 Found 241 hits with Last Name = 'kumar' and Initial = 'ss'
Found 241 hits with Last Name = 'kumar' and Initial = 'ss' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
DNA topoisomerase 1
(Homo sapiens (Human)) | BDBM50034013

((S)-9-Bromo-4-ethyl-4-hydroxy-11-methyl-1,12-dihyd...)Show SMILES CC[C@@]1(O)C(=O)OCc2c1cc1-c3nc4cnc(Br)cc4c(C)c3Cn1c2=O Show InChI InChI=1S/C20H16BrN3O4/c1-3-20(27)13-5-15-17-11(7-24(15)18(25)12(13)8-28-19(20)26)9(2)10-4-16(21)22-6-14(10)23-17/h4-6,27H,3,7-8H2,1-2H3/t20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc.
Curated by ChEMBL
| Assay Description
In vitro fragmentation of DNA in the presence of excess calf thymus topoisomerase. |
J Med Chem 38: 1106-18 (1995)
BindingDB Entry DOI: 10.7270/Q2X067P4 |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 1
(Homo sapiens (Human)) | BDBM50034009
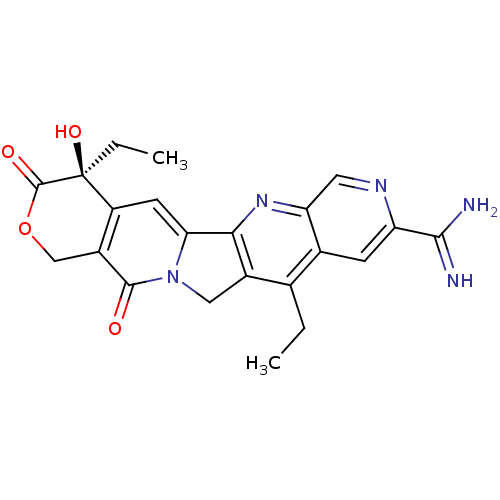
((S)-4,11-Diethyl-4-hydroxy-3,13-dioxo-3,4,12,13-te...)Show SMILES CCc1c2Cn3c(cc4c(COC(=O)[C@]4(O)CC)c3=O)-c2nc2cnc(cc12)C(N)=N Show InChI InChI=1S/C22H21N5O4/c1-3-10-11-5-15(19(23)24)25-7-16(11)26-18-12(10)8-27-17(18)6-14-13(20(27)28)9-31-21(29)22(14,30)4-2/h5-7,30H,3-4,8-9H2,1-2H3,(H3,23,24)/t22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc.
Curated by ChEMBL
| Assay Description
Minimum concentration that produced 50% fragmentation of DNA was measured in the presence of excess calf thymus topoisomerase. |
J Med Chem 38: 1106-18 (1995)
BindingDB Entry DOI: 10.7270/Q2X067P4 |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 1
(Homo sapiens (Human)) | BDBM50034018
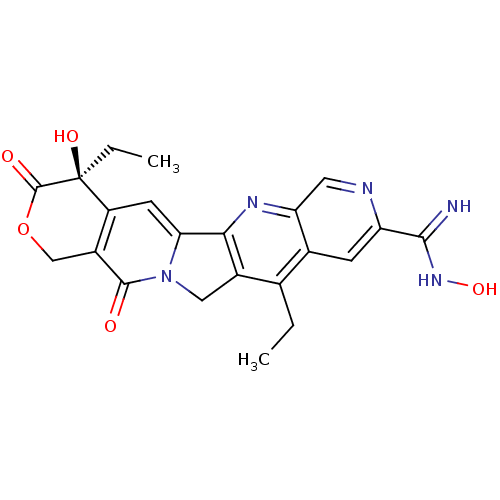
((S)-4,11-Diethyl-4,N-dihydroxy-3,13-dioxo-3,4,12,1...)Show SMILES CCc1c2Cn3c(cc4c(COC(=O)[C@]4(O)CC)c3=O)-c2nc2cnc(cc12)C(=N)NO Show InChI InChI=1S/C22H21N5O5/c1-3-10-11-5-15(19(23)26-31)24-7-16(11)25-18-12(10)8-27-17(18)6-14-13(20(27)28)9-32-21(29)22(14,30)4-2/h5-7,30-31H,3-4,8-9H2,1-2H3,(H2,23,26)/t22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc.
Curated by ChEMBL
| Assay Description
Minimum concentration that produced 50% fragmentation of DNA was measured in the presence of excess calf thymus topoisomerase. |
J Med Chem 38: 1106-18 (1995)
BindingDB Entry DOI: 10.7270/Q2X067P4 |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 1
(Homo sapiens (Human)) | BDBM50034019
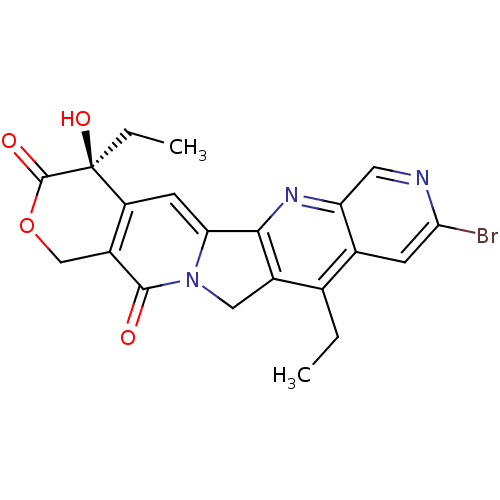
((S)-9-Bromo-4,11-diethyl-4-hydroxy-1,12-dihydro-4H...)Show SMILES CCc1c2Cn3c(cc4c(COC(=O)[C@]4(O)CC)c3=O)-c2nc2cnc(Br)cc12 Show InChI InChI=1S/C21H18BrN3O4/c1-3-10-11-5-17(22)23-7-15(11)24-18-12(10)8-25-16(18)6-14-13(19(25)26)9-29-20(27)21(14,28)4-2/h5-7,28H,3-4,8-9H2,1-2H3/t21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 122 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc.
Curated by ChEMBL
| Assay Description
In vitro fragmentation of DNA in the presence of excess calf thymus topoisomerase. |
J Med Chem 38: 1106-18 (1995)
BindingDB Entry DOI: 10.7270/Q2X067P4 |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 1
(Homo sapiens (Human)) | BDBM50034014
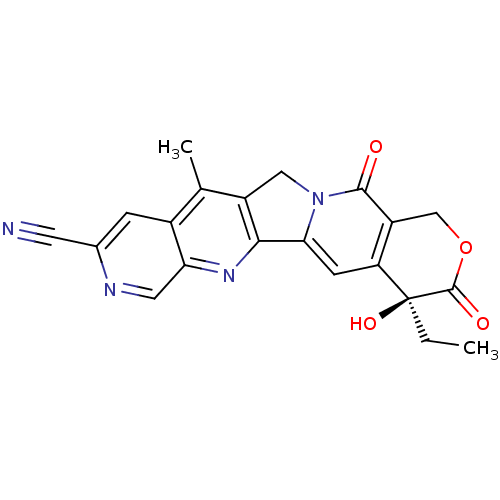
((S)-4-Ethyl-4-hydroxy-11-methyl-3,13-dioxo-3,4,12,...)Show SMILES CC[C@@]1(O)C(=O)OCc2c1cc1-c3nc4cnc(cc4c(C)c3Cn1c2=O)C#N Show InChI InChI=1S/C21H16N4O4/c1-3-21(28)15-5-17-18-13(8-25(17)19(26)14(15)9-29-20(21)27)10(2)12-4-11(6-22)23-7-16(12)24-18/h4-5,7,28H,3,8-9H2,1-2H3/t21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 143 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc.
