

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50029593 (CHEMBL7162 | N-(2-(cyclohexyloxy)-4-nitrophenyl)me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoires Innothera Curated by ChEMBL | Assay Description The compound was evaluated for its inhibitory activity against Prostaglandin G/H synthase 2 using osteosarcomes cells | Bioorg Med Chem Lett 12: 779-82 (2002) BindingDB Entry DOI: 10.7270/Q20864MC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM22369 (4-(4-methanesulfonylphenyl)-3-phenyl-2,5-dihydrofu...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoires Innothera Curated by ChEMBL | Assay Description The compound was evaluated for prostaglandin E2 inhibition using recombinant Prostaglandin G/H synthase 2 | Bioorg Med Chem Lett 12: 779-82 (2002) BindingDB Entry DOI: 10.7270/Q20864MC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50110484 (3-((3-fluoro-5-(1-methoxycyclohexyl)phenoxy)methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoires Innothera Curated by ChEMBL | Assay Description The compound was evaluated for its inhibitory activity against 5-lipoxygenase using granulocytes-type cells | Bioorg Med Chem Lett 12: 779-82 (2002) BindingDB Entry DOI: 10.7270/Q20864MC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50110484 (3-((3-fluoro-5-(1-methoxycyclohexyl)phenoxy)methyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoires Innothera Curated by ChEMBL | Assay Description The compound was evaluated for its inhibitory activity against Prostaglandin G/H synthase 2 using osteosarcomes cells | Bioorg Med Chem Lett 12: 779-82 (2002) BindingDB Entry DOI: 10.7270/Q20864MC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50110483 (2-[3-Fluoro-5-(4-methoxy-tetrahydro-pyran-4-yl)-ph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoires Innothera Curated by ChEMBL | Assay Description The compound was evaluated for prostaglandin E2 inhibition using recombinant Prostaglandin G/H synthase 2 | Bioorg Med Chem Lett 12: 779-82 (2002) BindingDB Entry DOI: 10.7270/Q20864MC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50110485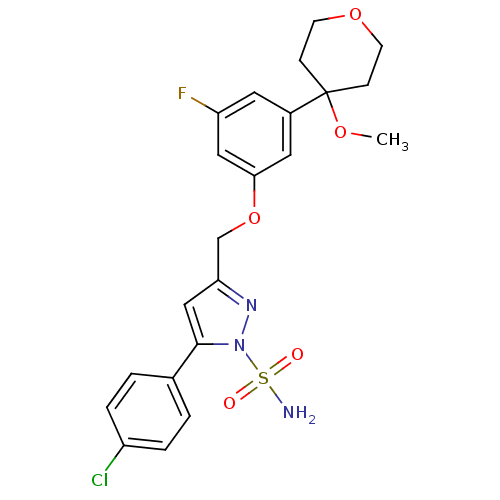 (5-(4-Chloro-phenyl)-3-[3-fluoro-5-(4-methoxy-tetra...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoires Innothera Curated by ChEMBL | Assay Description The compound was evaluated for prostaglandin E2 inhibition using recombinant Prostaglandin G/H synthase 2 | Bioorg Med Chem Lett 12: 779-82 (2002) BindingDB Entry DOI: 10.7270/Q20864MC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50110485 (5-(4-Chloro-phenyl)-3-[3-fluoro-5-(4-methoxy-tetra...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoires Innothera Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase activity of compound evaluated as determined by the inhibition of calcium ionophore-induced leukotriene B4 production in... | Bioorg Med Chem Lett 12: 779-82 (2002) BindingDB Entry DOI: 10.7270/Q20864MC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50110483 (2-[3-Fluoro-5-(4-methoxy-tetrahydro-pyran-4-yl)-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoires Innothera Curated by ChEMBL | Assay Description The 5-lipoxygenase activity of the compound was determined by the inhibition of calcium ionophore-induced leukotriene B4 production in human blood. | Bioorg Med Chem Lett 12: 779-82 (2002) BindingDB Entry DOI: 10.7270/Q20864MC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50110483 (2-[3-Fluoro-5-(4-methoxy-tetrahydro-pyran-4-yl)-ph...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoires Innothera Curated by ChEMBL | Assay Description The compound was evaluated for prostaglandin E2 inhibition using recombinant Prostaglandin G/H synthase 1 | Bioorg Med Chem Lett 12: 779-82 (2002) BindingDB Entry DOI: 10.7270/Q20864MC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50000541 ((+-)-1-(1-Benzo[b]thien-2-ylethyl)-1-hydroxyurea |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | DrugBank PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoires Innothera Curated by ChEMBL | Assay Description The compound was evaluated for its inhibitory activity against 5-lipoxygenase using granulocytes-type cells | Bioorg Med Chem Lett 12: 779-82 (2002) BindingDB Entry DOI: 10.7270/Q20864MC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50029593 (CHEMBL7162 | N-(2-(cyclohexyloxy)-4-nitrophenyl)me...