 Found 42 hits with Last Name = 'pass' and Initial = 'sl'
Found 42 hits with Last Name = 'pass' and Initial = 'sl' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50348452
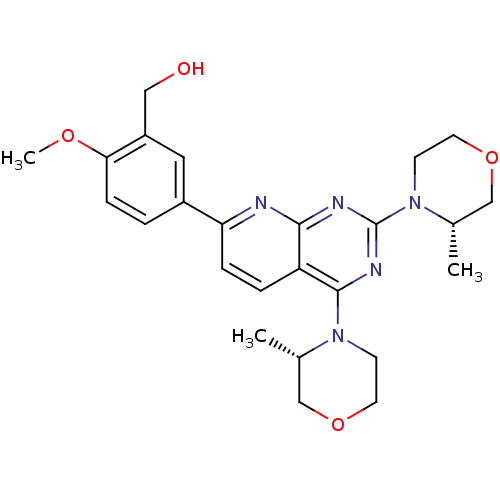
(AZD-8055 | CHEMBL1801204 | US9102670, 1a)Show SMILES COc1ccc(cc1CO)-c1ccc2c(nc(nc2n1)N1CCOC[C@@H]1C)N1CCOC[C@@H]1C |r| Show InChI InChI=1S/C25H31N5O4/c1-16-14-33-10-8-29(16)24-20-5-6-21(18-4-7-22(32-3)19(12-18)13-31)26-23(20)27-25(28-24)30-9-11-34-15-17(30)2/h4-7,12,16-17,31H,8-11,13-15H2,1-3H3/t16-,17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant FLAG-tagged mTOR (1362 to 2549) (unknown origin) expressed in HEK293 cells |
Bioorg Med Chem Lett 23: 1212-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.01.019
BindingDB Entry DOI: 10.7270/Q2N29Z97 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50072961
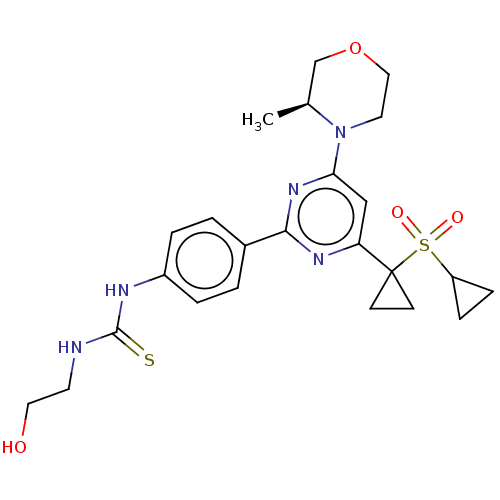
(CHEMBL3410672)Show SMILES C[C@H]1COCCN1c1cc(nc(n1)-c1ccc(NC(=S)NCCO)cc1)C1(CC1)S(=O)(=O)C1CC1 |r| Show InChI InChI=1S/C24H31N5O4S2/c1-16-15-33-13-11-29(16)21-14-20(24(8-9-24)35(31,32)19-6-7-19)27-22(28-21)17-2-4-18(5-3-17)26-23(34)25-10-12-30/h2-5,14,16,19,30H,6-13,15H2,1H3,(H2,25,26,34)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant truncated FLAG-tagged mTOR (1362 to 2549 aa) (unknown origin) expressed in HEK293 cells using biotinylated p70 peptide as s... |
J Med Chem 58: 2326-49 (2015)
Article DOI: 10.1021/jm501778s
BindingDB Entry DOI: 10.7270/Q2DF6SW8 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50310989
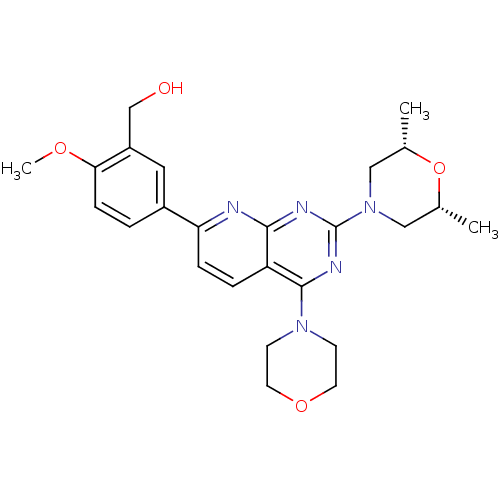
((5-(2-((2R,6S)-2,6-dimethylmorpholino)-4-morpholin...)Show SMILES COc1ccc(cc1CO)-c1ccc2c(nc(nc2n1)N1C[C@H](C)O[C@H](C)C1)N1CCOCC1 |r| Show InChI InChI=1S/C25H31N5O4/c1-16-13-30(14-17(2)34-16)25-27-23-20(24(28-25)29-8-10-33-11-9-29)5-6-21(26-23)18-4-7-22(32-3)19(12-18)15-31/h4-7,12,16-17,31H,8-11,13-15H2,1-3H3/t16-,17+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant FLAG-tagged mTOR (1362 to 2549) (unknown origin) expressed in HEK293 cells |
Bioorg Med Chem Lett 23: 1212-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.01.019
BindingDB Entry DOI: 10.7270/Q2N29Z97 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50429701
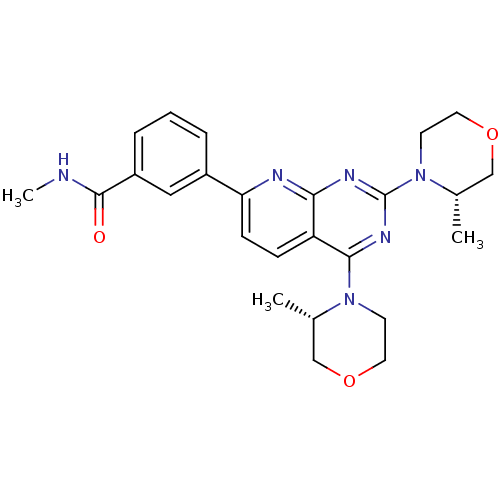
(AZD-2014 | CHEMBL2336325 | US9102670, 1ap)Show SMILES CNC(=O)c1cccc(c1)-c1ccc2c(nc(nc2n1)N1CCOC[C@@H]1C)N1CCOC[C@@H]1C |r| Show InChI InChI=1S/C25H30N6O3/c1-16-14-33-11-9-30(16)23-20-7-8-21(18-5-4-6-19(13-18)24(32)26-3)27-22(20)28-25(29-23)31-10-12-34-15-17(31)2/h4-8,13,16-17H,9-12,14-15H2,1-3H3,(H,26,32)/t16-,17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant FLAG-tagged mTOR (1362 to 2549) (unknown origin) expressed in HEK293 cells |
Bioorg Med Chem Lett 23: 1212-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.01.019
BindingDB Entry DOI: 10.7270/Q2N29Z97 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50072963
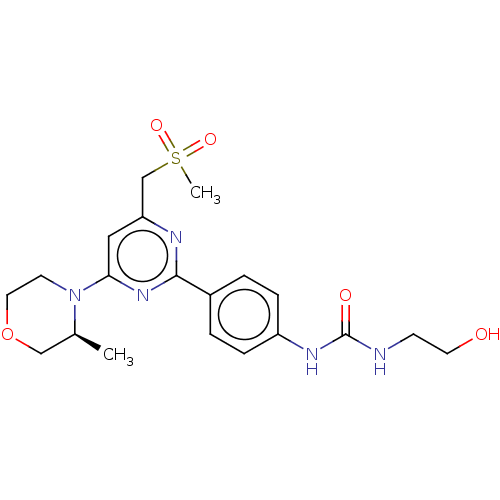
(CHEMBL3410548)Show SMILES C[C@H]1COCCN1c1cc(CS(C)(=O)=O)nc(n1)-c1ccc(NC(=O)NCCO)cc1 |r| Show InChI InChI=1S/C20H27N5O5S/c1-14-12-30-10-8-25(14)18-11-17(13-31(2,28)29)22-19(24-18)15-3-5-16(6-4-15)23-20(27)21-7-9-26/h3-6,11,14,26H,7-10,12-13H2,1-2H3,(H2,21,23,27)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant truncated FLAG-tagged mTOR (1362 to 2549 aa) (unknown origin) expressed in HEK293 cells using biotinylated p70 peptide as s... |
J Med Chem 58: 2326-49 (2015)
Article DOI: 10.1021/jm501778s
BindingDB Entry DOI: 10.7270/Q2DF6SW8 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50384248
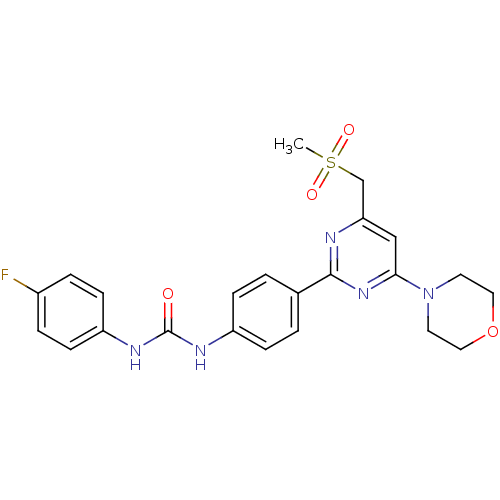
(CHEMBL2030451)Show SMILES CS(=O)(=O)Cc1cc(nc(n1)-c1ccc(NC(=O)Nc2ccc(F)cc2)cc1)N1CCOCC1 Show InChI InChI=1S/C23H24FN5O4S/c1-34(31,32)15-20-14-21(29-10-12-33-13-11-29)28-22(25-20)16-2-6-18(7-3-16)26-23(30)27-19-8-4-17(24)5-9-19/h2-9,14H,10-13,15H2,1H3,(H2,26,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant truncated FLAG-tagged mTOR (1362 to 2549 aa) (unknown origin) expressed in HEK293 cells using biotinylated p70 peptide as s... |
J Med Chem 58: 2326-49 (2015)
Article DOI: 10.1021/jm501778s
BindingDB Entry DOI: 10.7270/Q2DF6SW8 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50384247
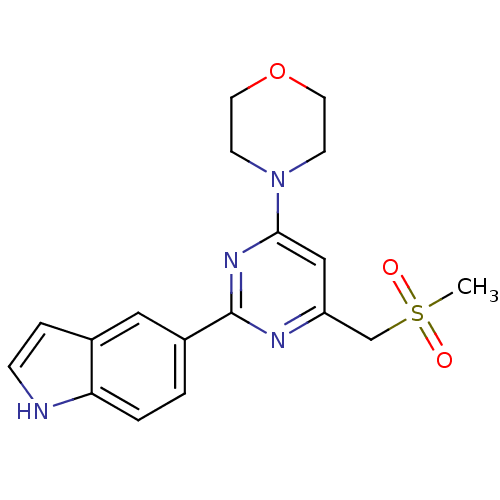
(CHEMBL2030436)Show SMILES CS(=O)(=O)Cc1cc(nc(n1)-c1ccc2[nH]ccc2c1)N1CCOCC1 Show InChI InChI=1S/C18H20N4O3S/c1-26(23,24)12-15-11-17(22-6-8-25-9-7-22)21-18(20-15)14-2-3-16-13(10-14)4-5-19-16/h2-5,10-11,19H,6-9,12H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 288 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant truncated FLAG-tagged mTOR (1362 to 2549 aa) (unknown origin) expressed in HEK293 cells using biotinylated p70 peptide as s... |
J Med Chem 58: 2326-49 (2015)
Article DOI: 10.1021/jm501778s
BindingDB Entry DOI: 10.7270/Q2DF6SW8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50384248

