 Found 65 hits with Last Name = 'miller' and Initial = 'sg'
Found 65 hits with Last Name = 'miller' and Initial = 'sg' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50374091
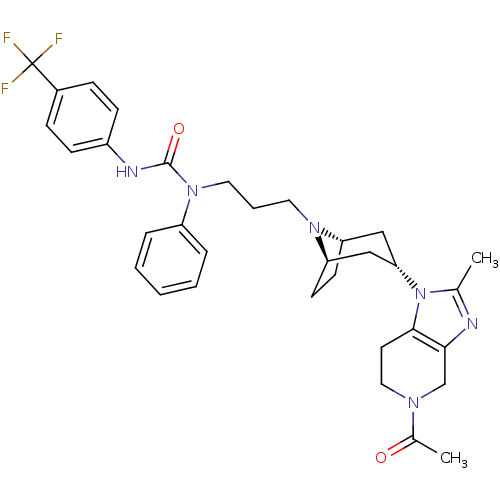
(CHEMBL257000)Show SMILES CC(=O)N1CCc2c(C1)nc(C)n2[C@H]1C[C@@H]2CC[C@H](C1)N2CCCN(C(=O)Nc1ccc(cc1)C(F)(F)F)c1ccccc1 |THB:21:20:14.13.19:17.16| Show InChI InChI=1S/C33H39F3N6O2/c1-22-37-30-21-39(23(2)43)18-15-31(30)42(22)29-19-27-13-14-28(20-29)40(27)16-6-17-41(26-7-4-3-5-8-26)32(44)38-25-11-9-24(10-12-25)33(34,35)36/h3-5,7-12,27-29H,6,13-21H2,1-2H3,(H,38,44)/t27-,28+,29- | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
K£mia, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at CCR5 receptor by [35S]GTP-gamma-S binding assay |
Bioorg Med Chem Lett 18: 1498-501 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.058
BindingDB Entry DOI: 10.7270/Q2V125PH |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50374072
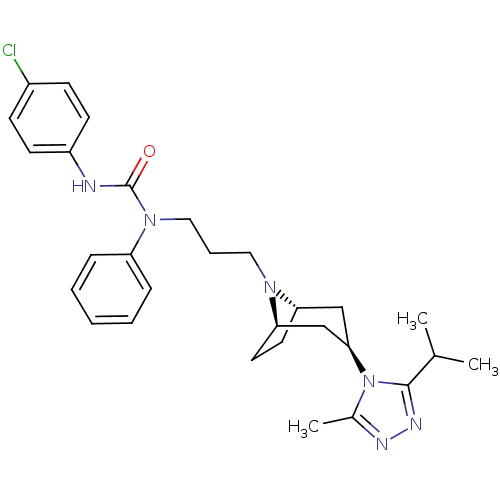
(CHEMBL272716)Show SMILES CC(C)c1nnc(C)n1[C@@H]1C[C@@H]2CC[C@H](C1)N2CCCN(C(=O)Nc1ccc(Cl)cc1)c1ccccc1 |THB:17:16:10.9.15:13.12| Show InChI InChI=1S/C29H37ClN6O/c1-20(2)28-33-32-21(3)36(28)27-18-25-14-15-26(19-27)34(25)16-7-17-35(24-8-5-4-6-9-24)29(37)31-23-12-10-22(30)11-13-23/h4-6,8-13,20,25-27H,7,14-19H2,1-3H3,(H,31,37)/t25-,26+,27+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
K£mia, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]MIP1beta from human recombinant CCR5 receptor expressed in CHOK1 cells |
Bioorg Med Chem Lett 18: 1498-501 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.058
BindingDB Entry DOI: 10.7270/Q2V125PH |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50374084
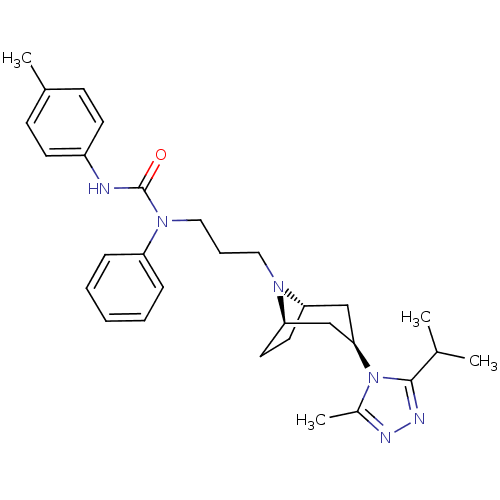
(CHEMBL257081)Show SMILES CC(C)c1nnc(C)n1[C@@H]1C[C@@H]2CC[C@H](C1)N2CCCN(C(=O)Nc1ccc(C)cc1)c1ccccc1 |THB:17:16:10.9.15:13.12| Show InChI InChI=1S/C30H40N6O/c1-21(2)29-33-32-23(4)36(29)28-19-26-15-16-27(20-28)34(26)17-8-18-35(25-9-6-5-7-10-25)30(37)31-24-13-11-22(3)12-14-24/h5-7,9-14,21,26-28H,8,15-20H2,1-4H3,(H,31,37)/t26-,27+,28+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
K£mia, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]MIP1beta from human recombinant CCR5 receptor expressed in CHOK1 cells |
Bioorg Med Chem Lett 18: 1498-501 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.058
BindingDB Entry DOI: 10.7270/Q2V125PH |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50374080
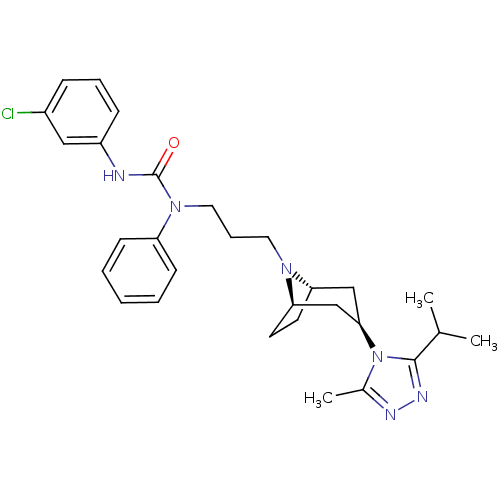
(CHEMBL271075)Show SMILES CC(C)c1nnc(C)n1[C@@H]1C[C@@H]2CC[C@H](C1)N2CCCN(C(=O)Nc1cccc(Cl)c1)c1ccccc1 |THB:17:16:10.9.15:13.12| Show InChI InChI=1S/C29H37ClN6O/c1-20(2)28-33-32-21(3)36(28)27-18-25-13-14-26(19-27)34(25)15-8-16-35(24-11-5-4-6-12-24)29(37)31-23-10-7-9-22(30)17-23/h4-7,9-12,17,20,25-27H,8,13-16,18-19H2,1-3H3,(H,31,37)/t25-,26+,27+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
K£mia, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]MIP1beta from human recombinant CCR5 receptor expressed in CHOK1 cells |
Bioorg Med Chem Lett 18: 1498-501 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.058
BindingDB Entry DOI: 10.7270/Q2V125PH |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50374087
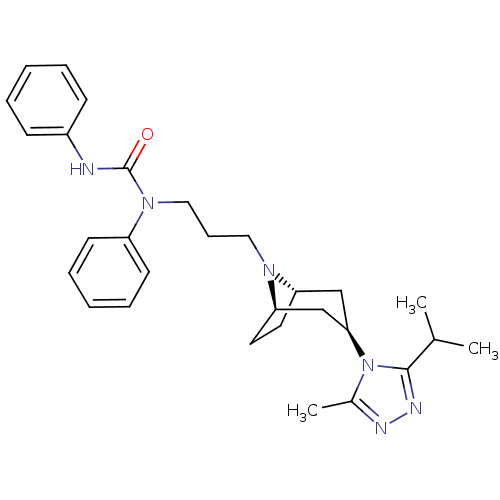
(CHEMBL272490)Show SMILES CC(C)c1nnc(C)n1[C@@H]1C[C@@H]2CC[C@H](C1)N2CCCN(C(=O)Nc1ccccc1)c1ccccc1 |THB:17:16:10.9.15:13.12| Show InChI InChI=1S/C29H38N6O/c1-21(2)28-32-31-22(3)35(28)27-19-25-15-16-26(20-27)33(25)17-10-18-34(24-13-8-5-9-14-24)29(36)30-23-11-6-4-7-12-23/h4-9,11-14,21,25-27H,10,15-20H2,1-3H3,(H,30,36)/t25-,26+,27+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
K£mia, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]MIP1beta from human recombinant CCR5 receptor expressed in CHOK1 cells |
Bioorg Med Chem Lett 18: 1498-501 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.058
BindingDB Entry DOI: 10.7270/Q2V125PH |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50374082
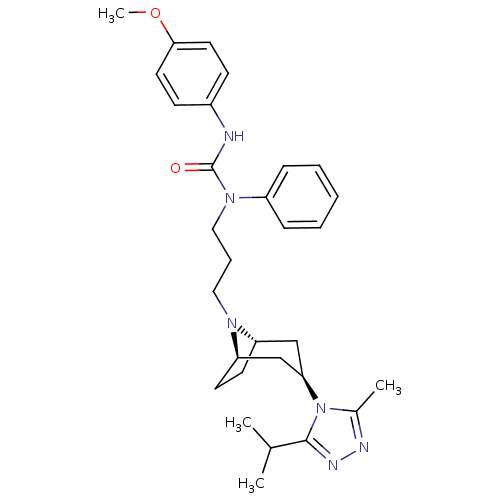
(CHEMBL402242)Show SMILES COc1ccc(NC(=O)N(CCCN2[C@H]3CC[C@@H]2C[C@@H](C3)n2c(C)nnc2C(C)C)c2ccccc2)cc1 |THB:12:13:20.19.18:16.15| Show InChI InChI=1S/C30H40N6O2/c1-21(2)29-33-32-22(3)36(29)27-19-25-13-14-26(20-27)34(25)17-8-18-35(24-9-6-5-7-10-24)30(37)31-23-11-15-28(38-4)16-12-23/h5-7,9-12,15-16,21,25-27H,8,13-14,17-20H2,1-4H3,(H,31,37)/t25-,26+,27+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
