

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chymase (Homo sapiens (Human)) | BDBM134267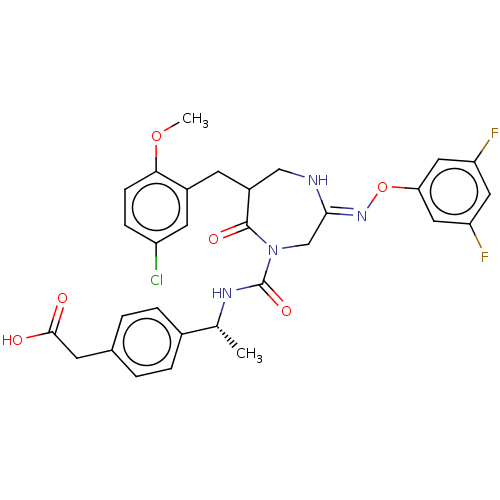 (US8846660, 41) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Daiichi Sankyo Company, Ltd. US Patent | Assay Description The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor... | US Patent US8846660 (2014) BindingDB Entry DOI: 10.7270/Q22V2DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM134262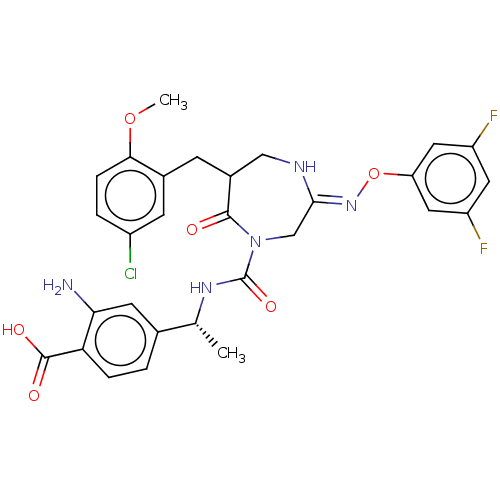 (US8846660, 30) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Daiichi Sankyo Company, Ltd. US Patent | Assay Description The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor... | US Patent US8846660 (2014) BindingDB Entry DOI: 10.7270/Q22V2DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM134278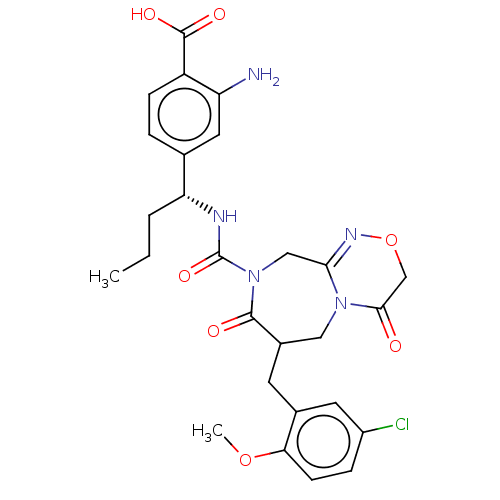 (US8846660, 96) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Daiichi Sankyo Company, Ltd. US Patent | Assay Description The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor... | US Patent US8846660 (2014) BindingDB Entry DOI: 10.7270/Q22V2DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50452609 (CHEMBL4206099) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Asubio Pharma Co., Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human chymase pre-incubated for 10 mins before Suc-Ala-Ala-Pro-Phe-MCA substrate addition and measured after 10 mins by flu... | Bioorg Med Chem Lett 28: 188-192 (2018) Article DOI: 10.1016/j.bmcl.2017.11.031 BindingDB Entry DOI: 10.7270/Q2T72M14 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM134276 (US8846660, 76) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Daiichi Sankyo Company, Ltd. US Patent | Assay Description The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor... | US Patent US8846660 (2014) BindingDB Entry DOI: 10.7270/Q22V2DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM134263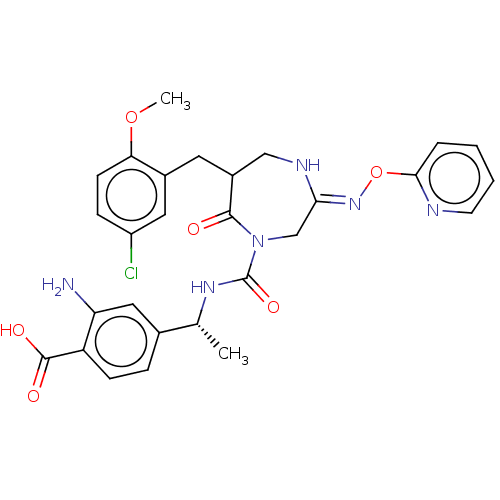 (US8846660, 31) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Daiichi Sankyo Company, Ltd. US Patent | Assay Description The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor... | US Patent US8846660 (2014) BindingDB Entry DOI: 10.7270/Q22V2DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM134261 (US8846660, 29) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Daiichi