 Found 40 hits with Last Name = 'mahmoud' and Initial = 's'
Found 40 hits with Last Name = 'mahmoud' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Replicase polyprotein 1ab
(2019-nCoV) | BDBM419133
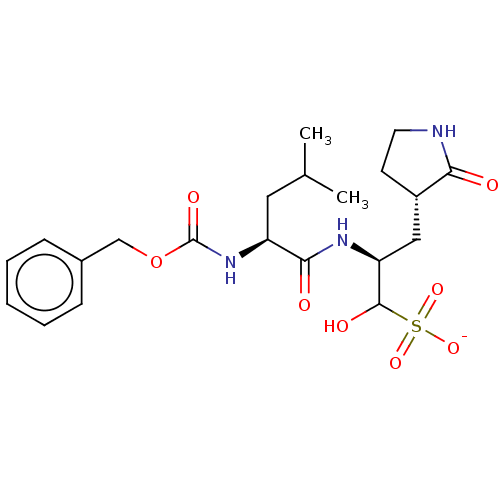
(BDBM429386 | GC376)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](C[C@@H]1CCNC1=O)C(O)S([O-])(=O)=O Show InChI InChI=1S/C21H31N3O8S/c1-13(2)10-16(24-21(28)32-12-14-6-4-3-5-7-14)19(26)23-17(20(27)33(29,30)31)11-15-8-9-22-18(15)25/h3-7,13,15-17,20,27H,8-12H2,1-2H3,(H,22,25)(H,23,26)(H,24,28)(H,29,30,31)/p-1/t15?,16-,17-,20?/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| | n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50572153
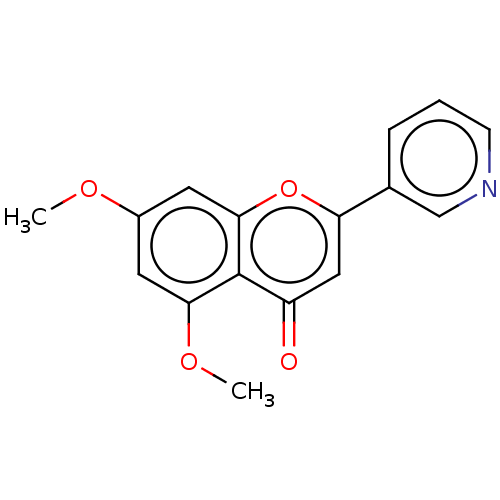
(CHEMBL1529347) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at PPARgamma (unknown origin) expressed in human LNCaP cells assessed as suppression of pioglitazone-induced PPAR response elemen... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116368
BindingDB Entry DOI: 10.7270/Q2X3527W |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50572152
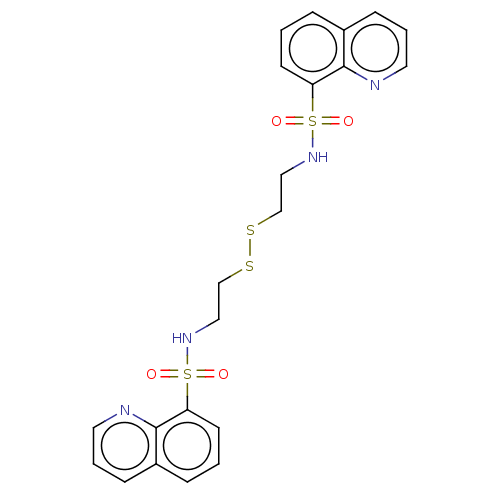
(CHEMBL4864404)Show SMILES O=S(=O)(NCCSSCCNS(=O)(=O)c1cccc2cccnc12)c1cccc2cccnc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at PPARgamma (unknown origin) expressed in human LNCaP cells assessed as suppression of pioglitazone-induced PPAR response elemen... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116368
BindingDB Entry DOI: 10.7270/Q2X3527W |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50572154
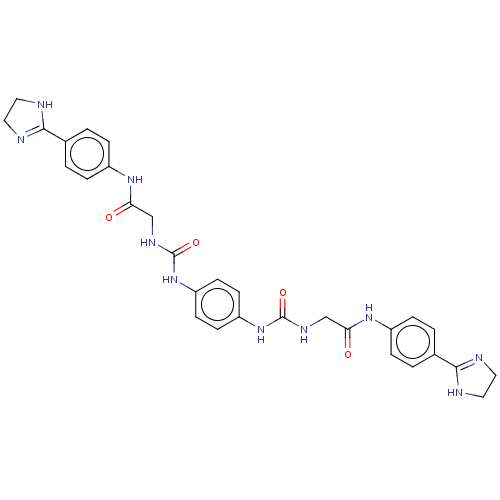
(CHEMBL4858300)Show SMILES O=C(CNC(=O)Nc1ccc(NC(=O)NCC(=O)Nc2ccc(cc2)C2=NCCN2)cc1)Nc1ccc(cc1)C1=NCCN1 |t:26,43| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at PPARgamma (unknown origin) expressed in human LNCaP cells assessed as suppression of pioglitazone-induced PPAR response elemen... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116368
BindingDB Entry DOI: 10.7270/Q2X3527W |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM429505
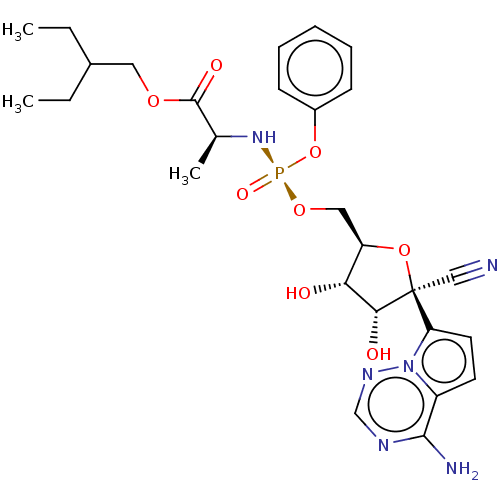