K£mia, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]MIP1beta from human recombinant CCR5 receptor expressed in CHOK1 cells |
Bioorg Med Chem Lett 18: 1498-501 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.058
BindingDB Entry DOI: 10.7270/Q2V125PH |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50374074
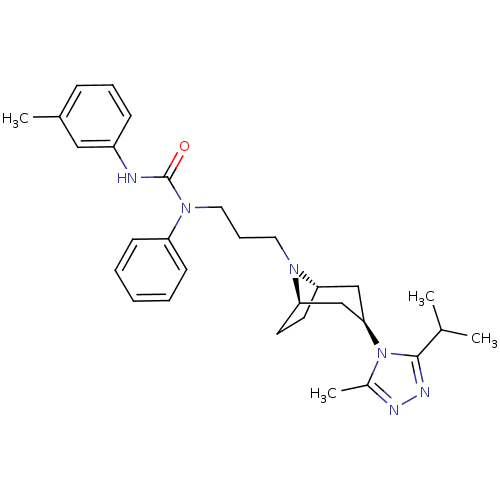
(CHEMBL257728)Show SMILES CC(C)c1nnc(C)n1[C@@H]1C[C@@H]2CC[C@H](C1)N2CCCN(C(=O)Nc1cccc(C)c1)c1ccccc1 |THB:17:16:10.9.15:13.12| Show InChI InChI=1S/C30H40N6O/c1-21(2)29-33-32-23(4)36(29)28-19-26-14-15-27(20-28)34(26)16-9-17-35(25-12-6-5-7-13-25)30(37)31-24-11-8-10-22(3)18-24/h5-8,10-13,18,21,26-28H,9,14-17,19-20H2,1-4H3,(H,31,37)/t26-,27+,28+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
K£mia, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]MIP1beta from human recombinant CCR5 receptor expressed in CHOK1 cells |
Bioorg Med Chem Lett 18: 1498-501 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.058
BindingDB Entry DOI: 10.7270/Q2V125PH |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50374090

(CHEMBL256584)Show SMILES CC(C)c1nnc(C)n1[C@@H]1C[C@@H]2CC[C@H](C1)N2CCCN(C(=O)NC1CCCCC1)c1ccccc1 |THB:17:16:10.9.15:13.12| Show InChI InChI=1S/C29H44N6O/c1-21(2)28-32-31-22(3)35(28)27-19-25-15-16-26(20-27)33(25)17-10-18-34(24-13-8-5-9-14-24)29(36)30-23-11-6-4-7-12-23/h5,8-9,13-14,21,23,25-27H,4,6-7,10-12,15-20H2,1-3H3,(H,30,36)/t25-,26+,27+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
K£mia, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]MIP1beta from human recombinant CCR5 receptor expressed in CHOK1 cells |
Bioorg Med Chem Lett 18: 1498-501 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.058
BindingDB Entry DOI: 10.7270/Q2V125PH |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50374078
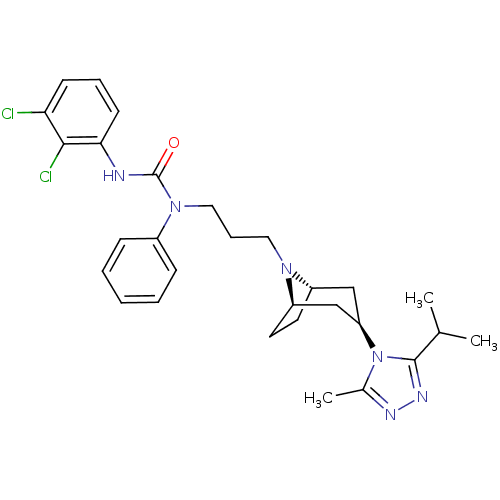
(CHEMBL272064)Show SMILES CC(C)c1nnc(C)n1[C@@H]1C[C@@H]2CC[C@H](C1)N2CCCN(C(=O)Nc1cccc(Cl)c1Cl)c1ccccc1 |THB:17:16:10.9.15:13.12| Show InChI InChI=1S/C29H36Cl2N6O/c1-19(2)28-34-33-20(3)37(28)24-17-22-13-14-23(18-24)35(22)15-8-16-36(21-9-5-4-6-10-21)29(38)32-26-12-7-11-25(30)27(26)31/h4-7,9-12,19,22-24H,8,13-18H2,1-3H3,(H,32,38)/t22-,23+,24+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
K£mia, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]MIP1beta from human recombinant CCR5 receptor expressed in CHOK1 cells |
Bioorg Med Chem Lett 18: 1498-501 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.058
BindingDB Entry DOI: 10.7270/Q2V125PH |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50374073
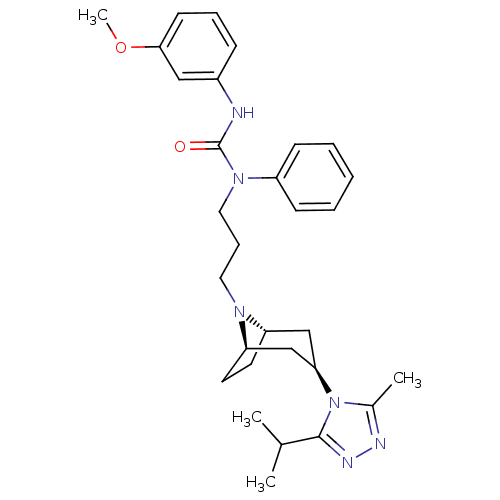
(CHEMBL255854)Show SMILES COc1cccc(NC(=O)N(CCCN2[C@H]3CC[C@@H]2C[C@@H](C3)n2c(C)nnc2C(C)C)c2ccccc2)c1 |THB:13:14:21.20.19:17.16| Show InChI InChI=1S/C30H40N6O2/c1-21(2)29-33-32-22(3)36(29)27-19-25-14-15-26(20-27)34(25)16-9-17-35(24-11-6-5-7-12-24)30(37)31-23-10-8-13-28(18-23)38-4/h5-8,10-13,18,21,25-27H,9,14-17,19-20H2,1-4H3,(H,31,37)/t25-,26+,27+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
K£mia, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]MIP1beta from human recombinant CCR5 receptor expressed in CHOK1 cells |
Bioorg Med Chem Lett 18: 1498-501 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.058
BindingDB Entry DOI: 10.7270/Q2V125PH |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50374089
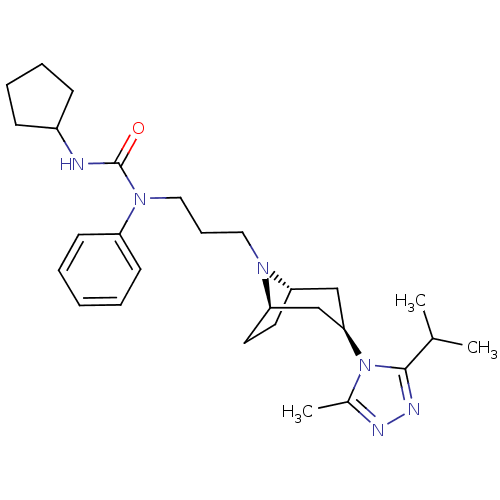
(CHEMBL272556)Show SMILES CC(C)c1nnc(C)n1[C@@H]1C[C@@H]2CC[C@H](C1)N2CCCN(C(=O)NC1CCCC1)c1ccccc1 |THB:17:16:10.9.15:13.12| Show InChI InChI=1S/C28H42N6O/c1-20(2)27-31-30-21(3)34(27)26-18-24-14-15-25(19-26)32(24)16-9-17-33(23-12-5-4-6-13-23)28(35)29-22-10-7-8-11-22/h4-6,12-13,20,22,24-26H,7-11,14-19H2,1-3H3,(H,29,35)/t24-,25+,26+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
K£mia, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]MIP1beta from human recombinant CCR5 receptor expressed in CHOK1 cells |
Bioorg Med Chem Lett 18: 1498-501 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.058
BindingDB Entry DOI: 10.7270/Q2V125PH |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50374075
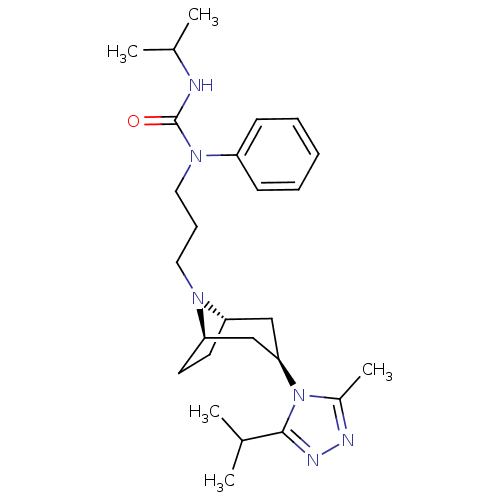
(CHEMBL402776)Show SMILES CC(C)NC(=O)N(CCCN1[C@H]2CC[C@@H]1C[C@@H](C2)n1c(C)nnc1C(C)C)c1ccccc1 |THB:9:10:17.16.15:13.12| Show InChI InChI=1S/C26H40N6O/c1-18(2)25-29-28-20(5)32(25)24-16-22-12-13-23(17-24)30(22)14-9-15-31(26(33)27-19(3)4)21-10-7-6-8-11-21/h6-8,10-11,18-19,22-24H,9,12-17H2,1-5H3,(H,27,33)/t22-,23+,24+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 96 | n/a | n/a | n/a | n/a | n/a | n/a |