Curated by ChEMBL
| Assay Description
In vitro fragmentation of DNA in the presence of excess calf thymus topoisomerase. |
J Med Chem 38: 1106-18 (1995)
BindingDB Entry DOI: 10.7270/Q2X067P4 |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 1
(Homo sapiens (Human)) | BDBM50034015
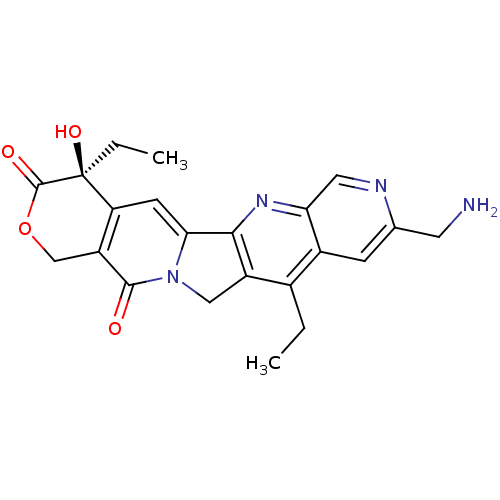
((S)-9-Aminomethyl-4,11-diethyl-4-hydroxy-1,12-dihy...)Show SMILES CCc1c2Cn3c(cc4c(COC(=O)[C@]4(O)CC)c3=O)-c2nc2cnc(CN)cc12 Show InChI InChI=1S/C22H22N4O4/c1-3-12-13-5-11(7-23)24-8-17(13)25-19-14(12)9-26-18(19)6-16-15(20(26)27)10-30-21(28)22(16,29)4-2/h5-6,8,29H,3-4,7,9-10,23H2,1-2H3/t22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 167 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc.
Curated by ChEMBL
| Assay Description
In vitro fragmentation of DNA in the presence of excess calf thymus topoisomerase. |
J Med Chem 38: 1106-18 (1995)
BindingDB Entry DOI: 10.7270/Q2X067P4 |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 1
(Homo sapiens (Human)) | BDBM50034020
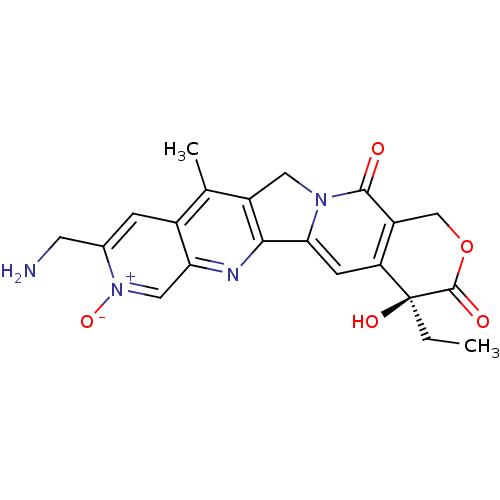
((S)-9-Aminomethyl-4-ethyl-4-hydroxy-11-methyl-8-ox...)Show SMILES CC[C@@]1(O)C(=O)OCc2c1cc1-c3nc4c[n+]([O-])c(CN)cc4c(C)c3Cn1c2=O Show InChI InChI=1S/C21H20N4O5/c1-3-21(28)15-5-17-18-13(7-24(17)19(26)14(15)9-30-20(21)27)10(2)12-4-11(6-22)25(29)8-16(12)23-18/h4-5,8,28H,3,6-7,9,22H2,1-2H3/t21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc.
Curated by ChEMBL
| Assay Description
Minimum concentration that produced 50% fragmentation of DNA was measured in the presence of excess calf thymus topoisomerase. |
J Med Chem 38: 1106-18 (1995)
BindingDB Entry DOI: 10.7270/Q2X067P4 |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 1
(Homo sapiens (Human)) | BDBM50034024
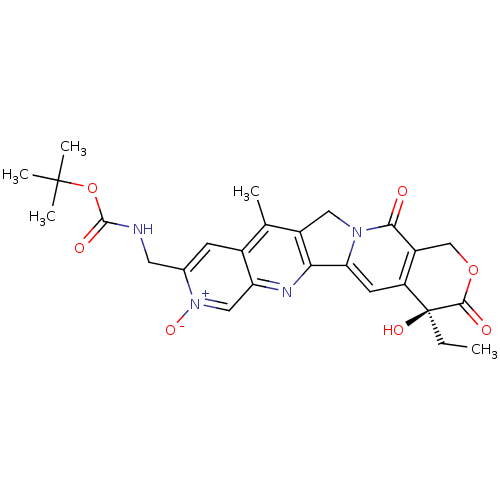
(((S)-4-Ethyl-4-hydroxy-11-methyl-3,13-dioxo-8-oxy-...)Show SMILES CC[C@@]1(O)C(=O)OCc2c1cc1-c3nc4c[n+]([O-])c(CNC(=O)OC(C)(C)C)cc4c(C)c3Cn1c2=O Show InChI InChI=1S/C26H28N4O7/c1-6-26(34)18-8-20-21-16(10-29(20)22(31)17(18)12-36-23(26)32)13(2)15-7-14(30(35)11-19(15)28-21)9-27-24(33)37-25(3,4)5/h7-8,11,34H,6,9-10,12H2,1-5H3,(H,27,33)/t26-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 217 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc.
Curated by ChEMBL
| Assay Description
In vitro fragmentation of DNA in the presence of excess calf thymus topoisomerase. |
J Med Chem 38: 1106-18 (1995)
BindingDB Entry DOI: 10.7270/Q2X067P4 |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 1
(Homo sapiens (Human)) | BDBM50034010
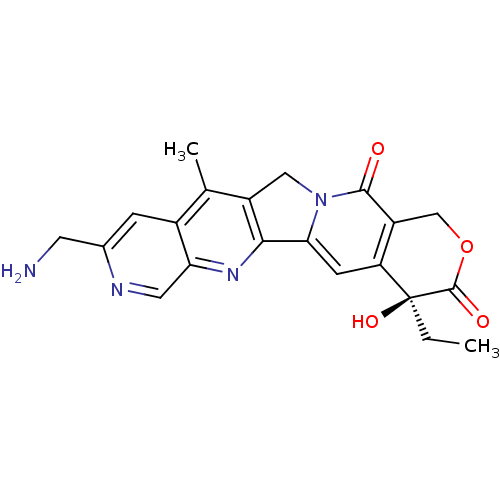
((S)-9-Aminomethyl-4-ethyl-4-hydroxy-11-methyl-1,12...)Show SMILES CC[C@@]1(O)C(=O)OCc2c1cc1-c3nc4cnc(CN)cc4c(C)c3Cn1c2=O Show InChI InChI=1S/C21H20N4O4/c1-3-21(28)15-5-17-18-13(8-25(17)19(26)14(15)9-29-20(21)27)10(2)12-4-11(6-22)23-7-16(12)24-18/h4-5,7,28H,3,6,8-9,22H2,1-2H3/t21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 232 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc.
Curated by ChEMBL
| Assay Description
Minimum concentration that produced 50% fragmentation of DNA was measured in the presence of excess calf thymus topoisomerase. |
J Med Chem 38: 1106-18 (1995)
BindingDB Entry DOI: 10.7270/Q2X067P4 |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 1
(Homo sapiens (Human)) | BDBM50034016