Sankyo Company, Ltd. US Patent | Assay Description The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor... | US Patent US8846660 (2014) BindingDB Entry DOI: 10.7270/Q22V2DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM134266 (US8846660, 40) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Daiichi Sankyo Company, Ltd. US Patent | Assay Description The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor... | US Patent US8846660 (2014) BindingDB Entry DOI: 10.7270/Q22V2DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50210107 (7-chloro-3-(4-chlorophenylsulfonyl)quinazoline-2,4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Asubio Pharma Co., Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant chymase | Bioorg Med Chem Lett 17: 3431-4 (2007) Article DOI: 10.1016/j.bmcl.2007.03.038 BindingDB Entry DOI: 10.7270/Q2G160H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50058973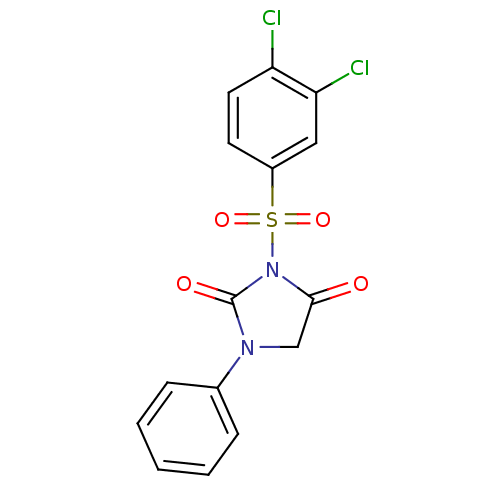 (3-(3,4-Dichloro-benzenesulfonyl)-1-phenyl-imidazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Suntory Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against human heart chymase in vitro. | J Med Chem 40: 2156-63 (1997) Article DOI: 10.1021/jm960793t BindingDB Entry DOI: 10.7270/Q2QJ7GDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM100749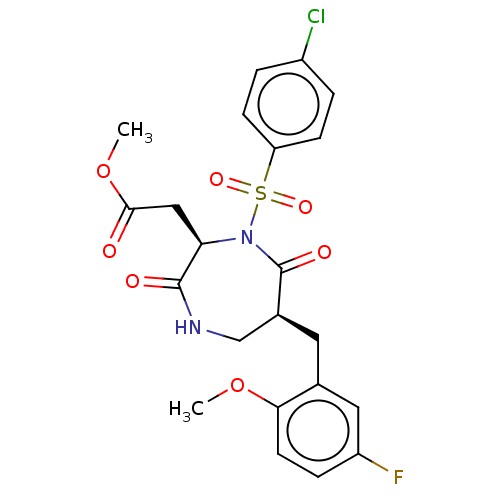 (US8507714, 214) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Company, Limited US Patent | Assay Description Inhibitory activity of the compounds for recombinant human chymase was measured by method of Pasztor et al. (Pasztor et al., Acta Biol. Hung. 42:285-... | US Patent US8507714 (2013) BindingDB Entry DOI: 10.7270/Q2H130N8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM100743 (US8507714, 67) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Company, Limited US Patent | Assay Description Inhibitory activity of the compounds for recombinant human chymase was measured by method of Pasztor et al. (Pasztor et al., Acta Biol. Hung. 42:285-... | US Patent US8507714 (2013) BindingDB Entry DOI: 10.7270/Q2H130N8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM134253 (US8846660, 103 | US8846660, 12) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Daiichi Sankyo Company, Ltd. US Patent | Assay Description