K£mia, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]MIP1beta from human recombinant CCR5 receptor expressed in CHOK1 cells |
Bioorg Med Chem Lett 18: 1498-501 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.058
BindingDB Entry DOI: 10.7270/Q2V125PH |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50374081
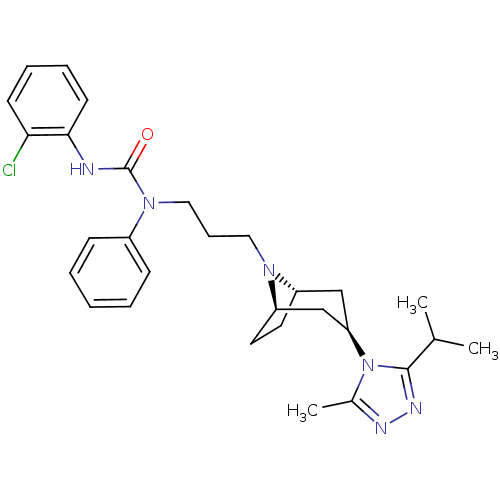
(CHEMBL271076)Show SMILES CC(C)c1nnc(C)n1[C@@H]1C[C@@H]2CC[C@H](C1)N2CCCN(C(=O)Nc1ccccc1Cl)c1ccccc1 |THB:17:16:10.9.15:13.12| Show InChI InChI=1S/C29H37ClN6O/c1-20(2)28-33-32-21(3)36(28)25-18-23-14-15-24(19-25)34(23)16-9-17-35(22-10-5-4-6-11-22)29(37)31-27-13-8-7-12-26(27)30/h4-8,10-13,20,23-25H,9,14-19H2,1-3H3,(H,31,37)/t23-,24+,25+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
K£mia, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]MIP1beta from human recombinant CCR5 receptor expressed in CHOK1 cells |
Bioorg Med Chem Lett 18: 1498-501 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.058
BindingDB Entry DOI: 10.7270/Q2V125PH |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50374088

(CHEMBL411640)Show SMILES CCCNC(=O)N(CCCN1[C@H]2CC[C@@H]1C[C@@H](C2)n1c(C)nnc1C(C)C)c1ccccc1 |THB:9:10:17.16.15:13.12| Show InChI InChI=1S/C26H40N6O/c1-5-14-27-26(33)31(21-10-7-6-8-11-21)16-9-15-30-22-12-13-23(30)18-24(17-22)32-20(4)28-29-25(32)19(2)3/h6-8,10-11,19,22-24H,5,9,12-18H2,1-4H3,(H,27,33)/t22-,23+,24+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 271 | n/a | n/a | n/a | n/a | n/a | n/a |
K£mia, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]MIP1beta from human recombinant CCR5 receptor expressed in CHOK1 cells |
Bioorg Med Chem Lett 18: 1498-501 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.058
BindingDB Entry DOI: 10.7270/Q2V125PH |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50374085

(CHEMBL405634)Show SMILES CC(C)c1nnc(C)n1[C@@H]1C[C@@H]2CC[C@H](C1)N2CCCN(C(=O)Nc1ccccc1C)c1ccccc1 |THB:17:16:10.9.15:13.12| Show InChI InChI=1S/C30H40N6O/c1-21(2)29-33-32-23(4)36(29)27-19-25-15-16-26(20-27)34(25)17-10-18-35(24-12-6-5-7-13-24)30(37)31-28-14-9-8-11-22(28)3/h5-9,11-14,21,25-27H,10,15-20H2,1-4H3,(H,31,37)/t25-,26+,27+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 271 | n/a | n/a | n/a | n/a | n/a | n/a |
K£mia, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]MIP1beta from human recombinant CCR5 receptor expressed in CHOK1 cells |
Bioorg Med Chem Lett 18: 1498-501 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.058
BindingDB Entry DOI: 10.7270/Q2V125PH |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50374076

(CHEMBL256792)Show SMILES CC(C)c1nnc(C)n1[C@@H]1C[C@@H]2CC[C@H](C1)N2CCCN(C(=O)C1CCCCC1)c1ccccc1 |THB:17:16:10.9.15:13.12| Show InChI InChI=1S/C29H43N5O/c1-21(2)28-31-30-22(3)34(28)27-19-25-15-16-26(20-27)32(25)17-10-18-33(24-13-8-5-9-14-24)29(35)23-11-6-4-7-12-23/h5,8-9,13-14,21,23,25-27H,4,6-7,10-12,15-20H2,1-3H3/t25-,26+,27+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
K£mia, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]MIP1beta from human recombinant CCR5 receptor expressed in CHOK1 cells |
Bioorg Med Chem Lett 18: 1498-501 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.058
BindingDB Entry DOI: 10.7270/Q2V125PH |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50374086
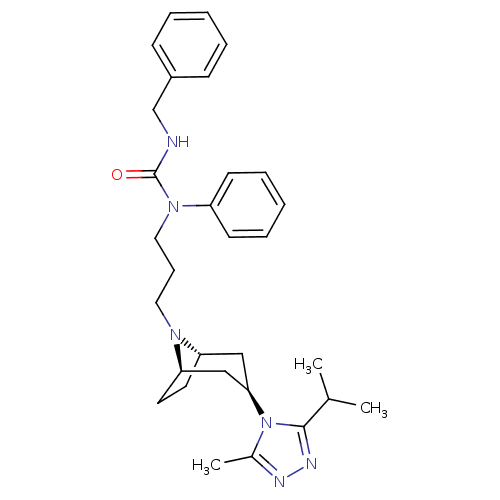
(CHEMBL272581)Show SMILES CC(C)c1nnc(C)n1[C@@H]1C[C@@H]2CC[C@H](C1)N2CCCN(C(=O)NCc1ccccc1)c1ccccc1 |THB:17:16:10.9.15:13.12| Show InChI InChI=1S/C30H40N6O/c1-22(2)29-33-32-23(3)36(29)28-19-26-15-16-27(20-28)34(26)17-10-18-35(25-13-8-5-9-14-25)30(37)31-21-24-11-6-4-7-12-24/h4-9,11-14,22,26-28H,10,15-21H2,1-3H3,(H,31,37)/t26-,27+,28+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 323 | n/a | n/a | n/a | n/a | n/a | n/a |
K£mia, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]MIP1beta from human recombinant CCR5 receptor expressed in CHOK1 cells |
Bioorg Med Chem Lett 18: 1498-501 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.058
BindingDB Entry DOI: 10.7270/Q2V125PH |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50374083

(CHEMBL255855)Show SMILES COc1ccccc1NC(=O)N(CCCN1[C@H]2CC[C@@H]1C[C@@H](C2)n1c(C)nnc1C(C)C)c1ccccc1 |THB:14:15:22.21.20:18.17| Show InChI InChI=1S/C30H40N6O2/c1-21(2)29-33-32-22(3)36(29)26-19-24-15-16-25(20-26)34(24)17-10-18-35(23-11-6-5-7-12-23)30(37)31-27-13-8-9-14-28(27)38-4/h5-9,11-14,21,24-26H,10,15-20H2,1-4H3,(H,31,37)/t24-,25+,26+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 381 | n/a | n/a | n/a | n/a | n/a | n/a |