((S)-4,11-Diethyl-4-hydroxy-3,13-dioxo-3,4,12,13-te...)Show SMILES CCc1c2Cn3c(cc4c(COC(=O)[C@]4(O)CC)c3=O)-c2nc2cnc(cc12)C#N Show InChI InChI=1S/C22H18N4O4/c1-3-12-13-5-11(7-23)24-8-17(13)25-19-14(12)9-26-18(19)6-16-15(20(26)27)10-30-21(28)22(16,29)4-2/h5-6,8,29H,3-4,9-10H2,1-2H3/t22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 268 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc.
Curated by ChEMBL
| Assay Description
In vitro fragmentation of DNA in the presence of excess calf thymus topoisomerase. |
J Med Chem 38: 1106-18 (1995)
BindingDB Entry DOI: 10.7270/Q2X067P4 |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 1
(Homo sapiens (Human)) | BDBM50034011
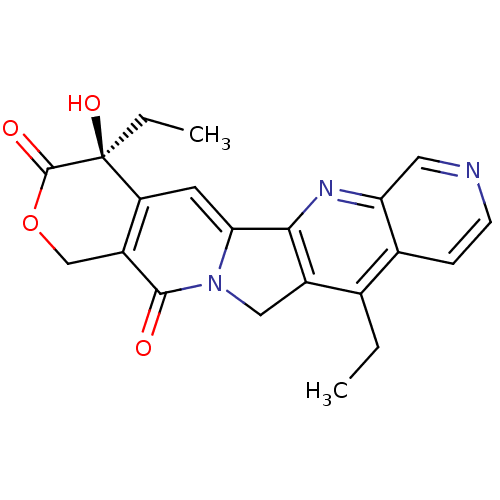
((S)-4,11-Diethyl-4-hydroxy-1,12-dihydro-4H-2-oxa-6...)Show SMILES CCc1c2Cn3c(cc4c(COC(=O)[C@]4(O)CC)c3=O)-c2nc2cnccc12 Show InChI InChI=1S/C21H19N3O4/c1-3-11-12-5-6-22-8-16(12)23-18-13(11)9-24-17(18)7-15-14(19(24)25)10-28-20(26)21(15,27)4-2/h5-8,27H,3-4,9-10H2,1-2H3/t21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 336 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc.
Curated by ChEMBL
| Assay Description
In vitro fragmentation of DNA in the presence of excess calf thymus topoisomerase. |
J Med Chem 38: 1106-18 (1995)
BindingDB Entry DOI: 10.7270/Q2X067P4 |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 1
(Homo sapiens (Human)) | BDBM50034030