(CHEMBL2030451)Show SMILES CS(=O)(=O)Cc1cc(nc(n1)-c1ccc(NC(=O)Nc2ccc(F)cc2)cc1)N1CCOCC1 Show InChI InChI=1S/C23H24FN5O4S/c1-34(31,32)15-20-14-21(29-10-12-33-13-11-29)28-22(25-20)16-2-6-18(7-3-16)26-23(30)27-19-8-4-17(24)5-9-19/h2-9,14H,10-13,15H2,1H3,(H2,26,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 562 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kalpha (unknown origin) using biotinylated PIP2 as substrate |
J Med Chem 58: 2326-49 (2015)
Article DOI: 10.1021/jm501778s
BindingDB Entry DOI: 10.7270/Q2DF6SW8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50072962
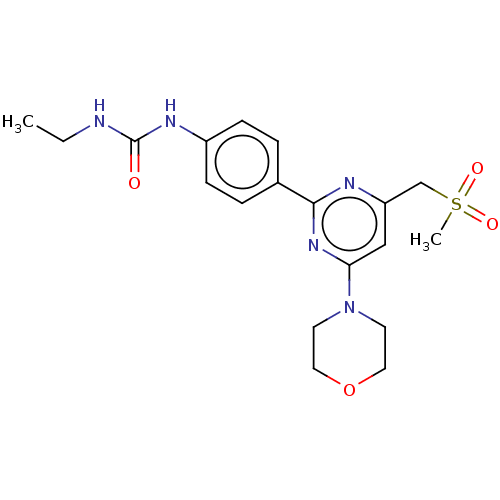
(CHEMBL3410675)Show SMILES CCNC(=O)Nc1ccc(cc1)-c1nc(CS(C)(=O)=O)cc(n1)N1CCOCC1 Show InChI InChI=1S/C19H25N5O4S/c1-3-20-19(25)22-15-6-4-14(5-7-15)18-21-16(13-29(2,26)27)12-17(23-18)24-8-10-28-11-9-24/h4-7,12H,3,8-11,13H2,1-2H3,(H2,20,22,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 562 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kalpha (unknown origin) using biotinylated PIP2 as substrate |
J Med Chem 58: 2326-49 (2015)
Article DOI: 10.1021/jm501778s
BindingDB Entry DOI: 10.7270/Q2DF6SW8 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50072964
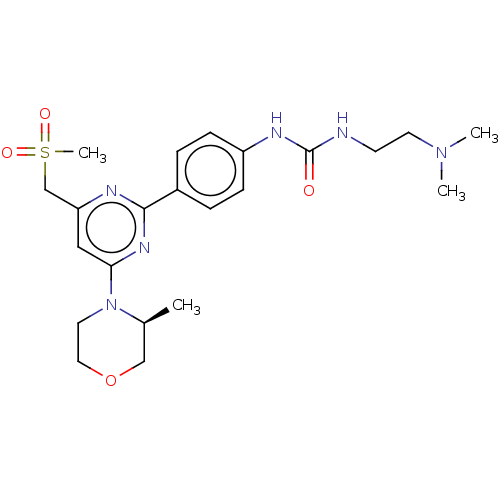
(CHEMBL3410642)Show SMILES C[C@H]1COCCN1c1cc(CS(C)(=O)=O)nc(n1)-c1ccc(NC(=O)NCCN(C)C)cc1 |r| Show InChI InChI=1S/C22H32N6O4S/c1-16-14-32-12-11-28(16)20-13-19(15-33(4,30)31)24-21(26-20)17-5-7-18(8-6-17)25-22(29)23-9-10-27(2)3/h5-8,13,16H,9-12,14-15H2,1-4H3,(H2,23,25,29)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 759 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant truncated FLAG-tagged mTOR (1362 to 2549 aa) (unknown origin) expressed in HEK293 cells using biotinylated p70 peptide as s... |
J Med Chem 58: 2326-49 (2015)
Article DOI: 10.1021/jm501778s
BindingDB Entry DOI: 10.7270/Q2DF6SW8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50072961