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoires Innothera Curated by ChEMBL | Assay Description The compound was evaluated for its inhibitory activity against Prostaglandin G/H synthase 1 using monocytes-like cells | Bioorg Med Chem Lett 12: 779-82 (2002) BindingDB Entry DOI: 10.7270/Q20864MC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50110486 (3-Butyl-1-(4-methanesulfonyl-phenyl)-5-phenyl-1H-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoires Innothera Curated by ChEMBL | Assay Description The compound was evaluated for its inhibitory activity against 5-lipoxygenase using granulocytes-type cells | Bioorg Med Chem Lett 12: 779-82 (2002) BindingDB Entry DOI: 10.7270/Q20864MC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50110481 (1-(4-Methanesulfonyl-phenyl)-5-phenyl-1H-pyrazole-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoires Innothera Curated by ChEMBL | Assay Description The compound was evaluated for its inhibitory activity against Prostaglandin G/H synthase 2 using osteosarcomes cells | Bioorg Med Chem Lett 12: 779-82 (2002) BindingDB Entry DOI: 10.7270/Q20864MC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50110480 (CHEMBL166156 | [1-(4-Methanesulfonyl-phenyl)-5-phe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoires Innothera Curated by ChEMBL | Assay Description The compound was evaluated for its inhibitory activity against Prostaglandin G/H synthase 2 using osteosarcomes cells | Bioorg Med Chem Lett 12: 779-82 (2002) BindingDB Entry DOI: 10.7270/Q20864MC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50110481 (1-(4-Methanesulfonyl-phenyl)-5-phenyl-1H-pyrazole-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoires Innothera Curated by ChEMBL | Assay Description The compound was evaluated for its inhibitory activity against 5-lipoxygenase using granulocytes-type cells | Bioorg Med Chem Lett 12: 779-82 (2002) BindingDB Entry DOI: 10.7270/Q20864MC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM22369 (4-(4-methanesulfonylphenyl)-3-phenyl-2,5-dihydrofu...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoires Innothera Curated by ChEMBL | Assay Description The compound was evaluated for its inhibitory activity against Prostaglandin G/H synthase 1 using monocytes-like cells | Bioorg Med Chem Lett 12: 779-82 (2002) BindingDB Entry DOI: 10.7270/Q20864MC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50000541 ((+-)-1-(1-Benzo[b]thien-2-ylethyl)-1-hydroxyurea |...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoires Innothera Curated by ChEMBL | Assay Description The compound was evaluated for its inhibitory activity against Prostaglandin G/H synthase 1 using monocytes-like cells | Bioorg Med Chem Lett 12: 779-82 (2002) BindingDB Entry DOI: 10.7270/Q20864MC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50110486 (3-Butyl-1-(4-methanesulfonyl-phenyl)-5-phenyl-1H-p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoires Innothera Curated by ChEMBL | Assay Description The compound was evaluated for its inhibitory activity against Prostaglandin G/H synthase 2 using osteosarcomes cells | Bioorg Med Chem Lett 12: 779-82 (2002) BindingDB Entry DOI: 10.7270/Q20864MC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50029593 (CHEMBL7162 | N-(2-(cyclohexyloxy)-4-nitrophenyl)me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoires Innothera Curated by ChEMBL | Assay Description The compound was evaluated for its inhibitory activity against 5-lipoxygenase using granulocytes-type cells | Bioorg Med Chem Lett 12: 779-82 (2002) BindingDB Entry DOI: 10.7270/Q20864MC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50110480 (CHEMBL166156 | [1-(4-Methanesulfonyl-phenyl)-5-phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoires Innothera Curated by ChEMBL | Assay Description The compound was evaluated for its inhibitory activity against 5-lipoxygenase using granulocytes-type cells | Bioorg Med Chem Lett 12: 779-82 (2002) BindingDB Entry DOI: 10.7270/Q20864MC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50110480 (CHEMBL166156 | [1-(4-Methanesulfonyl-phenyl)-5-phe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoires Innothera Curated by ChEMBL | Assay Description The compound was evaluated for its inhibitory activity against Prostaglandin G/H synthase 1 using monocytes-like cells | Bioorg Med Chem Lett 12: 779-82 (2002) BindingDB Entry DOI: 10.7270/Q20864MC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50110486 (3-Butyl-1-(4-methanesulfonyl-phenyl)-5-phenyl-1H-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoires Innothera Curated by ChEMBL | Assay Description The compound was evaluated for its inhibitory activity against Prostaglandin G/H synthase 1 using monocytes-like cells | Bioorg Med Chem Lett 12: 779-82 (2002) BindingDB Entry DOI: 10.7270/Q20864MC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50110484 (3-((3-fluoro-5-(1-methoxycyclohexyl)phenoxy)methyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoires Innothera Curated by ChEMBL | Assay Description The compound was evaluated for its inhibitory activity against Prostaglandin G/H synthase 1 using monocytes-like cells | Bioorg Med Chem Lett 12: 779-82 (2002) BindingDB Entry DOI: 10.7270/Q20864MC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50110482 (1-(4-Methanesulfonyl-phenyl)-3-methyl-5-phenyl-1H-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoires Innothera Curated by ChEMBL | Assay Description The compound was evaluated for its inhibitory activity against Prostaglandin G/H synthase 1 using monocytes-like cells | Bioorg Med Chem Lett 12: 779-82 (2002) BindingDB Entry DOI: 10.7270/Q20864MC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM22369 (4-(4-methanesulfonylphenyl)-3-phenyl-2,5-dihydrofu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoires Innothera Curated by ChEMBL | Assay Description The compound was evaluated for its inhibitory activity against 5-lipoxygenase using granulocytes-type cells | Bioorg Med Chem Lett 12: 779-82 (2002) BindingDB Entry DOI: 10.7270/Q20864MC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50110482 (1-(4-Methanesulfonyl-phenyl)-3-methyl-5-phenyl-1H-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoires Innothera Curated by ChEMBL | Assay Description The compound was evaluated for its inhibitory activity against Prostaglandin G/H synthase 2 using osteosarcomes cells | Bioorg Med Chem Lett 12: 779-82 (2002) BindingDB Entry DOI: 10.7270/Q20864MC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50110481 (1-(4-Methanesulfonyl-phenyl)-5-phenyl-1H-pyrazole-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoires Innothera Curated by ChEMBL | Assay Description The compound was evaluated for its inhibitory activity against Prostaglandin G/H synthase 1 using monocytes-like cells | Bioorg Med Chem Lett 12: 779-82 (2002) BindingDB Entry DOI: 10.7270/Q20864MC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50000541 ((+-)-1-(1-Benzo[b]thien-2-ylethyl)-1-hydroxyurea |...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoires Innothera Curated by ChEMBL | Assay Description The compound was evaluated for its inhibitory activity against Prostaglandin G/H synthase 2 using osteosarcomes cells | Bioorg Med Chem Lett 12: 779-82 (2002) BindingDB Entry DOI: 10.7270/Q20864MC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50110485 (5-(4-Chloro-phenyl)-3-[3-fluoro-5-(4-methoxy-tetra...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 3.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoires Innothera Curated by ChEMBL | Assay Description The compound was evaluated for prostaglandin E2 inhibition using recombinant Prostaglandin G/H synthase 1 | Bioorg Med Chem Lett 12: 779-82 (2002) BindingDB Entry DOI: 10.7270/Q20864MC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50110482 (1-(4-Methanesulfonyl-phenyl)-3-methyl-5-phenyl-1H-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoires Innothera Curated by ChEMBL | Assay Description The compound was evaluated for its inhibitory activity against 5-lipoxygenase using granulocytes-type cells | Bioorg Med Chem Lett 12: 779-82 (2002) BindingDB Entry DOI: 10.7270/Q20864MC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||