

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thioredoxin glutathione reductase (Schistosoma mansoni) | BDBM50049873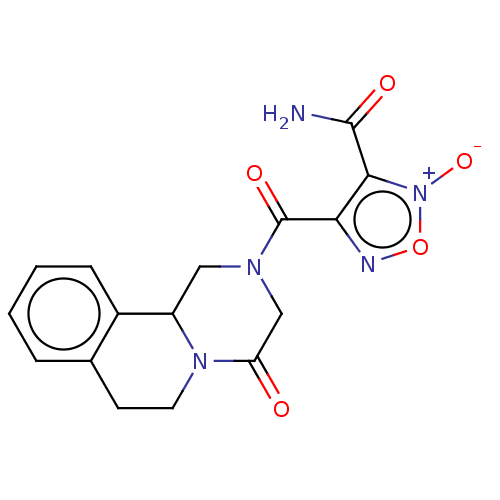 (CHEMBL3322287) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ of Torino Curated by ChEMBL | Assay Description Inhibition of Schistosoma mansoni recombinant thioredoxin glutathione reductase assessed as 5-thio-2nitrobezoic acid formation after 3 mins | Eur J Med Chem 84: 135-45 (2014) Article DOI: 10.1016/j.ejmech.2014.07.007 BindingDB Entry DOI: 10.7270/Q21G0NW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thioredoxin glutathione reductase (Schistosoma mansoni) | BDBM50049868 (CHEMBL3322292) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ of Torino Curated by ChEMBL | Assay Description Inhibition of Schistosoma mansoni recombinant thioredoxin glutathione reductase assessed as 5-thio-2nitrobezoic acid formation after 3 mins | Eur J Med Chem 84: 135-45 (2014) Article DOI: 10.1016/j.ejmech.2014.07.007 BindingDB Entry DOI: 10.7270/Q21G0NW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thioredoxin glutathione reductase (Schistosoma mansoni) | BDBM50049874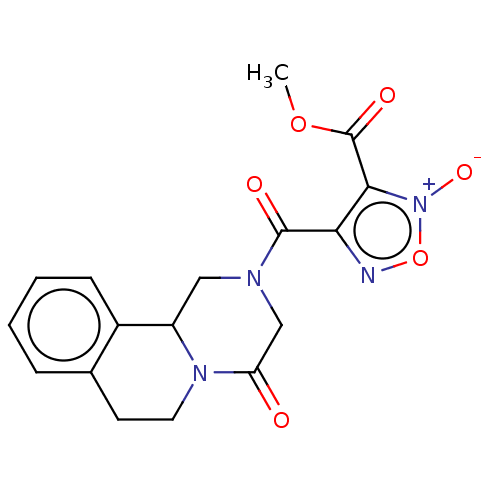 (CHEMBL3322286) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 148 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ of Torino Curated by ChEMBL | Assay Description Inhibition of Schistosoma mansoni recombinant thioredoxin glutathione reductase assessed as 5-thio-2nitrobezoic acid formation after 3 mins | Eur J Med Chem 84: 135-45 (2014) Article DOI: 10.1016/j.ejmech.2014.07.007 BindingDB Entry DOI: 10.7270/Q21G0NW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thioredoxin glutathione reductase (Schistosoma mansoni) | BDBM50049872 (CHEMBL3322288) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 316 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ of Torino Curated by ChEMBL | Assay Description Inhibition of Schistosoma mansoni recombinant thioredoxin glutathione reductase assessed as 5-thio-2nitrobezoic acid formation after 3 mins | Eur J Med Chem 84: 135-45 (2014) Article DOI: 10.1016/j.ejmech.2014.07.007 BindingDB Entry DOI: 10.7270/Q21G0NW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thioredoxin glutathione reductase (Schistosoma mansoni) | BDBM50049867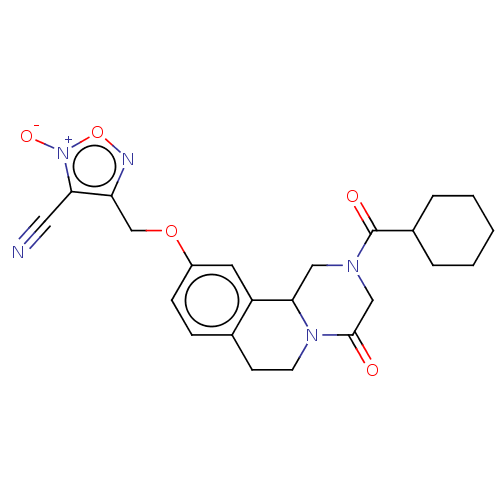 (CHEMBL3322293) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ of Torino Curated by ChEMBL | Assay Description Inhibition of Schistosoma mansoni recombinant thioredoxin glutathione reductase assessed as 5-thio-2nitrobezoic acid formation after 3 mins | Eur J Med Chem 84: 135-45 (2014) Article DOI: 10.1016/j.ejmech.2014.07.007 BindingDB Entry DOI: 10.7270/Q21G0NW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM50464816 (CHEMBL4285001) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Curated by ChEMBL | Assay Description Inhibition of FLAG-tagged HDAC2 (unknown origin) expressed in HEK293T cells using acetylated lysine side chain as substrate after 1 hr by colorimetri... | Eur J Med Chem 144: 612-625 (2018) Article DOI: 10.1016/j.ejmech.2017.12.047 BindingDB Entry DOI: 10.7270/Q2KP84T7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM50464815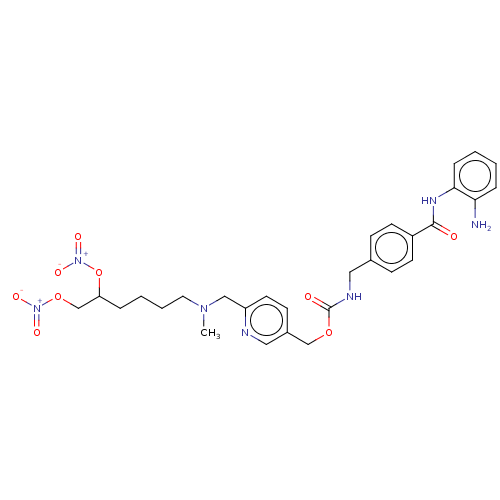 (CHEMBL4281580) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Curated by ChEMBL | Assay Description Inhibition of FLAG-tagged HDAC2 (unknown origin) expressed in HEK293T cells using acetylated lysine side chain as substrate after 1 hr by colorimetri... | Eur J Med Chem 144: 612-625 (2018) Article DOI: 10.1016/j.ejmech.2017.12.047 BindingDB Entry DOI: 10.7270/Q2KP84T7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM19410 (CHEMBL27759 | MS-275 | US11377423, MS-275 | US1167...