(Remdesivir | US20240034730, Compound Remdesivir | ...)Show SMILES CCC(CC)COC(=O)[C@H](C)N[P@](=O)(OC[C@H]1O[C@](C#N)([C@H](O)[C@@H]1O)c1ccc2c(N)ncnn12)Oc1ccccc1 Show InChI InChI=1S/C27H35N6O8P/c1-4-18(5-2)13-38-26(36)17(3)32-42(37,41-19-9-7-6-8-10-19)39-14-21-23(34)24(35)27(15-28,40-21)22-12-11-20-25(29)30-16-31-33(20)22/h6-12,16-18,21,23-24,34-35H,4-5,13-14H2,1-3H3,(H,32,37)(H2,29,30,31)/t17-,21+,23+,24+,27-,42-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | n/a | n/a | 1.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50572141
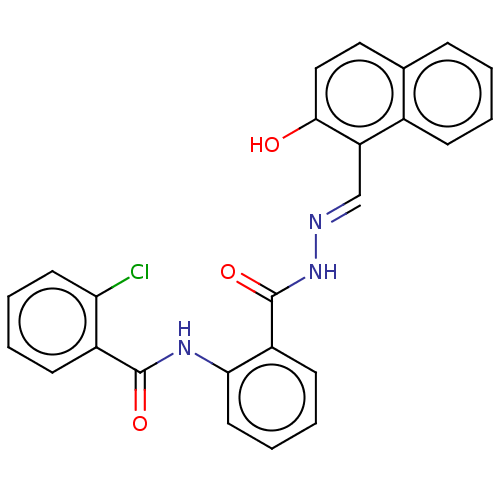
(CHEMBL4866821)Show SMILES Oc1ccc2ccccc2c1\C=N\NC(=O)c1ccccc1NC(=O)c1ccccc1Cl | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at PPARgamma (unknown origin) expressed in human LNCaP cells assessed as suppression of pioglitazone-induced PPAR response elemen... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116368
BindingDB Entry DOI: 10.7270/Q2X3527W |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50572149
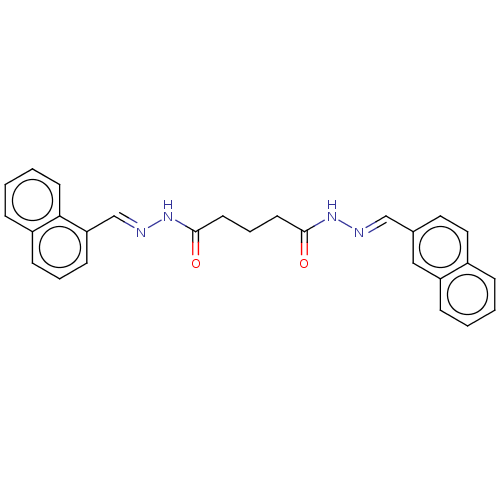
(CHEMBL4876787)Show SMILES O=C(CCCC(=O)N\N=C\c1cccc2ccccc12)N\N=C\c1ccc2ccccc2c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at PPARgamma (unknown origin) expressed in human LNCaP cells assessed as suppression of pioglitazone-induced PPAR response elemen... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116368
BindingDB Entry DOI: 10.7270/Q2X3527W |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50572148
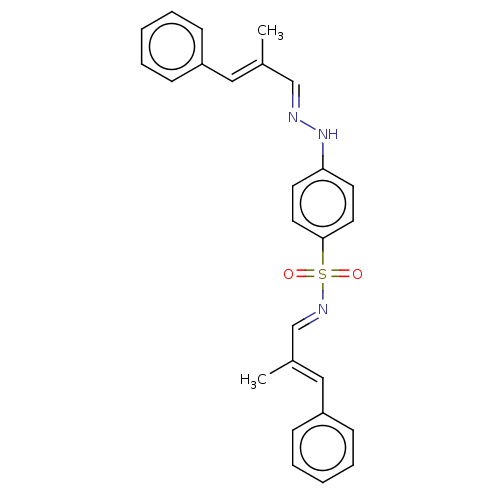
(CHEMBL4876358)Show SMILES C\C(\C=N\Nc1ccc(cc1)S(=O)(=O)\N=C\C(\C)=C\c1ccccc1)=C/c1ccccc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at PPARgamma (unknown origin) expressed in human LNCaP cells assessed as suppression of pioglitazone-induced PPAR response elemen... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116368
BindingDB Entry DOI: 10.7270/Q2X3527W |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50572143
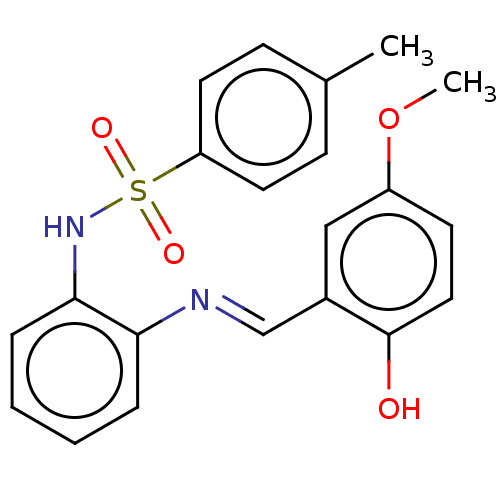
(CHEMBL4850980)Show SMILES COc1ccc(O)c(\C=N\c2ccccc2NS(=O)(=O)c2ccc(C)cc2)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at PPARgamma (unknown origin) expressed in human LNCaP cells assessed as suppression of pioglitazone-induced PPAR response elemen... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116368
BindingDB Entry DOI: 10.7270/Q2X3527W |
More data for this
Ligand-Target Pair | |
DNA polymerase alpha catalytic subunit
(Homo sapiens (Human)) | BDBM50090910