(CHEMBL3410672)Show SMILES C[C@H]1COCCN1c1cc(nc(n1)-c1ccc(NC(=S)NCCO)cc1)C1(CC1)S(=O)(=O)C1CC1 |r| Show InChI InChI=1S/C24H31N5O4S2/c1-16-15-33-13-11-29(16)21-14-20(24(8-9-24)35(31,32)19-6-7-19)27-22(28-21)17-2-4-18(5-3-17)26-23(34)25-10-12-30/h2-5,14,16,19,30H,6-13,15H2,1H3,(H2,25,26,34)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 912 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kalpha (unknown origin) using biotinylated PIP2 as substrate |
J Med Chem 58: 2326-49 (2015)
Article DOI: 10.1021/jm501778s
BindingDB Entry DOI: 10.7270/Q2DF6SW8 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50384255
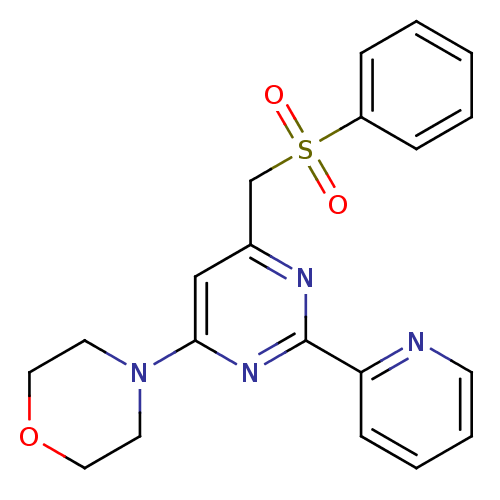
(CHEMBL1312643)Show SMILES O=S(=O)(Cc1cc(nc(n1)-c1ccccn1)N1CCOCC1)c1ccccc1 Show InChI InChI=1S/C20H20N4O3S/c25-28(26,17-6-2-1-3-7-17)15-16-14-19(24-10-12-27-13-11-24)23-20(22-16)18-8-4-5-9-21-18/h1-9,14H,10-13,15H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant truncated FLAG-tagged mTOR (1362 to 2549 aa) (unknown origin) expressed in HEK293 cells using biotinylated p70 peptide as s... |
J Med Chem 58: 2326-49 (2015)
Article DOI: 10.1021/jm501778s
BindingDB Entry DOI: 10.7270/Q2DF6SW8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50072963

(CHEMBL3410548)Show SMILES C[C@H]1COCCN1c1cc(CS(C)(=O)=O)nc(n1)-c1ccc(NC(=O)NCCO)cc1 |r| Show InChI InChI=1S/C20H27N5O5S/c1-14-12-30-10-8-25(14)18-11-17(13-31(2,28)29)22-19(24-18)15-3-5-16(6-4-15)23-20(27)21-7-9-26/h3-6,11,14,26H,7-10,12-13H2,1-2H3,(H2,21,23,27)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kalpha (unknown origin) using biotinylated PIP2 as substrate |
J Med Chem 58: 2326-49 (2015)
Article DOI: 10.1021/jm501778s
BindingDB Entry DOI: 10.7270/Q2DF6SW8 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50429717
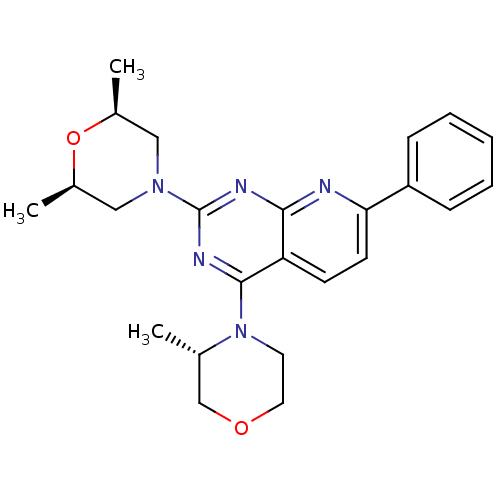
(CHEMBL2336331 | US9102670, 1j)Show SMILES C[C@H]1CN(C[C@@H](C)O1)c1nc(N2CCOC[C@@H]2C)c2ccc(nc2n1)-c1ccccc1 |r| Show InChI InChI=1S/C24H29N5O2/c1-16-15-30-12-11-29(16)23-20-9-10-21(19-7-5-4-6-8-19)25-22(20)26-24(27-23)28-13-17(2)31-18(3)14-28/h4-10,16-18H,11-15H2,1-3H3/t16-,17-,18+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 23: 1212-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.01.019
BindingDB Entry DOI: 10.7270/Q2N29Z97 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50310989