The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor... | US Patent US8846660 (2014) BindingDB Entry DOI: 10.7270/Q22V2DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM134256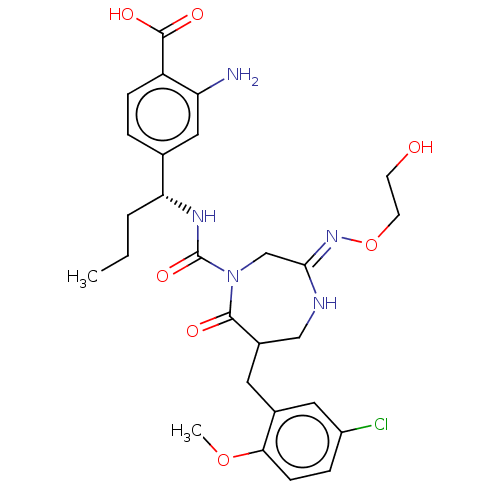 (US8846660, 18) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Daiichi Sankyo Company, Ltd. US Patent | Assay Description The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor... | US Patent US8846660 (2014) BindingDB Entry DOI: 10.7270/Q22V2DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50058984 (3-(3,4-Dichloro-benzenesulfonyl)-1-(3,4-dimethyl-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Suntory Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against human heart chymase in vitro. | J Med Chem 40: 2156-63 (1997) Article DOI: 10.1021/jm960793t BindingDB Entry DOI: 10.7270/Q2QJ7GDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM134272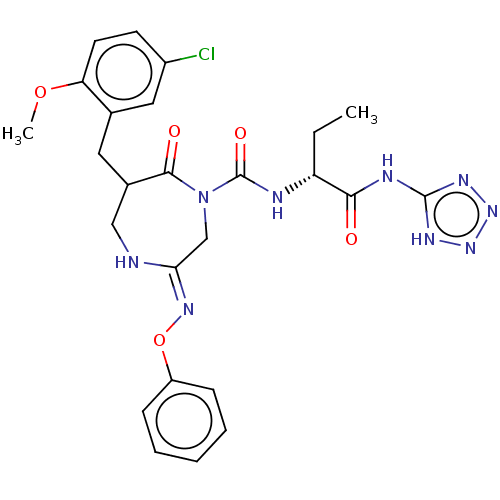 (US8846660, 58) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Daiichi Sankyo Company, Ltd. US Patent | Assay Description The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor... | US Patent US8846660 (2014) BindingDB Entry DOI: 10.7270/Q22V2DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM134260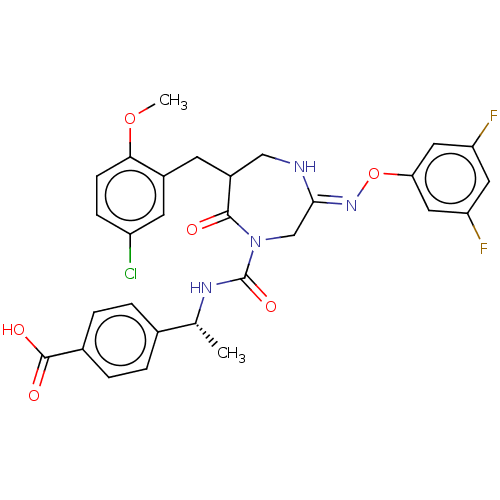 (US8846660, 27) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Daiichi Sankyo Company, Ltd. US Patent | Assay Description The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor... | US Patent US8846660 (2014) BindingDB Entry DOI: 10.7270/Q22V2DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM134269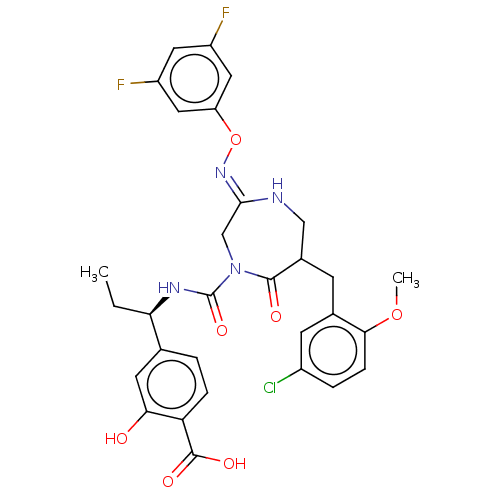 (US8846660, 44) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Daiichi Sankyo Company, Ltd. US Patent | Assay Description The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor... | US Patent US8846660 (2014) BindingDB Entry DOI: 10.7270/Q22V2DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM134268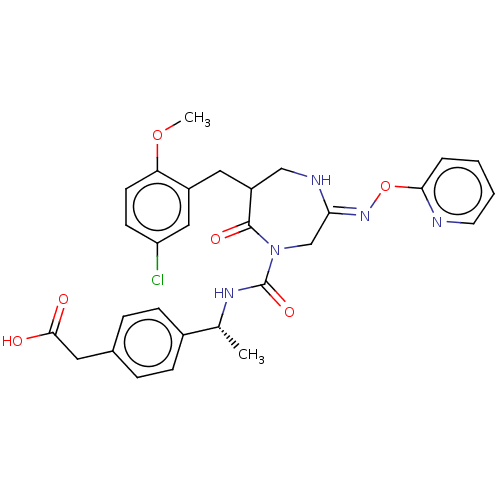 (US8846660, 42) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Daiichi Sankyo Company, Ltd. US Patent | Assay Description The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor... | US Patent US8846660 (2014) BindingDB Entry DOI: 10.7270/Q22V2DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50058990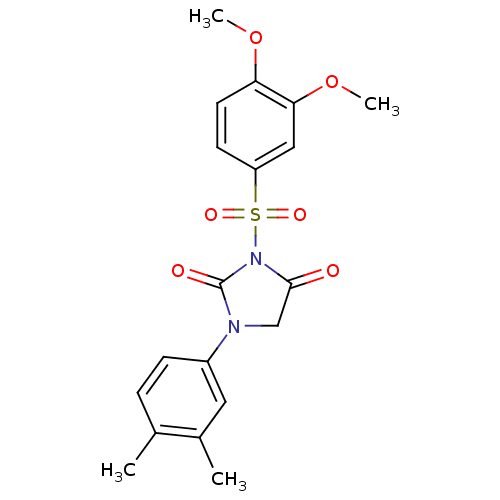 (3-(3,4-Dimethoxy-benzenesulfonyl)-1-(3,4-dimethyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Suntory Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against human heart chymase in vitro. | J Med Chem 40: 2156-63 (1997) Article DOI: 10.1021/jm960793t BindingDB Entry DOI: 10.7270/Q2QJ7GDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50210098 (6-(4,5-dichloro-2-methoxybenzyl)-4-(4-chlorophenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Asubio Pharma Co., Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant chymase | Bioorg Med Chem Lett 17: 3431-4 (2007) Article DOI: 10.1016/j.bmcl.2007.03.038 BindingDB Entry DOI: 10.7270/Q2G160H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50058996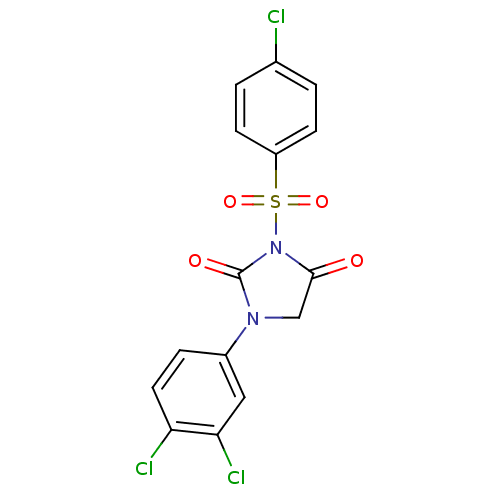 (3-(4-Chloro-benzenesulfonyl)-1-(3,4-dichloro-pheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Suntory Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against human heart chymase in vitro. | J Med Chem 40: 2156-63 (1997) Article DOI: 10.1021/jm960793t BindingDB Entry DOI: 10.7270/Q2QJ7GDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM100752 (US8507714, 269) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Company, Limited US Patent | Assay Description Inhibitory activity of the compounds for recombinant human chymase was measured by method of Pasztor et al. (Pasztor et al., Acta Biol. Hung. 42:285-... | US Patent US8507714 (2013) BindingDB Entry DOI: 10.7270/Q2H130N8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50210116 (6-(5-fluoro-2-methoxybenzyl)-4-(4-chlorophenylsulf...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Asubio Pharma Co., Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant chymase | Bioorg Med Chem Lett 17: 3431-4 (2007) Article DOI: 10.1016/j.bmcl.2007.03.038 BindingDB Entry DOI: 10.7270/Q2G160H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50210120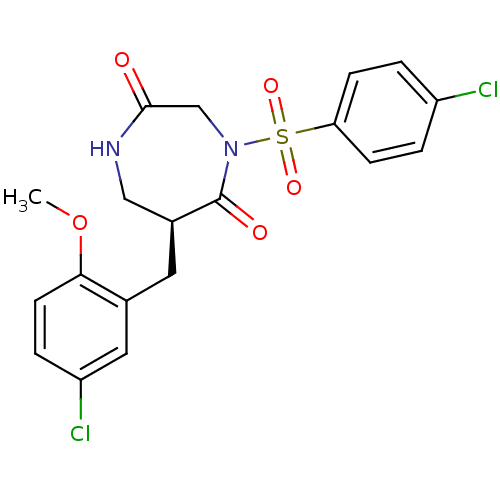 ((S)-6-(5-chloro-2-methoxybenzyl)-4-(4-chlorophenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Asubio Pharma Co., Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant chymase | Bioorg Med Chem Lett 17: 3431-4 (2007) Article DOI: 10.1016/j.bmcl.2007.03.038 BindingDB Entry DOI: 10.7270/Q2G160H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50210094 (3-((1-(4-chlorophenylsulfonyl)-3,7-dioxo-1,4-diaze...