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 740 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Curated by ChEMBL | Assay Description Inhibition of FLAG-tagged HDAC2 (unknown origin) expressed in HEK293T cells using acetylated lysine side chain as substrate after 1 hr by colorimetri... | Eur J Med Chem 144: 612-625 (2018) Article DOI: 10.1016/j.ejmech.2017.12.047 BindingDB Entry DOI: 10.7270/Q2KP84T7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50464816 (CHEMBL4285001) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Curated by ChEMBL | Assay Description Inhibition of human FLAG-tagged HDAC1 expressed in HEK293T cells using acetylated lysine side chain as substrate after 1 hr by colorimetric detection... | Eur J Med Chem 144: 612-625 (2018) Article DOI: 10.1016/j.ejmech.2017.12.047 BindingDB Entry DOI: 10.7270/Q2KP84T7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50464815 (CHEMBL4281580) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Curated by ChEMBL | Assay Description Inhibition of human FLAG-tagged HDAC1 expressed in HEK293T cells using acetylated lysine side chain as substrate after 1 hr by colorimetric detection... | Eur J Med Chem 144: 612-625 (2018) Article DOI: 10.1016/j.ejmech.2017.12.047 BindingDB Entry DOI: 10.7270/Q2KP84T7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM19410 (CHEMBL27759 | MS-275 | US11377423, MS-275 | US1167...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Curated by ChEMBL | Assay Description Inhibition of human FLAG-tagged HDAC1 expressed in HEK293T cells using acetylated lysine side chain as substrate after 1 hr by colorimetric detection... | Eur J Med Chem 144: 612-625 (2018) Article DOI: 10.1016/j.ejmech.2017.12.047 BindingDB Entry DOI: 10.7270/Q2KP84T7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM50464816 (CHEMBL4285001) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Curated by ChEMBL | Assay Description Inhibition of human FLAG-tagged HDAC3 expressed in HEK293T cells using acetylated lysine side chain as substrate after 1 hr by colorimetric detection... | Eur J Med Chem 144: 612-625 (2018) Article DOI: 10.1016/j.ejmech.2017.12.047 BindingDB Entry DOI: 10.7270/Q2KP84T7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM50464815 (CHEMBL4281580) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Curated by ChEMBL | Assay Description Inhibition of human FLAG-tagged HDAC3 expressed in HEK293T cells using acetylated lysine side chain as substrate after 1 hr by colorimetric detection... | Eur J Med Chem 144: 612-625 (2018) Article DOI: 10.1016/j.ejmech.2017.12.047 BindingDB Entry DOI: 10.7270/Q2KP84T7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM19410 (CHEMBL27759 | MS-275 | US11377423, MS-275 | US1167...