((S)-4-Ethyl-4-hydroxy-1,12-dihydro-4H-2-oxa-6,8,12...)Show SMILES CC[C@@]1(O)C(=O)OCc2c1cc1-c3nc4cnccc4cc3Cn1c2=O Show InChI InChI=1S/C19H15N3O4/c1-2-19(25)13-6-15-16-11(5-10-3-4-20-7-14(10)21-16)8-22(15)17(23)12(13)9-26-18(19)24/h3-7,25H,2,8-9H2,1H3/t19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 383 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc.
Curated by ChEMBL
| Assay Description
In vitro fragmentation of DNA in the presence of excess calf thymus topoisomerase. |
J Med Chem 38: 1106-18 (1995)
BindingDB Entry DOI: 10.7270/Q2X067P4 |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 1
(Homo sapiens (Human)) | BDBM50034012
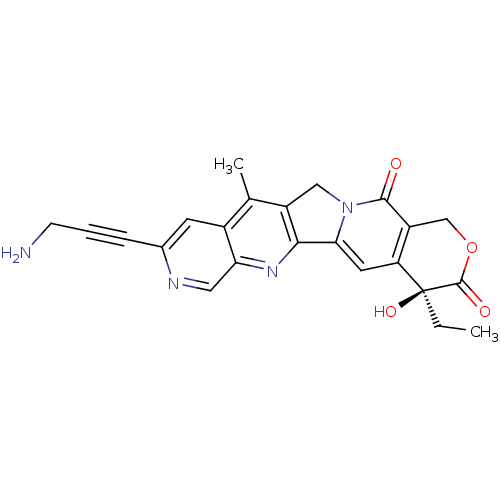
((S)-9-(3-Amino-prop-1-ynyl)-4-ethyl-4-hydroxy-11-m...)Show SMILES CC[C@@]1(O)C(=O)OCc2c1cc1-c3nc4cnc(cc4c(C)c3Cn1c2=O)C#CCN Show InChI InChI=1S/C23H20N4O4/c1-3-23(30)17-8-19-20-15(10-27(19)21(28)16(17)11-31-22(23)29)12(2)14-7-13(5-4-6-24)25-9-18(14)26-20/h7-9,30H,3,6,10-11,24H2,1-2H3/t23-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 505 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc.
Curated by ChEMBL
| Assay Description
In vitro fragmentation of DNA in the presence of excess calf thymus topoisomerase. |
J Med Chem 38: 1106-18 (1995)
BindingDB Entry DOI: 10.7270/Q2X067P4 |
More data for this
Ligand-Target Pair | |
Apolipoprotein L1
(Homo sapiens) | BDBM615196
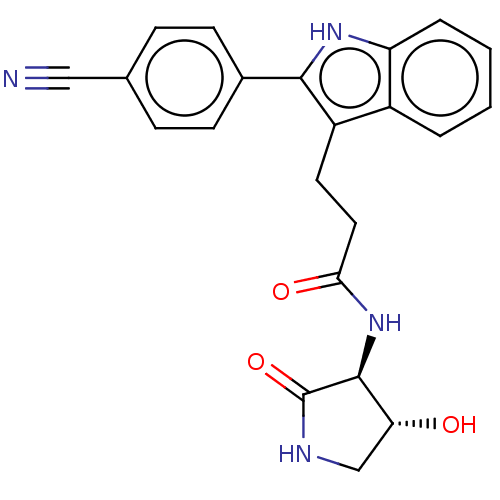
(3-[2-(4-cyanophenyl)-1H-indol-3-yl]-N-[(3S,4R)-4-h...)Show SMILES O[C@@H]1CNC(=O)[C@H]1NC(=O)CCc1c([nH]c2ccccc12)-c1ccc(cc1)C#N |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc.
US Patent
| |
US Patent US9725441 (2017)
BindingDB Entry DOI: 10.7270/Q2FJ2MXG |
More data for this
Ligand-Target Pair | |
Apolipoprotein L1
(Homo sapiens) | BDBM615197
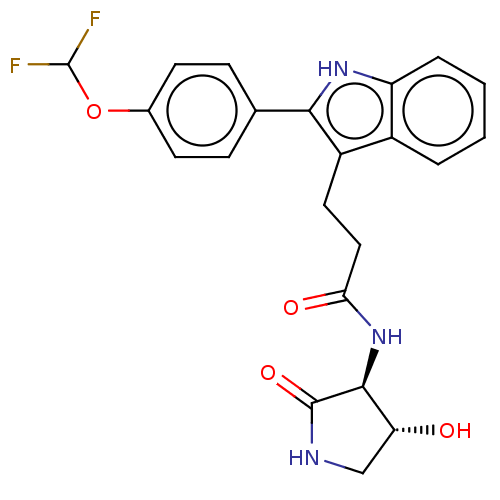
(US20230271945, Compound 117)Show SMILES O[C@@H]1CNC(=O)[C@H]1NC(=O)CCc1c([nH]c2ccccc12)-c1ccc(OC(F)F)cc1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc.
US Patent
| |
US Patent US9725441 (2017)
BindingDB Entry DOI: 10.7270/Q2FJ2MXG |
More data for this
Ligand-Target Pair | |
Apolipoprotein L1
(Homo sapiens) | BDBM615198
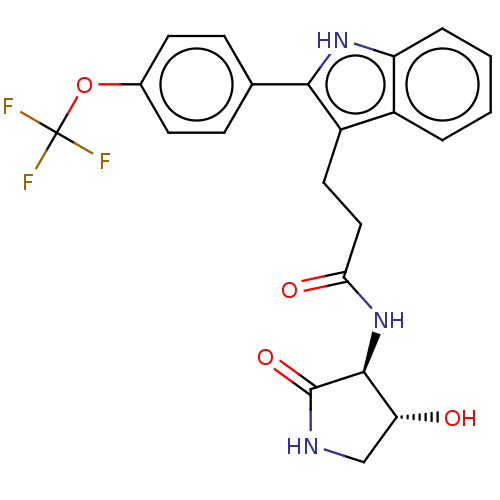
(US20230271945, Compound 118)Show SMILES O[C@@H]1CNC(=O)[C@H]1NC(=O)CCc1c([nH]c2ccccc12)-c1ccc(OC(F)(F)F)cc1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc.
US Patent
| |
US Patent US9725441 (2017)
BindingDB Entry DOI: 10.7270/Q2FJ2MXG |
More data for this
Ligand-Target Pair | |
Apolipoprotein L1
(Homo sapiens) | BDBM615198

(US20230271945, Compound 118)Show SMILES O[C@@H]1CNC(=O)[C@H]1NC(=O)CCc1c([nH]c2ccccc12)-c1ccc(OC(F)(F)F)cc1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc.
US Patent
| |
US Patent US9725441 (2017)
BindingDB Entry DOI: 10.7270/Q2FJ2MXG |
More data for this
Ligand-Target Pair | |
Apolipoprotein L1
(Homo sapiens) | BDBM615200
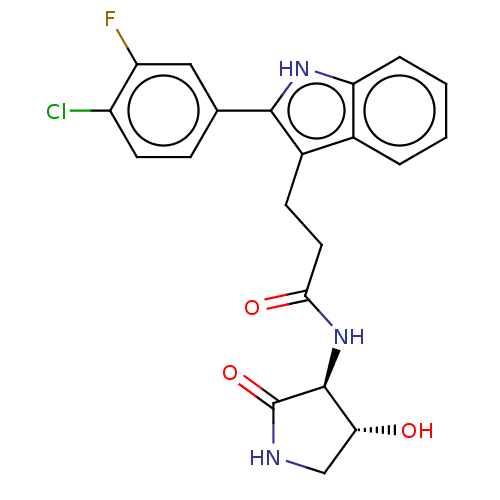
(US20230271945, Compound 120)Show SMILES O[C@@H]1CNC(=O)[C@H]1NC(=O)CCc1c([nH]c2ccccc12)-c1ccc(Cl)c(F)c1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc.
US Patent
| |
US Patent US9725441 (2017)
BindingDB Entry DOI: 10.7270/Q2FJ2MXG |
More data for this
Ligand-Target Pair | |
Apolipoprotein L1
(Homo sapiens) | BDBM615200