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Asubio Pharma Co., Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant chymase | Bioorg Med Chem Lett 17: 3431-4 (2007) Article DOI: 10.1016/j.bmcl.2007.03.038 BindingDB Entry DOI: 10.7270/Q2G160H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50059007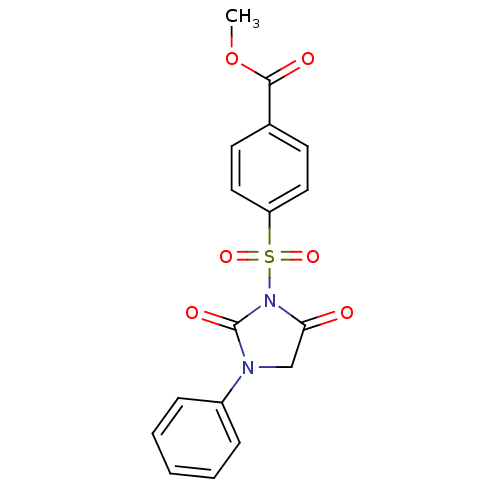 (4-(2,5-Dioxo-3-phenyl-imidazolidine-1-sulfonyl)-be...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Suntory Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against human heart chymase in vitro. | J Med Chem 40: 2156-63 (1997) Article DOI: 10.1021/jm960793t BindingDB Entry DOI: 10.7270/Q2QJ7GDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM134252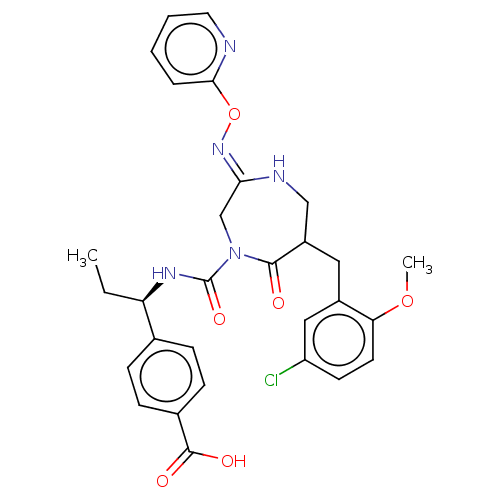 (US8846660, 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Daiichi Sankyo Company, Ltd. US Patent | Assay Description The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor... | US Patent US8846660 (2014) BindingDB Entry DOI: 10.7270/Q22V2DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM134275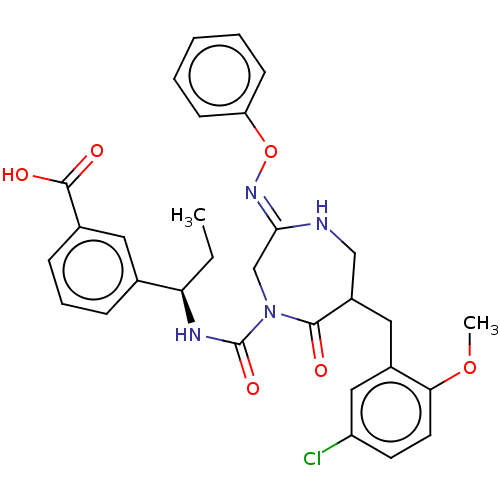 (US8846660, 73) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Daiichi Sankyo Company, Ltd. US Patent | Assay Description The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor... | US Patent US8846660 (2014) BindingDB Entry DOI: 10.7270/Q22V2DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM134264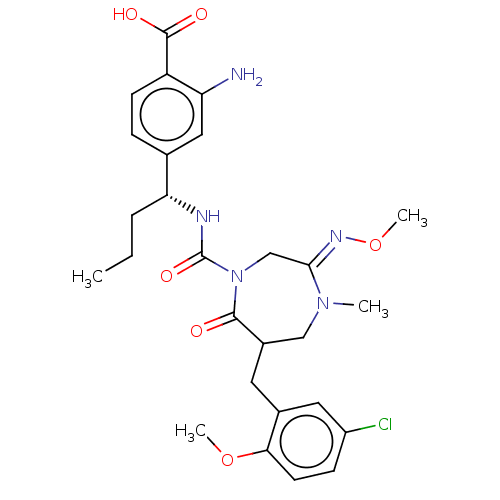 (US8846660, 34) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Daiichi Sankyo Company, Ltd. US Patent | Assay Description The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor... | US Patent US8846660 (2014) BindingDB Entry DOI: 10.7270/Q22V2DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM134273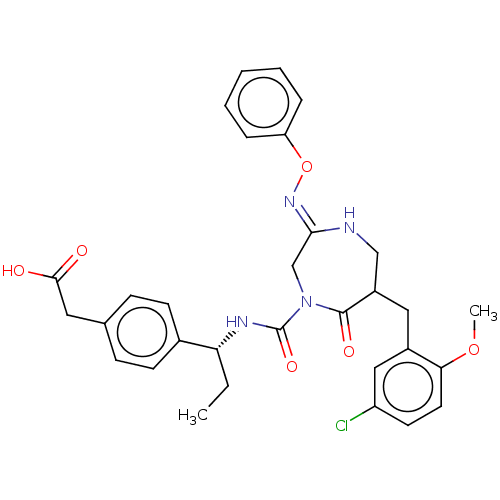 (US8846660, 64) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Daiichi Sankyo Company, Ltd. US Patent | Assay Description The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor... | US Patent US8846660 (2014) BindingDB Entry DOI: 10.7270/Q22V2DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM134270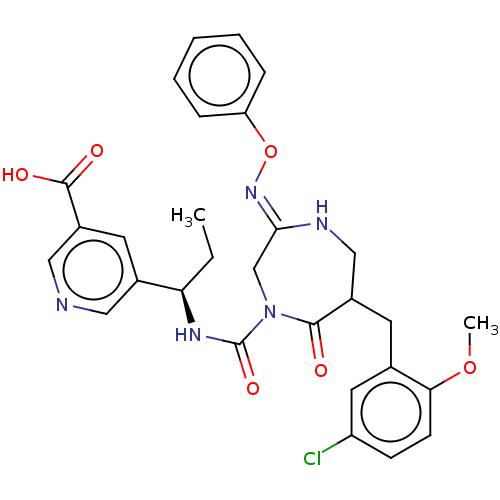 (US8846660, 49) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Daiichi Sankyo Company, Ltd. US Patent | Assay Description