(3alpha,16,17,18-tetrahydroxyaphidicolone | 6,13-di...)Show SMILES C[C@@]1(CO)[C@H](O)CC[C@@]2(C)[C@H]1CC[C@H]1C[C@@H]3C[C@]21CC[C@]3(O)CO |r| Show InChI InChI=1S/C20H34O4/c1-17(11-21)15-4-3-13-9-14-10-19(13,7-8-20(14,24)12-22)18(15,2)6-5-16(17)23/h13-16,21-24H,3-12H2,1-2H3/t13-,14+,15-,16+,17-,18-,19-,20-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human DNA polymerase alpha using 5' end radiolabeled 24nt DNA/48nt DNA as primer/template after 5 mins by PAGE analysis |
J Med Chem 60: 5424-5437 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00067
BindingDB Entry DOI: 10.7270/Q2BZ6873 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50572151
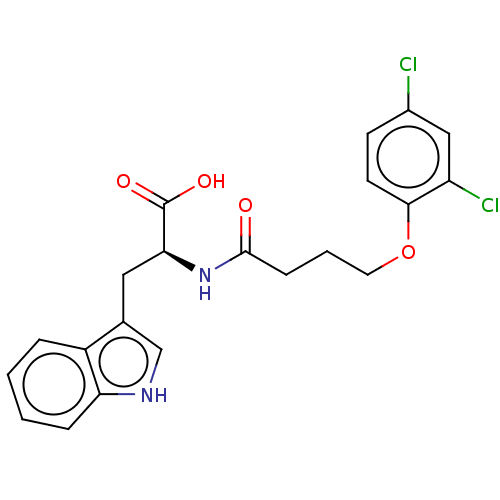
(CHEMBL4876041)Show SMILES OC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CCCOc1ccc(Cl)cc1Cl |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at PPARgamma (unknown origin) expressed in human LNCaP cells assessed as suppression of pioglitazone-induced PPAR response elemen... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116368
BindingDB Entry DOI: 10.7270/Q2X3527W |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50572146
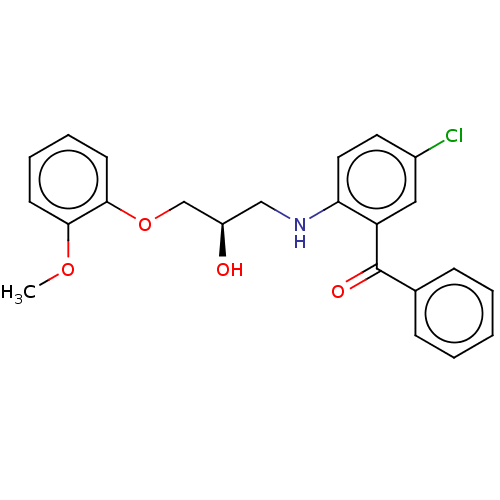
(CHEMBL4851842)Show SMILES COc1ccccc1OC[C@H](O)CNc1ccc(Cl)cc1C(=O)c1ccccc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at PPARgamma (unknown origin) expressed in human LNCaP cells assessed as suppression of pioglitazone-induced PPAR response elemen... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116368
BindingDB Entry DOI: 10.7270/Q2X3527W |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50060925
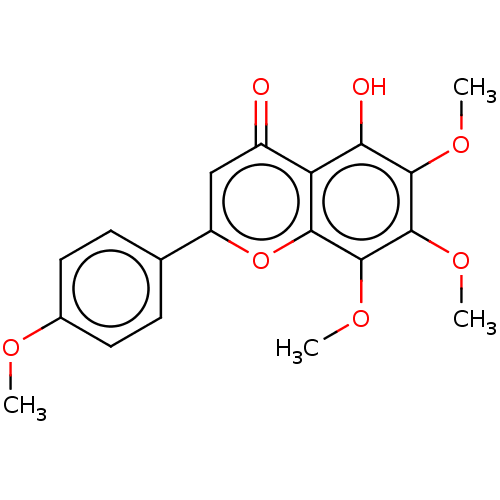
(CHEBI:79628 | GARDENIN B)Show SMILES COc1ccc(cc1)-c1cc(=O)c2c(O)c(OC)c(OC)c(OC)c2o1 Show InChI InChI=1S/C19H18O7/c1-22-11-7-5-10(6-8-11)13-9-12(20)14-15(21)17(23-2)19(25-4)18(24-3)16(14)26-13/h5-9,21H,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at PPARgamma (unknown origin) expressed in human LNCaP cells assessed as suppression of pioglitazone-induced PPAR response elemen... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116368
BindingDB Entry DOI: 10.7270/Q2X3527W |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50572142
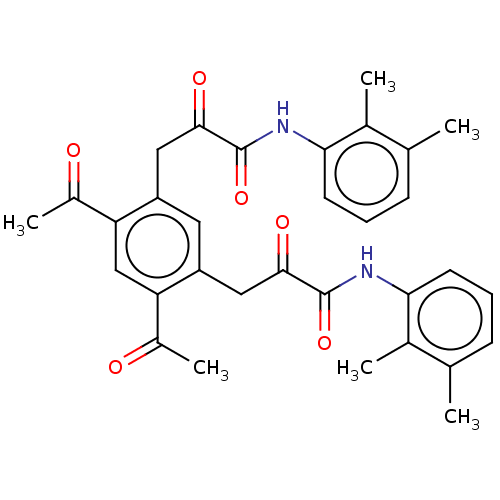
(CHEMBL4862243)Show SMILES CC(=O)c1cc(C(C)=O)c(CC(=O)C(=O)Nc2cccc(C)c2C)cc1CC(=O)C(=O)Nc1cccc(C)c1C | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at PPARgamma (unknown origin) expressed in human LNCaP cells assessed as suppression of pioglitazone-induced PPAR response elemen... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116368
BindingDB Entry DOI: 10.7270/Q2X3527W |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50572147
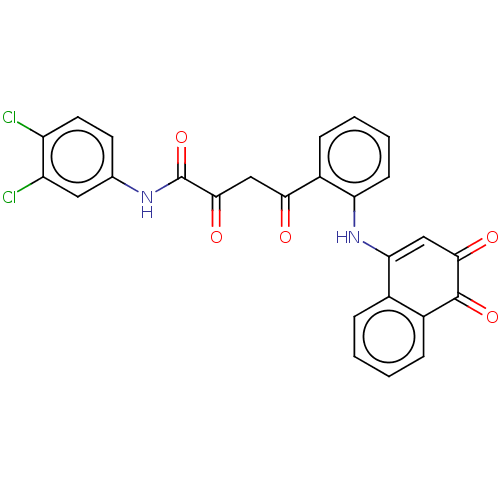
(CHEMBL1990669)Show SMILES Clc1ccc(NC(=O)C(=O)CC(=O)c2ccccc2NC2=CC(=O)C(=O)c3ccccc23)cc1Cl |t:21| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at PPARgamma (unknown origin) expressed in human LNCaP cells assessed as suppression of pioglitazone-induced PPAR response elemen... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116368
BindingDB Entry DOI: 10.7270/Q2X3527W |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM50615193
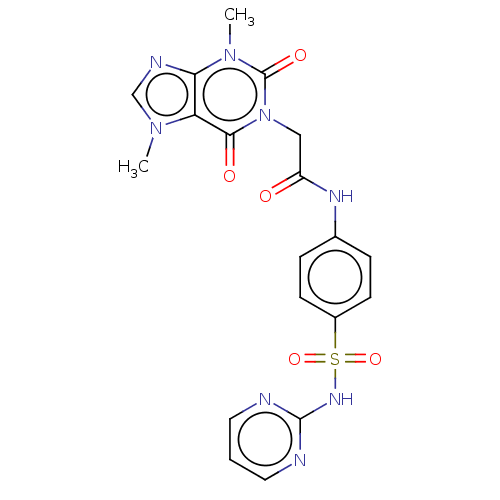
(CHEMBL5275584)Show SMILES Cn1cnc2n(C)c(=O)n(CC(=O)Nc3ccc(cc3)S(=O)(=O)Nc3ncccn3)c(=O)c12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 3.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50322668
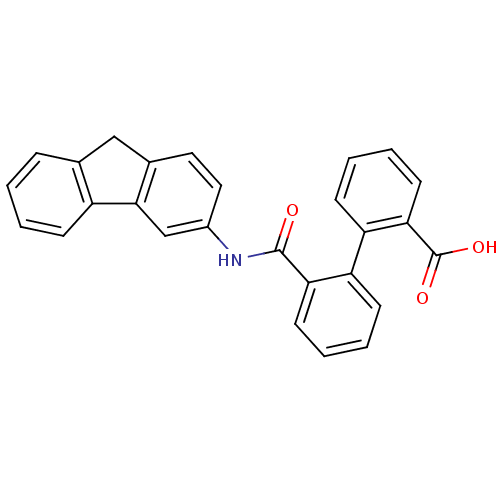
(2'-(9H-fluoren-3-ylcarbamoyl)biphenyl-2-carboxylic...)Show SMILES OC(=O)c1ccccc1-c1ccccc1C(=O)Nc1ccc2Cc3ccccc3-c2c1 Show InChI InChI=1S/C27H19NO3/c29-26(23-11-5-3-9-21(23)22-10-4-6-12-24(22)27(30)31)28-19-14-13-18-15-17-7-1-2-8-20(17)25(18)16-19/h1-14,16H,15H2,(H,28,29)(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at PPARgamma (unknown origin) expressed in human LNCaP cells assessed as suppression of pioglitazone-induced PPAR response elemen... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116368
BindingDB Entry DOI: 10.7270/Q2X3527W |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50572150