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Curated by ChEMBL | Assay Description Inhibition of human FLAG-tagged HDAC3 expressed in HEK293T cells using acetylated lysine side chain as substrate after 1 hr by colorimetric detection... | Eur J Med Chem 144: 612-625 (2018) Article DOI: 10.1016/j.ejmech.2017.12.047 BindingDB Entry DOI: 10.7270/Q2KP84T7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thioredoxin glutathione reductase (Schistosoma mansoni) | BDBM50038412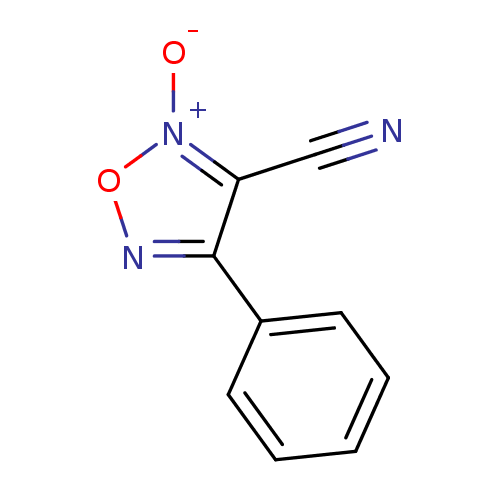 (2-Oxy-4-phenyl-furazan-3-carbonitrile | 3-cyano-4-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ of Torino Curated by ChEMBL | Assay Description Inhibition of Schistosoma mansoni recombinant thioredoxin glutathione reductase assessed as 5-thio-2nitrobezoic acid formation after 3 mins | Eur J Med Chem 84: 135-45 (2014) Article DOI: 10.1016/j.ejmech.2014.07.007 BindingDB Entry DOI: 10.7270/Q21G0NW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thioredoxin glutathione reductase (Schistosoma mansoni) | BDBM50049864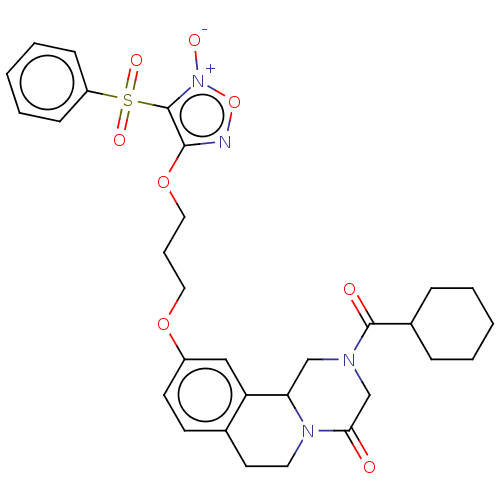 (CHEMBL3322356) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ of Torino Curated by ChEMBL | Assay Description Inhibition of Schistosoma mansoni recombinant thioredoxin glutathione reductase assessed as 5-thio-2nitrobezoic acid formation after 3 mins | Eur J Med Chem 84: 135-45 (2014) Article DOI: 10.1016/j.ejmech.2014.07.007 BindingDB Entry DOI: 10.7270/Q21G0NW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50250181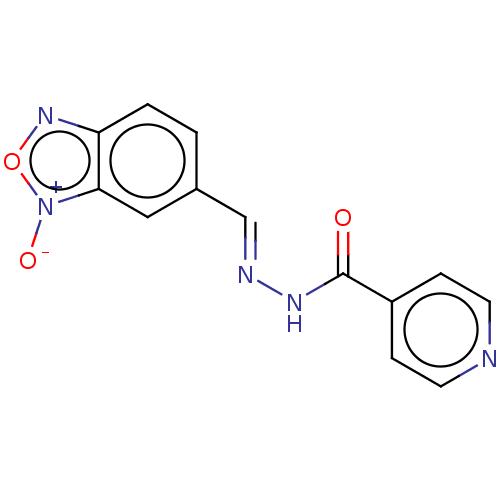 (CHEMBL2338421 | N''-(5-Benzofuroxanylmethylidene)I...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.53E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
São Paulo State University (UNESP) , Institute of Chemistry, Araraquara 14800060, Brazil. Curated by ChEMBL | Assay Description Inhibition of CYP2C19 in human liver microsomes in presence of NADPH by LC-MS/MS analysis | J Med Chem 60: 8647-8660 (2017) Article DOI: 10.1021/acs.jmedchem.7b01332 BindingDB Entry DOI: 10.7270/Q20G3NK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50250181 (CHEMBL2338421 | N''-(5-Benzofuroxanylmethylidene)I...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
São Paulo State University (UNESP) , Institute of Chemistry, Araraquara 14800060, Brazil. Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes in presence of NADPH by LC-MS/MS analysis | J Med Chem 60: 8647-8660 (2017) Article DOI: 10.1021/acs.jmedchem.7b01332 BindingDB Entry DOI: 10.7270/Q20G3NK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2B6 (Homo sapiens (Human)) | BDBM50250181 (CHEMBL2338421 | N''-(5-Benzofuroxanylmethylidene)I...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
São Paulo State University (UNESP) , Institute of Chemistry, Araraquara 14800060, Brazil. Curated by ChEMBL | Assay Description Inhibition of CYP2B6 in human liver microsomes in presence of NADPH by LC-MS/MS analysis | J Med Chem 60: 8647-8660 (2017) Article DOI: 10.1021/acs.jmedchem.7b01332 BindingDB Entry DOI: 10.7270/Q20G3NK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C8 (Homo sapiens (Human)) | BDBM50250181 (CHEMBL2338421 | N''-(5-Benzofuroxanylmethylidene)I...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
