 Found 829 hits with Last Name = 'rani' and Initial = 's'
Found 829 hits with Last Name = 'rani' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Nicotinamide N-methyltransferase
(Homo sapiens (Human)) | BDBM50530711
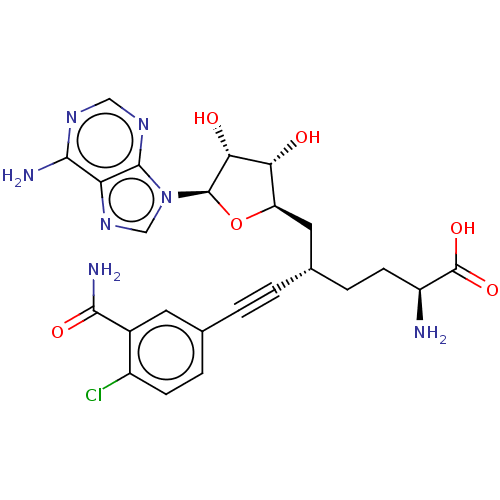
(CHEMBL4553052)Show SMILES N[C@@H](CC[C@@H](C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C#Cc1ccc(Cl)c(c1)C(N)=O)C(O)=O |r| Show InChI InChI=1S/C24H26ClN7O6/c25-14-5-3-11(7-13(14)21(28)35)1-2-12(4-6-15(26)24(36)37)8-16-18(33)19(34)23(38-16)32-10-31-17-20(27)29-9-30-22(17)32/h3,5,7,9-10,12,15-16,18-19,23,33-34H,4,6,8,26H2,(H2,28,35)(H,36,37)(H2,27,29,30)/t12-,15-,16+,18+,19+,23+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.191 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioKin Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged wild type human NNMT expressed in Escherichia coli NiCo21(DE3) assessed as reduction in 1-methylquinolinium leve... |
J Med Chem 62: 9837-9873 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01238
BindingDB Entry DOI: 10.7270/Q2D50RDJ |
More data for this
Ligand-Target Pair | |
Nicotinamide N-methyltransferase
(Homo sapiens (Human)) | BDBM50530711

(CHEMBL4553052)Show SMILES N[C@@H](CC[C@@H](C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C#Cc1ccc(Cl)c(c1)C(N)=O)C(O)=O |r| Show InChI InChI=1S/C24H26ClN7O6/c25-14-5-3-11(7-13(14)21(28)35)1-2-12(4-6-15(26)24(36)37)8-16-18(33)19(34)23(38-16)32-10-31-17-20(27)29-9-30-22(17)32/h3,5,7,9-10,12,15-16,18-19,23,33-34H,4,6,8,26H2,(H2,28,35)(H,36,37)(H2,27,29,30)/t12-,15-,16+,18+,19+,23+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.191 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioKin Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged wild type human NNMT expressed in Escherichia coli NiCo21(DE3) assessed as reduction in 1-methylquinolinium leve... |
J Med Chem 62: 9837-9873 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01238
BindingDB Entry DOI: 10.7270/Q2D50RDJ |
More data for this
Ligand-Target Pair | |
Nicotinamide N-methyltransferase
(Homo sapiens (Human)) | BDBM50530731
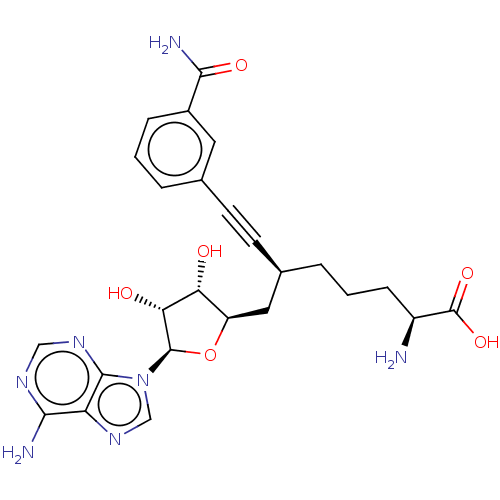
(CHEMBL4580446)Show SMILES N[C@@H](CCC[C@@H](C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C#Cc1cccc(c1)C(N)=O)C(O)=O |r| Show InChI InChI=1S/C25H29N7O6/c26-16(25(36)37)6-2-4-14(8-7-13-3-1-5-15(9-13)22(28)35)10-17-19(33)20(34)24(38-17)32-12-31-18-21(27)29-11-30-23(18)32/h1,3,5,9,11-12,14,16-17,19-20,24,33-34H,2,4,6,10,26H2,(H2,28,35)(H,36,37)(H2,27,29,30)/t14-,16+,17-,19-,20-,24-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioKin Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged wild type human NNMT expressed in Escherichia coli NiCo21(DE3) assessed as reduction in 1-methylquinolinium leve... |
J Med Chem 62: 9837-9873 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01238
BindingDB Entry DOI: 10.7270/Q2D50RDJ |
More data for this
Ligand-Target Pair | |
Nicotinamide N-methyltransferase
(Homo sapiens (Human)) | BDBM50530731