(US20230271945, Compound 120)Show SMILES O[C@@H]1CNC(=O)[C@H]1NC(=O)CCc1c([nH]c2ccccc12)-c1ccc(Cl)c(F)c1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc.
US Patent
| |
US Patent US9725441 (2017)
BindingDB Entry DOI: 10.7270/Q2FJ2MXG |
More data for this
Ligand-Target Pair | |
Apolipoprotein L1
(Homo sapiens) | BDBM50547880
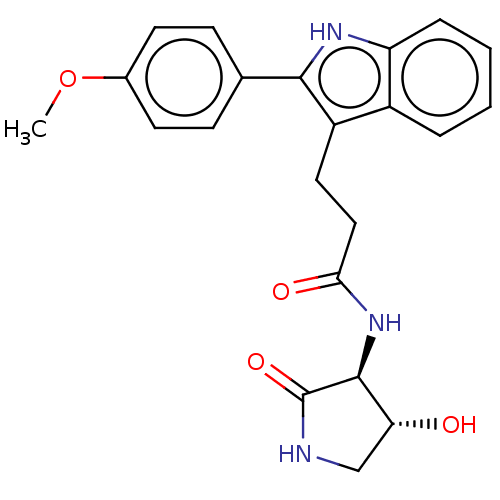
(CHEMBL4789274 | US20230271945, Compound 121)Show SMILES COc1ccc(cc1)-c1[nH]c2ccccc2c1CCC(=O)N[C@H]1[C@H](O)CNC1=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc.
US Patent
| |
US Patent US9725441 (2017)
BindingDB Entry DOI: 10.7270/Q2FJ2MXG |
More data for this
Ligand-Target Pair | |
Apolipoprotein L1
(Homo sapiens) | BDBM50547880

(CHEMBL4789274 | US20230271945, Compound 121)Show SMILES COc1ccc(cc1)-c1[nH]c2ccccc2c1CCC(=O)N[C@H]1[C@H](O)CNC1=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc.
US Patent
| |
US Patent US9725441 (2017)
BindingDB Entry DOI: 10.7270/Q2FJ2MXG |
More data for this
Ligand-Target Pair | |
Apolipoprotein L1
(Homo sapiens) | BDBM615202
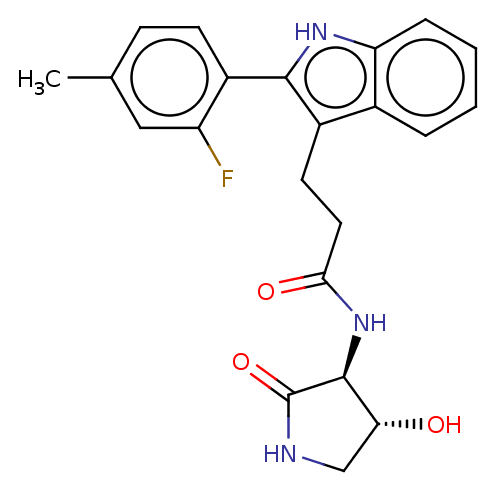
(US20230271945, Compound 122)Show SMILES Cc1ccc(-c2[nH]c3ccccc3c2CCC(=O)N[C@H]2[C@H](O)CNC2=O)c(F)c1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc.
US Patent
| |
US Patent US9725441 (2017)
BindingDB Entry DOI: 10.7270/Q2FJ2MXG |
More data for this
Ligand-Target Pair | |
Apolipoprotein L1
(Homo sapiens) | BDBM615202

(US20230271945, Compound 122)Show SMILES Cc1ccc(-c2[nH]c3ccccc3c2CCC(=O)N[C@H]2[C@H](O)CNC2=O)c(F)c1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc.
US Patent
| |
US Patent US9725441 (2017)
BindingDB Entry DOI: 10.7270/Q2FJ2MXG |
More data for this
Ligand-Target Pair | |
Apolipoprotein L1
(Homo sapiens) | BDBM615203
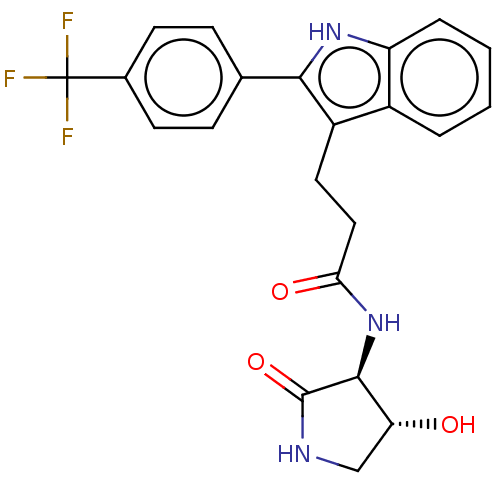
(US20230271945, Compound 123)Show SMILES O[C@@H]1CNC(=O)[C@H]1NC(=O)CCc1c([nH]c2ccccc12)-c1ccc(cc1)C(F)(F)F |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc.
US Patent
| |
US Patent US9725441 (2017)
BindingDB Entry DOI: 10.7270/Q2FJ2MXG |
More data for this
Ligand-Target Pair | |
Apolipoprotein L1
(Homo sapiens) | BDBM615203