(CHEMBL4847026)Show SMILES CCN(CC)c1ccc(CN(CC(=O)c2ccc(Cl)c(Cl)c2)Cc2ccccc2)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at PPARgamma (unknown origin) expressed in human LNCaP cells assessed as suppression of pioglitazone-induced PPAR response elemen... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116368
BindingDB Entry DOI: 10.7270/Q2X3527W |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM512828
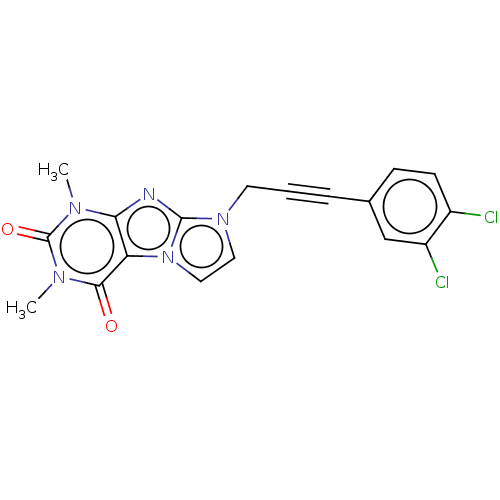
(acs.jmedchem.1c00409_ST.423)Show SMILES Cn1c2nc3n(CC#Cc4ccc(Cl)c(Cl)c4)ccn3c2c(=O)n(C)c1=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50412279