(CHEMBL4580446)Show SMILES N[C@@H](CCC[C@@H](C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C#Cc1cccc(c1)C(N)=O)C(O)=O |r| Show InChI InChI=1S/C25H29N7O6/c26-16(25(36)37)6-2-4-14(8-7-13-3-1-5-15(9-13)22(28)35)10-17-19(33)20(34)24(38-17)32-12-31-18-21(27)29-11-30-23(18)32/h1,3,5,9,11-12,14,16-17,19-20,24,33-34H,2,4,6,10,26H2,(H2,28,35)(H,36,37)(H2,27,29,30)/t14-,16+,17-,19-,20-,24-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioKin Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged wild type human NNMT expressed in Escherichia coli NiCo21(DE3) assessed as reduction in 1-methylquinolinium leve... |
J Med Chem 62: 9837-9873 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01238
BindingDB Entry DOI: 10.7270/Q2D50RDJ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM11625
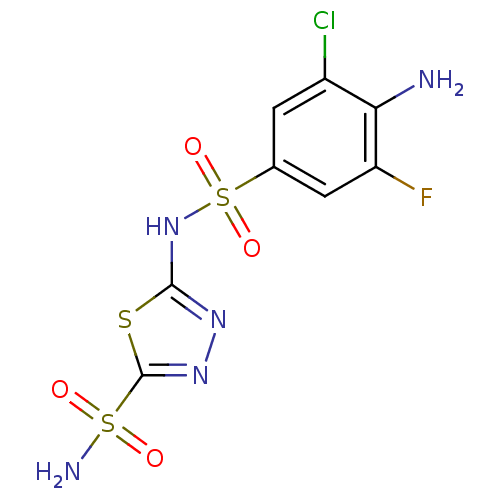
(2-N-(4-amino-3-chloro-5-fluorobenzene)-1,3,4-thiad...)Show SMILES Nc1c(F)cc(cc1Cl)S(=O)(=O)Nc1nnc(s1)S(N)(=O)=O Show InChI InChI=1S/C8H7ClFN5O4S3/c9-4-1-3(2-5(10)6(4)11)22(18,19)15-7-13-14-8(20-7)21(12,16)17/h1-2H,11H2,(H,13,15)(H2,12,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Universit£ Montpellier II
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase II by spectrophotometry at pH 7.5 |
Bioorg Med Chem 19: 1172-8 (2011)
Article DOI: 10.1016/j.bmc.2010.12.048
BindingDB Entry DOI: 10.7270/Q2C53M44 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nicotinamide N-methyltransferase
(Homo sapiens (Human)) | BDBM50530712
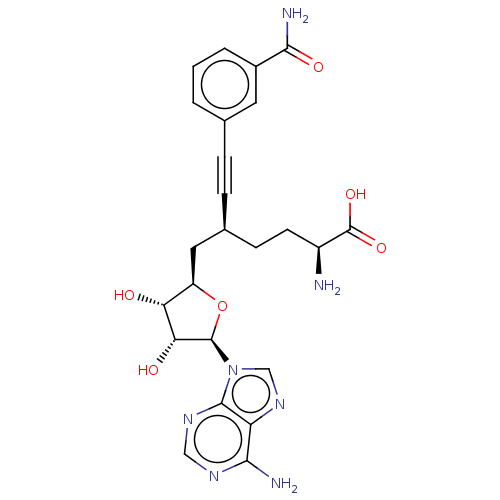
(CHEMBL4591248)Show SMILES N[C@@H](CC[C@@H](C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C#Cc1cccc(c1)C(N)=O)C(O)=O |r| Show InChI InChI=1S/C24H27N7O6/c25-15(24(35)36)7-6-13(5-4-12-2-1-3-14(8-12)21(27)34)9-16-18(32)19(33)23(37-16)31-11-30-17-20(26)28-10-29-22(17)31/h1-3,8,10-11,13,15-16,18-19,23,32-33H,6-7,9,25H2,(H2,27,34)(H,35,36)(H2,26,28,29)/t13-,15-,16+,18+,19+,23+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioKin Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged wild type human NNMT expressed in Escherichia coli NiCo21(DE3) assessed as reduction in 1-methylquinolinium leve... |
J Med Chem 62: 9837-9873 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01238
BindingDB Entry DOI: 10.7270/Q2D50RDJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nicotinamide N-methyltransferase
(Homo sapiens (Human)) | BDBM50530712

(CHEMBL4591248)Show SMILES N[C@@H](CC[C@@H](C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C#Cc1cccc(c1)C(N)=O)C(O)=O |r| Show InChI InChI=1S/C24H27N7O6/c25-15(24(35)36)7-6-13(5-4-12-2-1-3-14(8-12)21(27)34)9-16-18(32)19(33)23(37-16)31-11-30-17-20(26)28-10-29-22(17)31/h1-3,8,10-11,13,15-16,18-19,23,32-33H,6-7,9,25H2,(H2,27,34)(H,35,36)(H2,26,28,29)/t13-,15-,16+,18+,19+,23+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioKin Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged wild type human NNMT expressed in Escherichia coli NiCo21(DE3) assessed as reduction in 1-methylquinolinium leve... |
J Med Chem 62: 9837-9873 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01238
BindingDB Entry DOI: 10.7270/Q2D50RDJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nicotinamide N-methyltransferase
(Homo sapiens (Human)) | BDBM50530712

(CHEMBL4591248)Show SMILES N[C@@H](CC[C@@H](C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C#Cc1cccc(c1)C(N)=O)C(O)=O |r| Show InChI InChI=1S/C24H27N7O6/c25-15(24(35)36)7-6-13(5-4-12-2-1-3-14(8-12)21(27)34)9-16-18(32)19(33)23(37-16)31-11-30-17-20(26)28-10-29-22(17)31/h1-3,8,10-11,13,15-16,18-19,23,32-33H,6-7,9,25H2,(H2,27,34)(H,35,36)(H2,26,28,29)/t13-,15-,16+,18+,19+,23+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioKin Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged wild type human NNMT expressed in Escherichia coli NiCo21(DE3) assessed as reduction in 1-methylquinolinium leve... |
J Med Chem 62: 9837-9873 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01238
BindingDB Entry DOI: 10.7270/Q2D50RDJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nicotinamide N-methyltransferase
(Homo sapiens (Human)) | BDBM50530712

(CHEMBL4591248)Show SMILES N[C@@H](CC[C@@H](C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C#Cc1cccc(c1)C(N)=O)C(O)=O |r| Show InChI InChI=1S/C24H27N7O6/c25-15(24(35)36)7-6-13(5-4-12-2-1-3-14(8-12)21(27)34)9-16-18(32)19(33)23(37-16)31-11-30-17-20(26)28-10-29-22(17)31/h1-3,8,10-11,13,15-16,18-19,23,32-33H,6-7,9,25H2,(H2,27,34)(H,35,36)(H2,26,28,29)/t13-,15-,16+,18+,19+,23+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioKin Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged wild type human NNMT expressed in Escherichia coli NiCo21(DE3) assessed as reduction in 1-methylquinolinium leve... |
J Med Chem 62: 9837-9873 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01238
BindingDB Entry DOI: 10.7270/Q2D50RDJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50587810
(CHEMBL5193353)Show SMILES OC(=O)CC[C@H](NC(=O)N[C@@H](CCCCN(Cc1cccc(Cl)c1)C(=O)CCCCCNC(=O)CCC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)NCCCN=[N+]=[N-])C(O)=O)C(O)=O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01935
BindingDB Entry DOI: 10.7270/Q2NS0ZWP |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50098102
(CHEMBL23350 | Sulfamic acid 4-chloro-phenyl ester)Show InChI InChI=1S/C6H6ClNO3S/c7-5-1-3-6(4-2-5)11-12(8,9)10/h1-4H,(H2,8,9,10) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Universit£ Montpellier II
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase II by spectrophotometry at pH 7.5 |
Bioorg Med Chem 19: 1172-8 (2011)
Article DOI: 10.1016/j.bmc.2010.12.048
BindingDB Entry DOI: 10.7270/Q2C53M44 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM11625