((5-(2-((2R,6S)-2,6-dimethylmorpholino)-4-morpholin...)Show SMILES COc1ccc(cc1CO)-c1ccc2c(nc(nc2n1)N1C[C@H](C)O[C@H](C)C1)N1CCOCC1 |r| Show InChI InChI=1S/C25H31N5O4/c1-16-13-30(14-17(2)34-16)25-27-23-20(24(28-25)29-8-10-33-11-9-29)5-6-21(26-23)18-4-7-22(32-3)19(12-18)15-31/h4-7,12,16-17,31H,8-11,13-15H2,1-3H3/t16-,17+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using lipid PIP2 as substrate |
Bioorg Med Chem Lett 23: 1212-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.01.019
BindingDB Entry DOI: 10.7270/Q2N29Z97 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50072961

(CHEMBL3410672)Show SMILES C[C@H]1COCCN1c1cc(nc(n1)-c1ccc(NC(=S)NCCO)cc1)C1(CC1)S(=O)(=O)C1CC1 |r| Show InChI InChI=1S/C24H31N5O4S2/c1-16-15-33-13-11-29(16)21-14-20(24(8-9-24)35(31,32)19-6-7-19)27-22(28-21)17-2-4-18(5-3-17)26-23(34)25-10-12-30/h2-5,14,16,19,30H,6-13,15H2,1H3,(H2,25,26,34)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kbeta (unknown origin) using biotinylated PIP2 as substrate |
J Med Chem 58: 2326-49 (2015)
Article DOI: 10.1021/jm501778s
BindingDB Entry DOI: 10.7270/Q2DF6SW8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50072961

(CHEMBL3410672)Show SMILES C[C@H]1COCCN1c1cc(nc(n1)-c1ccc(NC(=S)NCCO)cc1)C1(CC1)S(=O)(=O)C1CC1 |r| Show InChI InChI=1S/C24H31N5O4S2/c1-16-15-33-13-11-29(16)21-14-20(24(8-9-24)35(31,32)19-6-7-19)27-22(28-21)17-2-4-18(5-3-17)26-23(34)25-10-12-30/h2-5,14,16,19,30H,6-13,15H2,1H3,(H2,25,26,34)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 6.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kgamma (unknown origin) using biotinylated PIP2 as substrate |
J Med Chem 58: 2326-49 (2015)
Article DOI: 10.1021/jm501778s
BindingDB Entry DOI: 10.7270/Q2DF6SW8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50072964

(CHEMBL3410642)Show SMILES C[C@H]1COCCN1c1cc(CS(C)(=O)=O)nc(n1)-c1ccc(NC(=O)NCCN(C)C)cc1 |r| Show InChI InChI=1S/C22H32N6O4S/c1-16-14-32-12-11-28(16)20-13-19(15-33(4,30)31)24-21(26-20)17-5-7-18(8-6-17)25-22(29)23-9-10-27(2)3/h5-8,13,16H,9-12,14-15H2,1-4H3,(H2,23,25,29)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kalpha (unknown origin) using biotinylated PIP2 as substrate |
J Med Chem 58: 2326-49 (2015)
Article DOI: 10.1021/jm501778s
BindingDB Entry DOI: 10.7270/Q2DF6SW8 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50429716
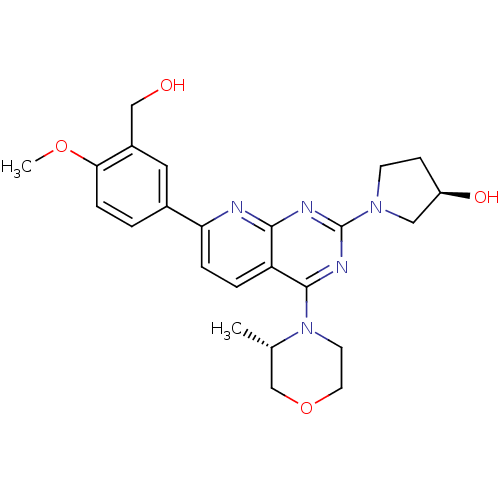
(CHEMBL2336332 | US9102670, 18bn)Show SMILES COc1ccc(cc1CO)-c1ccc2c(nc(nc2n1)N1CC[C@@H](O)C1)N1CCOC[C@@H]1C |r| Show InChI InChI=1S/C24H29N5O4/c1-15-14-33-10-9-29(15)23-19-4-5-20(16-3-6-21(32-2)17(11-16)13-30)25-22(19)26-24(27-23)28-8-7-18(31)12-28/h3-6,11,15,18,30-31H,7-10,12-14H2,1-2H3/t15-,18+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 23: 1212-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.01.019
BindingDB Entry DOI: 10.7270/Q2N29Z97 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50310989

((5-(2-((2R,6S)-2,6-dimethylmorpholino)-4-morpholin...)Show SMILES COc1ccc(cc1CO)-c1ccc2c(nc(nc2n1)N1C[C@H](C)O[C@H](C)C1)N1CCOCC1 |r| Show InChI InChI=1S/C25H31N5O4/c1-16-13-30(14-17(2)34-16)25-27-23-20(24(28-25)29-8-10-33-11-9-29)5-6-21(26-23)18-4-7-22(32-3)19(12-18)15-31/h4-7,12,16-17,31H,8-11,13-15H2,1-3H3/t16-,17+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 23: 1212-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.01.019
BindingDB Entry DOI: 10.7270/Q2N29Z97 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50310989

((5-(2-((2R,6S)-2,6-dimethylmorpholino)-4-morpholin...)Show SMILES COc1ccc(cc1CO)-c1ccc2c(nc(nc2n1)N1C[C@H](C)O[C@H](C)C1)N1CCOCC1 |r| Show InChI InChI=1S/C25H31N5O4/c1-16-13-30(14-17(2)34-16)25-27-23-20(24(28-25)29-8-10-33-11-9-29)5-6-21(26-23)18-4-7-22(32-3)19(12-18)15-31/h4-7,12,16-17,31H,8-11,13-15H2,1-3H3/t16-,17+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using lipid PIP2 as substrate |
Bioorg Med Chem Lett 23: 1212-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.01.019
BindingDB Entry DOI: 10.7270/Q2N29Z97 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50072961