(US20230271945, Compound 123)Show SMILES O[C@@H]1CNC(=O)[C@H]1NC(=O)CCc1c([nH]c2ccccc12)-c1ccc(cc1)C(F)(F)F |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc.
US Patent
| |
US Patent US9725441 (2017)
BindingDB Entry DOI: 10.7270/Q2FJ2MXG |
More data for this
Ligand-Target Pair | |
Apolipoprotein L1
(Homo sapiens) | BDBM615204
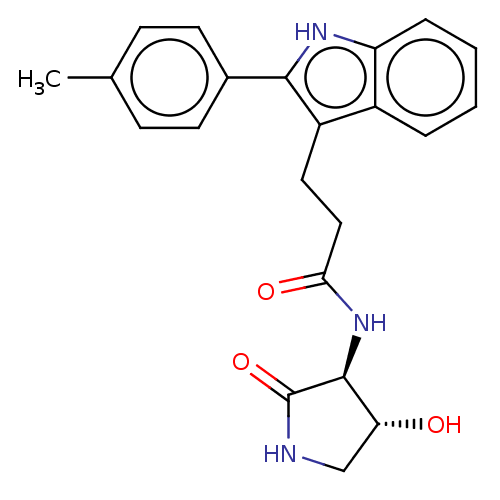
(US20230271945, Compound 124)Show SMILES Cc1ccc(cc1)-c1[nH]c2ccccc2c1CCC(=O)N[C@H]1[C@H](O)CNC1=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc.
US Patent
| |
US Patent US9725441 (2017)
BindingDB Entry DOI: 10.7270/Q2FJ2MXG |
More data for this
Ligand-Target Pair | |
Apolipoprotein L1
(Homo sapiens) | BDBM615204

(US20230271945, Compound 124)Show SMILES Cc1ccc(cc1)-c1[nH]c2ccccc2c1CCC(=O)N[C@H]1[C@H](O)CNC1=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc.
US Patent
| |
US Patent US9725441 (2017)
BindingDB Entry DOI: 10.7270/Q2FJ2MXG |
More data for this
Ligand-Target Pair | |
Apolipoprotein L1
(Homo sapiens) | BDBM615205
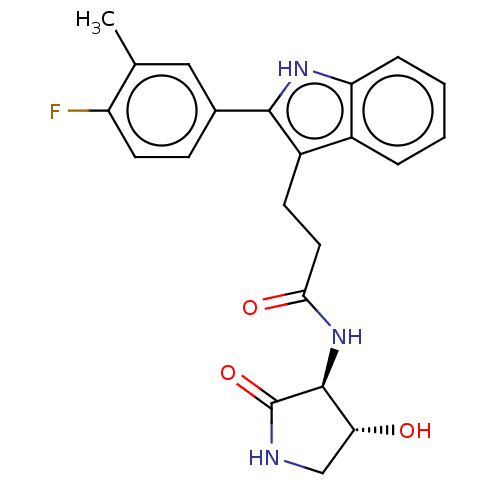
(US20230271945, Compound 125)Show SMILES Cc1cc(ccc1F)-c1[nH]c2ccccc2c1CCC(=O)N[C@H]1[C@H](O)CNC1=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc.
US Patent
| |
US Patent US9725441 (2017)
BindingDB Entry DOI: 10.7270/Q2FJ2MXG |
More data for this
Ligand-Target Pair | |
Apolipoprotein L1
(Homo sapiens) | BDBM615205

(US20230271945, Compound 125)Show SMILES Cc1cc(ccc1F)-c1[nH]c2ccccc2c1CCC(=O)N[C@H]1[C@H](O)CNC1=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc.
US Patent
| |
US Patent US9725441 (2017)
BindingDB Entry DOI: 10.7270/Q2FJ2MXG |
More data for this
Ligand-Target Pair | |
Apolipoprotein L1
(Homo sapiens) | BDBM615206
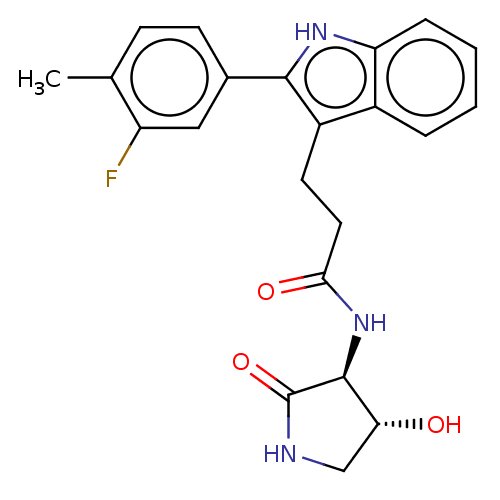
(US20230271945, Compound 126)Show SMILES Cc1ccc(cc1F)-c1[nH]c2ccccc2c1CCC(=O)N[C@H]1[C@H](O)CNC1=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc.
US Patent
| |
US Patent US9725441 (2017)
BindingDB Entry DOI: 10.7270/Q2FJ2MXG |
More data for this
Ligand-Target Pair | |
Apolipoprotein L1
(Homo sapiens) | BDBM615206

(US20230271945, Compound 126)Show SMILES Cc1ccc(cc1F)-c1[nH]c2ccccc2c1CCC(=O)N[C@H]1[C@H](O)CNC1=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc.
US Patent
| |
US Patent US9725441 (2017)
BindingDB Entry DOI: 10.7270/Q2FJ2MXG |
More data for this
Ligand-Target Pair | |
Apolipoprotein L1
(Homo sapiens) | BDBM615208
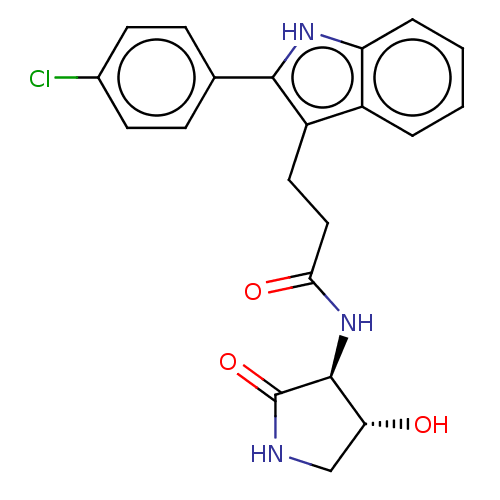
(3-[2-(4-chlorophenyl)-1H-indol-3-yl]-N-[(3S,4R)-4-...)Show SMILES O[C@@H]1CNC(=O)[C@H]1NC(=O)CCc1c([nH]c2ccccc12)-c1ccc(Cl)cc1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc.
US Patent
| |
US Patent US9725441 (2017)
BindingDB Entry DOI: 10.7270/Q2FJ2MXG |
More data for this
Ligand-Target Pair | |
Apolipoprotein L1
(Homo sapiens) | BDBM615208

