 Found 932 hits with Last Name = 'stokes' and Initial = 's'
Found 932 hits with Last Name = 'stokes' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM50534816

(CHEMBL4591500)Show SMILES COc1nnc2ccc(nn12)N1CCC(CC1)c1ccc(OCCN2[C@@H](C)CN(C[C@H]2C)C(C)=O)cc1 |r| Show InChI InChI=1S/C27H37N7O3/c1-19-17-32(21(3)35)18-20(2)33(19)15-16-37-24-7-5-22(6-8-24)23-11-13-31(14-12-23)26-10-9-25-28-29-27(36-4)34(25)30-26/h5-10,19-20,23H,11-18H2,1-4H3/t19-,20+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of Alexa647-labeled JQ1 derivative from wild type BRD4 tandem domain (44 to 460 residues) (unknown origin) incubated for 1 hr by fluores... |
J Med Chem 59: 7801-17 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00070
BindingDB Entry DOI: 10.7270/Q2697721 |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-beta
(Homo sapiens (Human)) | BDBM50394058
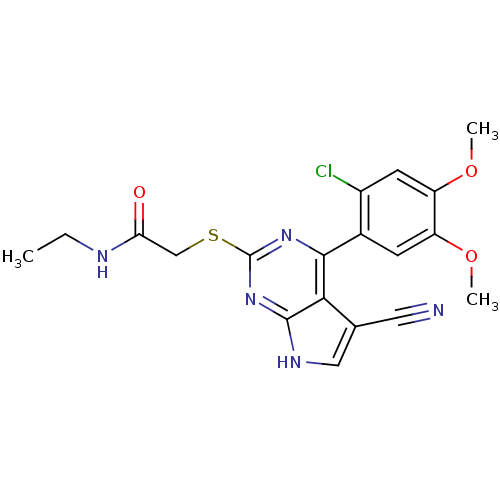
(CHEMBL2158570)Show SMILES CCNC(=O)CSc1nc(-c2cc(OC)c(OC)cc2Cl)c2c(c[nH]c2n1)C#N Show InChI InChI=1S/C19H18ClN5O3S/c1-4-22-15(26)9-29-19-24-17(16-10(7-21)8-23-18(16)25-19)11-5-13(27-2)14(28-3)6-12(11)20/h5-6,8H,4,9H2,1-3H3,(H,22,26)(H,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of fluorescently labeled VER51001 binding to full length human HSP90beta after 30 mins by fluorescence polarization assay |
Bioorg Med Chem 20: 6770-89 (2012)
Article DOI: 10.1016/j.bmc.2012.08.050
BindingDB Entry DOI: 10.7270/Q2KS6SN4 |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-beta
(Homo sapiens (Human)) | BDBM50394065
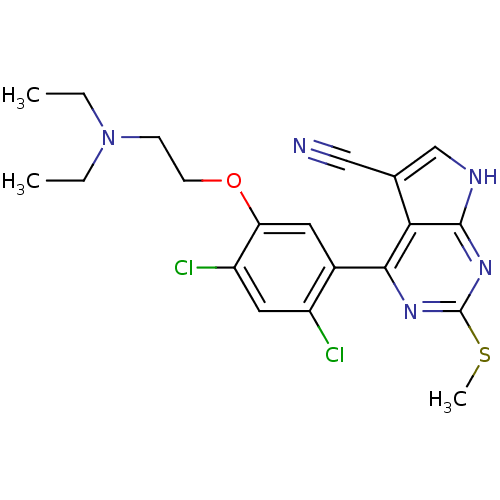
(CHEMBL2158626)Show SMILES CCN(CC)CCOc1cc(c(Cl)cc1Cl)-c1nc(SC)nc2[nH]cc(C#N)c12 Show InChI InChI=1S/C20H21Cl2N5OS/c1-4-27(5-2)6-7-28-16-8-13(14(21)9-15(16)22)18-17-12(10-23)11-24-19(17)26-20(25-18)29-3/h8-9,11H,4-7H2,1-3H3,(H,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of fluorescently labeled VER51001 binding to full length human HSP90beta after 30 mins by fluorescence polarization assay |
Bioorg Med Chem 20: 6770-89 (2012)
Article DOI: 10.1016/j.bmc.2012.08.050
BindingDB Entry DOI: 10.7270/Q2KS6SN4 |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-beta
(Homo sapiens (Human)) | BDBM50394056
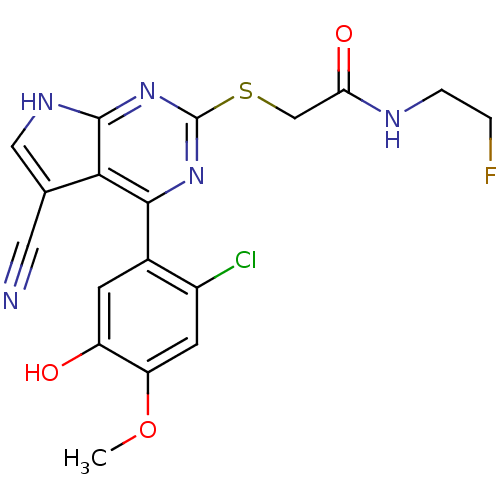
(CHEMBL2158577)Show SMILES COc1cc(Cl)c(cc1O)-c1nc(SCC(=O)NCCF)nc2[nH]cc(C#N)c12 Show InChI InChI=1S/C18H15ClFN5O3S/c1-28-13-5-11(19)10(4-12(13)26)16-15-9(6-21)7-23-17(15)25-18(24-16)29-8-14(27)22-3-2-20/h4-5,7,26H,2-3,8H2,1H3,(H,22,27)(H,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of fluorescently labeled VER51001 binding to full length human HSP90beta after 30 mins by fluorescence polarization assay |
Bioorg Med Chem 20: 6770-89 (2012)
Article DOI: 10.1016/j.bmc.2012.08.050
BindingDB Entry DOI: 10.7270/Q2KS6SN4 |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-beta
(Homo sapiens (Human)) | BDBM50394064
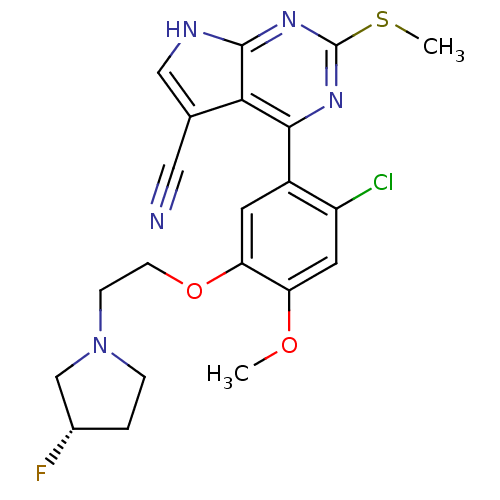
(CHEMBL2158627)Show SMILES COc1cc(Cl)c(cc1OCCN1CC[C@H](F)C1)-c1nc(SC)nc2[nH]cc(C#N)c12 |r| Show InChI InChI=1S/C21H21ClFN5O2S/c1-29-16-8-15(22)14(7-17(16)30-6-5-28-4-3-13(23)11-28)19-18-12(9-24)10-25-20(18)27-21(26-19)31-2/h7-8,10,13H,3-6,11H2,1-2H3,(H,25,26,27)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of fluorescently labeled VER51001 binding to full length human HSP90beta after 30 mins by fluorescence polarization assay |
Bioorg Med Chem 20: 6770-89 (2012)
Article DOI: 10.1016/j.bmc.2012.08.050
BindingDB Entry DOI: 10.7270/Q2KS6SN4 |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-beta
(Homo sapiens (Human)) | BDBM50394059
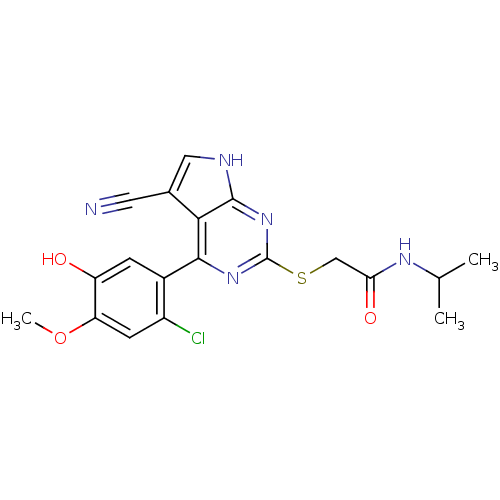
(CHEMBL2158569)Show SMILES COc1cc(Cl)c(cc1O)-c1nc(SCC(=O)NC(C)C)nc2[nH]cc(C#N)c12 Show InChI InChI=1S/C19H18ClN5O3S/c1-9(2)23-15(27)8-29-19-24-17(16-10(6-21)7-22-18(16)25-19)11-4-13(26)14(28-3)5-12(11)20/h4-5,7,9,26H,8H2,1-3H3,(H,23,27)(H,22,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of fluorescently labeled VER51001 binding to full length human HSP90beta after 30 mins by fluorescence polarization assay |
Bioorg Med Chem 20: 6770-89 (2012)
Article DOI: 10.1016/j.bmc.2012.08.050
BindingDB Entry DOI: 10.7270/Q2KS6SN4 |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM50534829