The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor... | US Patent US8846660 (2014) BindingDB Entry DOI: 10.7270/Q22V2DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50058964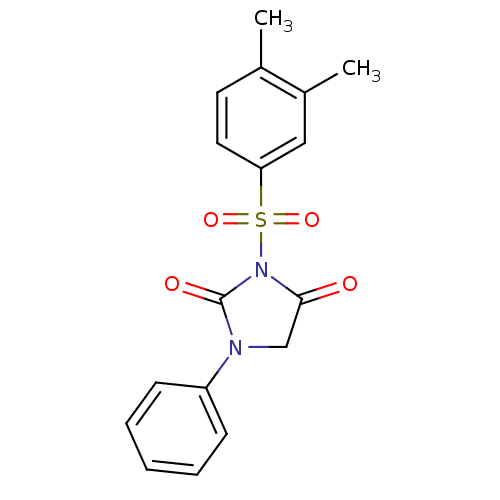 (3-(3,4-Dimethyl-benzenesulfonyl)-1-phenyl-imidazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Suntory Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against human heart chymase in vitro. | J Med Chem 40: 2156-63 (1997) Article DOI: 10.1021/jm960793t BindingDB Entry DOI: 10.7270/Q2QJ7GDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM134254 (US8846660, 14) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Daiichi Sankyo Company, Ltd. US Patent | Assay Description The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor... | US Patent US8846660 (2014) BindingDB Entry DOI: 10.7270/Q22V2DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM134255 (US8846660, 15) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Daiichi Sankyo Company, Ltd. US Patent | Assay Description The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor... | US Patent US8846660 (2014) BindingDB Entry DOI: 10.7270/Q22V2DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM134257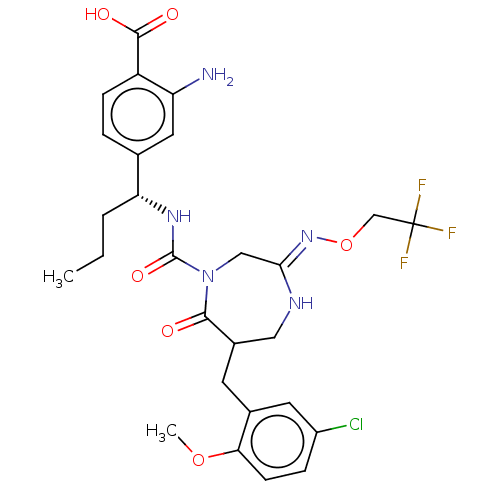 (US8846660, 20) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Daiichi Sankyo Company, Ltd. US Patent | Assay Description The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor... | US Patent US8846660 (2014) BindingDB Entry DOI: 10.7270/Q22V2DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM134280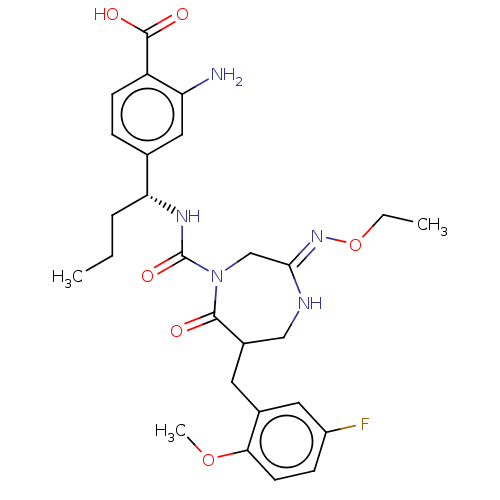 (US8846660, 108) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Daiichi Sankyo Company, Ltd. US Patent | Assay Description The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor... | US Patent US8846660 (2014) BindingDB Entry DOI: 10.7270/Q22V2DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50210743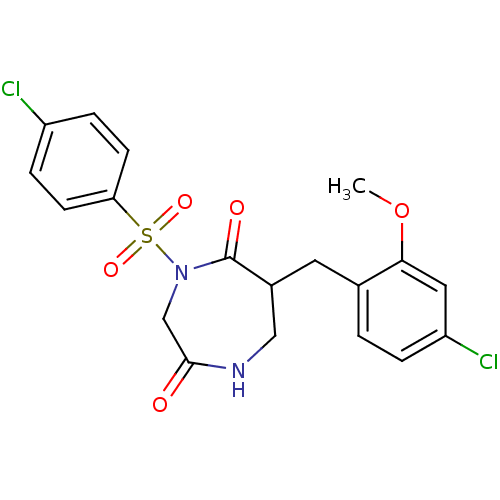 (6-(4-chloro-2-methoxybenzyl)-4-(4-chlorophenylsulf...