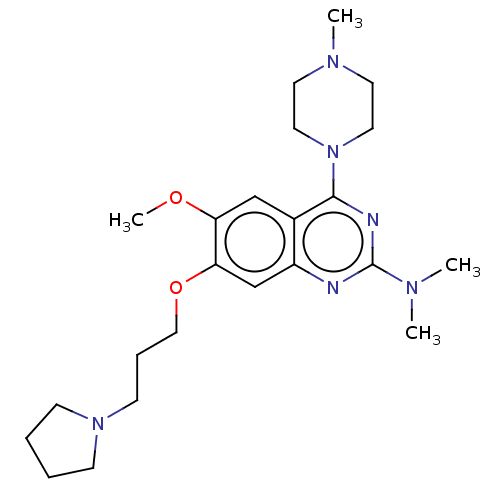 (CHEMBL4284607) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Biology Curated by ChEMBL | Assay Description Antagonist activity at human TLR9 expressed in HEK-Blue cells assessed as reduction in CpGB-induced NF-kappaB levels after 24 hrs by spectrophotometr... | Eur J Med Chem 159: 187-205 (2018) Article DOI: 10.1016/j.ejmech.2018.09.058 BindingDB Entry DOI: 10.7270/Q2C2504D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toll-like receptor 9 (Homo sapiens (Human)) | BDBM50467772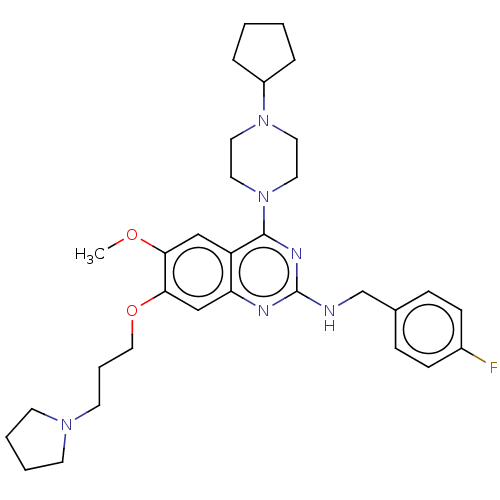 (CHEMBL4284496) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Biology Curated by ChEMBL | Assay Description Antagonist activity at human TLR9 expressed in HEK-Blue cells assessed as reduction in CpGB-induced NF-kappaB levels after 24 hrs by spectrophotometr... | Eur J Med Chem 159: 187-205 (2018) Article DOI: 10.1016/j.ejmech.2018.09.058 BindingDB Entry DOI: 10.7270/Q2C2504D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toll-like receptor 9 (Homo sapiens (Human)) | BDBM50467796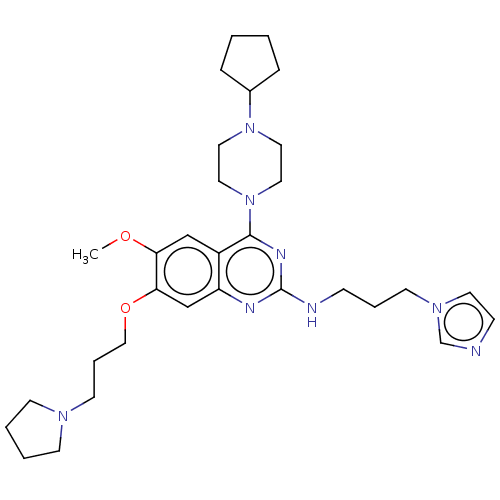 (CHEMBL4285558) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Biology Curated by ChEMBL | Assay Description Antagonist activity at human TLR9 expressed in HEK-Blue cells assessed as reduction in CpGB-induced NF-kappaB levels after 24 hrs by spectrophotometr... | Eur J Med Chem 159: 187-205 (2018) Article DOI: 10.1016/j.ejmech.2018.09.058 BindingDB Entry DOI: 10.7270/Q2C2504D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toll-like receptor 9 (Homo sapiens (Human)) | BDBM50467778 (CHEMBL4280997) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Biology Curated by ChEMBL | Assay Description Antagonist activity at human TLR9 expressed in HEK-Blue cells assessed as reduction in CpGB-induced NF-kappaB levels after 24 hrs by spectrophotometr... | Eur J Med Chem 159: 187-205 (2018) Article DOI: 10.1016/j.ejmech.2018.09.058 BindingDB Entry DOI: 10.7270/Q2C2504D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetolactate synthase 2, chloroplastic (Nicotiana tabacum) | BDBM50004717 (CHEMBL2269025) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul Women's University Curated by ChEMBL | Assay Description Inhibition of wild type Nicotiana tabacum (tobacco) GST-tagged acetolactate synthase expressed in Escherichia coli by modified Westerfeld method | Biochem Biophys Res Commun 258: 797-801 (1999) Article DOI: 10.1006/bbrc.1999.0708 BindingDB Entry DOI: 10.7270/Q2CR5VV2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toll-like receptor 9 (Homo sapiens (Human)) | BDBM50467771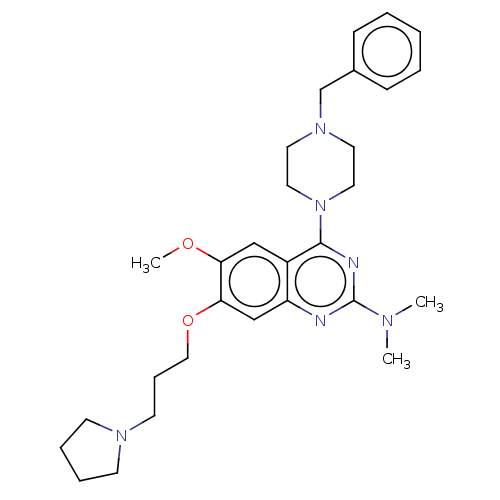 (CHEMBL4291815) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Biology Curated by ChEMBL | Assay Description Antagonist activity at human TLR9 expressed in HEK-Blue cells assessed as reduction in CpGB-induced NF-kappaB levels after 24 hrs by spectrophotometr... | Eur J Med Chem 159: 187-205 (2018) Article DOI: 10.1016/j.ejmech.2018.09.058 BindingDB Entry DOI: 10.7270/Q2C2504D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toll-like receptor 9 (Homo sapiens (Human)) | BDBM50467789 (CHEMBL4287813) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Biology Curated by ChEMBL | Assay Description Antagonist activity at human TLR9 expressed in HEK-Blue cells assessed as reduction in CpGB-induced NF-kappaB levels after 24 hrs by spectrophotometr... | Eur J Med Chem 159: 187-205 (2018) Article DOI: 10.1016/j.ejmech.2018.09.058 BindingDB Entry DOI: 10.7270/Q2C2504D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toll-like receptor 9 (Homo sapiens (Human)) | BDBM50467780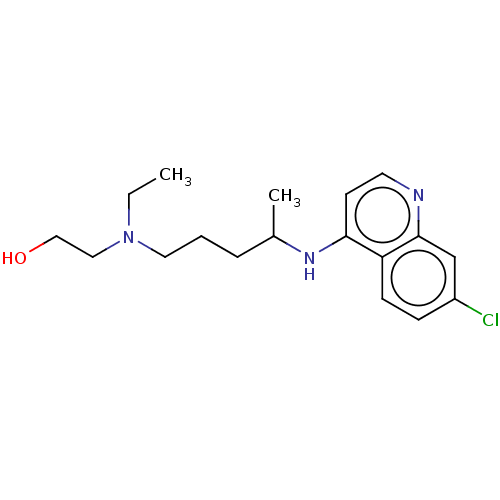 (CHEBI:5801 | Hydroxychloroquine | acs.jmedchem.1c0...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Biology Curated by ChEMBL | Assay Description Antagonist activity at human TLR9 expressed in HEK-Blue cells assessed as reduction in CpGB-induced NF-kappaB levels after 24 hrs by spectrophotometr... | Eur J Med Chem 159: 187-205 (2018) Article DOI: 10.1016/j.ejmech.2018.09.058 BindingDB Entry DOI: 10.7270/Q2C2504D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toll-like receptor 9 (Homo sapiens (Human)) | BDBM50467785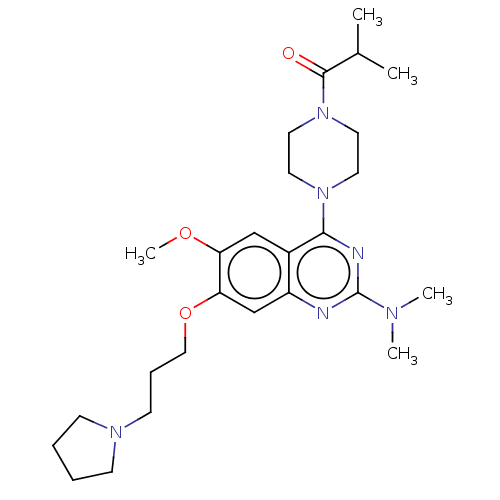 (CHEMBL4283550) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Biology Curated by ChEMBL | Assay Description Antagonist activity at human TLR9 expressed in HEK-Blue cells assessed as reduction in CpGB-induced NF-kappaB levels after 24 hrs by spectrophotometr... | Eur J Med Chem 159: 187-205 (2018) Article