(CHEMBL4447492)Show SMILES COc1nnc2ccc(nn12)N1CCC(CC1)c1ccc(CCN2CCN(C)C(=O)[C@H]2C)cc1 |r| Show InChI InChI=1S/C25H33N7O2/c1-18-24(33)29(2)16-17-30(18)13-10-19-4-6-20(7-5-19)21-11-14-31(15-12-21)23-9-8-22-26-27-25(34-3)32(22)28-23/h4-9,18,21H,10-17H2,1-3H3/t18-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of Alexa647-labeled JQ1 derivative from wild type BRD4 tandem domain (44 to 460 residues) (unknown origin) incubated for 1 hr by fluores... |
J Med Chem 59: 7801-17 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00070
BindingDB Entry DOI: 10.7270/Q2697721 |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-beta
(Homo sapiens (Human)) | BDBM50394066
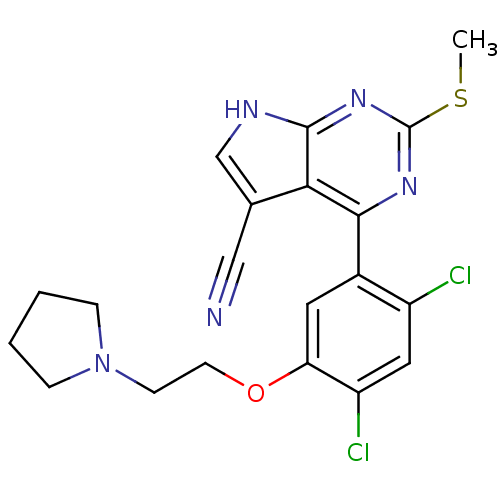
(CHEMBL2158625)Show SMILES CSc1nc(-c2cc(OCCN3CCCC3)c(Cl)cc2Cl)c2c(c[nH]c2n1)C#N Show InChI InChI=1S/C20H19Cl2N5OS/c1-29-20-25-18(17-12(10-23)11-24-19(17)26-20)13-8-16(15(22)9-14(13)21)28-7-6-27-4-2-3-5-27/h8-9,11H,2-7H2,1H3,(H,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of fluorescently labeled VER51001 binding to full length human HSP90beta after 30 mins by fluorescence polarization assay |
Bioorg Med Chem 20: 6770-89 (2012)
Article DOI: 10.1016/j.bmc.2012.08.050
BindingDB Entry DOI: 10.7270/Q2KS6SN4 |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM50534814
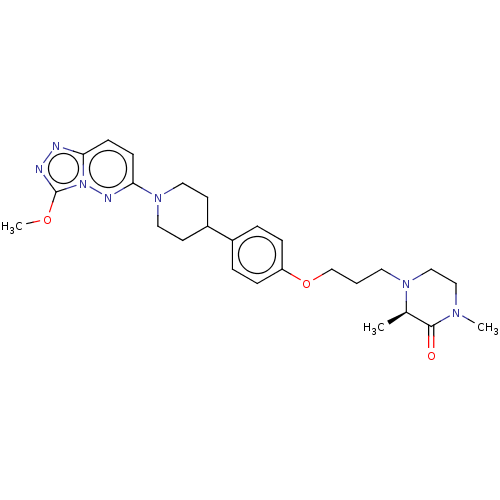
(CHEMBL4466676)Show SMILES COc1nnc2ccc(nn12)N1CCC(CC1)c1ccc(OCCCN2CCN(C)C(=O)[C@H]2C)cc1 |r| Show InChI InChI=1S/C26H35N7O3/c1-19-25(34)30(2)16-17-31(19)13-4-18-36-22-7-5-20(6-8-22)21-11-14-32(15-12-21)24-10-9-23-27-28-26(35-3)33(23)29-24/h5-10,19,21H,4,11-18H2,1-3H3/t19-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of Alexa647-labeled JQ1 derivative from wild type BRD4 tandem domain (44 to 460 residues) (unknown origin) incubated for 1 hr by fluores... |
J Med Chem 59: 7801-17 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00070
BindingDB Entry DOI: 10.7270/Q2697721 |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-beta
(Homo sapiens (Human)) | BDBM50394057
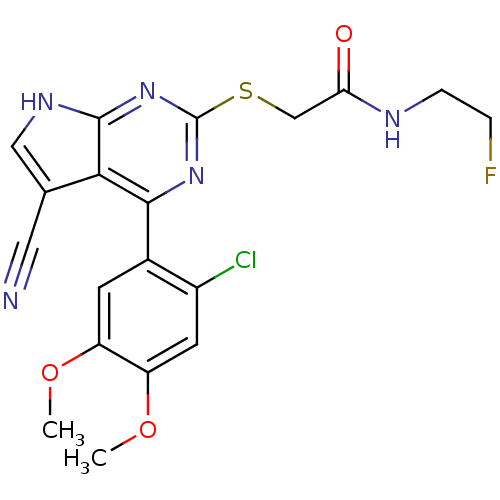
(CHEMBL2158576)Show SMILES COc1cc(Cl)c(cc1OC)-c1nc(SCC(=O)NCCF)nc2[nH]cc(C#N)c12 Show InChI InChI=1S/C19H17ClFN5O3S/c1-28-13-5-11(12(20)6-14(13)29-2)17-16-10(7-22)8-24-18(16)26-19(25-17)30-9-15(27)23-4-3-21/h5-6,8H,3-4,9H2,1-2H3,(H,23,27)(H,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of fluorescently labeled VER51001 binding to full length human HSP90beta after 30 mins by fluorescence polarization assay |
Bioorg Med Chem 20: 6770-89 (2012)
Article DOI: 10.1016/j.bmc.2012.08.050
BindingDB Entry DOI: 10.7270/Q2KS6SN4 |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM50260093
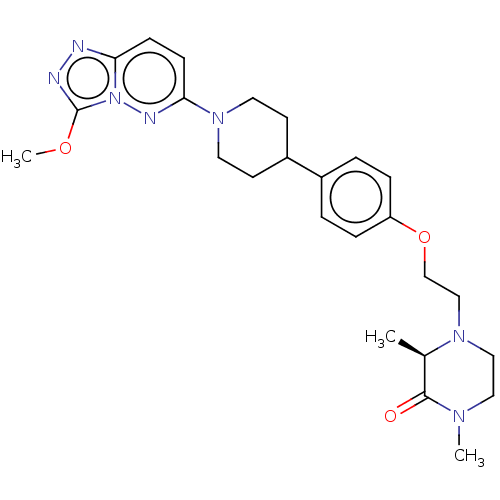
(CHEMBL4078100)Show SMILES COc1nnc2ccc(nn12)N1CCC(CC1)c1ccc(OCCN2CCN(C)C(=O)[C@H]2C)cc1 |r| Show InChI InChI=1S/C25H33N7O3/c1-18-24(33)29(2)14-15-30(18)16-17-35-21-6-4-19(5-7-21)20-10-12-31(13-11-20)23-9-8-22-26-27-25(34-3)32(22)28-23/h4-9,18,20H,10-17H2,1-3H3/t18-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of Alexa647-labeled JQ1 derivative from wild type BRD4 tandem domain (44 to 460 residues) (unknown origin) incubated for 1 hr by fluores... |
J Med Chem 59: 7801-17 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00070
BindingDB Entry DOI: 10.7270/Q2697721 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM50534827
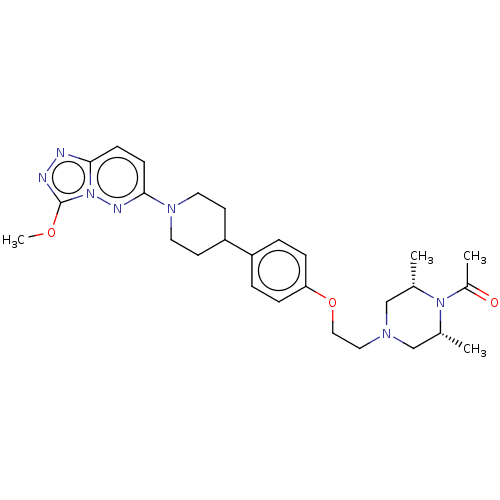
(CHEMBL4460682)Show SMILES COc1nnc2ccc(nn12)N1CCC(CC1)c1ccc(OCCN2C[C@H](C)N([C@H](C)C2)C(C)=O)cc1 |r| Show InChI InChI=1S/C27H37N7O3/c1-19-17-31(18-20(2)33(19)21(3)35)15-16-37-24-7-5-22(6-8-24)23-11-13-32(14-12-23)26-10-9-25-28-29-27(36-4)34(25)30-26/h5-10,19-20,23H,11-18H2,1-4H3/t19-,20+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of Alexa647-labeled JQ1 derivative from wild type BRD4 tandem domain (44 to 460 residues) (unknown origin) incubated for 1 hr by fluores... |
J Med Chem 59: 7801-17 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00070
BindingDB Entry DOI: 10.7270/Q2697721 |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM50534823
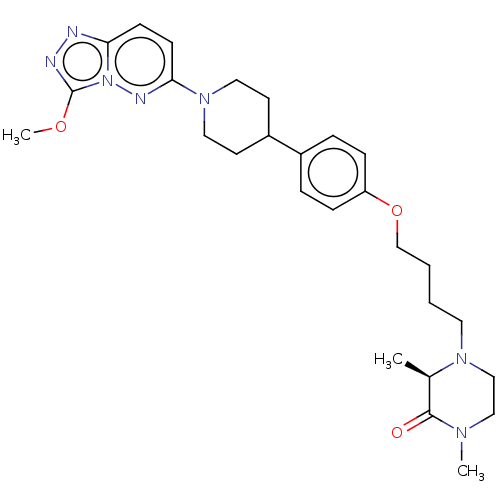
(CHEMBL4593653)Show SMILES COc1nnc2ccc(nn12)N1CCC(CC1)c1ccc(OCCCCN2CCN(C)C(=O)[C@H]2C)cc1 |r| Show InChI InChI=1S/C27H37N7O3/c1-20-26(35)31(2)17-18-32(20)14-4-5-19-37-23-8-6-21(7-9-23)22-12-15-33(16-13-22)25-11-10-24-28-29-27(36-3)34(24)30-25/h6-11,20,22H,4-5,12-19H2,1-3H3/t20-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of Alexa647-labeled JQ1 derivative from wild type BRD4 tandem domain (44 to 460 residues) (unknown origin) incubated for 1 hr by fluores... |
J Med Chem 59: 7801-17 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00070
BindingDB Entry DOI: 10.7270/Q2697721 |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-beta
(Homo sapiens (Human)) | BDBM50394062
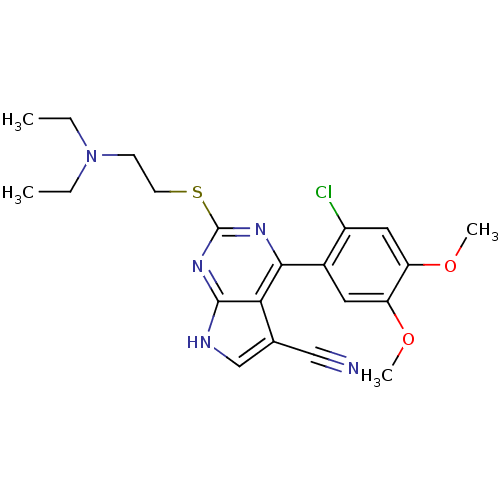
(CHEMBL2158630)Show SMILES CCN(CC)CCSc1nc(-c2cc(OC)c(OC)cc2Cl)c2c(c[nH]c2n1)C#N Show InChI InChI=1S/C21H24ClN5O2S/c1-5-27(6-2)7-8-30-21-25-19(18-13(11-23)12-24-20(18)26-21)14-9-16(28-3)17(29-4)10-15(14)22/h9-10,12H,5-8H2,1-4H3,(H,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of fluorescently labeled VER51001 binding to full length human HSP90beta after 30 mins by fluorescence polarization assay |
Bioorg Med Chem 20: 6770-89 (2012)
Article DOI: 10.1016/j.bmc.2012.08.050
BindingDB Entry DOI: 10.7270/Q2KS6SN4 |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM50534828