São Paulo State University (UNESP) , Institute of Chemistry, Araraquara 14800060, Brazil. Curated by ChEMBL | Assay Description Inhibition of CYP2C8 in human liver microsomes in presence of NADPH by LC-MS/MS analysis | J Med Chem 60: 8647-8660 (2017) Article DOI: 10.1021/acs.jmedchem.7b01332 BindingDB Entry DOI: 10.7270/Q20G3NK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50353899 (CHEMBL1829095) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Torino Curated by ChEMBL | Assay Description Antagonist activity at thromboxane receptor in human blood assessed as inhibition of U-46619-induced effect | Bioorg Med Chem 19: 5852-60 (2011) Article DOI: 10.1016/j.bmc.2011.08.018 BindingDB Entry DOI: 10.7270/Q29P321Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50353898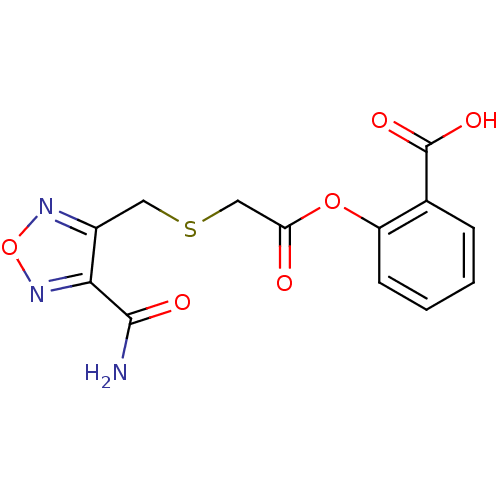 (CHEMBL1829096) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Torino Curated by ChEMBL | Assay Description Antagonist activity at thromboxane receptor in human blood assessed as inhibition of U-46619-induced effect | Bioorg Med Chem 19: 5852-60 (2011) Article DOI: 10.1016/j.bmc.2011.08.018 BindingDB Entry DOI: 10.7270/Q29P321Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thioredoxin glutathione reductase (Schistosoma mansoni) | BDBM50049863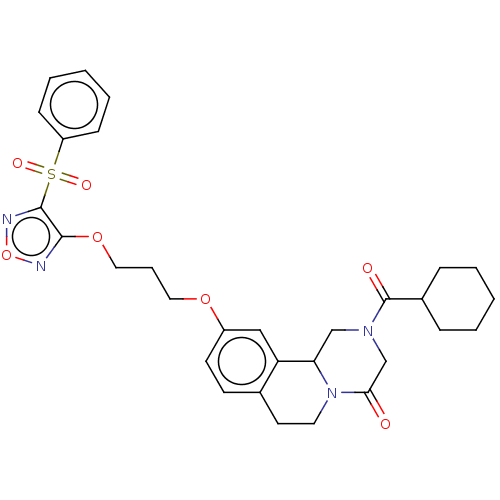 (CHEMBL3322357) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ of Torino Curated by ChEMBL | Assay Description Inhibition of Schistosoma mansoni recombinant thioredoxin glutathione reductase assessed as 5-thio-2nitrobezoic acid formation after 3 mins | Eur J Med Chem 84: 135-45 (2014) Article DOI: 10.1016/j.ejmech.2014.07.007 BindingDB Entry DOI: 10.7270/Q21G0NW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thioredoxin glutathione reductase (Schistosoma mansoni) | BDBM50049865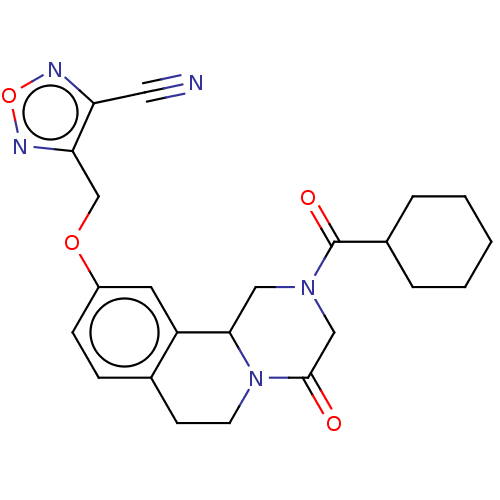 (CHEMBL3322355) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ of Torino Curated by ChEMBL | Assay Description Inhibition of Schistosoma mansoni recombinant thioredoxin glutathione reductase assessed as 5-thio-2nitrobezoic acid formation after 3 mins | Eur J Med Chem 84: 135-45 (2014) Article DOI: 10.1016/j.ejmech.2014.07.007 BindingDB Entry DOI: 10.7270/Q21G0NW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thioredoxin glutathione reductase (Schistosoma mansoni) | BDBM50049866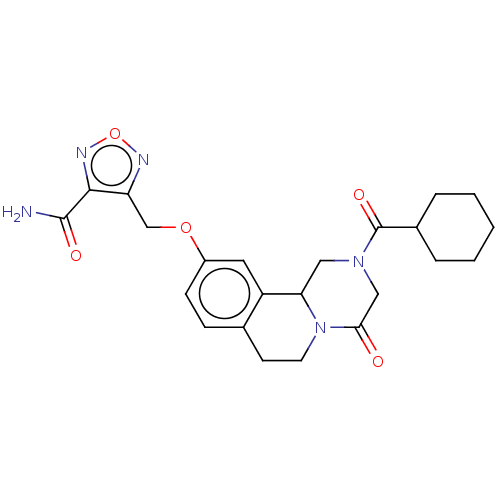 (CHEMBL3322294) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ of Torino Curated by ChEMBL | Assay Description Inhibition of Schistosoma mansoni recombinant thioredoxin glutathione reductase assessed as 5-thio-2nitrobezoic acid formation after 3 mins | Eur J Med Chem 84: 135-45 (2014) Article DOI: 10.1016/j.ejmech.2014.07.007 BindingDB Entry DOI: 10.7270/Q21G0NW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thioredoxin glutathione reductase (Schistosoma mansoni) | BDBM50049869 (CHEMBL3322291) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ of Torino Curated by ChEMBL | Assay Description Inhibition of Schistosoma mansoni recombinant thioredoxin glutathione reductase assessed as 5-thio-2nitrobezoic acid formation after 3 mins | Eur J Med Chem 84: 135-45 (2014) Article DOI: 10.1016/j.ejmech.2014.07.007 BindingDB Entry DOI: 10.7270/Q21G0NW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thioredoxin glutathione reductase (Schistosoma mansoni) | BDBM50049870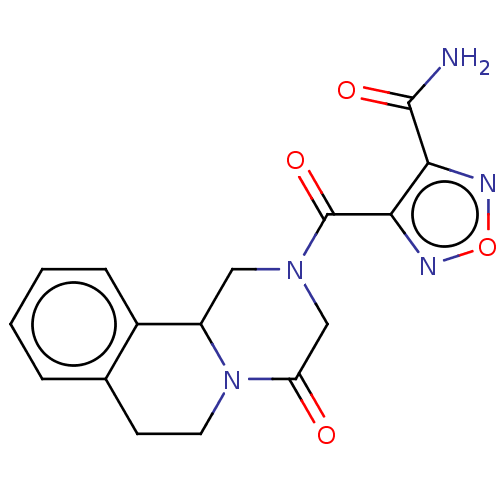 (CHEMBL3322290) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ of Torino Curated by ChEMBL | Assay Description Inhibition of Schistosoma mansoni recombinant thioredoxin glutathione reductase assessed as 5-thio-2nitrobezoic acid formation after 3 mins | Eur J Med Chem 84: 135-45 (2014) Article DOI: 10.1016/j.ejmech.2014.07.007 BindingDB Entry DOI: 10.7270/Q21G0NW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thioredoxin glutathione reductase (Schistosoma mansoni) | BDBM50049871 (CHEMBL3322289) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ of Torino Curated by ChEMBL | Assay Description Inhibition of Schistosoma mansoni recombinant thioredoxin glutathione reductase assessed as 5-thio-2nitrobezoic acid formation after 3 mins | Eur J Med Chem 84: 135-45 (2014) Article DOI: 10.1016/j.ejmech.2014.07.007 BindingDB Entry DOI: 10.7270/Q21G0NW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Multidrug resistance-associated protein 1 (Homo sapiens (Human)) | BDBM50324376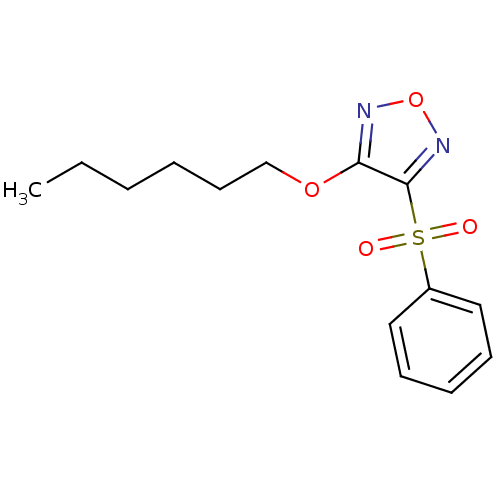 (3-Hexyloxy-4-phenylsulfonylfurazan | CHEMBL1215715) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Universita degli Studi di Torino Curated by ChEMBL | Assay Description Inhibition of MRP1 expressed in MDCK cells assessed as calcein AM accumulation by fluorescence assay | J Med Chem 53: 5467-75 (2010) Article DOI: 10.1021/jm100066y BindingDB Entry DOI: 10.7270/Q2JH3MC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Multidrug resistance-associated protein 1 (Homo sapiens (Human)) | BDBM50324349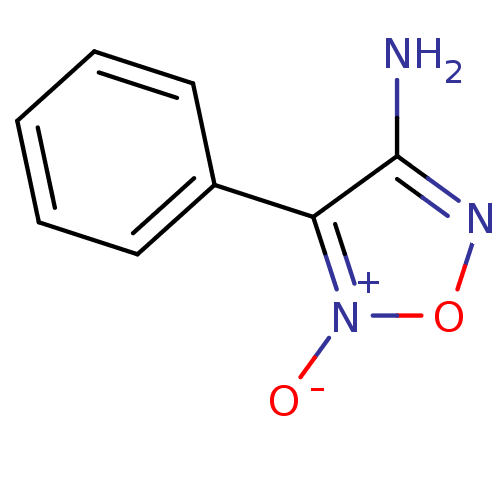 (4-amino-3-phenyl-1,2,5-oxadiazole 2-oxide | 5-Oxy-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Universita degli Studi di Torino Curated by ChEMBL | Assay Description Inhibition of MRP1 expressed in MDCK cells assessed as calcein AM accumulation by fluorescence assay | J Med Chem 53: 5467-75 (2010) Article DOI: 10.1021/jm100066y BindingDB Entry DOI: 10.7270/Q2JH3MC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Multidrug resistance-associated protein 1 (Homo sapiens (Human)) | BDBM50324355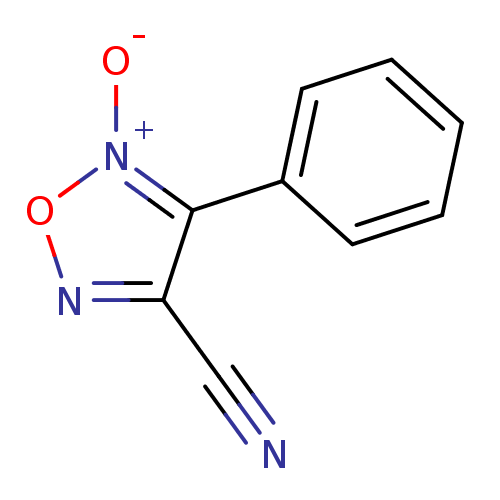 (4-cyano-3-phenyl-1,2,5-oxadiazole 2-oxide | 5-Oxy-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 8.50E+4 | n/a | n/a | n/a | n/a |
Universita degli Studi di Torino Curated by ChEMBL | Assay Description Inhibition of MRP1 expressed in MDCK cells assessed as calcein AM accumulation by fluorescence assay | J Med Chem 53: 5467-75 (2010) Article DOI: 10.1021/jm100066y BindingDB Entry DOI: 10.7270/Q2JH3MC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Multidrug resistance-associated protein 1 (Homo sapiens (Human)) | BDBM50324366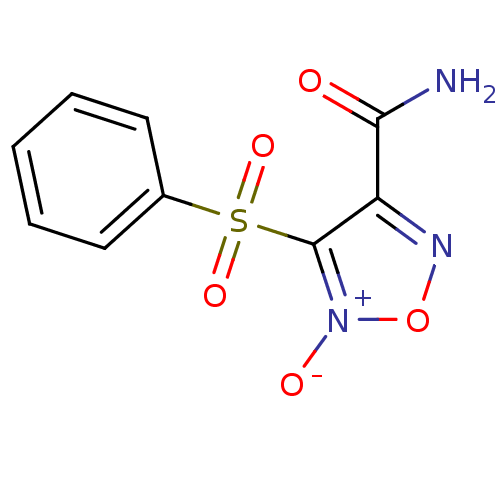 (3-Phenylsulfonylfuroxan-4-carboxamide | CHEMBL1215...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 8.58E+4 | n/a | n/a | n/a | n/a |
Universita degli Studi di Torino Curated by ChEMBL | Assay Description Induction of MRP1 expressed in MDCK cells assessed as calcein AM accumulation by fluorescence assay | J Med Chem 53: 5467-75 (2010) Article DOI: 10.1021/jm100066y BindingDB Entry DOI: 10.7270/Q2JH3MC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Multidrug resistance-associated protein 1 (Homo sapiens (Human)) | BDBM50324350 (2-Oxy-4-phenyl-furazan-3-ylamine | 3-amino-4-pheny...