DOI: 10.1016/j.ejmech.2018.09.058 BindingDB Entry DOI: 10.7270/Q2C2504D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toll-like receptor 9 (Homo sapiens (Human)) | BDBM50467777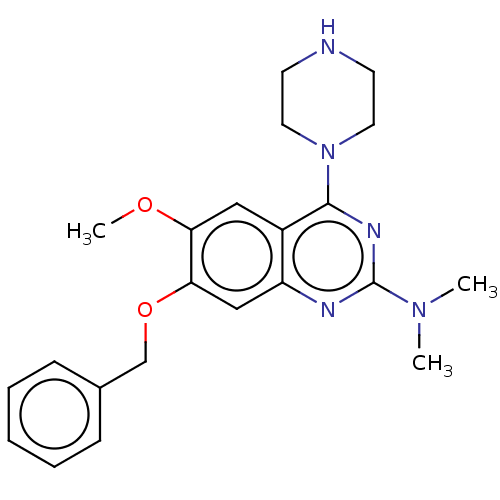 (CHEMBL4290193) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Biology Curated by ChEMBL | Assay Description Antagonist activity at human TLR9 expressed in HEK-Blue cells assessed as reduction in CpGB-induced NF-kappaB levels after 24 hrs by spectrophotometr... | Eur J Med Chem 159: 187-205 (2018) Article DOI: 10.1016/j.ejmech.2018.09.058 BindingDB Entry DOI: 10.7270/Q2C2504D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toll-like receptor 9 (Homo sapiens (Human)) | BDBM50467795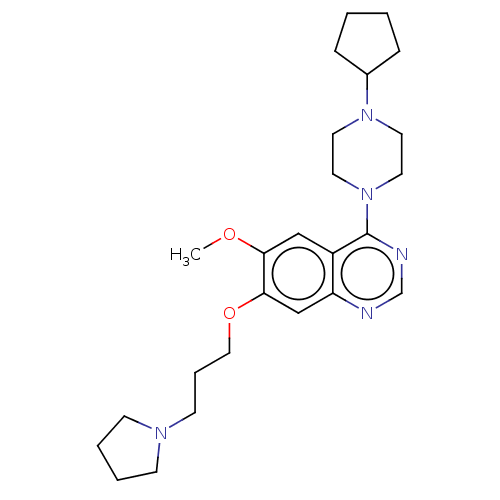 (CHEMBL4290016) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Biology Curated by ChEMBL | Assay Description Antagonist activity at human TLR9 expressed in HEK-Blue cells assessed as reduction in CpGB-induced NF-kappaB levels after 24 hrs by spectrophotometr... | Eur J Med Chem 159: 187-205 (2018) Article DOI: 10.1016/j.ejmech.2018.09.058 BindingDB Entry DOI: 10.7270/Q2C2504D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toll-like receptor 9 (Homo sapiens (Human)) | BDBM50467765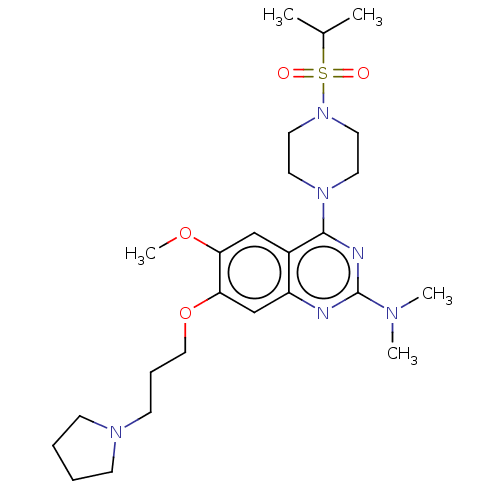 (CHEMBL4287984) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Biology Curated by ChEMBL | Assay Description Antagonist activity at human TLR9 expressed in HEK-Blue cells assessed as reduction in CpGB-induced NF-kappaB levels after 24 hrs by spectrophotometr... | Eur J Med Chem 159: 187-205 (2018) Article DOI: 10.1016/j.ejmech.2018.09.058 BindingDB Entry DOI: 10.7270/Q2C2504D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toll-like receptor 9 (Homo sapiens (Human)) | BDBM50467768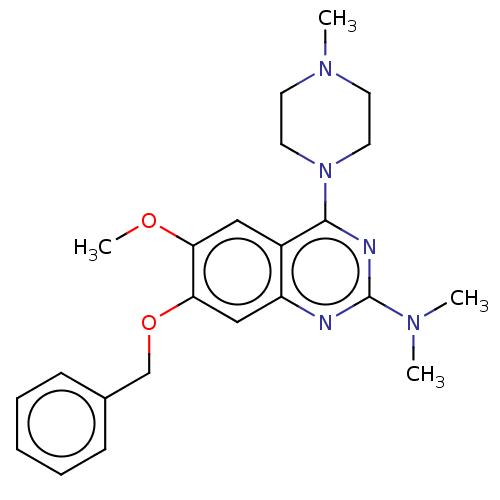 (CHEMBL4284406) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Biology Curated by ChEMBL | Assay Description Antagonist activity at human TLR9 expressed in HEK-Blue cells assessed as reduction in CpGB-induced NF-kappaB levels after 24 hrs by spectrophotometr... | Eur J Med Chem 159: 187-205 (2018) Article DOI: 10.1016/j.ejmech.2018.09.058 BindingDB Entry DOI: 10.7270/Q2C2504D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toll-like receptor 7 (Homo sapiens (Human)) | BDBM50568549 (CHEMBL4853223) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at TLR7 (unknown origin) | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112978 BindingDB Entry DOI: 10.7270/Q2W95DZ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toll-like receptor 7 (Homo sapiens (Human)) | BDBM50568548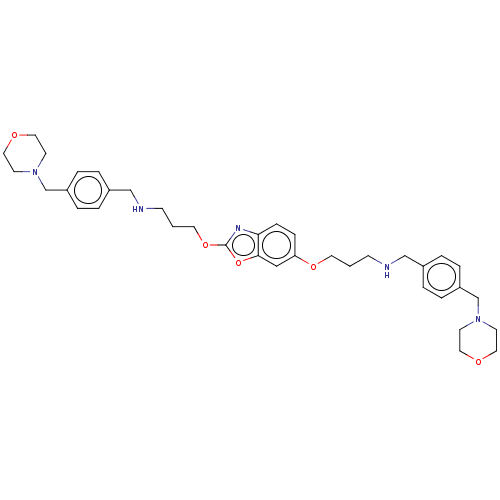 (CHEMBL4878190) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at TLR7 (unknown origin) | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112978 BindingDB Entry DOI: 10.7270/Q2W95DZ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toll-like receptor 9 (Homo sapiens (Human)) | BDBM50467779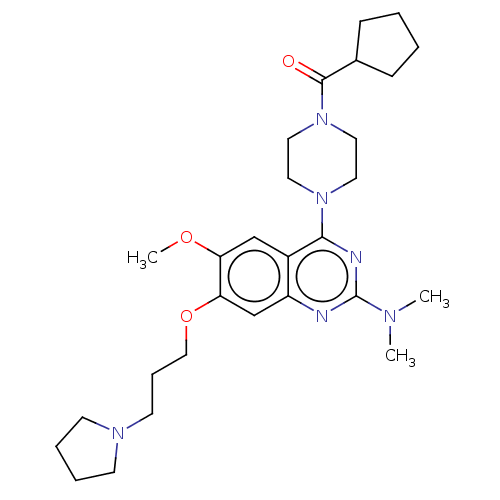 (CHEMBL4291391) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Biology Curated by ChEMBL | Assay Description Antagonist activity at human TLR9 expressed in HEK-Blue cells assessed as reduction in CpGB-induced NF-kappaB levels after 24 hrs by spectrophotometr... | Eur J Med Chem 159: 187-205 (2018) Article DOI: 10.1016/j.ejmech.2018.09.058 BindingDB Entry DOI: 10.7270/Q2C2504D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toll-like receptor 7 (Homo sapiens (Human)) | BDBM50568550 (CHEMBL4855190) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at TLR7 (unknown origin) | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112978 BindingDB Entry DOI: 10.7270/Q2W95DZ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toll-like receptor 7 (Homo sapiens (Human)) | BDBM50467786 (CHEMBL4281194) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Biology Curated by ChEMBL | Assay Description Antagonist activity at human TLR7 expressed in HEK-Blue cells assessed as reduction in CL264-induced NF-kappaB levels after 24 hrs by spectrophotomet... | Eur J Med Chem 159: 187-205 (2018) Article DOI: 10.1016/j.ejmech.2018.09.058 BindingDB Entry DOI: 10.7270/Q2C2504D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toll-like receptor 7 (Homo sapiens (Human)) | BDBM50467786 (CHEMBL4281194) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at TLR7 (unknown origin) | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112978 BindingDB Entry DOI: 10.7270/Q2W95DZ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 151 total ) | Next | Last >> |