(CHEMBL3410672)Show SMILES C[C@H]1COCCN1c1cc(nc(n1)-c1ccc(NC(=S)NCCO)cc1)C1(CC1)S(=O)(=O)C1CC1 |r| Show InChI InChI=1S/C24H31N5O4S2/c1-16-15-33-13-11-29(16)21-14-20(24(8-9-24)35(31,32)19-6-7-19)27-22(28-21)17-2-4-18(5-3-17)26-23(34)25-10-12-30/h2-5,14,16,19,30H,6-13,15H2,1H3,(H2,25,26,34)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 9.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kdelta (unknown origin) using biotinylated PIP2 as substrate |
J Med Chem 58: 2326-49 (2015)
Article DOI: 10.1021/jm501778s
BindingDB Entry DOI: 10.7270/Q2DF6SW8 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50429715
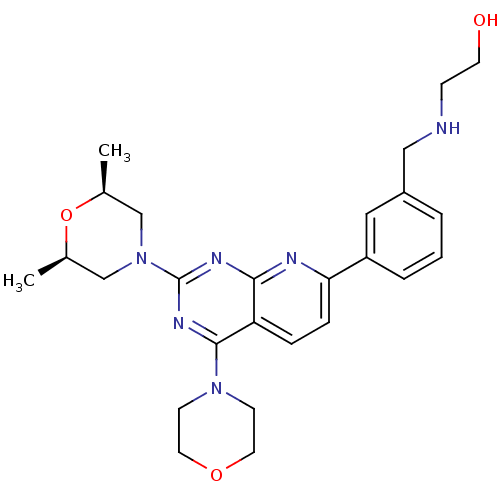
(CHEMBL2336328)Show SMILES C[C@H]1CN(C[C@@H](C)O1)c1nc(N2CCOCC2)c2ccc(nc2n1)-c1cccc(CNCCO)c1 |r| Show InChI InChI=1S/C26H34N6O3/c1-18-16-32(17-19(2)35-18)26-29-24-22(25(30-26)31-9-12-34-13-10-31)6-7-23(28-24)21-5-3-4-20(14-21)15-27-8-11-33/h3-7,14,18-19,27,33H,8-13,15-17H2,1-2H3/t18-,19+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 23: 1212-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.01.019
BindingDB Entry DOI: 10.7270/Q2N29Z97 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50429714
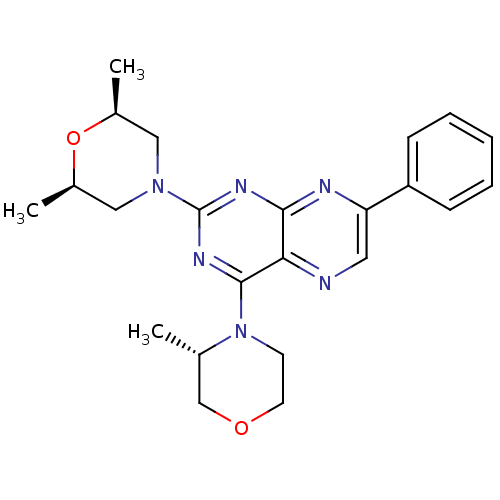
(CHEMBL2331605 | US9102670, 12a)Show SMILES C[C@H]1CN(C[C@@H](C)O1)c1nc(N2CCOC[C@@H]2C)c2ncc(nc2n1)-c1ccccc1 |r| Show InChI InChI=1S/C23H28N6O2/c1-15-14-30-10-9-29(15)22-20-21(25-19(11-24-20)18-7-5-4-6-8-18)26-23(27-22)28-12-16(2)31-17(3)13-28/h4-8,11,15-17H,9-10,12-14H2,1-3H3/t15-,16-,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.32E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 23: 1212-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.01.019
BindingDB Entry DOI: 10.7270/Q2N29Z97 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50429713
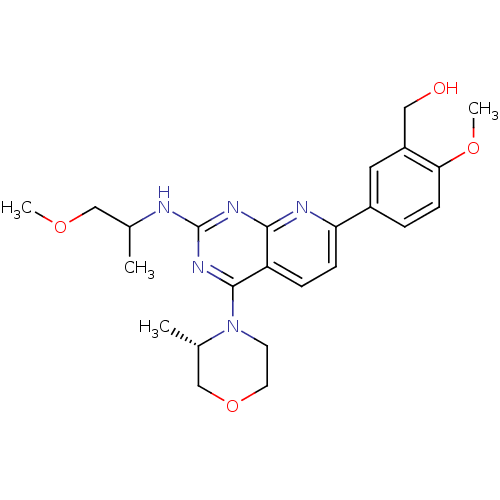
(CHEMBL2336334 | US9102670, 18ax)Show SMILES COCC(C)Nc1nc(N2CCOC[C@@H]2C)c2ccc(nc2n1)-c1ccc(OC)c(CO)c1 |r| Show InChI InChI=1S/C24H31N5O4/c1-15(13-31-3)25-24-27-22-19(23(28-24)29-9-10-33-14-16(29)2)6-7-20(26-22)17-5-8-21(32-4)18(11-17)12-30/h5-8,11,15-16,30H,9-10,12-14H2,1-4H3,(H,25,26,27,28)/t15?,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 23: 1212-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.01.019
BindingDB Entry DOI: 10.7270/Q2N29Z97 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50384255