(CHEMBL4448398)Show SMILES C[C@H]1N(CCOc2ccc(cc2)C2CCN(CC2)c2ccc3nnc(n3n2)C(F)(F)F)CCN(C)C1=O |r| Show InChI InChI=1S/C25H30F3N7O2/c1-17-23(36)32(2)13-14-33(17)15-16-37-20-5-3-18(4-6-20)19-9-11-34(12-10-19)22-8-7-21-29-30-24(25(26,27)28)35(21)31-22/h3-8,17,19H,9-16H2,1-2H3/t17-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of Alexa647-labeled JQ1 derivative from wild type BRD4 tandem domain (44 to 460 residues) (unknown origin) incubated for 1 hr by fluores... |
J Med Chem 59: 7801-17 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00070
BindingDB Entry DOI: 10.7270/Q2697721 |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM50534815
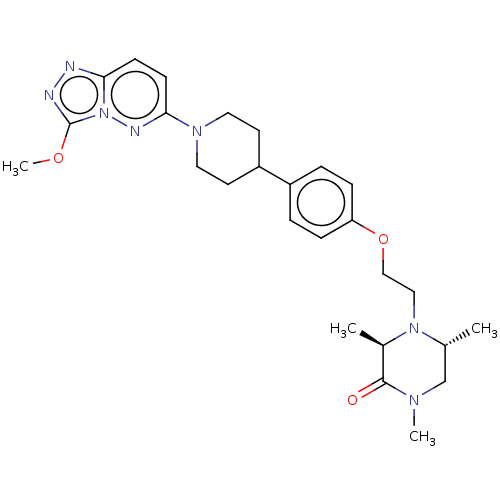
(CHEMBL4457035)Show SMILES COc1nnc2ccc(nn12)N1CCC(CC1)c1ccc(OCCN2[C@H](C)CN(C)C(=O)[C@H]2C)cc1 |r| Show InChI InChI=1S/C26H35N7O3/c1-18-17-30(3)25(34)19(2)32(18)15-16-36-22-7-5-20(6-8-22)21-11-13-31(14-12-21)24-10-9-23-27-28-26(35-4)33(23)29-24/h5-10,18-19,21H,11-17H2,1-4H3/t18-,19-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of Alexa647-labeled JQ1 derivative from wild type BRD4 tandem domain (44 to 460 residues) (unknown origin) incubated for 1 hr by fluores... |
J Med Chem 59: 7801-17 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00070
BindingDB Entry DOI: 10.7270/Q2697721 |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-beta
(Homo sapiens (Human)) | BDBM50394055
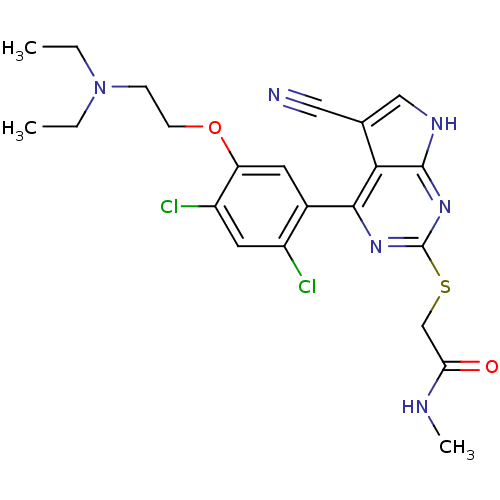
(CHEMBL2158563)Show SMILES CCN(CC)CCOc1cc(c(Cl)cc1Cl)-c1nc(SCC(=O)NC)nc2[nH]cc(C#N)c12 Show InChI InChI=1S/C22H24Cl2N6O2S/c1-4-30(5-2)6-7-32-17-8-14(15(23)9-16(17)24)20-19-13(10-25)11-27-21(19)29-22(28-20)33-12-18(31)26-3/h8-9,11H,4-7,12H2,1-3H3,(H,26,31)(H,27,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of fluorescently labeled VER51001 binding to full length human HSP90beta after 30 mins by fluorescence polarization assay |
Bioorg Med Chem 20: 6770-89 (2012)
Article DOI: 10.1016/j.bmc.2012.08.050
BindingDB Entry DOI: 10.7270/Q2KS6SN4 |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM50534813