K£mia, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]MIP1beta from human recombinant CCR5 receptor expressed in CHOK1 cells |
Bioorg Med Chem Lett 18: 1498-501 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.058
BindingDB Entry DOI: 10.7270/Q2V125PH |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50374079
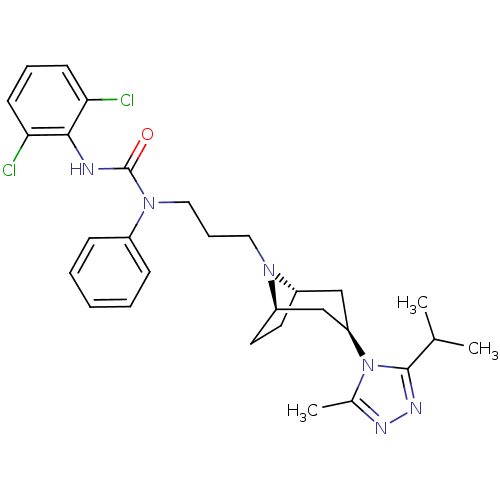
(CHEMBL404056)Show SMILES CC(C)c1nnc(C)n1[C@@H]1C[C@@H]2CC[C@H](C1)N2CCCN(C(=O)Nc1c(Cl)cccc1Cl)c1ccccc1 |THB:17:16:10.9.15:13.12| Show InChI InChI=1S/C29H36Cl2N6O/c1-19(2)28-34-33-20(3)37(28)24-17-22-13-14-23(18-24)35(22)15-8-16-36(21-9-5-4-6-10-21)29(38)32-27-25(30)11-7-12-26(27)31/h4-7,9-12,19,22-24H,8,13-18H2,1-3H3,(H,32,38)/t22-,23+,24+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 539 | n/a | n/a | n/a | n/a | n/a | n/a |
K£mia, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]MIP1beta from human recombinant CCR5 receptor expressed in CHOK1 cells |
Bioorg Med Chem Lett 18: 1498-501 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.058
BindingDB Entry DOI: 10.7270/Q2V125PH |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50374077

(CHEMBL255535)Show SMILES CC(C)c1nnc(C)n1[C@@H]1C[C@@H]2CC[C@H](C1)N2CCN(C(=O)C1CCCCC1)c1ccccc1 |THB:17:16:10.9.15:13.12| Show InChI InChI=1S/C28H41N5O/c1-20(2)27-30-29-21(3)33(27)26-18-24-14-15-25(19-26)31(24)16-17-32(23-12-8-5-9-13-23)28(34)22-10-6-4-7-11-22/h5,8-9,12-13,20,22,24-26H,4,6-7,10-11,14-19H2,1-3H3/t24-,25+,26+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 970 | n/a | n/a | n/a | n/a | n/a | n/a |
K£mia, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]MIP1beta from human recombinant CCR5 receptor expressed in CHOK1 cells |
Bioorg Med Chem Lett 18: 1498-501 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.058
BindingDB Entry DOI: 10.7270/Q2V125PH |
More data for this
Ligand-Target Pair | |
Thrombopoietin receptor
(Homo sapiens (Human)) | BDBM50105938

(4-{N'-[1-(3,4-Dimethyl-phenyl)-3-ethoxy-5-oxo-1,5-...)Show SMILES CCOc1[nH]n(-c2ccc(C)c(C)c2)c(=O)c1N=Nc1c(O)cc(c2ccccc12)S(O)(=O)=O |w:18.20| Show InChI InChI=1S/C23H22N4O6S/c1-4-33-22-21(23(29)27(26-22)15-10-9-13(2)14(3)11-15)25-24-20-17-8-6-5-7-16(17)19(12-18(20)28)34(30,31)32/h5-12,26,28H,4H2,1-3H3,(H,30,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 600 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Effective concentration for thrombopoietin luciferase activity was determined in BAF-3 cells |
J Med Chem 44: 3730-45 (2001)
BindingDB Entry DOI: 10.7270/Q2MP52KF |
More data for this
Ligand-Target Pair | |
Thrombopoietin receptor
(Homo sapiens (Human)) | BDBM50105936
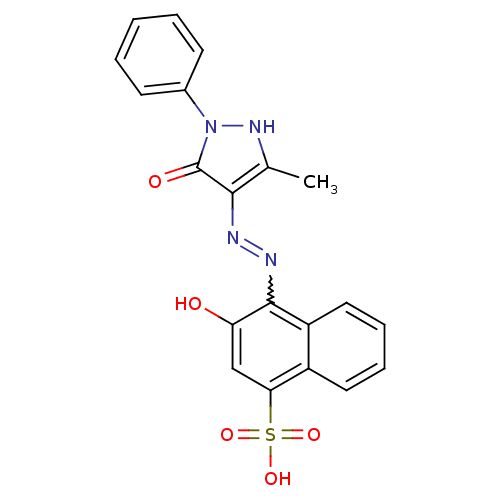
(3-Hydroxy-4-[N'-(3-methyl-5-oxo-1-phenyl-1,5-dihyd...)Show SMILES Cc1[nH]n(-c2ccccc2)c(=O)c1N=Nc1c(O)cc(c2ccccc12)S(O)(=O)=O |w:14.16| Show InChI InChI=1S/C20H16N4O5S/c1-12-18(20(26)24(23-12)13-7-3-2-4-8-13)21-22-19-15-10-6-5-9-14(15)17(11-16(19)25)30(27,28)29/h2-11,23,25H,1H3,(H,27,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Effective concentration for thrombopoietin luciferase activity was determined in BAF-3 cells |
J Med Chem 44: 3730-45 (2001)
BindingDB Entry DOI: 10.7270/Q2MP52KF |
More data for this
Ligand-Target Pair | |
Thrombopoietin receptor
(Homo sapiens (Human)) | BDBM50105937
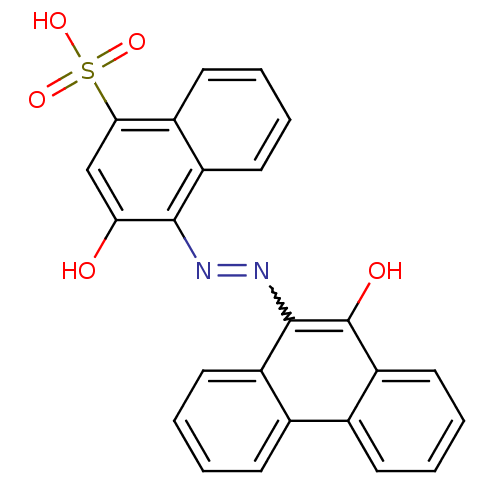
(3-Hydroxy-4-(10-hydroxy-phenanthren-9-ylazo)-napht...)Show SMILES Oc1cc(c2ccccc2c1N=Nc1c(O)c2ccccc2c2ccccc12)S(O)(=O)=O |w:12.14| Show InChI InChI=1S/C24H16N2O5S/c27-20-13-21(32(29,30)31)16-9-3-5-11-18(16)22(20)25-26-23-17-10-4-1-7-14(17)15-8-2-6-12-19(15)24(23)28/h1-13,27-28H,(H,29,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 200 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Effective concentration for thrombopoietin luciferase activity was determined in BAF-3 cells |
J Med Chem 44: 3730-45 (2001)
BindingDB Entry DOI: 10.7270/Q2MP52KF |
More data for this
Ligand-Target Pair | |
Thrombopoietin receptor
(Homo sapiens (Human)) | BDBM50105939
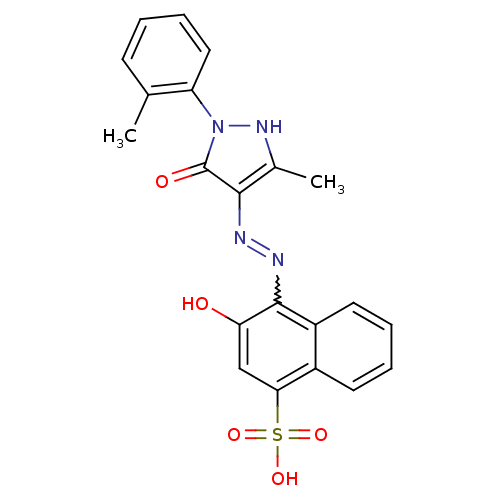
(3-Hydroxy-4-[N'-(3-methyl-5-oxo-1-o-tolyl-1,5-dihy...)Show SMILES Cc1[nH]n(-c2ccccc2C)c(=O)c1N=Nc1c(O)cc(c2ccccc12)S(O)(=O)=O |w:15.17| Show InChI InChI=1S/C21H18N4O5S/c1-12-7-3-6-10-16(12)25-21(27)19(13(2)24-25)22-23-20-15-9-5-4-8-14(15)18(11-17(20)26)31(28,29)30/h3-11,24,26H,1-2H3,(H,28,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Effective concentration for thrombopoietin luciferase activity was determined in BAF-3 cells |