(CHEMBL476121)Show SMILES COc1c(O)c2c(oc(cc2=O)-c2ccc(O)cc2)c(OC)c1OC Show InChI InChI=1S/C18H16O7/c1-22-16-14(21)13-11(20)8-12(9-4-6-10(19)7-5-9)25-15(13)17(23-2)18(16)24-3/h4-8,19,21H,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at PPARgamma (unknown origin) expressed in human LNCaP cells assessed as suppression of pioglitazone-induced PPAR response elemen... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116368
BindingDB Entry DOI: 10.7270/Q2X3527W |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50572144
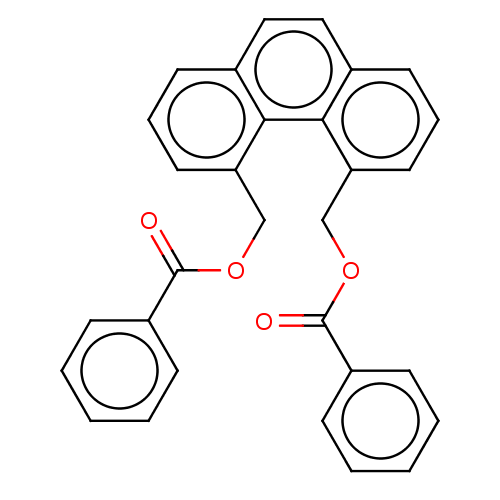
(CHEMBL4860371)Show SMILES O=C(OCc1cccc2ccc3cccc(COC(=O)c4ccccc4)c3c12)c1ccccc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at PPARgamma (unknown origin) expressed in human LNCaP cells assessed as suppression of pioglitazone-induced PPAR response elemen... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116368
BindingDB Entry DOI: 10.7270/Q2X3527W |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM50615194
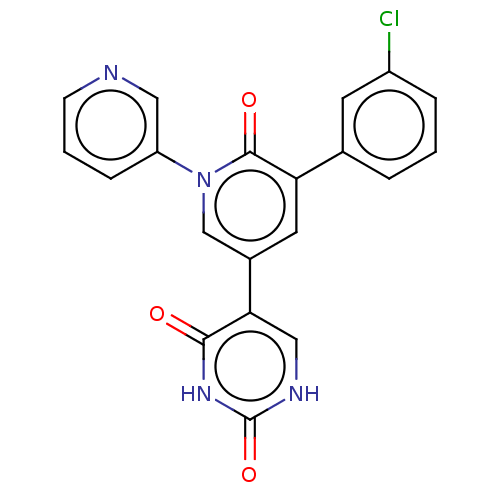
(CHEMBL5268256)Show SMILES Clc1cccc(c1)-c1cc(cn(-c2cccnc2)c1=O)-c1c[nH]c(=O)[nH]c1=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 6.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM294045
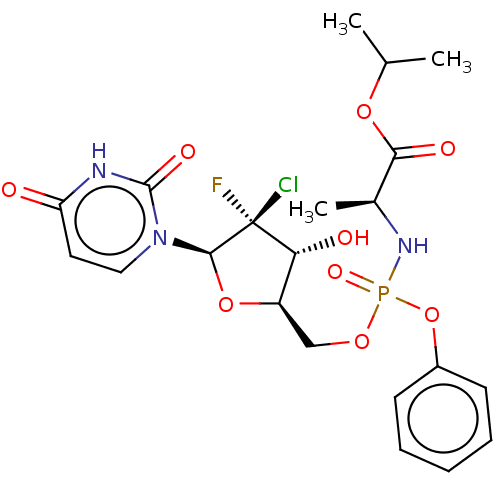
(US10106571, Example 2 | US10106571, Example 23)Show SMILES CC(C)OC(=O)[C@H](C)NP(=O)(OC[C@H]1O[C@@H](n2ccc(=O)[nH]c2=O)[C@@](F)(Cl)[C@@H]1O)Oc1ccccc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 using ketoconazole as substrate |
J Med Chem 60: 5424-5437 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00067
BindingDB Entry DOI: 10.7270/Q2BZ6873 |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM50615193