(2-N-(4-amino-3-chloro-5-fluorobenzene)-1,3,4-thiad...)Show SMILES Nc1c(F)cc(cc1Cl)S(=O)(=O)Nc1nnc(s1)S(N)(=O)=O Show InChI InChI=1S/C8H7ClFN5O4S3/c9-4-1-3(2-5(10)6(4)11)22(18,19)15-7-13-14-8(20-7)21(12,16)17/h1-2H,11H2,(H,13,15)(H2,12,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Universit£ Montpellier II
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase I by spectrophotometry at pH 7.5 |
Bioorg Med Chem 19: 1172-8 (2011)
Article DOI: 10.1016/j.bmc.2010.12.048
BindingDB Entry DOI: 10.7270/Q2C53M44 |
More data for this
Ligand-Target Pair | |
Nicotinamide N-methyltransferase
(Homo sapiens (Human)) | BDBM50530723

(CHEMBL4455627)Show SMILES N[C@@H](CC[C@@H](C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C#Cc1ccc(F)c(c1)C(N)=O)C(O)=O |r| Show InChI InChI=1S/C24H26FN7O6/c25-14-5-3-11(7-13(14)21(28)35)1-2-12(4-6-15(26)24(36)37)8-16-18(33)19(34)23(38-16)32-10-31-17-20(27)29-9-30-22(17)32/h3,5,7,9-10,12,15-16,18-19,23,33-34H,4,6,8,26H2,(H2,28,35)(H,36,37)(H2,27,29,30)/t12-,15-,16+,18+,19+,23+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioKin Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged wild type human NNMT expressed in Escherichia coli NiCo21(DE3) assessed as reduction in 1-methylquinolinium leve... |
J Med Chem 62: 9837-9873 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01238
BindingDB Entry DOI: 10.7270/Q2D50RDJ |
More data for this
Ligand-Target Pair | |
Nicotinamide N-methyltransferase
(Homo sapiens (Human)) | BDBM50530723

(CHEMBL4455627)Show SMILES N[C@@H](CC[C@@H](C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C#Cc1ccc(F)c(c1)C(N)=O)C(O)=O |r| Show InChI InChI=1S/C24H26FN7O6/c25-14-5-3-11(7-13(14)21(28)35)1-2-12(4-6-15(26)24(36)37)8-16-18(33)19(34)23(38-16)32-10-31-17-20(27)29-9-30-22(17)32/h3,5,7,9-10,12,15-16,18-19,23,33-34H,4,6,8,26H2,(H2,28,35)(H,36,37)(H2,27,29,30)/t12-,15-,16+,18+,19+,23+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioKin Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged wild type human NNMT expressed in Escherichia coli NiCo21(DE3) assessed as reduction in 1-methylquinolinium leve... |
J Med Chem 62: 9837-9873 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01238
BindingDB Entry DOI: 10.7270/Q2D50RDJ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10870

(2-N-(4-aminobenzene)-1,3,4-thiadiazole-2,5-disulfo...)Show InChI InChI=1S/C8H9N5O4S3/c9-5-1-3-6(4-2-5)20(16,17)13-7-11-12-8(18-7)19(10,14)15/h1-4H,9H2,(H,11,13)(H2,10,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Universit£ Montpellier II
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase II by spectrophotometry at pH 7.5 |
Bioorg Med Chem 19: 1172-8 (2011)
Article DOI: 10.1016/j.bmc.2010.12.048
BindingDB Entry DOI: 10.7270/Q2C53M44 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Nicotinamide N-methyltransferase
(Homo sapiens (Human)) | BDBM50530737

(CHEMBL4449355)Show SMILES N[C@@H](CC[C@@H](C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C#Cc1ccc(c(c1)C(N)=O)C(F)(F)F)C(O)=O |r| Show InChI InChI=1S/C25H26F3N7O6/c26-25(27,28)14-5-3-11(7-13(14)21(31)38)1-2-12(4-6-15(29)24(39)40)8-16-18(36)19(37)23(41-16)35-10-34-17-20(30)32-9-33-22(17)35/h3,5,7,9-10,12,15-16,18-19,23,36-37H,4,6,8,29H2,(H2,31,38)(H,39,40)(H2,30,32,33)/t12-,15-,16+,18+,19+,23+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioKin Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged wild type human NNMT expressed in Escherichia coli NiCo21(DE3) assessed as reduction in 1-methylquinolinium leve... |
J Med Chem 62: 9837-9873 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01238
BindingDB Entry DOI: 10.7270/Q2D50RDJ |
More data for this
Ligand-Target Pair | |
Nicotinamide N-methyltransferase
(Homo sapiens (Human)) | BDBM50530737

(CHEMBL4449355)Show SMILES N[C@@H](CC[C@@H](C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C#Cc1ccc(c(c1)C(N)=O)C(F)(F)F)C(O)=O |r| Show InChI InChI=1S/C25H26F3N7O6/c26-25(27,28)14-5-3-11(7-13(14)21(31)38)1-2-12(4-6-15(29)24(39)40)8-16-18(36)19(37)23(41-16)35-10-34-17-20(30)32-9-33-22(17)35/h3,5,7,9-10,12,15-16,18-19,23,36-37H,4,6,8,29H2,(H2,31,38)(H,39,40)(H2,30,32,33)/t12-,15-,16+,18+,19+,23+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioKin Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged wild type human NNMT expressed in Escherichia coli NiCo21(DE3) assessed as reduction in 1-methylquinolinium leve... |
J Med Chem 62: 9837-9873 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01238
BindingDB Entry DOI: 10.7270/Q2D50RDJ |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50587817
(CHEMBL5186540)Show SMILES CN1\C(=C\C=C\C=C\C2=[N+](CCCCCC(=O)NCc3cn(CCCNC(=O)[C@H](Cc4ccc(O)cc4)NC(=O)[C@H](Cc4ccccc4)NC(=O)CCC(=O)NCCCCCC(=O)N(CCCC[C@H](NC(=O)N[C@@H](CCC(O)=O)C(O)=O)C(O)=O)Cc4cccc(Cl)c4)nn3)c3ccc(cc3C2(C)C)S([O-])(=O)=O)C(C)(C)c2cc(ccc12)S(O)(=O)=O |r,c:8| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01935
BindingDB Entry DOI: 10.7270/Q2NS0ZWP |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10885