J Med Chem 44: 3730-45 (2001)
BindingDB Entry DOI: 10.7270/Q2MP52KF |
More data for this
Ligand-Target Pair | |
Thrombopoietin receptor
(Homo sapiens (Human)) | BDBM50105940
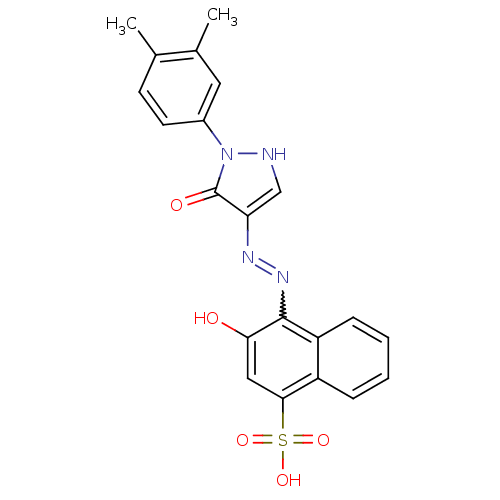
(4-{N'-[1-(3,4-Dimethyl-phenyl)-5-oxo-1,5-dihydro-p...)Show SMILES Cc1ccc(cc1C)-n1[nH]cc(N=Nc2c(O)cc(c3ccccc23)S(O)(=O)=O)c1=O |w:13.14| Show InChI InChI=1S/C21H18N4O5S/c1-12-7-8-14(9-13(12)2)25-21(27)17(11-22-25)23-24-20-16-6-4-3-5-15(16)19(10-18(20)26)31(28,29)30/h3-11,22,26H,1-2H3,(H,28,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 100 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Effective concentration for thrombopoietin luciferase activity was determined in BAF-3 cells |
J Med Chem 44: 3730-45 (2001)
BindingDB Entry DOI: 10.7270/Q2MP52KF |
More data for this
Ligand-Target Pair | |
Thrombopoietin receptor
(Homo sapiens (Human)) | BDBM50105942
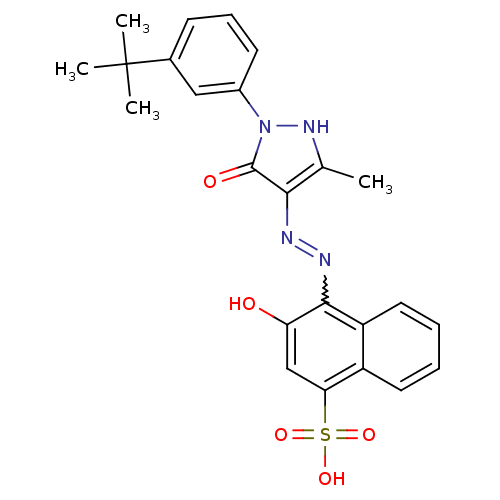
(4-{N'-[1-(3-tert-Butyl-phenyl)-3-methyl-5-oxo-1,5-...)Show SMILES Cc1[nH]n(-c2cccc(c2)C(C)(C)C)c(=O)c1N=Nc1c(O)cc(c2ccccc12)S(O)(=O)=O |w:18.20| Show InChI InChI=1S/C24H24N4O5S/c1-14-21(23(30)28(27-14)16-9-7-8-15(12-16)24(2,3)4)25-26-22-18-11-6-5-10-17(18)20(13-19(22)29)34(31,32)33/h5-13,27,29H,1-4H3,(H,31,32,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 70 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Effective concentration for thrombopoietin luciferase activity was determined in BAF-3 cells |
J Med Chem 44: 3730-45 (2001)
BindingDB Entry DOI: 10.7270/Q2MP52KF |
More data for this
Ligand-Target Pair | |
Thrombopoietin receptor
(Homo sapiens (Human)) | BDBM50105944
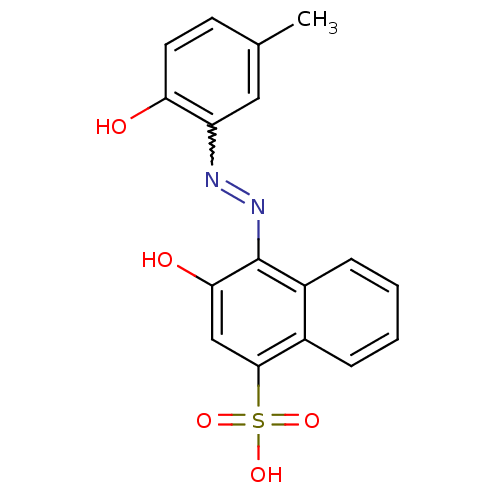
(3-Hydroxy-4-(2-hydroxy-5-methyl-phenylazo)-naphtha...)Show SMILES Cc1ccc(O)c(c1)N=Nc1c(O)cc(c2ccccc12)S(O)(=O)=O |w:8.8| Show InChI InChI=1S/C17H14N2O5S/c1-10-6-7-14(20)13(8-10)18-19-17-12-5-3-2-4-11(12)16(9-15(17)21)25(22,23)24/h2-9,20-21H,1H3,(H,22,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Effective concentration for thrombopoietin luciferase activity was determined in BAF-3 cells |
J Med Chem 44: 3730-45 (2001)
BindingDB Entry DOI: 10.7270/Q2MP52KF |
More data for this
Ligand-Target Pair | |
Thrombopoietin receptor
(Homo sapiens (Human)) | BDBM50105941

(4-(2,3-Dihydroxy-naphthalen-1-ylazo)-3-hydroxy-nap...)Show SMILES Oc1cc(c2ccccc2c1N=Nc1c(O)c(O)cc2ccccc12)S(O)(=O)=O |w:12.14| Show InChI InChI=1S/C20H14N2O6S/c23-15-10-17(29(26,27)28)13-7-3-4-8-14(13)18(15)21-22-19-12-6-2-1-5-11(12)9-16(24)20(19)25/h1-10,23-25H,(H,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Effective concentration for thrombopoietin luciferase activity was determined in BAF-3 cells |
J Med Chem 44: 3730-45 (2001)
BindingDB Entry DOI: 10.7270/Q2MP52KF |
More data for this
Ligand-Target Pair | |
Thrombopoietin receptor
(Homo sapiens (Human)) | BDBM50105943
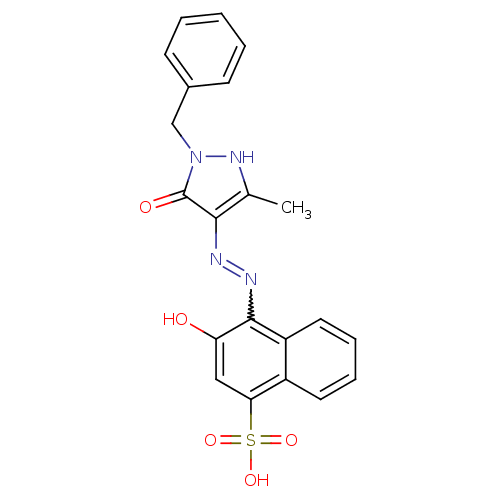
(4-[N'-(1-Benzyl-3-methyl-5-oxo-1,5-dihydro-pyrazol...)Show SMILES Cc1[nH]n(Cc2ccccc2)c(=O)c1N=Nc1c(O)cc(c2ccccc12)S(O)(=O)=O |w:15.17| Show InChI InChI=1S/C21H18N4O5S/c1-13-19(21(27)25(24-13)12-14-7-3-2-4-8-14)22-23-20-16-10-6-5-9-15(16)18(11-17(20)26)31(28,29)30/h2-11,24,26H,12H2,1H3,(H,28,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Effective concentration for thrombopoietin luciferase activity was determined in BAF-3 cells |
J Med Chem 44: 3730-45 (2001)
BindingDB Entry DOI: 10.7270/Q2MP52KF |
More data for this
Ligand-Target Pair | |
Thrombopoietin receptor
(Homo sapiens (Human)) | BDBM50105947
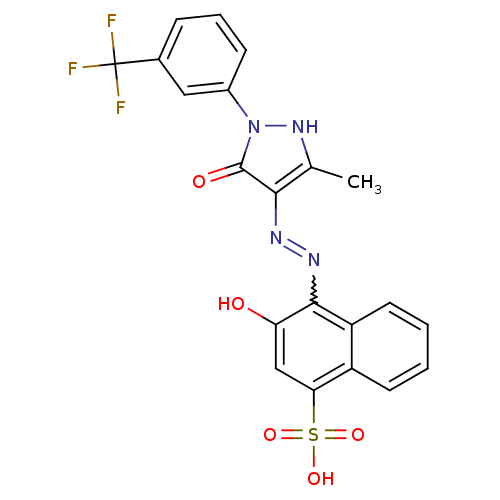
(3-Hydroxy-4-{N'-[3-methyl-5-oxo-1-(3-trifluorometh...)Show SMILES Cc1[nH]n(-c2cccc(c2)C(F)(F)F)c(=O)c1N=Nc1c(O)cc(c2ccccc12)S(O)(=O)=O |w:18.20| Show InChI InChI=1S/C21H15F3N4O5S/c1-11-18(20(30)28(27-11)13-6-4-5-12(9-13)21(22,23)24)25-26-19-15-8-3-2-7-14(15)17(10-16(19)29)34(31,32)33/h2-10,27,29H,1H3,(H,31,32,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 40 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Effective concentration for thrombopoietin luciferase activity was determined in BAF-3 cells |
J Med Chem 44: 3730-45 (2001)
BindingDB Entry DOI: 10.7270/Q2MP52KF |
More data for this
Ligand-Target Pair | |
Thrombopoietin receptor
(Homo sapiens (Human)) | BDBM50105946
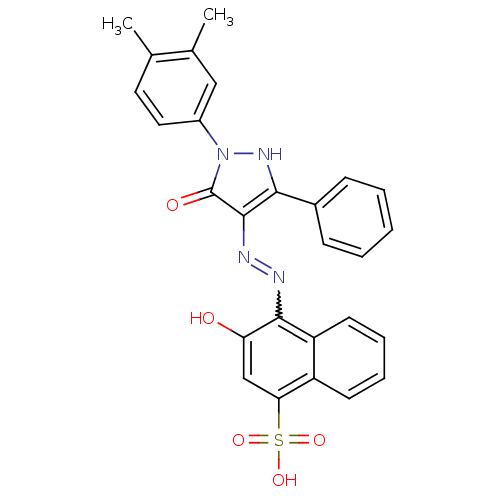