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Universita degli Studi di Torino Curated by ChEMBL | Assay Description Inhibition of MRP1 expressed in MDCK cells assessed as calcein AM accumulation by fluorescence assay | J Med Chem 53: 5467-75 (2010) Article DOI: 10.1021/jm100066y BindingDB Entry DOI: 10.7270/Q2JH3MC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Multidrug resistance-associated protein 1 (Homo sapiens (Human)) | BDBM50324362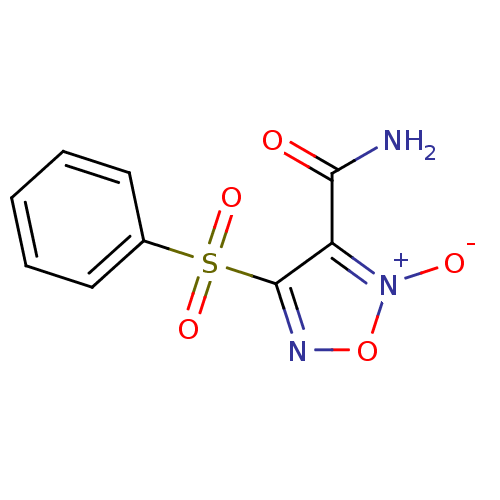 (4-Phenylsulfonylfuroxan-3-carboxamide | CHEMBL1215...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a |
Universita degli Studi di Torino Curated by ChEMBL | Assay Description Inhibition of MRP1 expressed in MDCK cells assessed as calcein AM accumulation by fluorescence assay | J Med Chem 53: 5467-75 (2010) Article DOI: 10.1021/jm100066y BindingDB Entry DOI: 10.7270/Q2JH3MC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Multidrug resistance-associated protein 1 (Homo sapiens (Human)) | BDBM50324368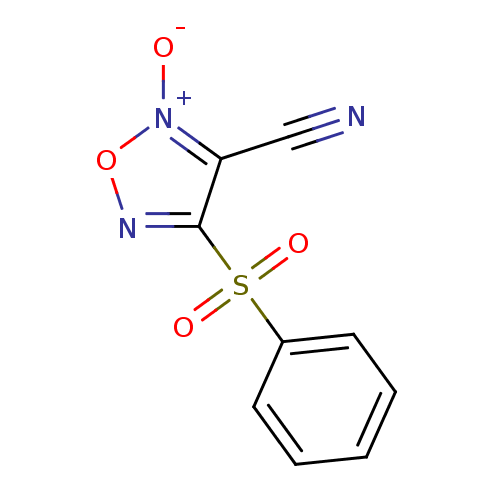 (4-Phenylsulfonylfuroxan-3-carbonitrile | CHEMBL121...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 7.20E+4 | n/a | n/a | n/a | n/a |
Universita degli Studi di Torino Curated by ChEMBL | Assay Description Induction of MRP1 expressed in MDCK cells assessed as calcein AM accumulation by fluorescence assay | J Med Chem 53: 5467-75 (2010) Article DOI: 10.1021/jm100066y BindingDB Entry DOI: 10.7270/Q2JH3MC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Multidrug resistance-associated protein 1 (Homo sapiens (Human)) | BDBM50324367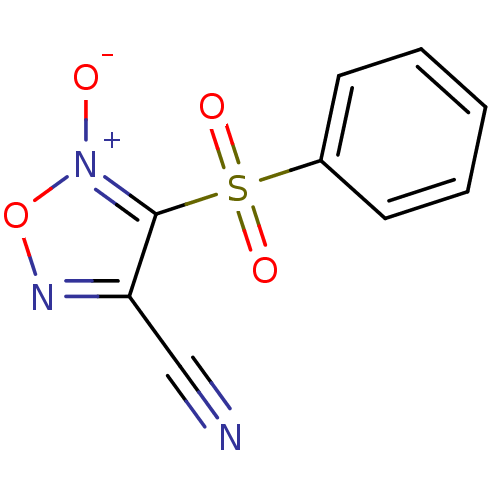 (3-Phenylsulfonylfuroxan-4-carbonitrile | CHEMBL121...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.95E+4 | n/a | n/a | n/a | n/a |
Universita degli Studi di Torino Curated by ChEMBL | Assay Description Induction of MRP1 expressed in MDCK cells assessed as calcein AM accumulation by fluorescence assay | J Med Chem 53: 5467-75 (2010) Article DOI: 10.1021/jm100066y BindingDB Entry DOI: 10.7270/Q2JH3MC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Multidrug resistance-associated protein 1 (Homo sapiens (Human)) | BDBM50324378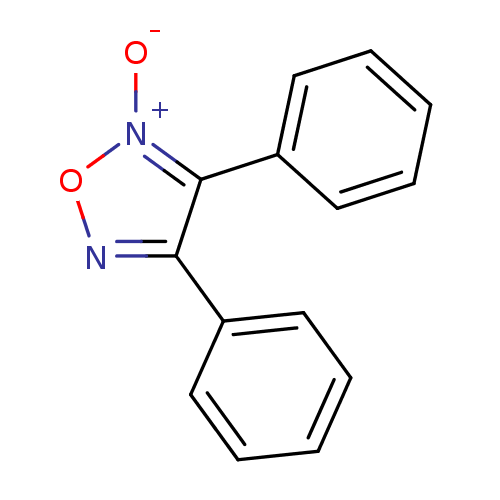 (3,4-diphenyl-1,2,5-oxadiazole 2-oxide | CHEMBL1212...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Universita degli Studi di Torino Curated by ChEMBL | Assay Description Inhibition of MRP1 expressed in MDCK cells assessed as calcein AM accumulation by fluorescence assay | J Med Chem 53: 5467-75 (2010) Article DOI: 10.1021/jm100066y BindingDB Entry DOI: 10.7270/Q2JH3MC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Multidrug resistance-associated protein 1 (Homo sapiens (Human)) | BDBM50324379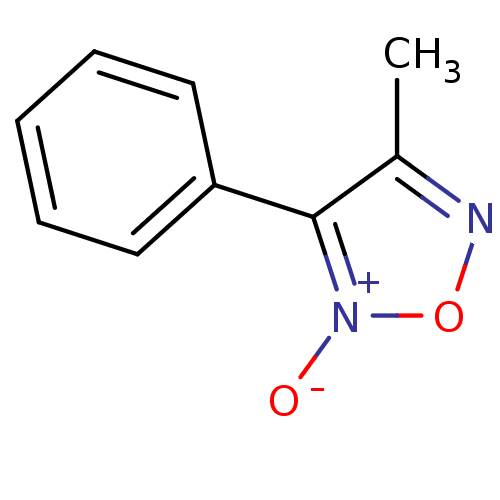 (4-methyl-3-phenyl-1,2,5-oxadiazole 2-oxide | CHEMB...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Universita degli Studi di Torino Curated by ChEMBL | Assay Description Inhibition of MRP1 expressed in MDCK cells assessed as calcein AM accumulation by fluorescence assay | J Med Chem 53: 5467-75 (2010) Article DOI: 10.1021/jm100066y BindingDB Entry DOI: 10.7270/Q2JH3MC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Multidrug resistance-associated protein 1 (Homo sapiens (Human)) | BDBM50324370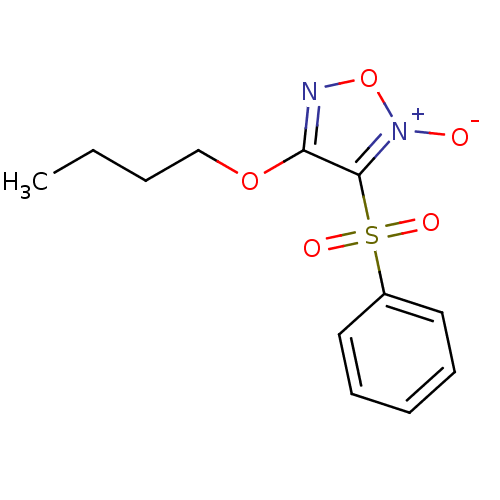 (4-Butoxy-3-phenylsulfonylfuroxan | 4-butoxy-3-(phe...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 7.61E+3 | n/a | n/a | n/a | n/a |