(CHEMBL5275584)Show SMILES Cn1cnc2n(C)c(=O)n(CC(=O)Nc3ccc(cc3)S(=O)(=O)Nc3ncccn3)c(=O)c12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 8.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50239940

(CHEBI:85083 | PSI-7977, GS-7977 | Sofosbuvir | Sov...)Show SMILES CC(C)OC(=O)[C@H](C)N[P@](=O)(OC[C@H]1O[C@@H](n2ccc(=O)[nH]c2=O)[C@](C)(F)[C@@H]1O)Oc1ccccc1 |r| Show InChI InChI=1S/C22H29FN3O9P/c1-13(2)33-19(29)14(3)25-36(31,35-15-8-6-5-7-9-15)32-12-16-18(28)22(4,23)20(34-16)26-11-10-17(27)24-21(26)30/h5-11,13-14,16,18,20,28H,12H2,1-4H3,(H,25,31)(H,24,27,30)/t14-,16+,18+,20+,22+,36-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 using ketoconazole as substrate |
J Med Chem 60: 5424-5437 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00067
BindingDB Entry DOI: 10.7270/Q2BZ6873 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50572145
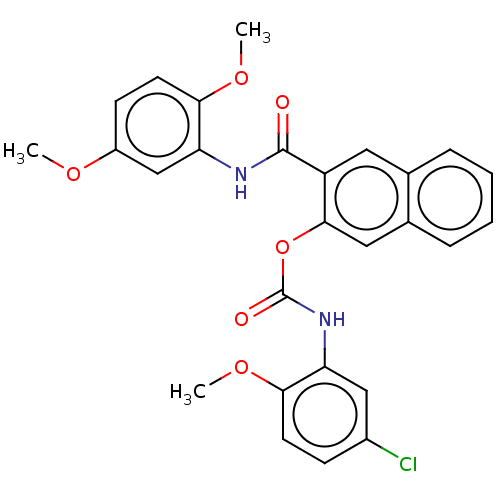
(CHEMBL4874261)Show SMILES COc1ccc(OC)c(NC(=O)c2cc3ccccc3cc2OC(=O)Nc2cc(Cl)ccc2OC)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at PPARgamma (unknown origin) expressed in human LNCaP cells assessed as suppression of pioglitazone-induced PPAR response elemen... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116368
BindingDB Entry DOI: 10.7270/Q2X3527W |
More data for this
Ligand-Target Pair | |
DNA polymerase beta
(Homo sapiens (Human)) | BDBM50020676

(CHEBI:41846 | CHEMBL566812)Show SMILES Cc1cn([C@H]2CC[C@@H](COP(O)(=O)OP(O)(=O)OP(O)(O)=O)O2)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C10H17N2O13P3/c1-6-4-12(10(14)11-9(6)13)8-3-2-7(23-8)5-22-27(18,19)25-28(20,21)24-26(15,16)17/h4,7-8H,2-3,5H2,1H3,(H,18,19)(H,20,21)(H,11,13,14)(H2,15,16,17)/t7-,8+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human DNA polymerase beta using 5' end radiolabeled 24nt DNA/48nt DNA as primer/template after 5 mins by PAGE analysis |
J Med Chem 60: 5424-5437 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00067
BindingDB Entry DOI: 10.7270/Q2BZ6873 |
More data for this
Ligand-Target Pair | |
DNA polymerase alpha catalytic subunit
(Homo sapiens (Human)) | BDBM50239939
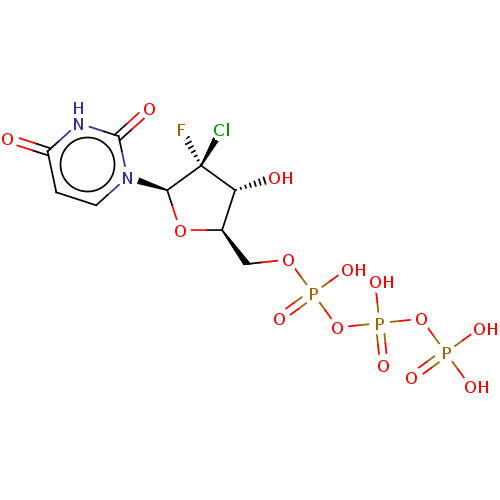
(CHEMBL4088430)Show SMILES O[C@@H]1[C@@H](COP(O)(=O)OP(O)(=O)OP(O)(O)=O)O[C@@H](n2ccc(=O)[nH]c2=O)[C@@]1(F)Cl |r| Show InChI InChI=1S/C9H13ClFN2O14P3/c10-9(11)6(15)4(25-7(9)13-2-1-5(14)12-8(13)16)3-24-29(20,21)27-30(22,23)26-28(17,18)19/h1-2,4,6-7,15H,3H2,(H,20,21)(H,22,23)(H,12,14,16)(H2,17,18,19)/t4-,6-,7-,9-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human DNA polymerase alpha using 5' end radiolabeled 24nt DNA/48nt DNA as primer/template after 5 mins by PAGE analysis |
J Med Chem 60: 5424-5437 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00067
BindingDB Entry DOI: 10.7270/Q2BZ6873 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM294045