(4-{N'-[1-(3,4-Dimethyl-phenyl)-5-oxo-3-phenyl-1,5-...)Show SMILES Cc1ccc(cc1C)-n1[nH]c(c(N=Nc2c(O)cc(c3ccccc23)S(O)(=O)=O)c1=O)-c1ccccc1 |w:13.14| Show InChI InChI=1S/C27H22N4O5S/c1-16-12-13-19(14-17(16)2)31-27(33)26(24(30-31)18-8-4-3-5-9-18)29-28-25-21-11-7-6-10-20(21)23(15-22(25)32)37(34,35)36/h3-15,30,32H,1-2H3,(H,34,35,36) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 900 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Effective concentration for thrombopoietin luciferase activity was determined in BAF-3 cells |
J Med Chem 44: 3730-45 (2001)
BindingDB Entry DOI: 10.7270/Q2MP52KF |
More data for this
Ligand-Target Pair | |
Thrombopoietin receptor
(Homo sapiens (Human)) | BDBM50105948
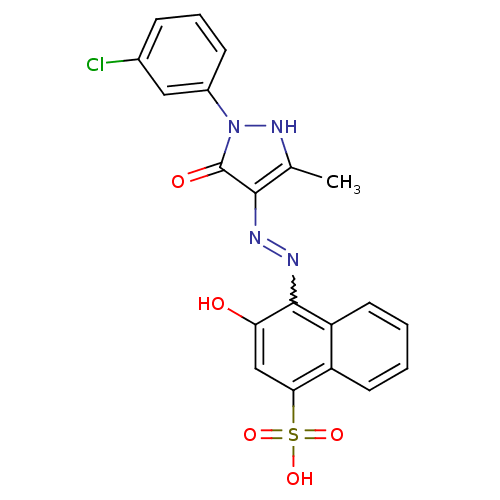
(4-{N'-[1-(3-Chloro-phenyl)-3-methyl-5-oxo-1,5-dihy...)Show SMILES Cc1[nH]n(-c2cccc(Cl)c2)c(=O)c1N=Nc1c(O)cc(c2ccccc12)S(O)(=O)=O |w:15.17| Show InChI InChI=1S/C20H15ClN4O5S/c1-11-18(20(27)25(24-11)13-6-4-5-12(21)9-13)22-23-19-15-8-3-2-7-14(15)17(10-16(19)26)31(28,29)30/h2-10,24,26H,1H3,(H,28,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Effective concentration for thrombopoietin luciferase activity was determined in BAF-3 cells |
J Med Chem 44: 3730-45 (2001)
BindingDB Entry DOI: 10.7270/Q2MP52KF |
More data for this
Ligand-Target Pair | |
Thrombopoietin receptor
(Homo sapiens (Human)) | BDBM50105949
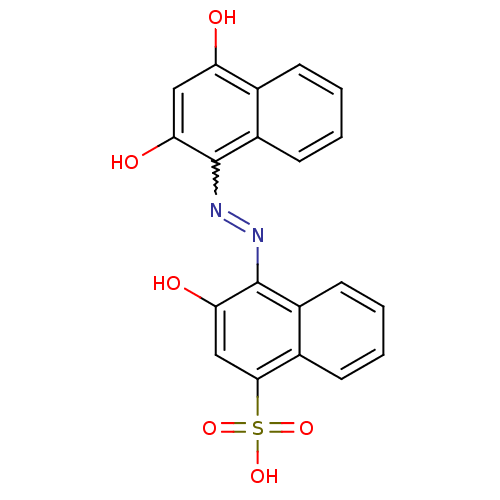
(4-(2,4-Dihydroxy-naphthalen-1-ylazo)-3-hydroxy-nap...)Show SMILES Oc1cc(O)c2ccccc2c1N=Nc1c(O)cc(c2ccccc12)S(O)(=O)=O |w:12.13| Show InChI InChI=1S/C20H14N2O6S/c23-15-9-16(24)19(13-7-3-1-5-11(13)15)21-22-20-14-8-4-2-6-12(14)18(10-17(20)25)29(26,27)28/h1-10,23-25H,(H,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Effective concentration for thrombopoietin luciferase activity was determined in BAF-3 cells |
J Med Chem 44: 3730-45 (2001)
BindingDB Entry DOI: 10.7270/Q2MP52KF |
More data for this
Ligand-Target Pair | |
Thrombopoietin receptor
(Homo sapiens (Human)) | BDBM50105951
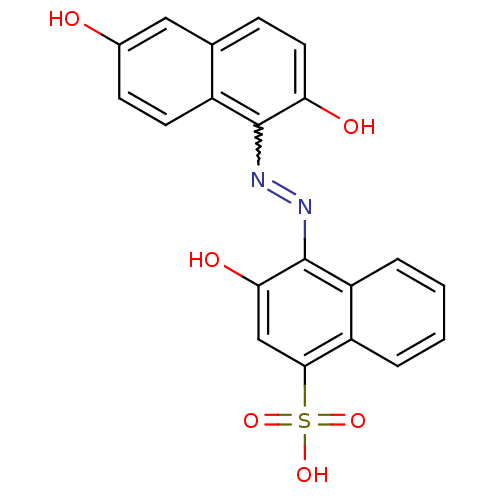
(4-(2,6-Dihydroxy-naphthalen-1-ylazo)-3-hydroxy-nap...)Show SMILES Oc1ccc2c(N=Nc3c(O)cc(c4ccccc34)S(O)(=O)=O)c(O)ccc2c1 |w:6.5| Show InChI InChI=1S/C20H14N2O6S/c23-12-6-7-13-11(9-12)5-8-16(24)19(13)21-22-20-15-4-2-1-3-14(15)18(10-17(20)25)29(26,27)28/h1-10,23-25H,(H,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Effective concentration for thrombopoietin luciferase activity was determined in BAF-3 cells |
J Med Chem 44: 3730-45 (2001)
BindingDB Entry DOI: 10.7270/Q2MP52KF |
More data for this
Ligand-Target Pair | |
Thrombopoietin receptor
(Homo sapiens (Human)) | BDBM50105952
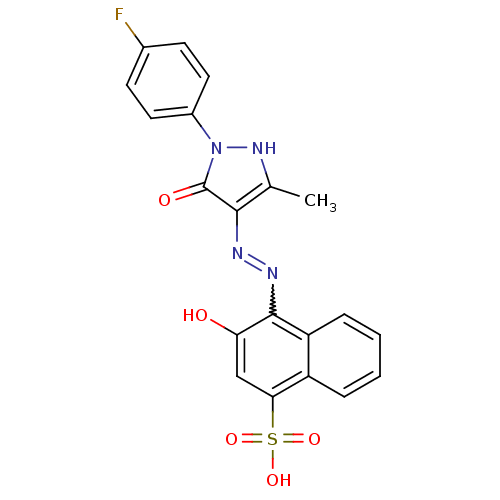
(4-{N'-[1-(4-Fluoro-phenyl)-3-methyl-5-oxo-1,5-dihy...)Show SMILES Cc1[nH]n(-c2ccc(F)cc2)c(=O)c1N=Nc1c(O)cc(c2ccccc12)S(O)(=O)=O |w:15.17| Show InChI InChI=1S/C20H15FN4O5S/c1-11-18(20(27)25(24-11)13-8-6-12(21)7-9-13)22-23-19-15-5-3-2-4-14(15)17(10-16(19)26)31(28,29)30/h2-10,24,26H,1H3,(H,28,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 500 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Effective concentration for thrombopoietin luciferase activity was determined in BAF-3 cells |
J Med Chem 44: 3730-45 (2001)
BindingDB Entry DOI: 10.7270/Q2MP52KF |
More data for this
Ligand-Target Pair | |
Thrombopoietin receptor
(Homo sapiens (Human)) | BDBM50105945
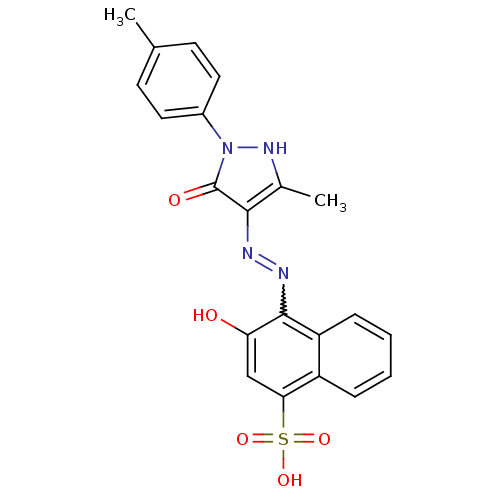
(3-Hydroxy-4-[N'-(3-methyl-5-oxo-1-p-tolyl-1,5-dihy...)Show SMILES Cc1[nH]n(-c2ccc(C)cc2)c(=O)c1N=Nc1c(O)cc(c2ccccc12)S(O)(=O)=O |w:15.17| Show InChI InChI=1S/C21H18N4O5S/c1-12-7-9-14(10-8-12)25-21(27)19(13(2)24-25)22-23-20-16-6-4-3-5-15(16)18(11-17(20)26)31(28,29)30/h3-11,24,26H,1-2H3,(H,28,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Effective concentration for thrombopoietin luciferase activity was determined in BAF-3 cells |
J Med Chem 44: 3730-45 (2001)
BindingDB Entry DOI: 10.7270/Q2MP52KF |
More data for this
Ligand-Target Pair | |
Thrombopoietin receptor
(Homo sapiens (Human)) | BDBM50105956
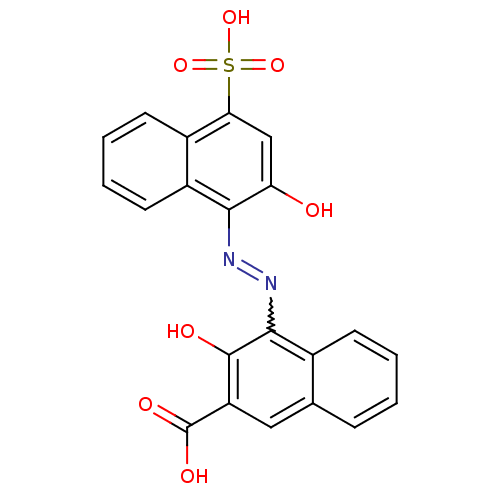
(3-Hydroxy-4-(2-hydroxy-4-sulfo-naphthalen-1-ylazo)...)Show SMILES OC(=O)c1cc2ccccc2c(N=Nc2c(O)cc(c3ccccc23)S(O)(=O)=O)c1O |w:12.12| Show InChI InChI=1S/C21H14N2O7S/c24-16-10-17(31(28,29)30)13-7-3-4-8-14(13)18(16)22-23-19-12-6-2-1-5-11(12)9-15(20(19)25)21(26)27/h1-10,24-25H,(H,26,27)(H,28,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Effective concentration for thrombopoietin luciferase activity was determined in BAF-3 cells |
J Med Chem 44: 3730-45 (2001)
BindingDB Entry DOI: 10.7270/Q2MP52KF |
More data for this
Ligand-Target Pair | |