(3-[2-(4-chlorophenyl)-1H-indol-3-yl]-N-[(3S,4R)-4-...)Show SMILES O[C@@H]1CNC(=O)[C@H]1NC(=O)CCc1c([nH]c2ccccc12)-c1ccc(Cl)cc1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc.
US Patent
| |
US Patent US9725441 (2017)
BindingDB Entry DOI: 10.7270/Q2FJ2MXG |
More data for this
Ligand-Target Pair | |
Apolipoprotein L1
(Homo sapiens) | BDBM615209
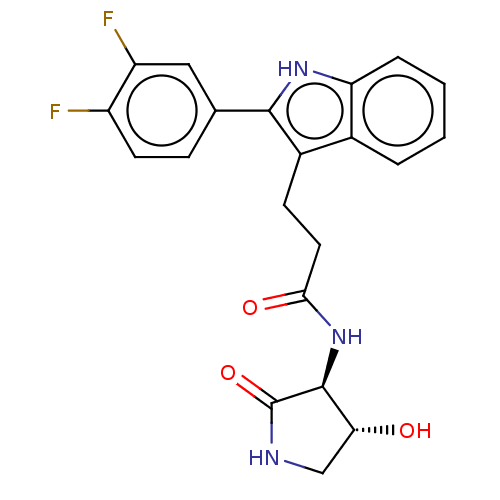
(3-[2-(3,4-difluorophenyl)-1H-indol-3-yl]-N-[(3S,4R...)Show SMILES O[C@@H]1CNC(=O)[C@H]1NC(=O)CCc1c([nH]c2ccccc12)-c1ccc(F)c(F)c1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc.
US Patent
| |
US Patent US9725441 (2017)
BindingDB Entry DOI: 10.7270/Q2FJ2MXG |
More data for this
Ligand-Target Pair | |
Apolipoprotein L1
(Homo sapiens) | BDBM615209

(3-[2-(3,4-difluorophenyl)-1H-indol-3-yl]-N-[(3S,4R...)Show SMILES O[C@@H]1CNC(=O)[C@H]1NC(=O)CCc1c([nH]c2ccccc12)-c1ccc(F)c(F)c1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc.
US Patent
| |
US Patent US9725441 (2017)
BindingDB Entry DOI: 10.7270/Q2FJ2MXG |
More data for this
Ligand-Target Pair | |
Apolipoprotein L1
(Homo sapiens) | BDBM615210
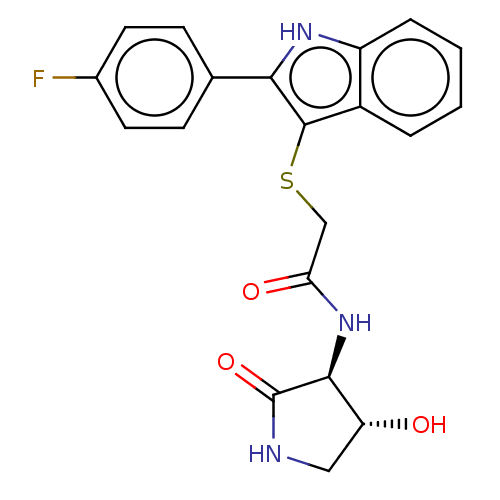
(2-[[2-(4-fluorophenyl)-1H-indol-3-yl]sulfanyl]-N-[...)Show SMILES O[C@@H]1CNC(=O)[C@H]1NC(=O)CSc1c([nH]c2ccccc12)-c1ccc(F)cc1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc.
US Patent
| |
US Patent US9725441 (2017)
BindingDB Entry DOI: 10.7270/Q2FJ2MXG |
More data for this
Ligand-Target Pair | |
Apolipoprotein L1
(Homo sapiens) | BDBM615124

(3-[2-(4-bromophenyl)-5-methyl-1H-indol-3-yl]-N-[(3...)Show SMILES Cc1ccc2[nH]c(c(CCC(=O)N[C@H]3CCNC3=O)c2c1)-c1ccc(Br)cc1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc.
US Patent
| |
US Patent US9725441 (2017)
BindingDB Entry DOI: 10.7270/Q2FJ2MXG |
More data for this
Ligand-Target Pair | |
Apolipoprotein L1
(Homo sapiens) | BDBM615124

(3-[2-(4-bromophenyl)-5-methyl-1H-indol-3-yl]-N-[(3...)Show SMILES Cc1ccc2[nH]c(c(CCC(=O)N[C@H]3CCNC3=O)c2c1)-c1ccc(Br)cc1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc.
US Patent
| |
US Patent US9725441 (2017)
BindingDB Entry DOI: 10.7270/Q2FJ2MXG |
More data for this
Ligand-Target Pair | |
Apolipoprotein L1
(Homo sapiens) | BDBM615125
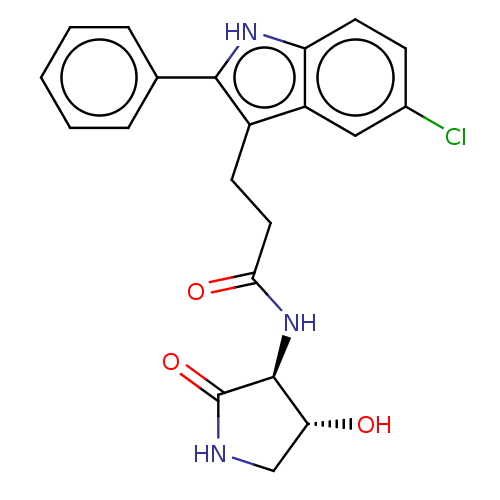
(3-(5-chloro-2-phenyl-1H-indol-3-yl)-N-[(3S,4R)-4-h...)Show SMILES O[C@@H]1CNC(=O)[C@H]1NC(=O)CCc1c([nH]c2ccc(Cl)cc12)-c1ccccc1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc.
US Patent
| |
US Patent US9725441 (2017)
BindingDB Entry DOI: 10.7270/Q2FJ2MXG |
More data for this
Ligand-Target Pair | |
Apolipoprotein L1
(Homo sapiens) | BDBM615125