Universita degli Studi di Torino Curated by ChEMBL | Assay Description Induction of MRP1 expressed in MDCK cells assessed as calcein AM accumulation by fluorescence assay | J Med Chem 53: 5467-75 (2010) Article DOI: 10.1021/jm100066y BindingDB Entry DOI: 10.7270/Q2JH3MC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Multidrug resistance-associated protein 1 (Homo sapiens (Human)) | BDBM50324361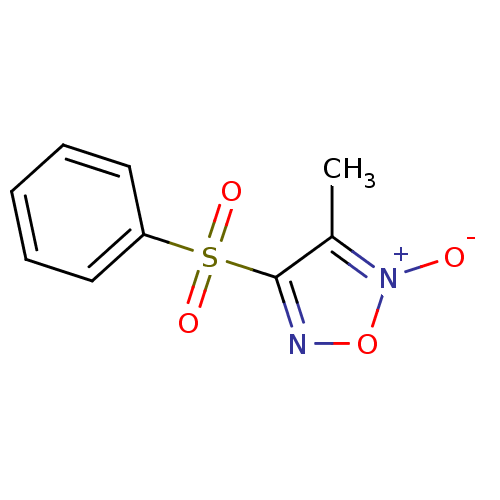 (3-methyl-4-(phenylsulfonyl)-1,2,5-oxadiazole 2-oxi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Universita degli Studi di Torino Curated by ChEMBL | Assay Description Inhibition of MRP1 expressed in MDCK cells assessed as calcein AM accumulation by fluorescence assay | J Med Chem 53: 5467-75 (2010) Article DOI: 10.1021/jm100066y BindingDB Entry DOI: 10.7270/Q2JH3MC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Multidrug resistance-associated protein 1 (Homo sapiens (Human)) | BDBM50324374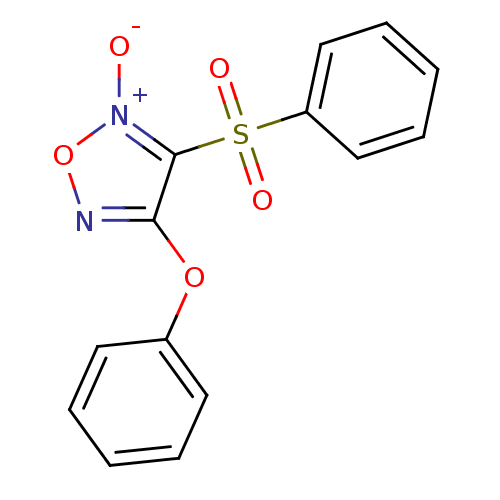 (4-Phenyloxy-3-phenylsulfonylfuroxan | CHEMBL121571...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.36E+4 | n/a | n/a | n/a | n/a |
Universita degli Studi di Torino Curated by ChEMBL | Assay Description Induction of MRP1 expressed in MDCK cells assessed as calcein AM accumulation by fluorescence assay | J Med Chem 53: 5467-75 (2010) Article DOI: 10.1021/jm100066y BindingDB Entry DOI: 10.7270/Q2JH3MC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Multidrug resistance-associated protein 1 (Homo sapiens (Human)) | BDBM50324373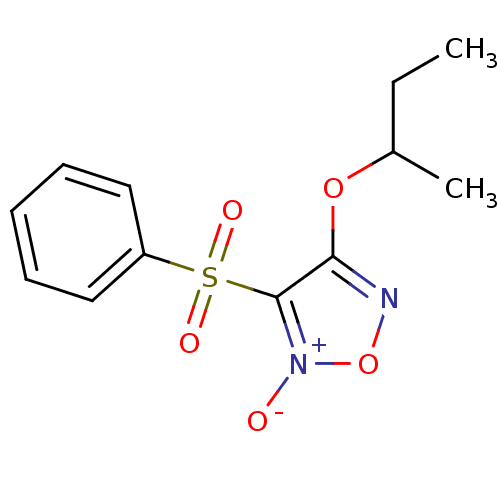 (4-Isobutoxy-3-phenylsulfonylfuroxan | 4-sec-butoxy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.60E+4 | n/a | n/a | n/a | n/a |
Universita degli Studi di Torino Curated by ChEMBL | Assay Description Induction of MRP1 expressed in MDCK cells assessed as calcein AM accumulation by fluorescence assay | J Med Chem 53: 5467-75 (2010) Article DOI: 10.1021/jm100066y BindingDB Entry DOI: 10.7270/Q2JH3MC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Multidrug resistance-associated protein 1 (Homo sapiens (Human)) | BDBM50300754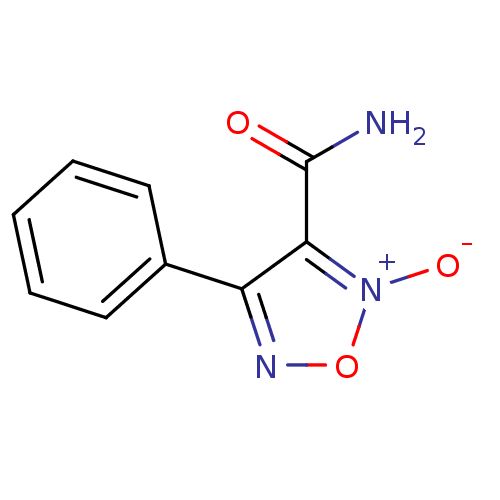 (2-Oxy-4-phenyl-furazan-3-carboxylic acid amide | 3...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Universita degli Studi di Torino Curated by ChEMBL | Assay Description Inhibition of MRP1 expressed in MDCK cells assessed as calcein AM accumulation by fluorescence assay | J Med Chem 53: 5467-75 (2010) Article DOI: 10.1021/jm100066y BindingDB Entry DOI: 10.7270/Q2JH3MC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Multidrug resistance-associated protein 1 (Homo sapiens (Human)) | BDBM50324375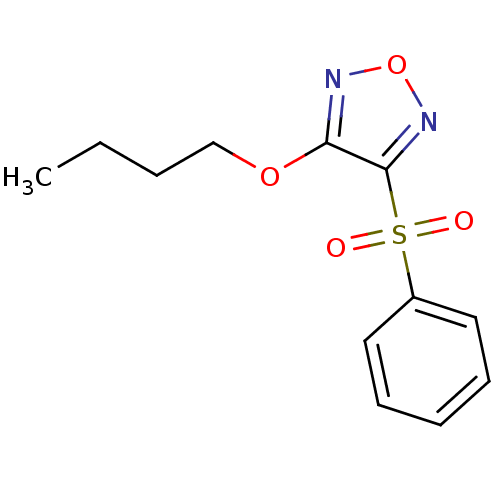 (3-Butoxy-4-phenylsulfonylfurazan | CHEMBL1215714) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Universita degli Studi di Torino Curated by ChEMBL | Assay Description Inhibition of MRP1 expressed in MDCK cells assessed as calcein AM accumulation by fluorescence assay | J Med Chem 53: 5467-75 (2010) Article DOI: 10.1021/jm100066y BindingDB Entry DOI: 10.7270/Q2JH3MC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Multidrug resistance-associated protein 1 (Homo sapiens (Human)) | BDBM50324351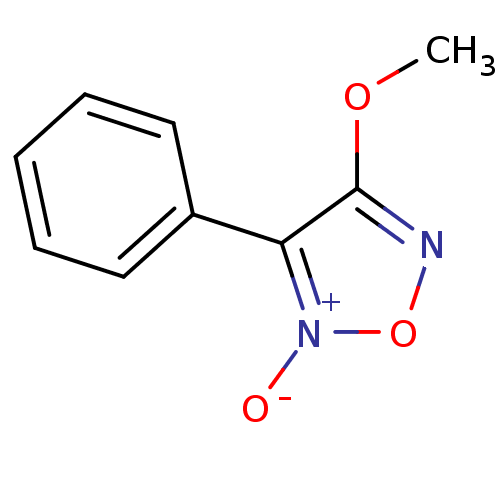 (4-methoxy-3-phenyl-1,2,5-oxadiazole 2-oxide | CHEM...