(CHEMBL4453435)Show SMILES COc1nnc2ccc(nn12)N1CCC(CC1)c1ccc(cc1)N1CC(CN2CCN(C)C(=O)[C@H]2C)C1 |r| Show InChI InChI=1S/C27H36N8O2/c1-19-26(36)31(2)14-15-33(19)16-20-17-34(18-20)23-6-4-21(5-7-23)22-10-12-32(13-11-22)25-9-8-24-28-29-27(37-3)35(24)30-25/h4-9,19-20,22H,10-18H2,1-3H3/t19-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of Alexa647-labeled JQ1 derivative from wild type BRD4 tandem domain (44 to 460 residues) (unknown origin) incubated for 1 hr by fluores... |
J Med Chem 59: 7801-17 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00070
BindingDB Entry DOI: 10.7270/Q2697721 |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-beta
(Homo sapiens (Human)) | BDBM50394063
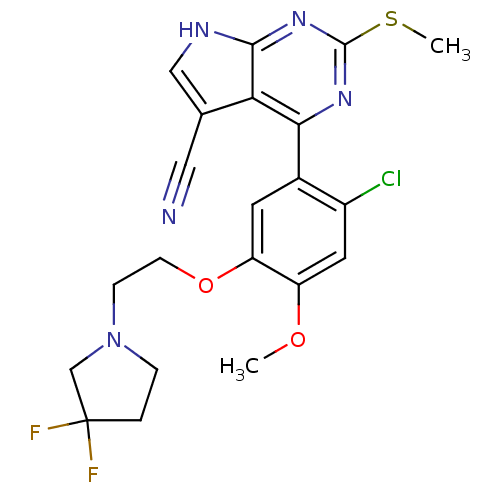
(CHEMBL2158628)Show SMILES COc1cc(Cl)c(cc1OCCN1CCC(F)(F)C1)-c1nc(SC)nc2[nH]cc(C#N)c12 Show InChI InChI=1S/C21H20ClF2N5O2S/c1-30-15-8-14(22)13(7-16(15)31-6-5-29-4-3-21(23,24)11-29)18-17-12(9-25)10-26-19(17)28-20(27-18)32-2/h7-8,10H,3-6,11H2,1-2H3,(H,26,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of fluorescently labeled VER51001 binding to full length human HSP90beta after 30 mins by fluorescence polarization assay |
Bioorg Med Chem 20: 6770-89 (2012)
Article DOI: 10.1016/j.bmc.2012.08.050
BindingDB Entry DOI: 10.7270/Q2KS6SN4 |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM50534819

(CHEMBL4470311)Show SMILES CSc1nnc2ccc(nn12)N1CCC(CC1)c1ccc(OCCN2CCN(C)C(=O)[C@H]2C)cc1 |r| Show InChI InChI=1S/C25H33N7O2S/c1-18-24(33)29(2)14-15-30(18)16-17-34-21-6-4-19(5-7-21)20-10-12-31(13-11-20)23-9-8-22-26-27-25(35-3)32(22)28-23/h4-9,18,20H,10-17H2,1-3H3/t18-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of Alexa647-labeled JQ1 derivative from wild type BRD4 tandem domain (44 to 460 residues) (unknown origin) incubated for 1 hr by fluores... |
J Med Chem 59: 7801-17 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00070
BindingDB Entry DOI: 10.7270/Q2697721 |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM50534825
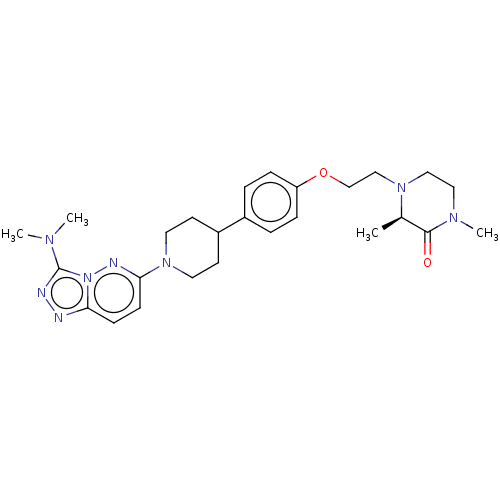
(CHEMBL4472759)Show SMILES C[C@H]1N(CCOc2ccc(cc2)C2CCN(CC2)c2ccc3nnc(N(C)C)n3n2)CCN(C)C1=O |r| Show InChI InChI=1S/C26H36N8O2/c1-19-25(35)31(4)15-16-32(19)17-18-36-22-7-5-20(6-8-22)21-11-13-33(14-12-21)24-10-9-23-27-28-26(30(2)3)34(23)29-24/h5-10,19,21H,11-18H2,1-4H3/t19-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of Alexa647-labeled JQ1 derivative from wild type BRD4 tandem domain (44 to 460 residues) (unknown origin) incubated for 1 hr by fluores... |
J Med Chem 59: 7801-17 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00070
BindingDB Entry DOI: 10.7270/Q2697721 |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM50534811
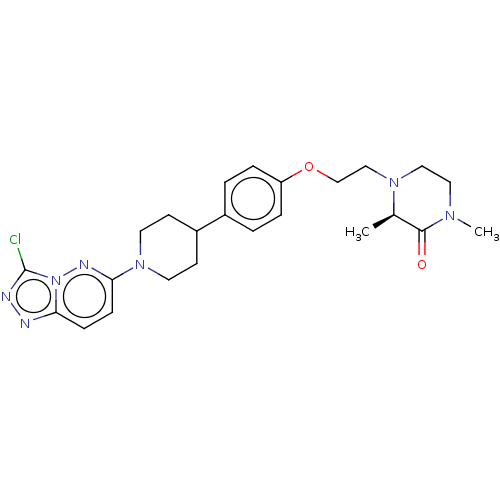
(CHEMBL4513991)Show SMILES C[C@H]1N(CCOc2ccc(cc2)C2CCN(CC2)c2ccc3nnc(Cl)n3n2)CCN(C)C1=O |r| Show InChI InChI=1S/C24H30ClN7O2/c1-17-23(33)29(2)13-14-30(17)15-16-34-20-5-3-18(4-6-20)19-9-11-31(12-10-19)22-8-7-21-26-27-24(25)32(21)28-22/h3-8,17,19H,9-16H2,1-2H3/t17-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of Alexa647-labeled JQ1 derivative from wild type BRD4 tandem domain (44 to 460 residues) (unknown origin) incubated for 1 hr by fluores... |
J Med Chem 59: 7801-17 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00070
BindingDB Entry DOI: 10.7270/Q2697721 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
[Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 1, mitochondrial
(Homo sapiens (Human)) | BDBM50236511
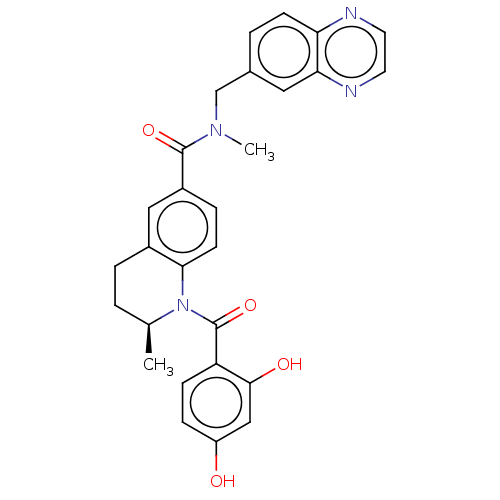
(CHEMBL3718319)Show SMILES C[C@H]1CCc2cc(ccc2N1C(=O)c1ccc(O)cc1O)C(=O)N(C)Cc1ccc2nccnc2c1 |r| Show InChI InChI=1S/C28H26N4O4/c1-17-3-5-19-14-20(6-10-25(19)32(17)28(36)22-8-7-21(33)15-26(22)34)27(35)31(2)16-18-4-9-23-24(13-18)30-12-11-29-23/h4,6-15,17,33-34H,3,5,16H2,1-2H3/t17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity towards human Dopamine receptor D2 (long) by [3H]-spiperone displacement. |
J Med Chem 60: 2271-2286 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01478
BindingDB Entry DOI: 10.7270/Q2XG9TDJ |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM50534816