Thrombopoietin receptor
(Homo sapiens (Human)) | BDBM50105953
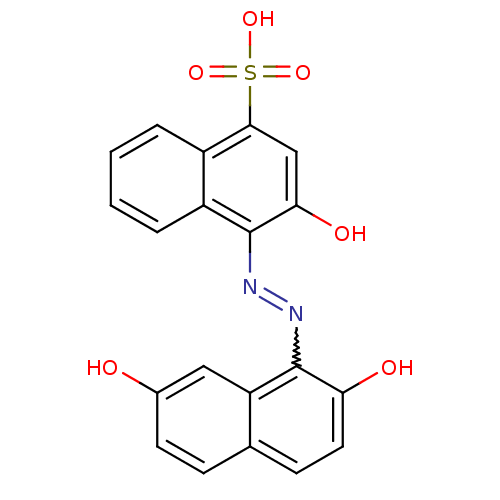
(4-(2,7-Dihydroxy-naphthalen-1-ylazo)-3-hydroxy-nap...)Show SMILES Oc1ccc2ccc(O)c(N=Nc3c(O)cc(c4ccccc34)S(O)(=O)=O)c2c1 |w:10.9| Show InChI InChI=1S/C20H14N2O6S/c23-12-7-5-11-6-8-16(24)20(15(11)9-12)22-21-19-14-4-2-1-3-13(14)18(10-17(19)25)29(26,27)28/h1-10,23-25H,(H,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Effective concentration for thrombopoietin luciferase activity was determined in BAF-3 cells |
J Med Chem 44: 3730-45 (2001)
BindingDB Entry DOI: 10.7270/Q2MP52KF |
More data for this
Ligand-Target Pair | |
Thrombopoietin receptor
(Homo sapiens (Human)) | BDBM50105955

(3-Hydroxy-4-{N'-[3-methyl-1-(2-nitro-phenyl)-5-oxo...)Show SMILES Cc1[nH]n(-c2ccccc2[N+]([O-])=O)c(=O)c1N=Nc1c(O)cc(c2ccccc12)S(O)(=O)=O |w:17.19| Show InChI InChI=1S/C20H15N5O7S/c1-11-18(20(27)24(23-11)14-8-4-5-9-15(14)25(28)29)21-22-19-13-7-3-2-6-12(13)17(10-16(19)26)33(30,31)32/h2-10,23,26H,1H3,(H,30,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Effective concentration for thrombopoietin luciferase activity was determined in BAF-3 cells |
J Med Chem 44: 3730-45 (2001)
BindingDB Entry DOI: 10.7270/Q2MP52KF |
More data for this
Ligand-Target Pair | |
Thrombopoietin receptor
(Homo sapiens (Human)) | BDBM50105954
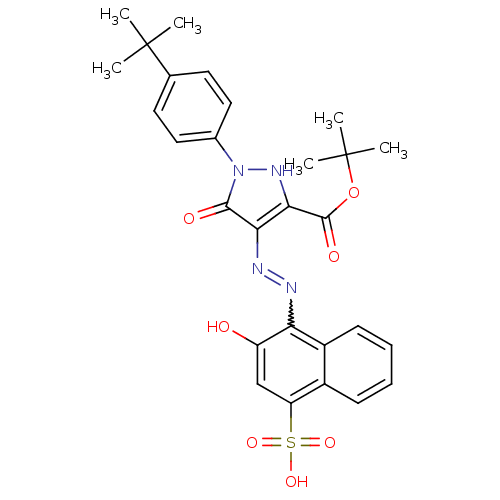
(1-(4-tert-Butyl-phenyl)-4-[(2-hydroxy-4-sulfo-naph...)Show SMILES CC(C)(C)OC(=O)c1[nH]n(-c2ccc(cc2)C(C)(C)C)c(=O)c1N=Nc1c(O)cc(c2ccccc12)S(O)(=O)=O |w:24.26| Show InChI InChI=1S/C28H30N4O7S/c1-27(2,3)16-11-13-17(14-12-16)32-25(34)23(24(31-32)26(35)39-28(4,5)6)30-29-22-19-10-8-7-9-18(19)21(15-20(22)33)40(36,37)38/h7-15,31,33H,1-6H3,(H,36,37,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 200 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Effective concentration for thrombopoietin luciferase activity was determined in BAF-3 cells |
J Med Chem 44: 3730-45 (2001)
BindingDB Entry DOI: 10.7270/Q2MP52KF |
More data for this
Ligand-Target Pair | |
Thrombopoietin receptor
(Homo sapiens (Human)) | BDBM50105959
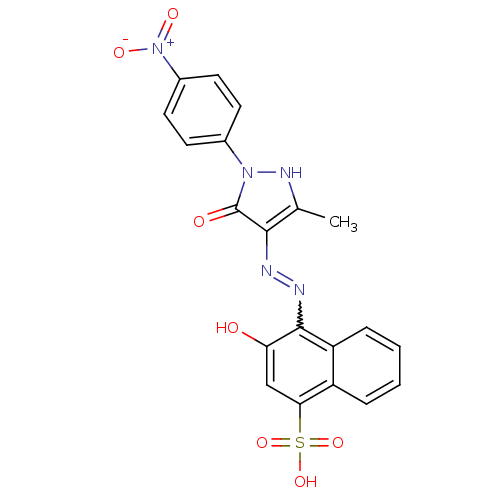
(3-Hydroxy-4-{N'-[3-methyl-1-(4-nitro-phenyl)-5-oxo...)Show SMILES Cc1[nH]n(-c2ccc(cc2)[N+]([O-])=O)c(=O)c1N=Nc1c(O)cc(c2ccccc12)S(O)(=O)=O |w:17.19| Show InChI InChI=1S/C20H15N5O7S/c1-11-18(20(27)24(23-11)12-6-8-13(9-7-12)25(28)29)21-22-19-15-5-3-2-4-14(15)17(10-16(19)26)33(30,31)32/h2-10,23,26H,1H3,(H,30,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 400 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Effective concentration for thrombopoietin luciferase activity was determined in BAF-3 cells |
J Med Chem 44: 3730-45 (2001)
BindingDB Entry DOI: 10.7270/Q2MP52KF |
More data for this
Ligand-Target Pair | |
Thrombopoietin receptor
(Homo sapiens (Human)) | BDBM50105958
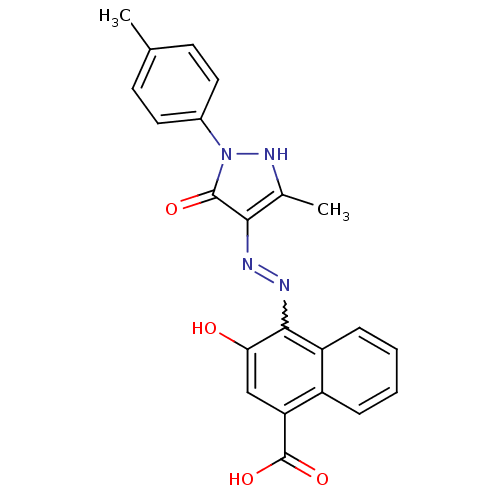
(3-Hydroxy-4-[N'-(3-methyl-5-oxo-1-p-tolyl-1,5-dihy...)Show SMILES Cc1[nH]n(-c2ccc(C)cc2)c(=O)c1N=Nc1c(O)cc(C(O)=O)c2ccccc12 |w:15.17| Show InChI InChI=1S/C22H18N4O4/c1-12-7-9-14(10-8-12)26-21(28)19(13(2)25-26)23-24-20-16-6-4-3-5-15(16)17(22(29)30)11-18(20)27/h3-11,25,27H,1-2H3,(H,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Effective concentration for thrombopoietin luciferase activity was determined in BAF-3 cells |
J Med Chem 44: 3730-45 (2001)
BindingDB Entry DOI: 10.7270/Q2MP52KF |
More data for this
Ligand-Target Pair | |
Thrombopoietin receptor
(Homo sapiens (Human)) | BDBM50105950
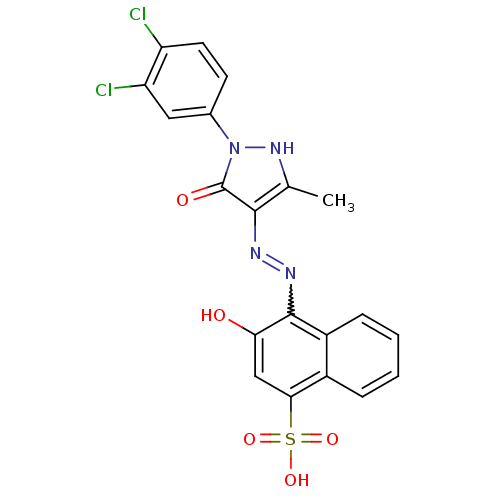
(4-{N'-[1-(3,4-Dichloro-phenyl)-3-methyl-5-oxo-1,5-...)Show SMILES Cc1[nH]n(-c2ccc(Cl)c(Cl)c2)c(=O)c1N=Nc1c(O)cc(c2ccccc12)S(O)(=O)=O |w:16.18| Show InChI InChI=1S/C20H14Cl2N4O5S/c1-10-18(20(28)26(25-10)11-6-7-14(21)15(22)8-11)23-24-19-13-5-3-2-4-12(13)17(9-16(19)27)32(29,30)31/h2-9,25,27H,1H3,(H,29,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 200 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Effective concentration for thrombopoietin luciferase activity was determined in BAF-3 cells |
J Med Chem 44: 3730-45 (2001)
BindingDB Entry DOI: 10.7270/Q2MP52KF |
More data for this
Ligand-Target Pair | |
Thrombopoietin receptor
(Homo sapiens (Human)) | BDBM50105957
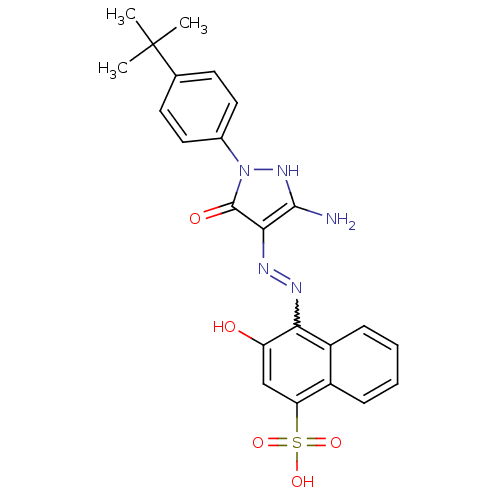
(4-{N'-[3-Amino-1-(4-tert-butyl-phenyl)-5-oxo-1,5-d...)Show SMILES CC(C)(C)c1ccc(cc1)-n1[nH]c(N)c(N=Nc2c(O)cc(c3ccccc23)S(O)(=O)=O)c1=O |w:16.17| Show InChI InChI=1S/C23H23N5O5S/c1-23(2,3)13-8-10-14(11-9-13)28-22(30)20(21(24)27-28)26-25-19-16-7-5-4-6-15(16)18(12-17(19)29)34(31,32)33/h4-12,27,29H,24H2,1-3H3,(H,31,32,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Effective concentration for thrombopoietin luciferase activity was determined in BAF-3 cells |