((4R)-4-(ethylamino)-2-(3-methoxypropyl)-1,1-dioxo-...)Show SMILES CCN[C@H]1CN(CCCOC)S(=O)(=O)c2sc(cc12)S(N)(=O)=O |r| Show InChI InChI=1S/C12H21N3O5S3/c1-3-14-10-8-15(5-4-6-20-2)23(18,19)12-9(10)7-11(21-12)22(13,16)17/h7,10,14H,3-6,8H2,1-2H3,(H2,13,16,17)/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Universit£ Montpellier II
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase II by spectrophotometry at pH 7.5 |
Bioorg Med Chem 19: 1172-8 (2011)
Article DOI: 10.1016/j.bmc.2010.12.048
BindingDB Entry DOI: 10.7270/Q2C53M44 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Nicotinamide N-methyltransferase
(Homo sapiens (Human)) | BDBM50530724
(CHEMBL4519184)Show SMILES Cc1ccc(cc1C(N)=O)C#C[C@@H](CC[C@H](N)C(O)=O)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C25H29N7O6/c1-12-2-3-13(8-15(12)22(28)35)4-5-14(6-7-16(26)25(36)37)9-17-19(33)20(34)24(38-17)32-11-31-18-21(27)29-10-30-23(18)32/h2-3,8,10-11,14,16-17,19-20,24,33-34H,6-7,9,26H2,1H3,(H2,28,35)(H,36,37)(H2,27,29,30)/t14-,16-,17+,19+,20+,24+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioKin Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged wild type human NNMT expressed in Escherichia coli NiCo21(DE3) assessed as reduction in 1-methylquinolinium leve... |
J Med Chem 62: 9837-9873 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01238
BindingDB Entry DOI: 10.7270/Q2D50RDJ |
More data for this
Ligand-Target Pair | |
Nicotinamide N-methyltransferase
(Homo sapiens (Human)) | BDBM50530724

(CHEMBL4519184)Show SMILES Cc1ccc(cc1C(N)=O)C#C[C@@H](CC[C@H](N)C(O)=O)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C25H29N7O6/c1-12-2-3-13(8-15(12)22(28)35)4-5-14(6-7-16(26)25(36)37)9-17-19(33)20(34)24(38-17)32-11-31-18-21(27)29-10-30-23(18)32/h2-3,8,10-11,14,16-17,19-20,24,33-34H,6-7,9,26H2,1H3,(H2,28,35)(H,36,37)(H2,27,29,30)/t14-,16-,17+,19+,20+,24+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioKin Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged wild type human NNMT expressed in Escherichia coli NiCo21(DE3) assessed as reduction in 1-methylquinolinium leve... |
J Med Chem 62: 9837-9873 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01238
BindingDB Entry DOI: 10.7270/Q2D50RDJ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50098102

(CHEMBL23350 | Sulfamic acid 4-chloro-phenyl ester)Show InChI InChI=1S/C6H6ClNO3S/c7-5-1-3-6(4-2-5)11-12(8,9)10/h1-4H,(H2,8,9,10) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Universit£ Montpellier II
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase I by spectrophotometry at pH 7.5 |
Bioorg Med Chem 19: 1172-8 (2011)
Article DOI: 10.1016/j.bmc.2010.12.048
BindingDB Entry DOI: 10.7270/Q2C53M44 |
More data for this
Ligand-Target Pair | |
Nicotinamide N-methyltransferase
(Homo sapiens (Human)) | BDBM50530735

(CHEMBL4435488)Show SMILES N[C@@H](CC[C@H](CCc1cccc(c1)C(N)=O)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(O)=O |r| Show InChI InChI=1S/C24H31N7O6/c25-15(24(35)36)7-6-13(5-4-12-2-1-3-14(8-12)21(27)34)9-16-18(32)19(33)23(37-16)31-11-30-17-20(26)28-10-29-22(17)31/h1-3,8,10-11,13,15-16,18-19,23,32-33H,4-7,9,25H2,(H2,27,34)(H,35,36)(H2,26,28,29)/t13-,15-,16+,18+,19+,23+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioKin Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged wild type human NNMT expressed in Escherichia coli NiCo21(DE3) assessed as reduction in 1-methylquinolinium leve... |
J Med Chem 62: 9837-9873 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01238
BindingDB Entry DOI: 10.7270/Q2D50RDJ |
More data for this
Ligand-Target Pair | |
Nicotinamide N-methyltransferase
(Homo sapiens (Human)) | BDBM50530735

(CHEMBL4435488)Show SMILES N[C@@H](CC[C@H](CCc1cccc(c1)C(N)=O)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(O)=O |r| Show InChI InChI=1S/C24H31N7O6/c25-15(24(35)36)7-6-13(5-4-12-2-1-3-14(8-12)21(27)34)9-16-18(32)19(33)23(37-16)31-11-30-17-20(26)28-10-29-22(17)31/h1-3,8,10-11,13,15-16,18-19,23,32-33H,4-7,9,25H2,(H2,27,34)(H,35,36)(H2,26,28,29)/t13-,15-,16+,18+,19+,23+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioKin Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged wild type human NNMT expressed in Escherichia coli NiCo21(DE3) assessed as reduction in 1-methylquinolinium leve... |
J Med Chem 62: 9837-9873 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01238
BindingDB Entry DOI: 10.7270/Q2D50RDJ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM11635