(US10106571, Example 2 | US10106571, Example 23)Show SMILES CC(C)OC(=O)[C@H](C)NP(=O)(OC[C@H]1O[C@@H](n2ccc(=O)[nH]c2=O)[C@@](F)(Cl)[C@@H]1O)Oc1ccccc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C9 using sulfaphenazole as substrate |
J Med Chem 60: 5424-5437 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00067
BindingDB Entry DOI: 10.7270/Q2BZ6873 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50239940

(CHEBI:85083 | PSI-7977, GS-7977 | Sofosbuvir | Sov...)Show SMILES CC(C)OC(=O)[C@H](C)N[P@](=O)(OC[C@H]1O[C@@H](n2ccc(=O)[nH]c2=O)[C@](C)(F)[C@@H]1O)Oc1ccccc1 |r| Show InChI InChI=1S/C22H29FN3O9P/c1-13(2)33-19(29)14(3)25-36(31,35-15-8-6-5-7-9-15)32-12-16-18(28)22(4,23)20(34-16)26-11-10-17(27)24-21(26)30/h5-11,13-14,16,18,20,28H,12H2,1-4H3,(H,25,31)(H,24,27,30)/t14-,16+,18+,20+,22+,36-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C9 using sulfaphenazole as substrate |
J Med Chem 60: 5424-5437 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00067
BindingDB Entry DOI: 10.7270/Q2BZ6873 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50239940

(CHEBI:85083 | PSI-7977, GS-7977 | Sofosbuvir | Sov...)Show SMILES CC(C)OC(=O)[C@H](C)N[P@](=O)(OC[C@H]1O[C@@H](n2ccc(=O)[nH]c2=O)[C@](C)(F)[C@@H]1O)Oc1ccccc1 |r| Show InChI InChI=1S/C22H29FN3O9P/c1-13(2)33-19(29)14(3)25-36(31,35-15-8-6-5-7-9-15)32-12-16-18(28)22(4,23)20(34-16)26-11-10-17(27)24-21(26)30/h5-11,13-14,16,18,20,28H,12H2,1-4H3,(H,25,31)(H,24,27,30)/t14-,16+,18+,20+,22+,36-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1A2 using alpha-naphthoflavone as substrate |
J Med Chem 60: 5424-5437 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00067
BindingDB Entry DOI: 10.7270/Q2BZ6873 |
More data for this
Ligand-Target Pair | |
DNA polymerase beta
(Homo sapiens (Human)) | BDBM50239939

(CHEMBL4088430)Show SMILES O[C@@H]1[C@@H](COP(O)(=O)OP(O)(=O)OP(O)(O)=O)O[C@@H](n2ccc(=O)[nH]c2=O)[C@@]1(F)Cl |r| Show InChI InChI=1S/C9H13ClFN2O14P3/c10-9(11)6(15)4(25-7(9)13-2-1-5(14)12-8(13)16)3-24-29(20,21)27-30(22,23)26-28(17,18)19/h1-2,4,6-7,15H,3H2,(H,20,21)(H,22,23)(H,12,14,16)(H2,17,18,19)/t4-,6-,7-,9-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human DNA polymerase beta using 5' end radiolabeled 24nt DNA/48nt DNA as primer/template after 5 mins by PAGE analysis |
J Med Chem 60: 5424-5437 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00067
BindingDB Entry DOI: 10.7270/Q2BZ6873 |
More data for this
Ligand-Target Pair | |
DNA polymerase beta
(Homo sapiens (Human)) | BDBM50333129
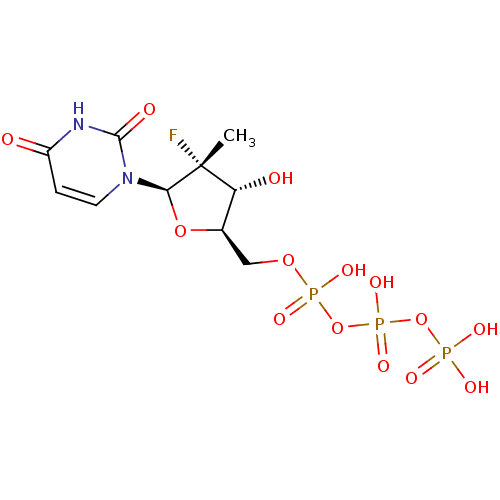
(((2R,3R,4R,5R)-5-(2,4-dioxo-3,4-dihydropyrimidin-1...)Show SMILES C[C@@]1(F)[C@H](O)[C@@H](COP(O)(=O)OP(O)(=O)OP(O)(O)=O)O[C@H]1n1ccc(=O)[nH]c1=O |r| Show InChI InChI=1S/C10H16FN2O14P3/c1-10(11)7(15)5(25-8(10)13-3-2-6(14)12-9(13)16)4-24-29(20,21)27-30(22,23)26-28(17,18)19/h2-3,5,7-8,15H,4H2,1H3,(H,20,21)(H,22,23)(H,12,14,16)(H2,17,18,19)/t5-,7-,8-,10-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human DNA polymerase beta using 5' end radiolabeled 24nt DNA/48nt DNA as primer/template after 5 mins by PAGE analysis |
J Med Chem 60: 5424-5437 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00067
BindingDB Entry DOI: 10.7270/Q2BZ6873 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM294045