(CHEMBL1312643)Show SMILES O=S(=O)(Cc1cc(nc(n1)-c1ccccn1)N1CCOCC1)c1ccccc1 Show InChI InChI=1S/C20H20N4O3S/c25-28(26,17-6-2-1-3-7-17)15-16-14-19(24-10-12-27-13-11-24)23-20(22-16)18-8-4-5-9-21-18/h1-9,14H,10-13,15H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.74E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kalpha (unknown origin) using biotinylated PIP2 as substrate |
J Med Chem 58: 2326-49 (2015)
Article DOI: 10.1021/jm501778s
BindingDB Entry DOI: 10.7270/Q2DF6SW8 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50429712
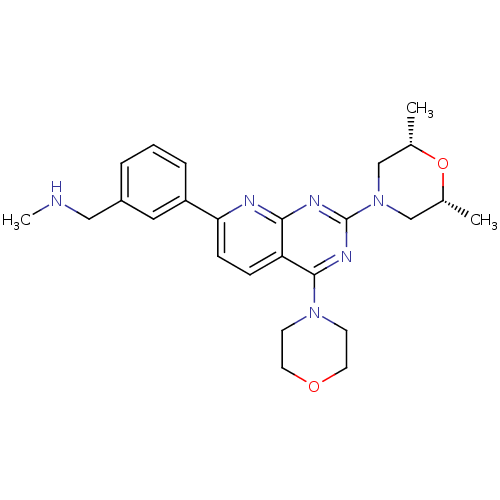
(CHEMBL2336329)Show SMILES CNCc1cccc(c1)-c1ccc2c(nc(nc2n1)N1C[C@H](C)O[C@H](C)C1)N1CCOCC1 |r| Show InChI InChI=1S/C25H32N6O2/c1-17-15-31(16-18(2)33-17)25-28-23-21(24(29-25)30-9-11-32-12-10-30)7-8-22(27-23)20-6-4-5-19(13-20)14-26-3/h4-8,13,17-18,26H,9-12,14-16H2,1-3H3/t17-,18+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.74E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 23: 1212-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.01.019
BindingDB Entry DOI: 10.7270/Q2N29Z97 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50429711
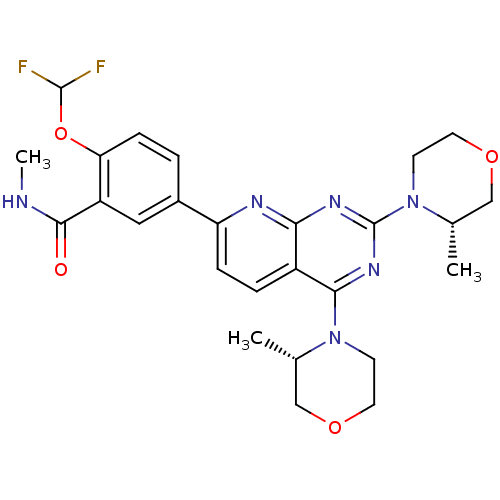
(CHEMBL2336323 | US9102670, 1dh)Show SMILES CNC(=O)c1cc(ccc1OC(F)F)-c1ccc2c(nc(nc2n1)N1CCOC[C@@H]1C)N1CCOC[C@@H]1C |r| Show InChI InChI=1S/C26H30F2N6O4/c1-15-13-36-10-8-33(15)23-18-5-6-20(17-4-7-21(38-25(27)28)19(12-17)24(35)29-3)30-22(18)31-26(32-23)34-9-11-37-14-16(34)2/h4-7,12,15-16,25H,8-11,13-14H2,1-3H3,(H,29,35)/t15-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.42E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 23: 1212-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.01.019
BindingDB Entry DOI: 10.7270/Q2N29Z97 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50384247

(CHEMBL2030436)Show SMILES CS(=O)(=O)Cc1cc(nc(n1)-c1ccc2[nH]ccc2c1)N1CCOCC1 Show InChI InChI=1S/C18H20N4O3S/c1-26(23,24)12-15-11-17(22-6-8-25-9-7-22)21-18(20-15)14-2-3-16-13(10-14)4-5-19-16/h2-5,10-11,19H,6-9,12H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.63E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kalpha (unknown origin) using biotinylated PIP2 as substrate |
J Med Chem 58: 2326-49 (2015)
Article DOI: 10.1021/jm501778s
BindingDB Entry DOI: 10.7270/Q2DF6SW8 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50429710
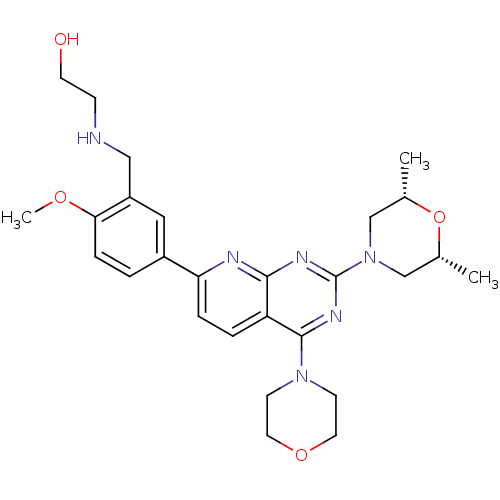
(CHEMBL2336327)Show SMILES COc1ccc(cc1CNCCO)-c1ccc2c(nc(nc2n1)N1C[C@H](C)O[C@H](C)C1)N1CCOCC1 |r| Show InChI InChI=1S/C27H36N6O4/c1-18-16-33(17-19(2)37-18)27-30-25-22(26(31-27)32-9-12-36-13-10-32)5-6-23(29-25)20-4-7-24(35-3)21(14-20)15-28-8-11-34/h4-7,14,18-19,28,34H,8-13,15-17H2,1-3H3/t18-,19+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 23: 1212-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.01.019
BindingDB Entry DOI: 10.7270/Q2N29Z97 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50310989

((5-(2-((2R,6S)-2,6-dimethylmorpholino)-4-morpholin...)Show SMILES COc1ccc(cc1CO)-c1ccc2c(nc(nc2n1)N1C[C@H](C)O[C@H](C)C1)N1CCOCC1 |r| Show InChI InChI=1S/C25H31N5O4/c1-16-13-30(14-17(2)34-16)25-27-23-20(24(28-25)29-8-10-33-11-9-29)5-6-21(26-23)18-4-7-22(32-3)19(12-18)15-31/h4-7,12,16-17,31H,8-11,13-15H2,1-3H3/t16-,17+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta (unknown origin) using lipid PIP2 as substrate |
Bioorg Med Chem Lett 23: 1212-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.01.019
BindingDB Entry DOI: 10.7270/Q2N29Z97 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50310989

((5-(2-((2R,6S)-2,6-dimethylmorpholino)-4-morpholin...)Show SMILES COc1ccc(cc1CO)-c1ccc2c(nc(nc2n1)N1C[C@H](C)O[C@H](C)C1)N1CCOCC1 |r| Show InChI InChI=1S/C25H31N5O4/c1-16-13-30(14-17(2)34-16)25-27-23-20(24(28-25)29-8-10-33-11-9-29)5-6-21(26-23)18-4-7-22(32-3)19(12-18)15-31/h4-7,12,16-17,31H,8-11,13-15H2,1-3H3/t16-,17+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using lipid PIP2 as substrate |
Bioorg Med Chem Lett 23: 1212-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.01.019
BindingDB Entry DOI: 10.7270/Q2N29Z97 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50348452