(4-sulfamoyl-N-[2-(4-sulfamoylphenyl)ethyl]benzamid...)Show SMILES NS(=O)(=O)c1ccc(CCNC(=O)c2ccc(cc2)S(N)(=O)=O)cc1 Show InChI InChI=1S/C15H17N3O5S2/c16-24(20,21)13-5-1-11(2-6-13)9-10-18-15(19)12-3-7-14(8-4-12)25(17,22)23/h1-8H,9-10H2,(H,18,19)(H2,16,20,21)(H2,17,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Universit£ Montpellier II
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase II by spectrophotometry at pH 7.5 |
Bioorg Med Chem 19: 1172-8 (2011)
Article DOI: 10.1016/j.bmc.2010.12.048
BindingDB Entry DOI: 10.7270/Q2C53M44 |
More data for this
Ligand-Target Pair | |
Nicotinamide N-methyltransferase
(Homo sapiens (Human)) | BDBM50530727
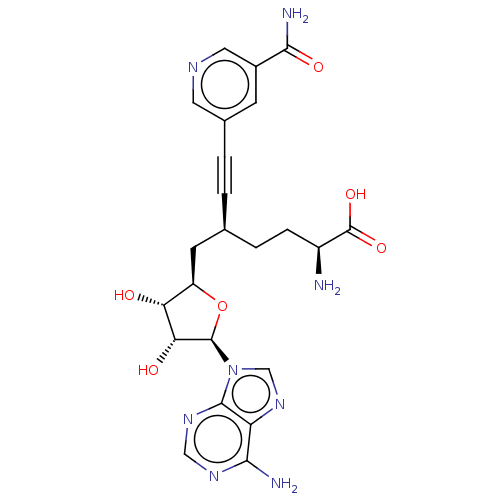
(CHEMBL4457046)Show SMILES N[C@@H](CC[C@@H](C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C#Cc1cncc(c1)C(N)=O)C(O)=O |r| Show InChI InChI=1S/C23H26N8O6/c24-14(23(35)36)4-3-11(1-2-12-5-13(20(26)34)8-27-7-12)6-15-17(32)18(33)22(37-15)31-10-30-16-19(25)28-9-29-21(16)31/h5,7-11,14-15,17-18,22,32-33H,3-4,6,24H2,(H2,26,34)(H,35,36)(H2,25,28,29)/t11-,14-,15+,17+,18+,22+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioKin Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged wild type human NNMT expressed in Escherichia coli NiCo21(DE3) assessed as reduction in 1-methylquinolinium leve... |
J Med Chem 62: 9837-9873 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01238
BindingDB Entry DOI: 10.7270/Q2D50RDJ |
More data for this
Ligand-Target Pair | |
Nicotinamide N-methyltransferase
(Homo sapiens (Human)) | BDBM50530727

(CHEMBL4457046)Show SMILES N[C@@H](CC[C@@H](C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C#Cc1cncc(c1)C(N)=O)C(O)=O |r| Show InChI InChI=1S/C23H26N8O6/c24-14(23(35)36)4-3-11(1-2-12-5-13(20(26)34)8-27-7-12)6-15-17(32)18(33)22(37-15)31-10-30-16-19(25)28-9-29-21(16)31/h5,7-11,14-15,17-18,22,32-33H,3-4,6,24H2,(H2,26,34)(H,35,36)(H2,25,28,29)/t11-,14-,15+,17+,18+,22+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioKin Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged wild type human NNMT expressed in Escherichia coli NiCo21(DE3) assessed as reduction in 1-methylquinolinium leve... |
J Med Chem 62: 9837-9873 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01238
BindingDB Entry DOI: 10.7270/Q2D50RDJ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Universit£ Montpellier II
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase XII by spectrophotometry at pH 7.5 |
Bioorg Med Chem 19: 1172-8 (2011)
Article DOI: 10.1016/j.bmc.2010.12.048
BindingDB Entry DOI: 10.7270/Q2C53M44 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM10870

(2-N-(4-aminobenzene)-1,3,4-thiadiazole-2,5-disulfo...)Show InChI InChI=1S/C8H9N5O4S3/c9-5-1-3-6(4-2-5)20(16,17)13-7-11-12-8(18-7)19(10,14)15/h1-4H,9H2,(H,11,13)(H2,10,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Universit£ Montpellier II
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase I by spectrophotometry at pH 7.5 |
Bioorg Med Chem 19: 1172-8 (2011)
Article DOI: 10.1016/j.bmc.2010.12.048
BindingDB Entry DOI: 10.7270/Q2C53M44 |
More data for this
Ligand-Target Pair | |
Nicotinamide N-methyltransferase
(Homo sapiens (Human)) | BDBM50530738
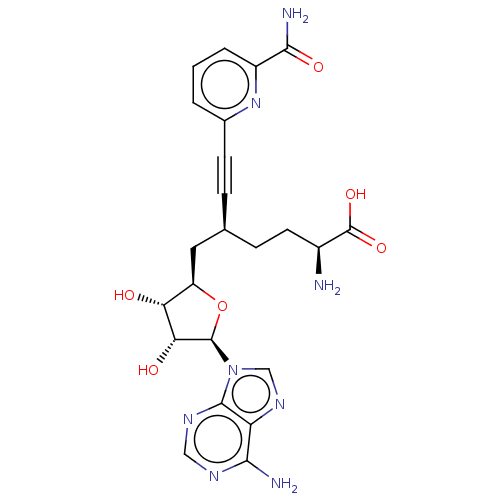
(CHEMBL4473236)Show SMILES N[C@@H](CC[C@@H](C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C#Cc1cccc(n1)C(N)=O)C(O)=O |r| Show InChI InChI=1S/C23H26N8O6/c24-13(23(35)36)7-5-11(4-6-12-2-1-3-14(30-12)20(26)34)8-15-17(32)18(33)22(37-15)31-10-29-16-19(25)27-9-28-21(16)31/h1-3,9-11,13,15,17-18,22,32-33H,5,7-8,24H2,(H2,26,34)(H,35,36)(H2,25,27,28)/t11-,13-,15+,17+,18+,22+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioKin Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged wild type human NNMT expressed in Escherichia coli NiCo21(DE3) assessed as reduction in 1-methylquinolinium leve... |
J Med Chem 62: 9837-9873 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01238
BindingDB Entry DOI: 10.7270/Q2D50RDJ |
More data for this
Ligand-Target Pair | |
Nicotinamide N-methyltransferase
(Homo sapiens (Human)) | BDBM50530738