(US10106571, Example 2 | US10106571, Example 23)Show SMILES CC(C)OC(=O)[C@H](C)NP(=O)(OC[C@H]1O[C@@H](n2ccc(=O)[nH]c2=O)[C@@](F)(Cl)[C@@H]1O)Oc1ccccc1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 using quinidine as substrate |
J Med Chem 60: 5424-5437 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00067
BindingDB Entry DOI: 10.7270/Q2BZ6873 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50239940

(CHEBI:85083 | PSI-7977, GS-7977 | Sofosbuvir | Sov...)Show SMILES CC(C)OC(=O)[C@H](C)N[P@](=O)(OC[C@H]1O[C@@H](n2ccc(=O)[nH]c2=O)[C@](C)(F)[C@@H]1O)Oc1ccccc1 |r| Show InChI InChI=1S/C22H29FN3O9P/c1-13(2)33-19(29)14(3)25-36(31,35-15-8-6-5-7-9-15)32-12-16-18(28)22(4,23)20(34-16)26-11-10-17(27)24-21(26)30/h5-11,13-14,16,18,20,28H,12H2,1-4H3,(H,25,31)(H,24,27,30)/t14-,16+,18+,20+,22+,36-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 using quinidine as substrate |
J Med Chem 60: 5424-5437 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00067
BindingDB Entry DOI: 10.7270/Q2BZ6873 |
More data for this
Ligand-Target Pair | |
DNA polymerase alpha catalytic subunit
(Homo sapiens (Human)) | BDBM50333129

(((2R,3R,4R,5R)-5-(2,4-dioxo-3,4-dihydropyrimidin-1...)Show SMILES C[C@@]1(F)[C@H](O)[C@@H](COP(O)(=O)OP(O)(=O)OP(O)(O)=O)O[C@H]1n1ccc(=O)[nH]c1=O |r| Show InChI InChI=1S/C10H16FN2O14P3/c1-10(11)7(15)5(25-8(10)13-3-2-6(14)12-9(13)16)4-24-29(20,21)27-30(22,23)26-28(17,18)19/h2-3,5,7-8,15H,4H2,1H3,(H,20,21)(H,22,23)(H,12,14,16)(H2,17,18,19)/t5-,7-,8-,10-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine
Curated by ChEMBL
| Assay Description
Competitive inhibition of leucine aminopeptidase; Ki value reporting the slope effect(Kis) |
J Med Chem 60: 5424-5437 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00067
BindingDB Entry DOI: 10.7270/Q2BZ6873 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM294045

(US10106571, Example 2 | US10106571, Example 23)Show SMILES CC(C)OC(=O)[C@H](C)NP(=O)(OC[C@H]1O[C@@H](n2ccc(=O)[nH]c2=O)[C@@](F)(Cl)[C@@H]1O)Oc1ccccc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1A2 using alpha-naphthoflavone as substrate |
J Med Chem 60: 5424-5437 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00067
BindingDB Entry DOI: 10.7270/Q2BZ6873 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50572138

(CHEMBL4878798)Show SMILES Cc1ccc(cc1)S(=O)(=O)N[C@@H](CCC(=O)Nc1ccccc1)C(O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at PPARgamma (unknown origin) expressed in human LNCaP cells assessed as suppression of pioglitazone-induced PPAR response elemen... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116368
BindingDB Entry DOI: 10.7270/Q2X3527W |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50572140
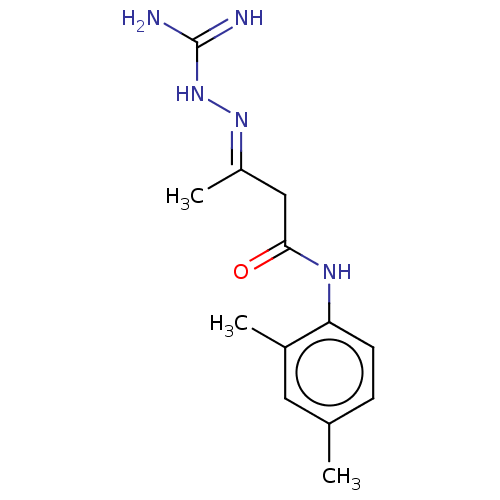
(CHEMBL4852057) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at PPARgamma (unknown origin) expressed in human LNCaP cells assessed as suppression of pioglitazone-induced PPAR response elemen... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116368
BindingDB Entry DOI: 10.7270/Q2X3527W |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50572139

(CHEMBL4869272)Show SMILES COc1ccc(NC(=O)CC[C@H](NS(=O)(=O)c2ccc(C)cc2)C(O)=O)cc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.75E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at PPARgamma (unknown origin) expressed in human LNCaP cells assessed as suppression of pioglitazone-induced PPAR response elemen... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116368
BindingDB Entry DOI: 10.7270/Q2X3527W |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data