(AZD-8055 | CHEMBL1801204 | US9102670, 1a)Show SMILES COc1ccc(cc1CO)-c1ccc2c(nc(nc2n1)N1CCOC[C@@H]1C)N1CCOC[C@@H]1C |r| Show InChI InChI=1S/C25H31N5O4/c1-16-14-33-10-8-29(16)24-20-5-6-21(18-4-7-22(32-3)19(12-18)13-31)26-23(20)27-25(28-24)30-9-11-34-15-17(30)2/h4-7,12,16-17,31H,8-11,13-15H2,1-3H3/t16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 23: 1212-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.01.019
BindingDB Entry DOI: 10.7270/Q2N29Z97 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50429707
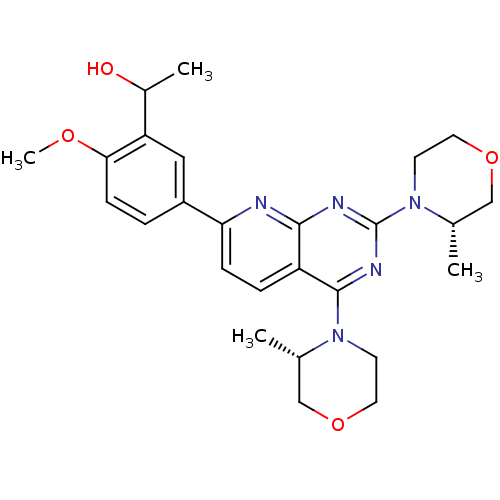
(CHEMBL2336319)Show SMILES COc1ccc(cc1C(C)O)-c1ccc2c(nc(nc2n1)N1CCOC[C@@H]1C)N1CCOC[C@@H]1C |r| Show InChI InChI=1S/C26H33N5O4/c1-16-14-34-11-9-30(16)25-20-6-7-22(19-5-8-23(33-4)21(13-19)18(3)32)27-24(20)28-26(29-25)31-10-12-35-15-17(31)2/h5-8,13,16-18,32H,9-12,14-15H2,1-4H3/t16-,17-,18?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 23: 1212-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.01.019
BindingDB Entry DOI: 10.7270/Q2N29Z97 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50429709
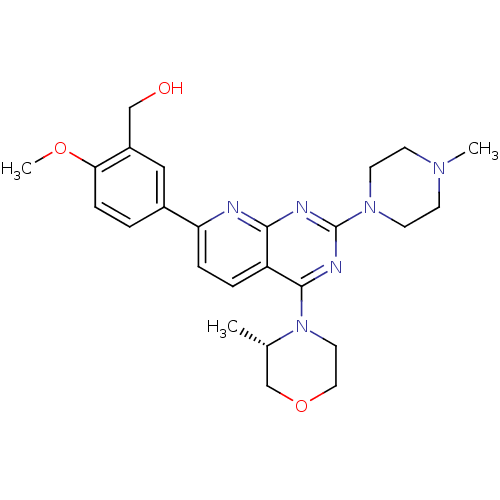
(CHEMBL2336333 | US9102670, 18cg)Show SMILES COc1ccc(cc1CO)-c1ccc2c(nc(nc2n1)N1CCN(C)CC1)N1CCOC[C@@H]1C |r| Show InChI InChI=1S/C25H32N6O3/c1-17-16-34-13-12-31(17)24-20-5-6-21(18-4-7-22(33-3)19(14-18)15-32)26-23(20)27-25(28-24)30-10-8-29(2)9-11-30/h4-7,14,17,32H,8-13,15-16H2,1-3H3/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 23: 1212-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.01.019
BindingDB Entry DOI: 10.7270/Q2N29Z97 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50429706
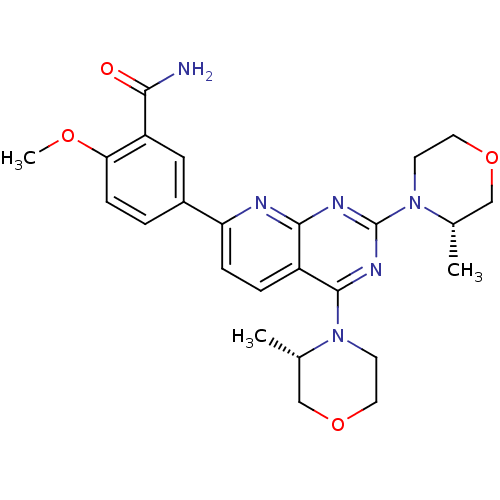
(CHEMBL2336320 | US9102670, 14b)Show SMILES COc1ccc(cc1C(N)=O)-c1ccc2c(nc(nc2n1)N1CCOC[C@@H]1C)N1CCOC[C@@H]1C |r| Show InChI InChI=1S/C25H30N6O4/c1-15-13-34-10-8-30(15)24-18-5-6-20(17-4-7-21(33-3)19(12-17)22(26)32)27-23(18)28-25(29-24)31-9-11-35-14-16(31)2/h4-7,12,15-16H,8-11,13-14H2,1-3H3,(H2,26,32)/t15-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 23: 1212-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.01.019
BindingDB Entry DOI: 10.7270/Q2N29Z97 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50429705
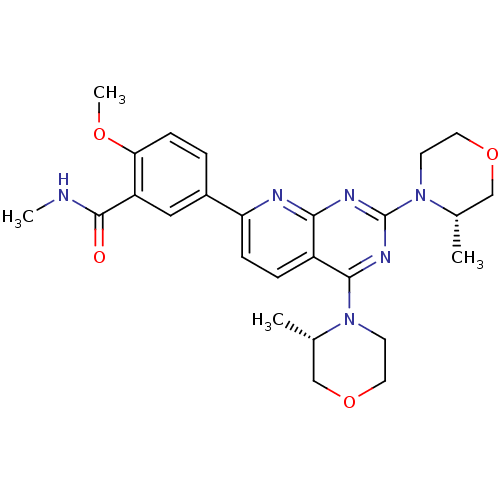
(CHEMBL2336321 | US9102670, 3ad)Show SMILES CNC(=O)c1cc(ccc1OC)-c1ccc2c(nc(nc2n1)N1CCOC[C@@H]1C)N1CCOC[C@@H]1C |r| Show InChI InChI=1S/C26H32N6O4/c1-16-14-35-11-9-31(16)24-19-6-7-21(18-5-8-22(34-4)20(13-18)25(33)27-3)28-23(19)29-26(30-24)32-10-12-36-15-17(32)2/h5-8,13,16-17H,9-12,14-15H2,1-4H3,(H,27,33)/t16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 23: 1212-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.01.019
BindingDB Entry DOI: 10.7270/Q2N29Z97 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50429704