(CHEMBL4473236)Show SMILES N[C@@H](CC[C@@H](C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C#Cc1cccc(n1)C(N)=O)C(O)=O |r| Show InChI InChI=1S/C23H26N8O6/c24-13(23(35)36)7-5-11(4-6-12-2-1-3-14(30-12)20(26)34)8-15-17(32)18(33)22(37-15)31-10-29-16-19(25)27-9-28-21(16)31/h1-3,9-11,13,15,17-18,22,32-33H,5,7-8,24H2,(H2,26,34)(H,35,36)(H2,25,27,28)/t11-,13-,15+,17+,18+,22+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioKin Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged wild type human NNMT expressed in Escherichia coli NiCo21(DE3) assessed as reduction in 1-methylquinolinium leve... |
J Med Chem 62: 9837-9873 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01238
BindingDB Entry DOI: 10.7270/Q2D50RDJ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10882

(6-ethoxy-1,3-benzothiazole-2-sulfonamide | CHEMBL1...)Show InChI InChI=1S/C9H10N2O3S2/c1-2-14-6-3-4-7-8(5-6)15-9(11-7)16(10,12)13/h3-5H,2H2,1H3,(H2,10,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Universit£ Montpellier II
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase II by spectrophotometry at pH 7.5 |
Bioorg Med Chem 19: 1172-8 (2011)
Article DOI: 10.1016/j.bmc.2010.12.048
BindingDB Entry DOI: 10.7270/Q2C53M44 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nicotinamide N-methyltransferase
(Homo sapiens (Human)) | BDBM50530726
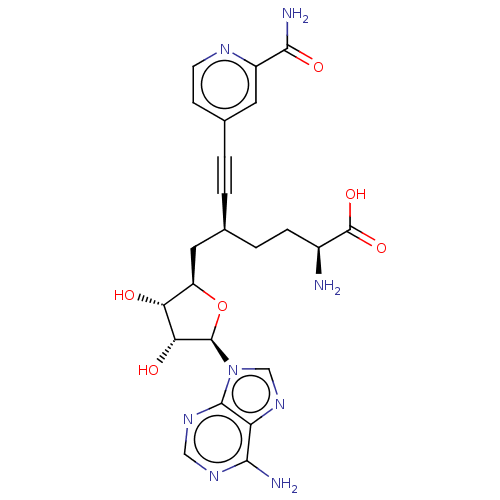
(CHEMBL4587553)Show SMILES N[C@@H](CC[C@@H](C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C#Cc1ccnc(c1)C(N)=O)C(O)=O |r| Show InChI InChI=1S/C23H26N8O6/c24-13(23(35)36)4-3-11(1-2-12-5-6-27-14(7-12)20(26)34)8-15-17(32)18(33)22(37-15)31-10-30-16-19(25)28-9-29-21(16)31/h5-7,9-11,13,15,17-18,22,32-33H,3-4,8,24H2,(H2,26,34)(H,35,36)(H2,25,28,29)/t11-,13-,15+,17+,18+,22+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioKin Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged wild type human NNMT expressed in Escherichia coli NiCo21(DE3) assessed as reduction in 1-methylquinolinium leve... |
J Med Chem 62: 9837-9873 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01238
BindingDB Entry DOI: 10.7270/Q2D50RDJ |
More data for this
Ligand-Target Pair | |
Nicotinamide N-methyltransferase
(Homo sapiens (Human)) | BDBM50530726

(CHEMBL4587553)Show SMILES N[C@@H](CC[C@@H](C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C#Cc1ccnc(c1)C(N)=O)C(O)=O |r| Show InChI InChI=1S/C23H26N8O6/c24-13(23(35)36)4-3-11(1-2-12-5-6-27-14(7-12)20(26)34)8-15-17(32)18(33)22(37-15)31-10-30-16-19(25)28-9-29-21(16)31/h5-7,9-11,13,15,17-18,22,32-33H,3-4,8,24H2,(H2,26,34)(H,35,36)(H2,25,28,29)/t11-,13-,15+,17+,18+,22+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioKin Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged wild type human NNMT expressed in Escherichia coli NiCo21(DE3) assessed as reduction in 1-methylquinolinium leve... |
J Med Chem 62: 9837-9873 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01238
BindingDB Entry DOI: 10.7270/Q2D50RDJ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10884

((2S,4S)-4-(ethylamino)-2-methyl-1,1-dioxo-2H,3H,4H...)Show SMILES CCN[C@H]1C[C@H](C)S(=O)(=O)c2sc(cc12)S(N)(=O)=O |r| Show InChI InChI=1S/C10H16N2O4S3/c1-3-12-8-4-6(2)18(13,14)10-7(8)5-9(17-10)19(11,15)16/h5-6,8,12H,3-4H2,1-2H3,(H2,11,15,16)/t6-,8-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Universit£ Montpellier II
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase II by spectrophotometry at pH 7.5 |
Bioorg Med Chem 19: 1172-8 (2011)
Article DOI: 10.1016/j.bmc.2010.12.048
BindingDB Entry DOI: 10.7270/Q2C53M44 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10886
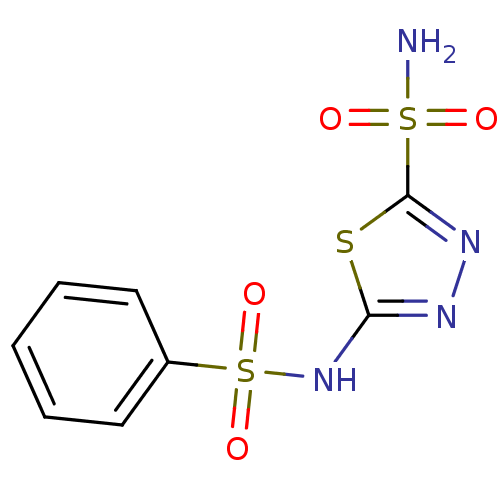
(2-N-benzene-1,3,4-thiadiazole-2,5-disulfonamide | ...)Show InChI InChI=1S/C8H8N4O4S3/c9-18(13,14)8-11-10-7(17-8)12-19(15,16)6-4-2-1-3-5-6/h1-5H,(H,10,12)(H2,9,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Universit£ Montpellier II
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase II by spectrophotometry at pH 7.5 |
Bioorg Med Chem 19: 1172-8 (2011)
Article DOI: 10.1016/j.bmc.2010.12.048
BindingDB Entry DOI: 10.7270/Q2C53M44 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10887