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Asubio Pharma Co., Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant chymase | Bioorg Med Chem Lett 17: 3435-9 (2007) Article DOI: 10.1016/j.bmcl.2007.03.085 BindingDB Entry DOI: 10.7270/Q2MK6CKH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM100740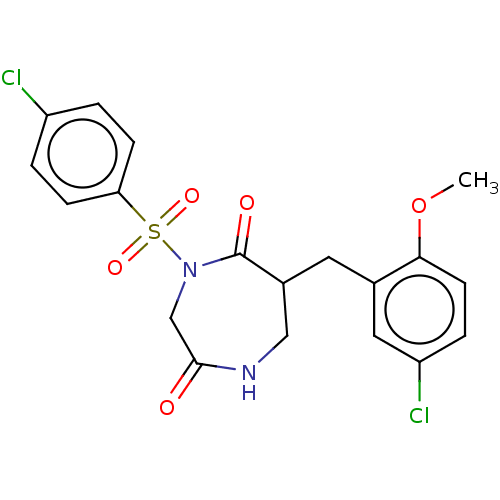 (CHEMBL391608 | US8507714, 29) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Asubio Pharma Co., Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant chymase | Bioorg Med Chem Lett 17: 3431-4 (2007) Article DOI: 10.1016/j.bmcl.2007.03.038 BindingDB Entry DOI: 10.7270/Q2G160H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM100740 (CHEMBL391608 | US8507714, 29) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Company, Limited US Patent | Assay Description Inhibitory activity of the compounds for recombinant human chymase was measured by method of Pasztor et al. (Pasztor et al., Acta Biol. Hung. 42:285-... | US Patent US8507714 (2013) BindingDB Entry DOI: 10.7270/Q2H130N8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50058969 (3-(4-Chloro-benzenesulfonyl)-1-(3,4-dimethyl-pheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Suntory Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against human heart chymase in vitro. | J Med Chem 40: 2156-63 (1997) Article DOI: 10.1021/jm960793t BindingDB Entry DOI: 10.7270/Q2QJ7GDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50210099 (CHEMBL246964 | rac-2q) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Asubio Pharma Co., Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant chymase | Bioorg Med Chem Lett 17: 3431-4 (2007) Article DOI: 10.1016/j.bmcl.2007.03.038 BindingDB Entry DOI: 10.7270/Q2G160H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50058972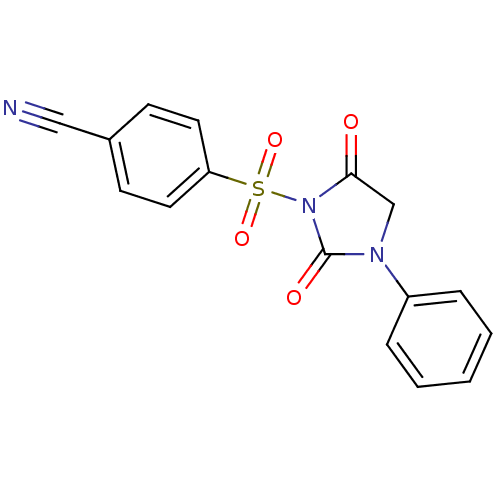 (4-(2,5-Dioxo-3-phenyl-imidazolidine-1-sulfonyl)-be...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Suntory Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against human heart chymase in vitro. | J Med Chem 40: 2156-63 (1997) Article DOI: 10.1021/jm960793t BindingDB Entry DOI: 10.7270/Q2QJ7GDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM134271 (US8846660, 53) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 40 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Daiichi Sankyo Company, Ltd. US Patent | Assay Description The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor... | US Patent US8846660 (2014) BindingDB Entry DOI: 10.7270/Q22V2DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM134258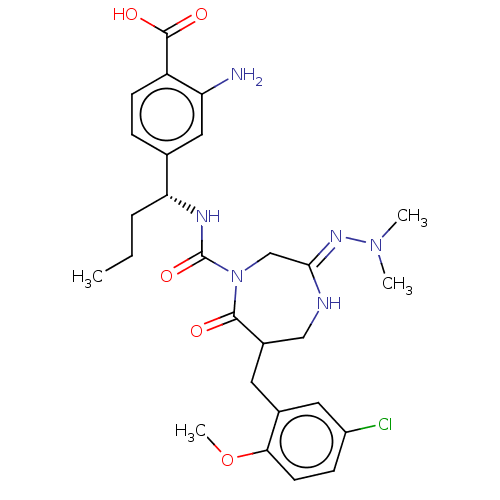 (US8846660, 22) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 