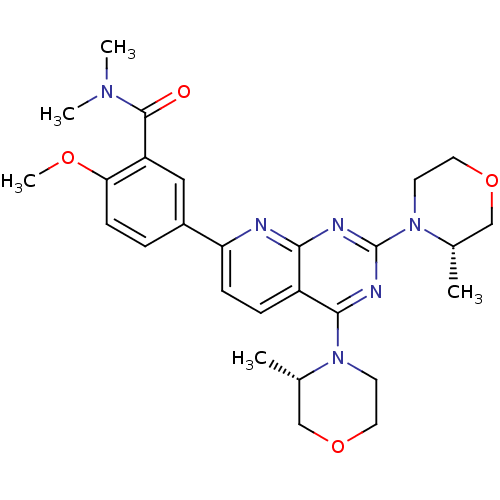
(CHEMBL2336322 | US9102670, 3t)Show SMILES COc1ccc(cc1C(=O)N(C)C)-c1ccc2c(nc(nc2n1)N1CCOC[C@@H]1C)N1CCOC[C@@H]1C |r| Show InChI InChI=1S/C27H34N6O4/c1-17-15-36-12-10-32(17)25-20-7-8-22(19-6-9-23(35-5)21(14-19)26(34)31(3)4)28-24(20)29-27(30-25)33-11-13-37-16-18(33)2/h6-9,14,17-18H,10-13,15-16H2,1-5H3/t17-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 23: 1212-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.01.019
BindingDB Entry DOI: 10.7270/Q2N29Z97 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50429703
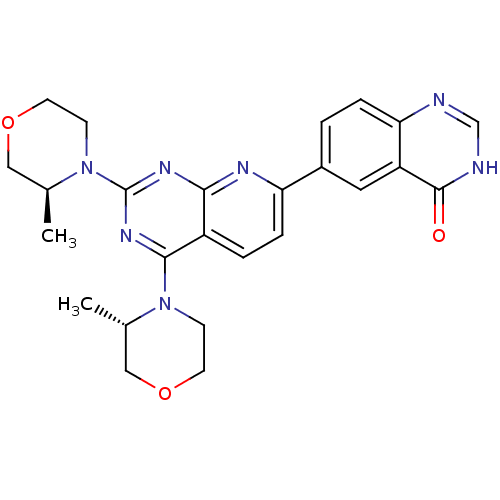
(CHEMBL2336324 | US9102670, 1cl)Show SMILES C[C@H]1COCCN1c1nc(N2CCOC[C@@H]2C)c2ccc(nc2n1)-c1ccc2nc[nH]c(=O)c2c1 |r| Show InChI InChI=1S/C25H27N7O3/c1-15-12-34-9-7-31(15)23-18-4-6-20(17-3-5-21-19(11-17)24(33)27-14-26-21)28-22(18)29-25(30-23)32-8-10-35-13-16(32)2/h3-6,11,14-16H,7-10,12-13H2,1-2H3,(H,26,27,33)/t15-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 23: 1212-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.01.019
BindingDB Entry DOI: 10.7270/Q2N29Z97 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50429702
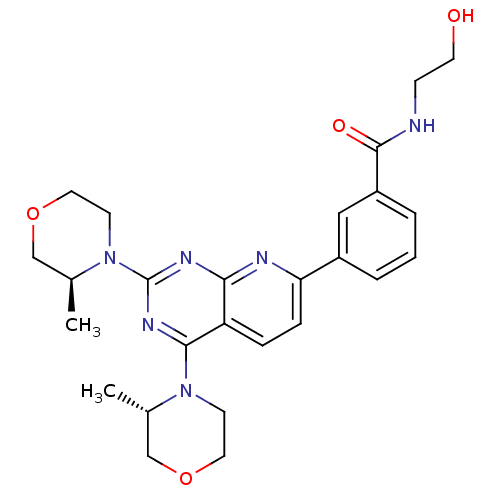
(CHEMBL2336326 | US9102670, 8a)Show SMILES C[C@H]1COCCN1c1nc(N2CCOC[C@@H]2C)c2ccc(nc2n1)-c1cccc(c1)C(=O)NCCO |r| Show InChI InChI=1S/C26H32N6O4/c1-17-15-35-12-9-31(17)24-21-6-7-22(19-4-3-5-20(14-19)25(34)27-8-11-33)28-23(21)29-26(30-24)32-10-13-36-16-18(32)2/h3-7,14,17-18,33H,8-13,15-16H2,1-2H3,(H,27,34)/t17-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 23: 1212-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.01.019
BindingDB Entry DOI: 10.7270/Q2N29Z97 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50429708
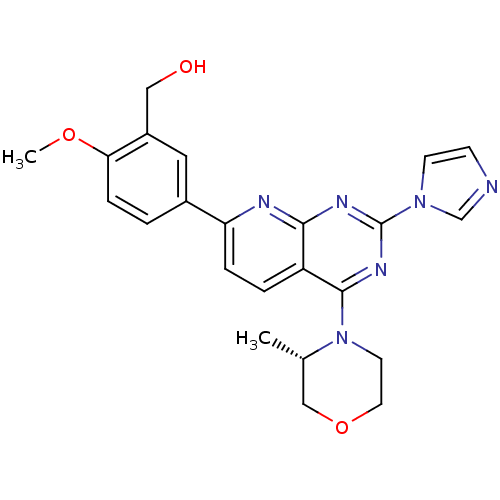
(CHEMBL2336318 | US9102670, 18ay)Show SMILES COc1ccc(cc1CO)-c1ccc2c(nc(nc2n1)-n1ccnc1)N1CCOC[C@@H]1C |r| Show InChI InChI=1S/C23H24N6O3/c1-15-13-32-10-9-29(15)22-18-4-5-19(16-3-6-20(31-2)17(11-16)12-30)25-21(18)26-23(27-22)28-8-7-24-14-28/h3-8,11,14-15,30H,9-10,12-13H2,1-2H3/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 23: 1212-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.01.019
BindingDB Entry DOI: 10.7270/Q2N29Z97 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50429701

(AZD-2014 | CHEMBL2336325 | US9102670, 1ap)Show SMILES CNC(=O)c1cccc(c1)-c1ccc2c(nc(nc2n1)N1CCOC[C@@H]1C)N1CCOC[C@@H]1C |r| Show InChI InChI=1S/C25H30N6O3/c1-16-14-33-11-9-30(16)23-20-7-8-21(18-5-4-6-19(13-18)24(32)26-3)27-22(20)28-25(29-23)31-10-12-34-15-17(31)2/h4-8,13,16-17H,9-12,14-15H2,1-3H3,(H,26,32)/t16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 23: 1212-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.01.019
BindingDB Entry DOI: 10.7270/Q2N29Z97 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data