(CHEMBL4591500)Show SMILES COc1nnc2ccc(nn12)N1CCC(CC1)c1ccc(OCCN2[C@@H](C)CN(C[C@H]2C)C(C)=O)cc1 |r| Show InChI InChI=1S/C27H37N7O3/c1-19-17-32(21(3)35)18-20(2)33(19)15-16-37-24-7-5-22(6-8-24)23-11-13-31(14-12-23)26-10-9-25-28-29-27(36-4)34(25)30-26/h5-10,19-20,23H,11-18H2,1-4H3/t19-,20+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of Alexa647-labeled JQ1 derivative from wild type BRD4 bromodomain 1 (44 to 460 residues) N140A mutant (unknown origin) incubated for 1 ... |
J Med Chem 59: 7801-17 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00070
BindingDB Entry DOI: 10.7270/Q2697721 |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM50534822

(CHEMBL4436251)Show SMILES C[C@H]1N(CCOc2ccc(cc2)C2CCN(CC2)c2ccc3nnc(C)n3n2)CCN(C)C1=O |r| Show InChI InChI=1S/C25H33N7O2/c1-18-25(33)29(3)14-15-30(18)16-17-34-22-6-4-20(5-7-22)21-10-12-31(13-11-21)24-9-8-23-27-26-19(2)32(23)28-24/h4-9,18,21H,10-17H2,1-3H3/t18-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of Alexa647-labeled JQ1 derivative from wild type BRD4 tandem domain (44 to 460 residues) (unknown origin) incubated for 1 hr by fluores... |
J Med Chem 59: 7801-17 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00070
BindingDB Entry DOI: 10.7270/Q2697721 |
More data for this
Ligand-Target Pair | |
[Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 1, mitochondrial
(Homo sapiens (Human)) | BDBM50236516
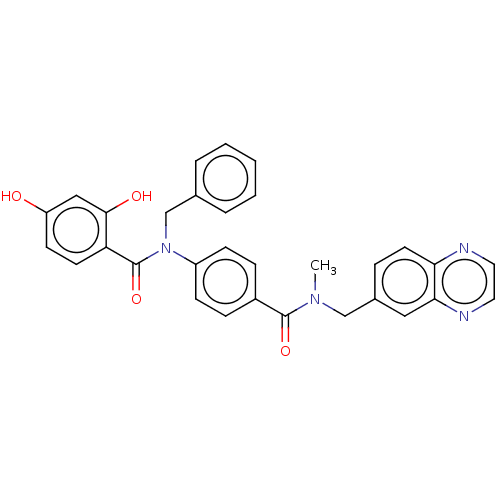
(CHEMBL3731789)Show SMILES CN(Cc1ccc2nccnc2c1)C(=O)c1ccc(cc1)N(Cc1ccccc1)C(=O)c1ccc(O)cc1O Show InChI InChI=1S/C31H26N4O4/c1-34(19-22-7-14-27-28(17-22)33-16-15-32-27)30(38)23-8-10-24(11-9-23)35(20-21-5-3-2-4-6-21)31(39)26-13-12-25(36)18-29(26)37/h2-18,36-37H,19-20H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of fluorescein-labelled VER160364 binding to PDHK1 (unknown origin) after 90 mins by fluorescence polarization assay |
J Med Chem 60: 2271-2286 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01478
BindingDB Entry DOI: 10.7270/Q2XG9TDJ |
More data for this
Ligand-Target Pair | |
[Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 1, mitochondrial
(Homo sapiens (Human)) | BDBM50236530
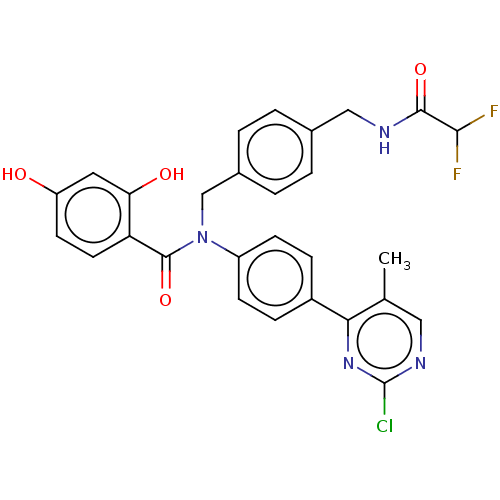
(CHEMBL3727577)Show SMILES Cc1cnc(Cl)nc1-c1ccc(cc1)N(Cc1ccc(CNC(=O)C(F)F)cc1)C(=O)c1ccc(O)cc1O Show InChI InChI=1S/C28H23ClF2N4O4/c1-16-13-33-28(29)34-24(16)19-6-8-20(9-7-19)35(27(39)22-11-10-21(36)12-23(22)37)15-18-4-2-17(3-5-18)14-32-26(38)25(30)31/h2-13,25,36-37H,14-15H2,1H3,(H,32,38) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of fluorescein-labelled VER160364 binding to PDHK1 (unknown origin) after 90 mins by fluorescence polarization assay |
J Med Chem 60: 2271-2286 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01478
BindingDB Entry DOI: 10.7270/Q2XG9TDJ |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-alpha
(Homo sapiens (Human)) | BDBM265209
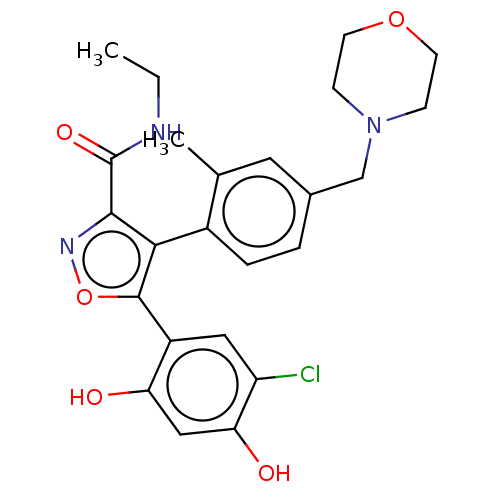
(US10413550, Example 41j | US11234987, Example 41j ...)Show SMILES CCNC(=O)c1noc(c1-c1ccc(CN2CCOCC2)cc1C)-c1cc(Cl)c(O)cc1O |(3.1,-6.74,;2.33,-5.41,;3.1,-4.07,;2.33,-2.74,;3.1,-1.41,;.79,-2.74,;-.12,-3.99,;-1.58,-3.51,;-1.58,-1.97,;-.12,-1.49,;.28,-.01,;-.81,1.08,;-.41,2.57,;1.08,2.97,;1.48,4.46,;2.96,4.85,;4.05,3.77,;5.54,4.16,;5.94,5.65,;4.85,6.74,;3.36,6.34,;2.17,1.88,;1.77,.39,;2.86,-.7,;-2.67,-.88,;-2.27,.61,;-3.36,1.7,;-2.96,3.18,;-4.85,1.3,;-5.94,2.39,;-5.25,-.19,;-4.16,-1.28,;-4.56,-2.77,)| Show InChI InChI=1S/C24H26ClN3O5/c1-3-26-24(31)22-21(23(33-27-22)17-11-18(25)20(30)12-19(17)29)16-5-4-15(10-14(16)2)13-28-6-8-32-9-7-28/h4-5,10-12,29-30H,3,6-9,13H2,1-2H3,(H,26,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd.
Curated by ChEMBL
| Assay Description
Displacement of fluorescein-labelled VER160364 from HSP90A (unknown origin) after 90 mins by fluorescence polarization assay |
J Med Chem 60: 2271-2286 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01478
BindingDB Entry DOI: 10.7270/Q2XG9TDJ |
More data for this
Ligand-Target Pair | |
[Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 1, mitochondrial
(Homo sapiens (Human)) | BDBM50236519
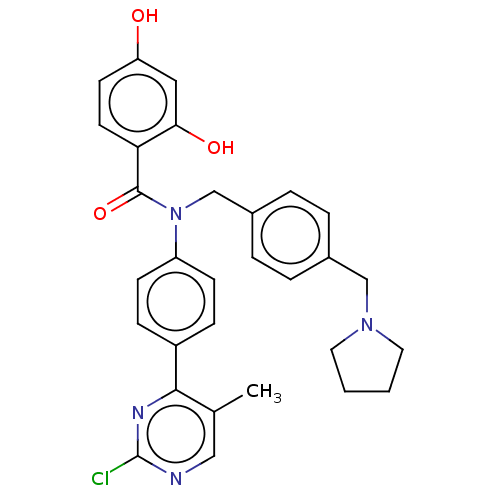
(CHEMBL3727843)Show SMILES Cc1cnc(Cl)nc1-c1ccc(cc1)N(Cc1ccc(CN2CCCC2)cc1)C(=O)c1ccc(O)cc1O Show InChI InChI=1S/C30H29ClN4O3/c1-20-17-32-30(31)33-28(20)23-8-10-24(11-9-23)35(29(38)26-13-12-25(36)16-27(26)37)19-22-6-4-21(5-7-22)18-34-14-2-3-15-34/h4-13,16-17,36-37H,2-3,14-15,18-19H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]WIN-35428 binding to dopamine transporter (DAT) of cynomolgus monkey caudate-putamen |
J Med Chem 60: 2271-2286 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01478
BindingDB Entry DOI: 10.7270/Q2XG9TDJ |
More data for this
Ligand-Target Pair | |
[Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 1, mitochondrial
(Homo sapiens (Human)) | BDBM50236514
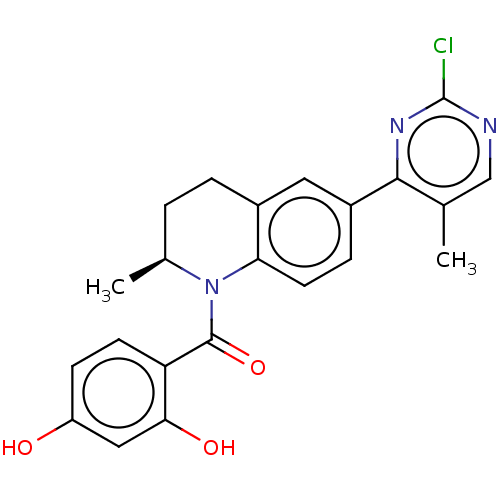
(CHEMBL4100504)Show SMILES C[C@H]1CCc2cc(ccc2N1C(=O)c1ccc(O)cc1O)-c1nc(Cl)ncc1C |r| Show InChI InChI=1S/C22H20ClN3O3/c1-12-11-24-22(23)25-20(12)15-5-8-18-14(9-15)4-3-13(2)26(18)21(29)17-7-6-16(27)10-19(17)28/h5-11,13,27-28H,3-4H2,1-2H3/t13-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of fluorescein-labelled VER160364 binding to PDHK1 (unknown origin) after 90 mins by fluorescence polarization assay |
J Med Chem 60: 2271-2286 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01478
BindingDB Entry DOI: 10.7270/Q2XG9TDJ |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM50365463