40 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Daiichi Sankyo Company, Ltd. US Patent | Assay Description The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor... | US Patent US8846660 (2014) BindingDB Entry DOI: 10.7270/Q22V2DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM134281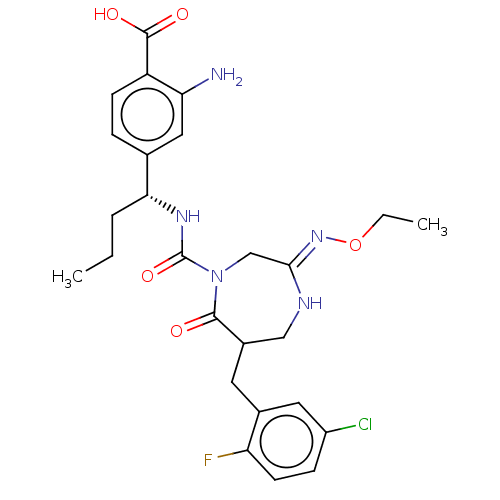 (US8846660, 113) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 40 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Daiichi Sankyo Company, Ltd. US Patent | Assay Description The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor... | US Patent US8846660 (2014) BindingDB Entry DOI: 10.7270/Q22V2DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50210096 (2-(4-chloro-2-((1-(4-chlorophenylsulfonyl)-3,7-dio...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Asubio Pharma Co., Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant chymase | Bioorg Med Chem Lett 17: 3431-4 (2007) Article DOI: 10.1016/j.bmcl.2007.03.038 BindingDB Entry DOI: 10.7270/Q2G160H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM87059 (CHEMBL247767 | Chymostatin) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Asubio Pharma Co., Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant chymase | Bioorg Med Chem Lett 17: 3431-4 (2007) Article DOI: 10.1016/j.bmcl.2007.03.038 BindingDB Entry DOI: 10.7270/Q2G160H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kallikrein-7 (Homo sapiens (Human)) | BDBM50460879 (CHEMBL4228441) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Asubio Pharma Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of recombinant human KLK7 using MOCAc-Arg-Pro-Lys-Pro-Val-Glu-Nva-Trp-Arg-Lys(Dnp)-NH2 as substrate preincubated for 10 mins followed by s... | Bioorg Med Chem Lett 28: 1371-1375 (2018) Article DOI: 10.1016/j.bmcl.2018.03.011 BindingDB Entry DOI: 10.7270/Q2222XD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50058977 (3-Benzoyl-1-phenyl-imidazolidine-2,4-dione | CHEMB...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Suntory Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against bovine pancreas alpha-chymotrypsin in vitro | J Med Chem 40: 2156-63 (1997) Article DOI: 10.1021/jm960793t BindingDB Entry DOI: 10.7270/Q2QJ7GDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 440 total ) | Next | Last >> |