(Sulfamate 7 | Topiramate (TPM) | US11535599, Examp...)Show SMILES CC1(C)O[C@@H]2CO[C@@]3(COS(N)(=O)=O)OC(C)(C)O[C@H]3[C@@H]2O1 |r| Show InChI InChI=1S/C12H21NO8S/c1-10(2)18-7-5-16-12(6-17-22(13,14)15)9(8(7)19-10)20-11(3,4)21-12/h7-9H,5-6H2,1-4H3,(H2,13,14,15)/t7-,8-,9+,12+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Universit£ Montpellier II
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase II by spectrophotometry at pH 7.5 |
Bioorg Med Chem 19: 1172-8 (2011)
Article DOI: 10.1016/j.bmc.2010.12.048
BindingDB Entry DOI: 10.7270/Q2C53M44 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50336639
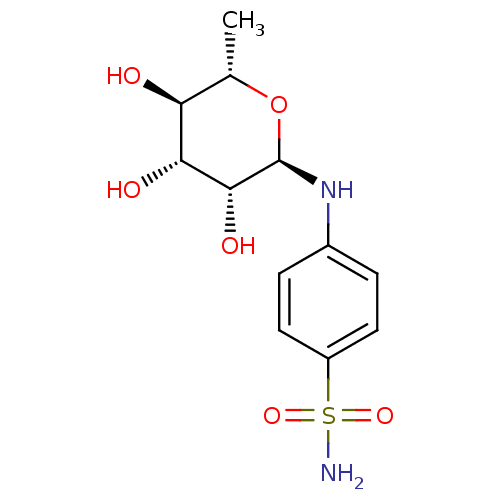
(4-((2R,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyltetra...)Show SMILES C[C@@H]1O[C@@H](Nc2ccc(cc2)S(N)(=O)=O)[C@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C12H18N2O6S/c1-6-9(15)10(16)11(17)12(20-6)14-7-2-4-8(5-3-7)21(13,18)19/h2-6,9-12,14-17H,1H3,(H2,13,18,19)/t6-,9-,10+,11+,12+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Universit£ Montpellier II
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase II by spectrophotometry at pH 7.5 |
Bioorg Med Chem 19: 1172-8 (2011)
Article DOI: 10.1016/j.bmc.2010.12.048
BindingDB Entry DOI: 10.7270/Q2C53M44 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Universit£ Montpellier II
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase II by spectrophotometry at pH 7.5 |
Bioorg Med Chem 19: 1172-8 (2011)
Article DOI: 10.1016/j.bmc.2010.12.048
BindingDB Entry DOI: 10.7270/Q2C53M44 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10875
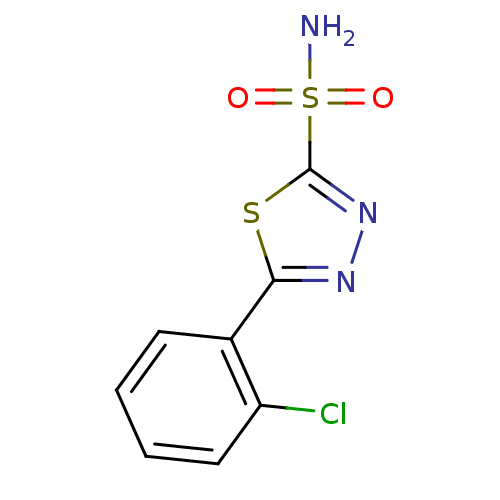
(5-(2-chlorophenyl)-1,3,4-thiadiazole-2-sulfonamide...)Show InChI InChI=1S/C8H6ClN3O2S2/c9-6-4-2-1-3-5(6)7-11-12-8(15-7)16(10,13)14/h1-4H,(H2,10,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
| MMDB
PDB
Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Universit£ Montpellier II
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase II by spectrophotometry at pH 7.5 |
Bioorg Med Chem 19: 1172-8 (2011)
Article DOI: 10.1016/j.bmc.2010.12.048
BindingDB Entry DOI: 10.7270/Q2C53M44 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10881

(CHEMBL288100 | MZA3 | Methazolamide | Methazolamid...)Show InChI InChI=1S/C5H8N4O3S2/c1-3(10)7-4-9(2)8-5(13-4)14(6,11)12/h1-2H3,(H2,6,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Universit£ Montpellier II
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase II by spectrophotometry at pH 7.5 |
Bioorg Med Chem 19: 1172-8 (2011)
Article DOI: 10.1016/j.bmc.2010.12.048
BindingDB Entry DOI: 10.7270/Q2C53M44 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM33281
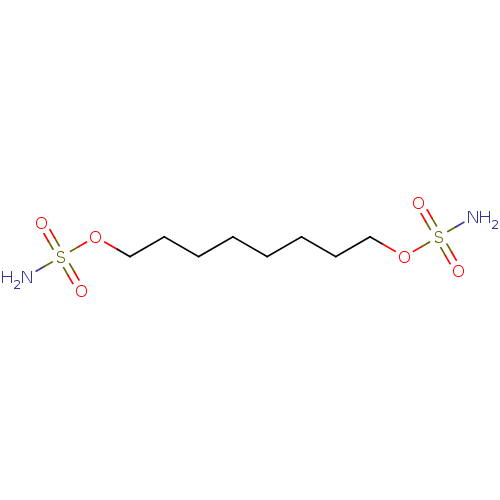
(CHEMBL182455 | bis-sulfamate, 3)Show InChI InChI=1S/C8H20N2O6S2/c9-17(11,12)15-7-5-3-1-2-4-6-8-16-18(10,13)14/h1-8H2,(H2,9,11,12)(H2,10,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 14.6 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Universit£ Montpellier II
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase II by spectrophotometry at pH 7.5 |
Bioorg Med Chem 19: 1172-8 (2011)
Article DOI: 10.1016/j.bmc.2010.12.048
BindingDB Entry DOI: 10.7270/Q2C53M44 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM10886

(2-N-benzene-1,3,4-thiadiazole-2,5-disulfonamide | ...)Show InChI InChI=1S/C8H8N4O4S3/c9-18(13,14)8-11-10-7(17-8)12-19(15,16)6-4-2-1-3-5-6/h1-5H,(H,10,12)(H2,9,13,14) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Universit£ Montpellier II
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase I by spectrophotometry at pH 7.5 |
Bioorg Med Chem 19: 1172-8 (2011)
Article DOI: 10.1016/j.bmc.2010.12.048
BindingDB Entry DOI: 10.7270/Q2C53M44 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10890