(CHEMBL1232461)Show SMILES CCNC(=O)C[C@@H]1N=C(c2ccc(Cl)cc2)c2cc(OC)ccc2-n2c(C)nnc12 |r,t:7| Show InChI InChI=1S/C22H22ClN5O2/c1-4-24-20(29)12-18-22-27-26-13(2)28(22)19-10-9-16(30-3)11-17(19)21(25-18)14-5-7-15(23)8-6-14/h5-11,18H,4,12H2,1-3H3,(H,24,29)/t18-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of Alexa647-labeled JQ1 derivative from wild type BRD4 bromodomain 2 (44 to 460 residues) N433A mutant (unknown origin) incubated for 1 ... |
J Med Chem 59: 7801-17 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00070
BindingDB Entry DOI: 10.7270/Q2697721 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM50534816

(CHEMBL4591500)Show SMILES COc1nnc2ccc(nn12)N1CCC(CC1)c1ccc(OCCN2[C@@H](C)CN(C[C@H]2C)C(C)=O)cc1 |r| Show InChI InChI=1S/C27H37N7O3/c1-19-17-32(21(3)35)18-20(2)33(19)15-16-37-24-7-5-22(6-8-24)23-11-13-31(14-12-23)26-10-9-25-28-29-27(36-4)34(25)30-26/h5-10,19-20,23H,11-18H2,1-4H3/t19-,20+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of Alexa647-labeled JQ1 derivative from wild type BRD4 bromodomain 2 (44 to 460 residues) N433A mutant (unknown origin) incubated for 1 ... |
J Med Chem 59: 7801-17 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00070
BindingDB Entry DOI: 10.7270/Q2697721 |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM50365463

(CHEMBL1232461)Show SMILES CCNC(=O)C[C@@H]1N=C(c2ccc(Cl)cc2)c2cc(OC)ccc2-n2c(C)nnc12 |r,t:7| Show InChI InChI=1S/C22H22ClN5O2/c1-4-24-20(29)12-18-22-27-26-13(2)28(22)19-10-9-16(30-3)11-17(19)21(25-18)14-5-7-15(23)8-6-14/h5-11,18H,4,12H2,1-3H3,(H,24,29)/t18-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of Alexa647-labeled JQ1 derivative from wild type BRD4 tandem domain (44 to 460 residues) (unknown origin) incubated for 1 hr by fluores... |
J Med Chem 59: 7801-17 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00070
BindingDB Entry DOI: 10.7270/Q2697721 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM50365463

(CHEMBL1232461)Show SMILES CCNC(=O)C[C@@H]1N=C(c2ccc(Cl)cc2)c2cc(OC)ccc2-n2c(C)nnc12 |r,t:7| Show InChI InChI=1S/C22H22ClN5O2/c1-4-24-20(29)12-18-22-27-26-13(2)28(22)19-10-9-16(30-3)11-17(19)21(25-18)14-5-7-15(23)8-6-14/h5-11,18H,4,12H2,1-3H3,(H,24,29)/t18-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of Alexa647-labeled JQ1 derivative from wild type BRD4 bromodomain 1 (44 to 460 residues) N140A mutant (unknown origin) incubated for 1 ... |
J Med Chem 59: 7801-17 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00070
BindingDB Entry DOI: 10.7270/Q2697721 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM50534824