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Universita degli Studi di Torino Curated by ChEMBL | Assay Description Inhibition of MRP1 expressed in MDCK cells assessed as calcein AM accumulation by fluorescence assay | J Med Chem 53: 5467-75 (2010) Article DOI: 10.1021/jm100066y BindingDB Entry DOI: 10.7270/Q2JH3MC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Multidrug resistance-associated protein 1 (Homo sapiens (Human)) | BDBM50324356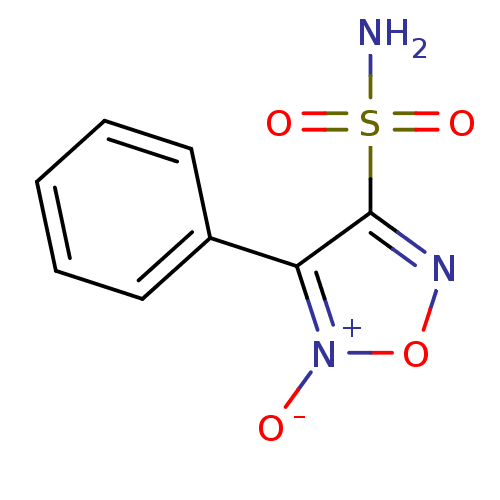 (3-phenyl-4-sulfamoyl-1,2,5-oxadiazole 2-oxide | CH...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Universita degli Studi di Torino Curated by ChEMBL | Assay Description Inhibition of MRP1 expressed in MDCK cells assessed as calcein AM accumulation by fluorescence assay | J Med Chem 53: 5467-75 (2010) Article DOI: 10.1021/jm100066y BindingDB Entry DOI: 10.7270/Q2JH3MC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Multidrug resistance-associated protein 1 (Homo sapiens (Human)) | BDBM50324357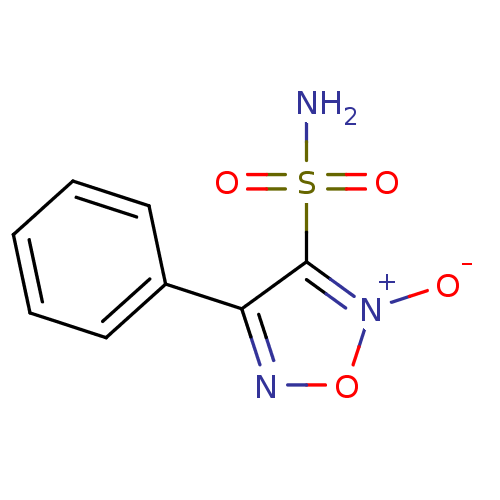 (4-phenyl-3-sulfamoyl-1,2,5-oxadiazole 2-oxide | CH...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Universita degli Studi di Torino Curated by ChEMBL | Assay Description Inhibition of MRP1 expressed in MDCK cells assessed as calcein AM accumulation by fluorescence assay | J Med Chem 53: 5467-75 (2010) Article DOI: 10.1021/jm100066y BindingDB Entry DOI: 10.7270/Q2JH3MC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Multidrug resistance-associated protein 1 (Homo sapiens (Human)) | BDBM50001285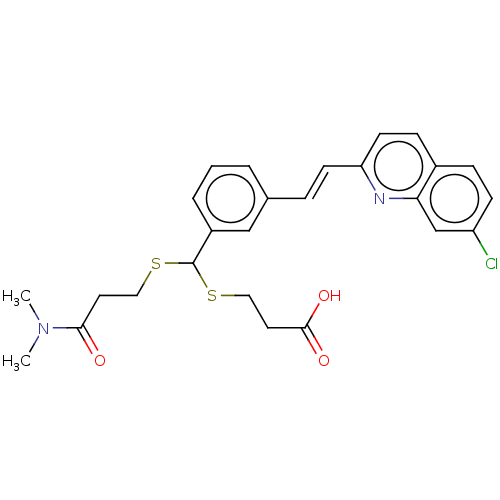 ((E)-3-((3-(2-(7-chloroquinolin-2-yl)vinyl)phenyl)(...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.85E+3 | n/a | n/a | n/a | n/a |
Universita degli Studi di Torino Curated by ChEMBL | Assay Description Inhibition of MRP1 expressed in MDCK cells assessed as calcein AM accumulation by fluorescence assay | J Med Chem 53: 5467-75 (2010) Article DOI: 10.1021/jm100066y BindingDB Entry DOI: 10.7270/Q2JH3MC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Multidrug resistance-associated protein 1 (Homo sapiens (Human)) | BDBM50324359 (3-Benzenesulfonyl-4-phenyl-furazan 2-oxide | 4-phe...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 6.40E+4 | n/a | n/a | n/a | n/a |
Universita degli Studi di Torino Curated by ChEMBL | Assay Description Inhibition of MRP1 expressed in MDCK cells assessed as calcein AM accumulation by fluorescence assay | J Med Chem 53: 5467-75 (2010) Article DOI: 10.1021/jm100066y BindingDB Entry DOI: 10.7270/Q2JH3MC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Multidrug resistance-associated protein 1 (Homo sapiens (Human)) | BDBM50324371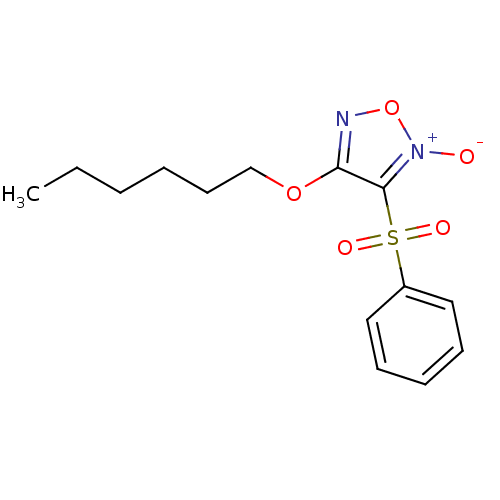 (4-Hexyloxy-3-phenylsulfonylfuroxan | CHEMBL1215644) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a |
Universita degli Studi di Torino Curated by ChEMBL | Assay Description Induction of MRP1 expressed in MDCK cells assessed as calcein AM accumulation by fluorescence assay | J Med Chem 53: 5467-75 (2010) Article DOI: 10.1021/jm100066y BindingDB Entry DOI: 10.7270/Q2JH3MC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 179 total ) | Next | Last >> |