(1-N-(3-chloro-1H-indol-7-yl)benzene-1,4-disulfonam...)Show SMILES NS(=O)(=O)c1ccc(cc1)S(=O)(=O)Nc1cccc2c(Cl)c[nH]c12 Show InChI InChI=1S/C14H12ClN3O4S2/c15-12-8-17-14-11(12)2-1-3-13(14)18-24(21,22)10-6-4-9(5-7-10)23(16,19)20/h1-8,17-18H,(H2,16,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Universit£ Montpellier II
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase II by spectrophotometry at pH 7.5 |
Bioorg Med Chem 19: 1172-8 (2011)
Article DOI: 10.1016/j.bmc.2010.12.048
BindingDB Entry DOI: 10.7270/Q2C53M44 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50314771
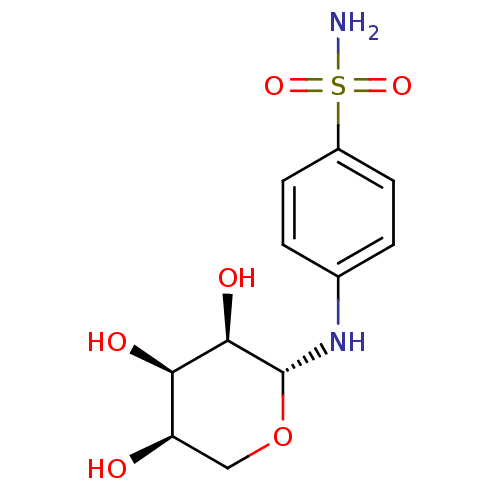
(4-((2R,3R,4R,5R)-3,4,5-trihydroxytetrahydro-2H-pyr...)Show SMILES NS(=O)(=O)c1ccc(N[C@@H]2OC[C@@H](O)[C@@H](O)[C@H]2O)cc1 |r| Show InChI InChI=1S/C11H16N2O6S/c12-20(17,18)7-3-1-6(2-4-7)13-11-10(16)9(15)8(14)5-19-11/h1-4,8-11,13-16H,5H2,(H2,12,17,18)/t8-,9-,10-,11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Universit£ Montpellier II
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase II by spectrophotometry at pH 7.5 |
Bioorg Med Chem 19: 1172-8 (2011)
Article DOI: 10.1016/j.bmc.2010.12.048
BindingDB Entry DOI: 10.7270/Q2C53M44 |
More data for this
Ligand-Target Pair | |
Nicotinamide N-methyltransferase
(Homo sapiens (Human)) | BDBM50530720

(CHEMBL4565052)Show SMILES N[C@@H](CC[C@@H](C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C#Cc1cccc(c1)C(N)=O)C(N)=O |r| Show InChI InChI=1S/C24H28N8O5/c25-15(22(28)36)7-6-13(5-4-12-2-1-3-14(8-12)21(27)35)9-16-18(33)19(34)24(37-16)32-11-31-17-20(26)29-10-30-23(17)32/h1-3,8,10-11,13,15-16,18-19,24,33-34H,6-7,9,25H2,(H2,27,35)(H2,28,36)(H2,26,29,30)/t13-,15-,16+,18+,19+,24+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioKin Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged wild type human NNMT expressed in Escherichia coli NiCo21(DE3) assessed as reduction in 1-methylquinolinium leve... |
J Med Chem 62: 9837-9873 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01238
BindingDB Entry DOI: 10.7270/Q2D50RDJ |
More data for this
Ligand-Target Pair | |
Nicotinamide N-methyltransferase
(Homo sapiens (Human)) | BDBM50530720

(CHEMBL4565052)Show SMILES N[C@@H](CC[C@@H](C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C#Cc1cccc(c1)C(N)=O)C(N)=O |r| Show InChI InChI=1S/C24H28N8O5/c25-15(22(28)36)7-6-13(5-4-12-2-1-3-14(8-12)21(27)35)9-16-18(33)19(34)24(37-16)32-11-31-17-20(26)29-10-30-23(17)32/h1-3,8,10-11,13,15-16,18-19,24,33-34H,6-7,9,25H2,(H2,27,35)(H2,28,36)(H2,26,29,30)/t13-,15-,16+,18+,19+,24+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioKin Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged wild type human NNMT expressed in Escherichia coli NiCo21(DE3) assessed as reduction in 1-methylquinolinium leve... |
J Med Chem 62: 9837-9873 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01238
BindingDB Entry DOI: 10.7270/Q2D50RDJ |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50587818

(CHEMBL5206903)Show SMILES CN1\C(=C\C=C2/CCCC(\C=C\C3=[N+](CCCCCC(=O)NCc4cn(CCCNC(=O)[C@H](Cc5ccc(O)cc5)NC(=O)[C@H](Cc5ccccc5)NC(=O)CCC(=O)NCCCCCC(=O)N(CCCC[C@H](NC(=O)N[C@@H](CCC(O)=O)C(O)=O)C(O)=O)Cc5cccc(Cl)c5)nn4)c4ccc(cc4C3(C)C)S([O-])(=O)=O)=C2)C(C)(C)c2cc(ccc12)S(O)(=O)=O |r,c:12,117| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01935
BindingDB Entry DOI: 10.7270/Q2NS0ZWP |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10869

(5-imino-4-methyl-4,5-dihydro-1,3,4-thiadiazole-2-s...)Show InChI InChI=1S/C3H6N4O2S2/c1-7-2(4)10-3(6-7)11(5,8)9/h4H,1H3,(H2,5,8,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Universit£ Montpellier II
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase II by spectrophotometry at pH 7.5 |
Bioorg Med Chem 19: 1172-8 (2011)
Article DOI: 10.1016/j.bmc.2010.12.048
BindingDB Entry DOI: 10.7270/Q2C53M44 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Universit£ Montpellier II
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase II by spectrophotometry at pH 7.5 |
Bioorg Med Chem 19: 1172-8 (2011)
Article DOI: 10.1016/j.bmc.2010.12.048
BindingDB Entry DOI: 10.7270/Q2C53M44 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data