(CHEMBL4438843)Show SMILES COc1nnc2ccc(nn12)N1CCC(CC1)c1ccc(cc1)N1CCC(CC1)N1CCN(C)C(=O)[C@H]1C |r| Show InChI InChI=1S/C28H38N8O2/c1-20-27(37)32(2)18-19-35(20)24-12-16-33(17-13-24)23-6-4-21(5-7-23)22-10-14-34(15-11-22)26-9-8-25-29-30-28(38-3)36(25)31-26/h4-9,20,22,24H,10-19H2,1-3H3/t20-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of Alexa647-labeled JQ1 derivative from wild type BRD4 tandem domain (44 to 460 residues) (unknown origin) incubated for 1 hr by fluores... |
J Med Chem 59: 7801-17 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00070
BindingDB Entry DOI: 10.7270/Q2697721 |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM50534827

(CHEMBL4460682)Show SMILES COc1nnc2ccc(nn12)N1CCC(CC1)c1ccc(OCCN2C[C@H](C)N([C@H](C)C2)C(C)=O)cc1 |r| Show InChI InChI=1S/C27H37N7O3/c1-19-17-31(18-20(2)33(19)21(3)35)15-16-37-24-7-5-22(6-8-24)23-11-13-32(14-12-23)26-10-9-25-28-29-27(36-4)34(25)30-26/h5-10,19-20,23H,11-18H2,1-4H3/t19-,20+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of Alexa647-labeled JQ1 derivative from wild type BRD4 bromodomain 2 (44 to 460 residues) N433A mutant (unknown origin) incubated for 1 ... |
J Med Chem 59: 7801-17 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00070
BindingDB Entry DOI: 10.7270/Q2697721 |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM50260093

(CHEMBL4078100)Show SMILES COc1nnc2ccc(nn12)N1CCC(CC1)c1ccc(OCCN2CCN(C)C(=O)[C@H]2C)cc1 |r| Show InChI InChI=1S/C25H33N7O3/c1-18-24(33)29(2)14-15-30(18)16-17-35-21-6-4-19(5-7-21)20-10-12-31(13-11-20)23-9-8-22-26-27-25(34-3)32(22)28-23/h4-9,18,20H,10-17H2,1-3H3/t18-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human BRD4 bromodomain 1 expressed in bacterial expression system |
J Med Chem 59: 7801-17 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00070
BindingDB Entry DOI: 10.7270/Q2697721 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neuronal acetylcholine receptor subunit alpha-4
(Homo sapiens (Human)) | BDBM50047420
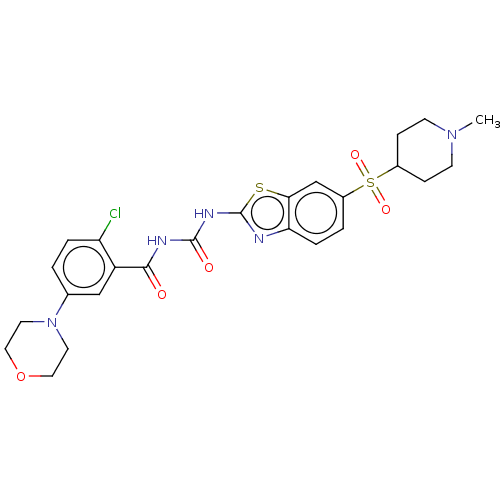
(CHEMBL3319405)Show SMILES CN1CCC(CC1)S(=O)(=O)c1ccc2nc(NC(=O)NC(=O)c3cc(ccc3Cl)N3CCOCC3)sc2c1 Show InChI InChI=1S/C25H28ClN5O5S2/c1-30-8-6-17(7-9-30)38(34,35)18-3-5-21-22(15-18)37-25(27-21)29-24(33)28-23(32)19-14-16(2-4-20(19)26)31-10-12-36-13-11-31/h2-5,14-15,17H,6-13H2,1H3,(H2,27,28,29,32,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of alpha4 nAChR (unknown origin) |
J Med Chem 57: 6128-40 (2014)
Article DOI: 10.1021/jm500610n
BindingDB Entry DOI: 10.7270/Q279469X |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-beta
(Homo sapiens (Human)) | BDBM50394061
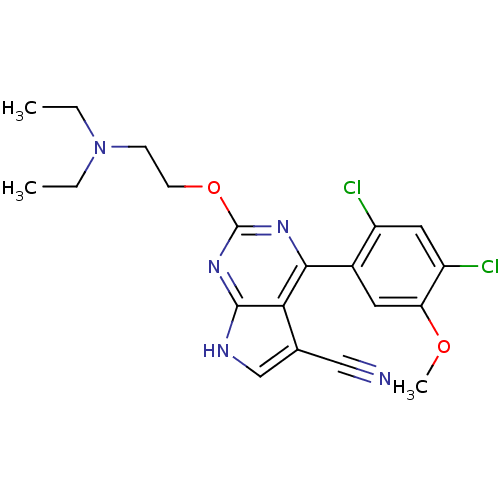
(CHEMBL2158008)Show SMILES CCN(CC)CCOc1nc(-c2cc(OC)c(Cl)cc2Cl)c2c(c[nH]c2n1)C#N Show InChI InChI=1S/C20H21Cl2N5O2/c1-4-27(5-2)6-7-29-20-25-18(17-12(10-23)11-24-19(17)26-20)13-8-16(28-3)15(22)9-14(13)21/h8-9,11H,4-7H2,1-3H3,(H,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of fluorescently labeled VER51001 binding to full length human HSP90beta after 30 mins by fluorescence polarization assay |
Bioorg Med Chem 20: 6770-89 (2012)
Article DOI: 10.1016/j.bmc.2012.08.050
BindingDB Entry DOI: 10.7270/Q2KS6SN4 |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM50534826

(CHEMBL4461618)Show SMILES COc1nnc2ccc(nn12)N1CCC(CC1)c1ccc(cc1)N1CC(C1)N1CCN(C)C(=O)[C@H]1C |r| Show InChI InChI=1S/C26H34N8O2/c1-18-25(35)30(2)14-15-33(18)22-16-32(17-22)21-6-4-19(5-7-21)20-10-12-31(13-11-20)24-9-8-23-27-28-26(36-3)34(23)29-24/h4-9,18,20,22H,10-17H2,1-3H3/t18-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of Alexa647-labeled JQ1 derivative from wild type BRD4 tandem domain (44 to 460 residues) (unknown origin) incubated for 1 hr by fluores... |
J Med Chem 59: 7801-17 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00070
BindingDB Entry DOI: 10.7270/Q2697721 |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 3
(Homo sapiens (Human)) | BDBM50260093

(CHEMBL4078100)Show SMILES COc1nnc2ccc(nn12)N1CCC(CC1)c1ccc(OCCN2CCN(C)C(=O)[C@H]2C)cc1 |r| Show InChI InChI=1S/C25H33N7O3/c1-18-24(33)29(2)14-15-30(18)16-17-35-21-6-4-19(5-7-21)20-10-12-31(13-11-20)23-9-8-22-26-27-25(34-3)32(22)28-23/h4-9,18,20H,10-17H2,1-3H3/t18-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human BRD3 bromodomain 1 expressed in bacterial expression system |
J Med Chem 59: 7801-17 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00070
BindingDB Entry DOI: 10.7270/Q2697721 |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM50534823

(CHEMBL4593653)Show SMILES COc1nnc2ccc(nn12)N1CCC(CC1)c1ccc(OCCCCN2CCN(C)C(=O)[C@H]2C)cc1 |r| Show InChI InChI=1S/C27H37N7O3/c1-20-26(35)31(2)17-18-32(20)14-4-5-19-37-23-8-6-21(7-9-23)22-12-15-33(16-13-22)25-11-10-24-28-29-27(36-3)34(24)30-25/h6-11,20,22H,4-5,12-19H2,1-3H3/t20-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of Alexa647-labeled JQ1 derivative from wild type BRD4 bromodomain 2 (44 to 460 residues) N433A mutant (unknown origin) incubated for 1 ... |
J Med Chem 59: 7801-17 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00070
BindingDB Entry DOI: 10.7270/Q2697721 |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM50534814