J Med Chem 44: 3730-45 (2001)
BindingDB Entry DOI: 10.7270/Q2MP52KF |
More data for this
Ligand-Target Pair | |
Thrombopoietin receptor
(Homo sapiens (Human)) | BDBM50105962
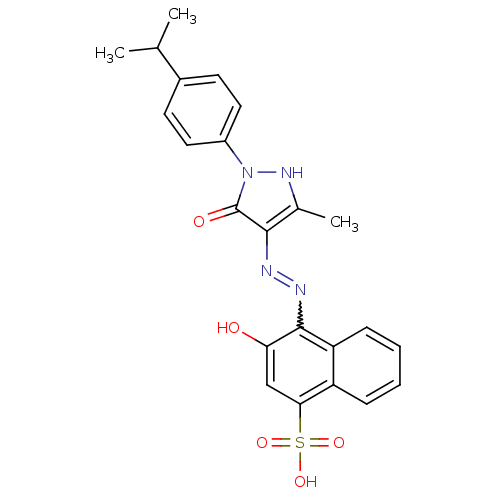
(3-Hydroxy-4-{N'-[1-(4-isopropyl-phenyl)-3-methyl-5...)Show SMILES CC(C)c1ccc(cc1)-n1[nH]c(C)c(N=Nc2c(O)cc(c3ccccc23)S(O)(=O)=O)c1=O |w:15.16| Show InChI InChI=1S/C23H22N4O5S/c1-13(2)15-8-10-16(11-9-15)27-23(29)21(14(3)26-27)24-25-22-18-7-5-4-6-17(18)20(12-19(22)28)33(30,31)32/h4-13,26,28H,1-3H3,(H,30,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 70 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Effective concentration for thrombopoietin luciferase activity was determined in BAF-3 cells |
J Med Chem 44: 3730-45 (2001)
BindingDB Entry DOI: 10.7270/Q2MP52KF |
More data for this
Ligand-Target Pair | |
Thrombopoietin receptor
(Homo sapiens (Human)) | BDBM50105960
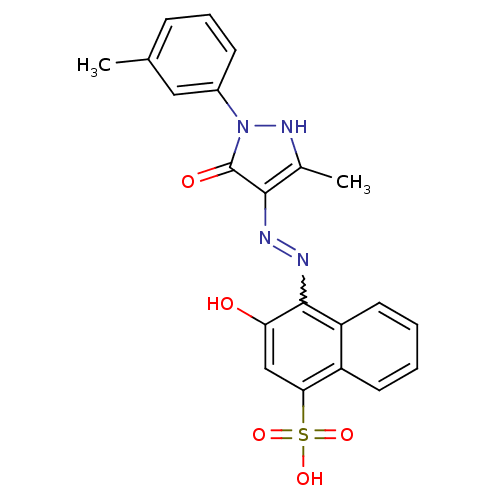
(3-Hydroxy-4-[N'-(3-methyl-5-oxo-1-m-tolyl-1,5-dihy...)Show SMILES Cc1[nH]n(-c2cccc(C)c2)c(=O)c1N=Nc1c(O)cc(c2ccccc12)S(O)(=O)=O |w:15.17| Show InChI InChI=1S/C21H18N4O5S/c1-12-6-5-7-14(10-12)25-21(27)19(13(2)24-25)22-23-20-16-9-4-3-8-15(16)18(11-17(20)26)31(28,29)30/h3-11,24,26H,1-2H3,(H,28,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 700 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Effective concentration for thrombopoietin luciferase activity was determined in BAF-3 cells |
J Med Chem 44: 3730-45 (2001)
BindingDB Entry DOI: 10.7270/Q2MP52KF |
More data for this
Ligand-Target Pair | |
Thrombopoietin receptor
(Homo sapiens (Human)) | BDBM50105961

(3-Hydroxy-4-(2-hydroxy-7-methoxy-naphthalen-1-ylaz...)Show SMILES COc1ccc2ccc(O)c(N=Nc3c(O)cc(c4ccccc34)S(O)(=O)=O)c2c1 |w:11.10| Show InChI InChI=1S/C21H16N2O6S/c1-29-13-8-6-12-7-9-17(24)21(16(12)10-13)23-22-20-15-5-3-2-4-14(15)19(11-18(20)25)30(26,27)28/h2-11,24-25H,1H3,(H,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 300 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Effective concentration for thrombopoietin luciferase activity was determined in BAF-3 cells |
J Med Chem 44: 3730-45 (2001)
BindingDB Entry DOI: 10.7270/Q2MP52KF |
More data for this
Ligand-Target Pair | |
Thrombopoietin receptor
(Homo sapiens (Human)) | BDBM50105964
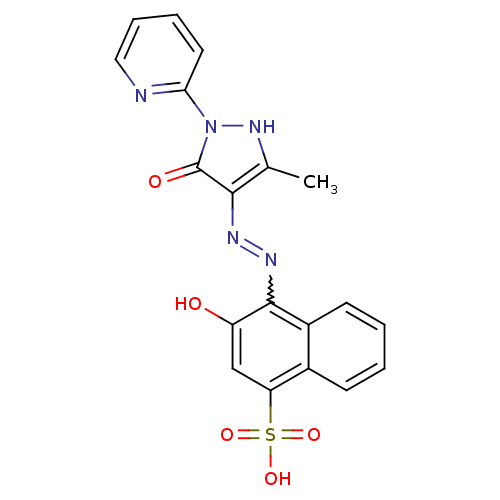
(3-Hydroxy-4-[N'-(3-methyl-5-oxo-1-pyridin-2-yl-1,5...)Show SMILES Cc1[nH]n(-c2ccccn2)c(=O)c1N=Nc1c(O)cc(c2ccccc12)S(O)(=O)=O |w:14.16| Show InChI InChI=1S/C19H15N5O5S/c1-11-17(19(26)24(23-11)16-8-4-5-9-20-16)21-22-18-13-7-3-2-6-12(13)15(10-14(18)25)30(27,28)29/h2-10,23,25H,1H3,(H,27,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Effective concentration for thrombopoietin luciferase activity was determined in BAF-3 cells |
J Med Chem 44: 3730-45 (2001)
BindingDB Entry DOI: 10.7270/Q2MP52KF |
More data for this
Ligand-Target Pair | |
Thrombopoietin receptor
(Homo sapiens (Human)) | BDBM50105967
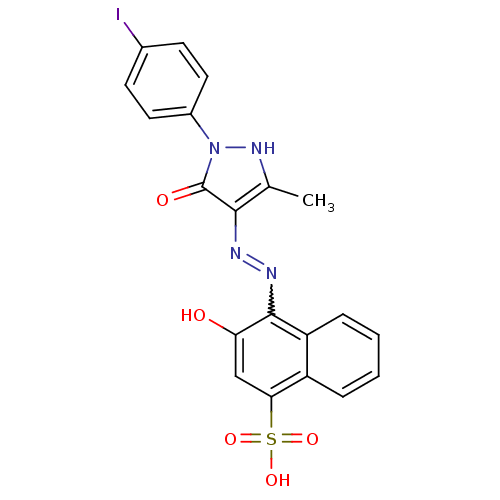
(3-Hydroxy-4-{N'-[1-(4-iodo-phenyl)-3-methyl-5-oxo-...)Show SMILES Cc1[nH]n(-c2ccc(I)cc2)c(=O)c1N=Nc1c(O)cc(c2ccccc12)S(O)(=O)=O |w:15.17| Show InChI InChI=1S/C20H15IN4O5S/c1-11-18(20(27)25(24-11)13-8-6-12(21)7-9-13)22-23-19-15-5-3-2-4-14(15)17(10-16(19)26)31(28,29)30/h2-10,24,26H,1H3,(H,28,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 300 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Effective concentration for thrombopoietin luciferase activity was determined in BAF-3 cells |
J Med Chem 44: 3730-45 (2001)
BindingDB Entry DOI: 10.7270/Q2MP52KF |
More data for this
Ligand-Target Pair | |
Thrombopoietin receptor
(Homo sapiens (Human)) | BDBM50105965
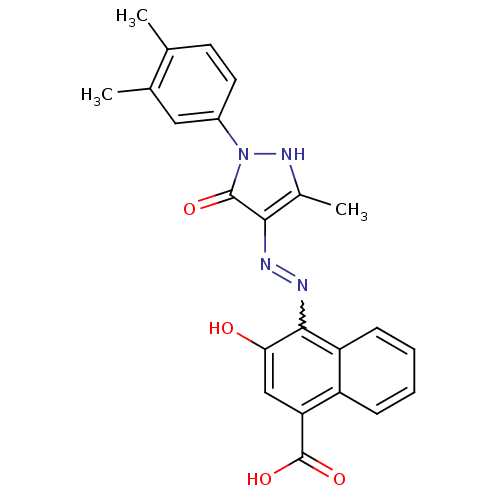
(4-{N'-[1-(3,4-Dimethyl-phenyl)-3-methyl-5-oxo-1,5-...)Show SMILES Cc1[nH]n(-c2ccc(C)c(C)c2)c(=O)c1N=Nc1c(O)cc(C(O)=O)c2ccccc12 |w:16.18| Show InChI InChI=1S/C23H20N4O4/c1-12-8-9-15(10-13(12)2)27-22(29)20(14(3)26-27)24-25-21-17-7-5-4-6-16(17)18(23(30)31)11-19(21)28/h4-11,26,28H,1-3H3,(H,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Effective concentration for thrombopoietin luciferase activity was determined in BAF-3 cells |
J Med Chem 44: 3730-45 (2001)
BindingDB Entry DOI: 10.7270/Q2MP52KF |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data