(CHEMBL4466676)Show SMILES COc1nnc2ccc(nn12)N1CCC(CC1)c1ccc(OCCCN2CCN(C)C(=O)[C@H]2C)cc1 |r| Show InChI InChI=1S/C26H35N7O3/c1-19-25(34)30(2)16-17-31(19)13-4-18-36-22-7-5-20(6-8-22)21-11-14-32(15-12-21)24-10-9-23-27-28-26(35-3)33(23)29-24/h5-10,19,21H,4,11-18H2,1-3H3/t19-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of Alexa647-labeled JQ1 derivative from wild type BRD4 bromodomain 1 (44 to 460 residues) N140A mutant (unknown origin) incubated for 1 ... |
J Med Chem 59: 7801-17 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00070
BindingDB Entry DOI: 10.7270/Q2697721 |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM50534828

(CHEMBL4448398)Show SMILES C[C@H]1N(CCOc2ccc(cc2)C2CCN(CC2)c2ccc3nnc(n3n2)C(F)(F)F)CCN(C)C1=O |r| Show InChI InChI=1S/C25H30F3N7O2/c1-17-23(36)32(2)13-14-33(17)15-16-37-20-5-3-18(4-6-20)19-9-11-34(12-10-19)22-8-7-21-29-30-24(25(26,27)28)35(21)31-22/h3-8,17,19H,9-16H2,1-2H3/t17-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of Alexa647-labeled JQ1 derivative from wild type BRD4 bromodomain 1 (44 to 460 residues) N140A mutant (unknown origin) incubated for 1 ... |
J Med Chem 59: 7801-17 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00070
BindingDB Entry DOI: 10.7270/Q2697721 |
More data for this
Ligand-Target Pair | |
[Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 1, mitochondrial
(Homo sapiens (Human)) | BDBM50236518
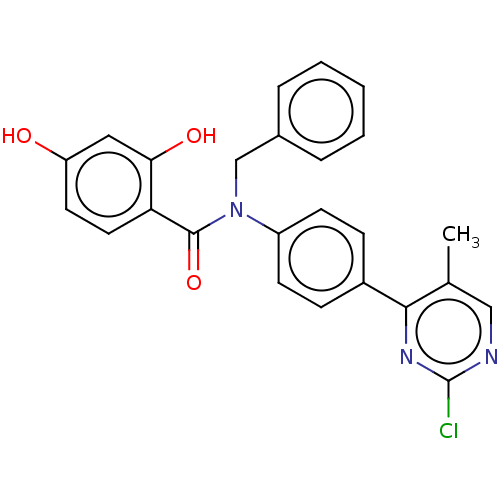
(CHEMBL3732469)Show SMILES Cc1cnc(Cl)nc1-c1ccc(cc1)N(Cc1ccccc1)C(=O)c1ccc(O)cc1O Show InChI InChI=1S/C25H20ClN3O3/c1-16-14-27-25(26)28-23(16)18-7-9-19(10-8-18)29(15-17-5-3-2-4-6-17)24(32)21-12-11-20(30)13-22(21)31/h2-14,30-31H,15H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 67 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of fluorescein-labelled VER160364 binding to PDHK1 (unknown origin) after 90 mins by fluorescence polarization assay |
J Med Chem 60: 2271-2286 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01478
BindingDB Entry DOI: 10.7270/Q2XG9TDJ |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM50534814

(CHEMBL4466676)Show SMILES COc1nnc2ccc(nn12)N1CCC(CC1)c1ccc(OCCCN2CCN(C)C(=O)[C@H]2C)cc1 |r| Show InChI InChI=1S/C26H35N7O3/c1-19-25(34)30(2)16-17-31(19)13-4-18-36-22-7-5-20(6-8-22)21-11-14-32(15-12-21)24-10-9-23-27-28-26(35-3)33(23)29-24/h5-10,19,21H,4,11-18H2,1-3H3/t19-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of Alexa647-labeled JQ1 derivative from wild type BRD4 bromodomain 2 (44 to 460 residues) N433A mutant (unknown origin) incubated for 1 ... |
J Med Chem 59: 7801-17 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00070
BindingDB Entry DOI: 10.7270/Q2697721 |
More data for this
Ligand-Target Pair | |
[Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 1, mitochondrial
(Homo sapiens (Human)) | BDBM50236521
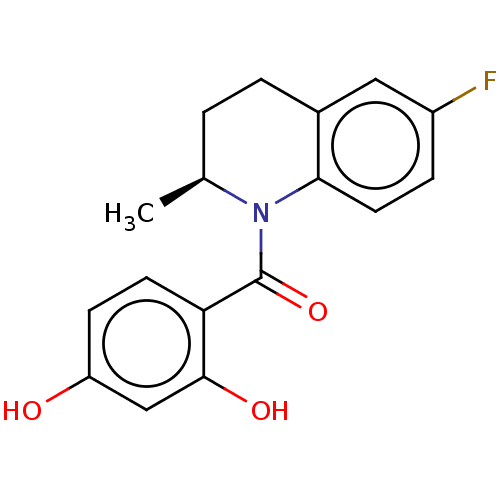
(CHEMBL3715843)Show SMILES C[C@H]1CCc2cc(F)ccc2N1C(=O)c1ccc(O)cc1O |r| Show InChI InChI=1S/C17H16FNO3/c1-10-2-3-11-8-12(18)4-7-15(11)19(10)17(22)14-6-5-13(20)9-16(14)21/h4-10,20-21H,2-3H2,1H3/t10-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity to PDHK1 (unknown origin) by isothermal titration calorimetry |
J Med Chem 60: 2271-2286 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01478
BindingDB Entry DOI: 10.7270/Q2XG9TDJ |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM50534823

(CHEMBL4593653)Show SMILES COc1nnc2ccc(nn12)N1CCC(CC1)c1ccc(OCCCCN2CCN(C)C(=O)[C@H]2C)cc1 |r| Show InChI InChI=1S/C27H37N7O3/c1-20-26(35)31(2)17-18-32(20)14-4-5-19-37-23-8-6-21(7-9-23)22-12-15-33(16-13-22)25-11-10-24-28-29-27(36-3)34(24)30-25/h6-11,20,22H,4-5,12-19H2,1-3H3/t20-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of Alexa647-labeled JQ1 derivative from wild type BRD4 bromodomain 1 (44 to 460 residues) N140A mutant (unknown origin) incubated for 1 ... |
J Med Chem 59: 7801-17 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00070
BindingDB Entry DOI: 10.7270/Q2697721 |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM50534829

(CHEMBL4447492)Show SMILES COc1nnc2ccc(nn12)N1CCC(CC1)c1ccc(CCN2CCN(C)C(=O)[C@H]2C)cc1 |r| Show InChI InChI=1S/C25H33N7O2/c1-18-24(33)29(2)16-17-30(18)13-10-19-4-6-20(7-5-19)21-11-14-31(15-12-21)23-9-8-22-26-27-25(34-3)32(22)28-23/h4-9,18,21H,10-17H2,1-3H3/t18-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of Alexa647-labeled JQ1 derivative from wild type BRD4 bromodomain 2 (44 to 460 residues) N433A mutant (unknown origin) incubated for 1 ... |
J Med Chem 59: 7801-17 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00070
BindingDB Entry DOI: 10.7270/Q2697721 |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 2
(Homo sapiens (Human)) | BDBM50260093

(CHEMBL4078100)Show SMILES COc1nnc2ccc(nn12)N1CCC(CC1)c1ccc(OCCN2CCN(C)C(=O)[C@H]2C)cc1 |r| Show InChI InChI=1S/C25H33N7O3/c1-18-24(33)29(2)14-15-30(18)16-17-35-21-6-4-19(5-7-21)20-10-12-31(13-11-20)23-9-8-22-26-27-25(34-3)32(22)28-23/h4-9,18,20H,10-17H2,1-3H3/t18-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human BRD2 bromodomain 1 expressed in bacterial expression system |
J Med Chem 59: 7801-17 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00070
BindingDB Entry DOI: 10.7270/Q2697721 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data