(3-(5-chloro-2-phenyl-1H-indol-3-yl)-N-[(3S,4R)-4-h...)Show SMILES O[C@@H]1CNC(=O)[C@H]1NC(=O)CCc1c([nH]c2ccc(Cl)cc12)-c1ccccc1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc.
US Patent
| |
US Patent US9725441 (2017)
BindingDB Entry DOI: 10.7270/Q2FJ2MXG |
More data for this
Ligand-Target Pair | |
Apolipoprotein L1
(Homo sapiens) | BDBM615126
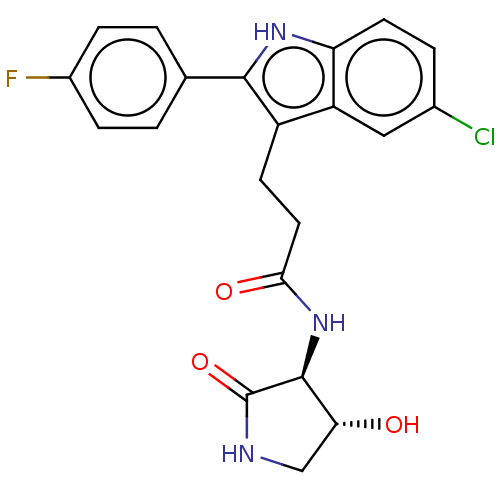
(3-[5-chloro-2-(4-fluorophenyl)-1H-indol-3-yl]-N-[(...)Show SMILES O[C@@H]1CNC(=O)[C@H]1NC(=O)CCc1c([nH]c2ccc(Cl)cc12)-c1ccc(F)cc1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc.
US Patent
| |
US Patent US9725441 (2017)
BindingDB Entry DOI: 10.7270/Q2FJ2MXG |
More data for this
Ligand-Target Pair | |
Apolipoprotein L1
(Homo sapiens) | BDBM615127
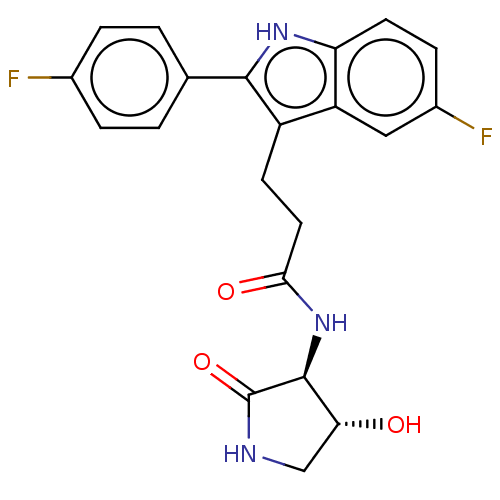
(US20230271945, Compound 46)Show SMILES O[C@@H]1CNC(=O)[C@H]1NC(=O)CCc1c([nH]c2ccc(F)cc12)-c1ccc(F)cc1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc.
US Patent
| |
US Patent US9725441 (2017)
BindingDB Entry DOI: 10.7270/Q2FJ2MXG |
More data for this
Ligand-Target Pair | |
Apolipoprotein L1
(Homo sapiens) | BDBM615127

(US20230271945, Compound 46)Show SMILES O[C@@H]1CNC(=O)[C@H]1NC(=O)CCc1c([nH]c2ccc(F)cc12)-c1ccc(F)cc1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc.
US Patent
| |
US Patent US9725441 (2017)
BindingDB Entry DOI: 10.7270/Q2FJ2MXG |
More data for this
Ligand-Target Pair | |
Apolipoprotein L1
(Homo sapiens) | BDBM615128
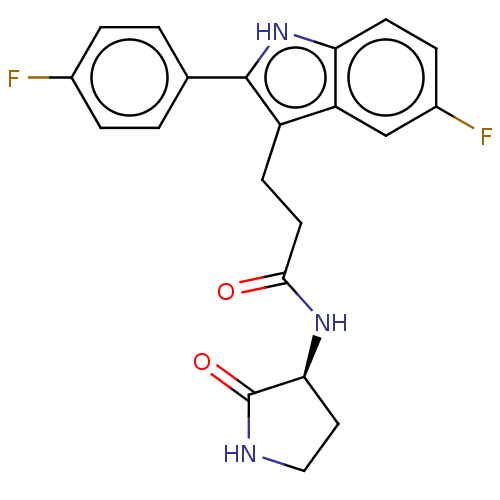
(US20230271945, Compound 47)Show SMILES Fc1ccc(cc1)-c1[nH]c2ccc(F)cc2c1CCC(=O)N[C@H]1CCNC1=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc.
US Patent
| |
US Patent US9725441 (2017)
BindingDB Entry DOI: 10.7270/Q2FJ2MXG |
More data for this
Ligand-Target Pair | |
Apolipoprotein L1
(Homo sapiens) | BDBM615128

(US20230271945, Compound 47)Show SMILES Fc1ccc(cc1)-c1[nH]c2ccc(F)cc2c1CCC(=O)N[C@H]1CCNC1=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc.
US Patent
| |
US Patent US9725441 (2017)
BindingDB Entry DOI: 10.7270/Q2FJ2MXG |
More data for this
Ligand-Target Pair | |
Apolipoprotein L1
(Homo sapiens) | BDBM615129
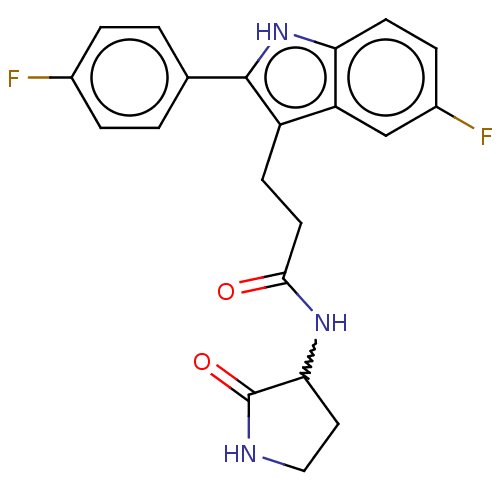
(US20230271945, Compound 48)Show SMILES Fc1ccc(cc1)-c1[nH]c2ccc(F)cc2c1CCC(=O)NC1CCNC1=O |w:21.24| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc.
US Patent
| |
US Patent US9725441 (2017)
BindingDB Entry DOI: 10.7270/Q2FJ2MXG |
More data for this
Ligand-Target Pair | |
Apolipoprotein L1
(Homo sapiens) | BDBM615129

(US20230271945, Compound 48)Show SMILES Fc1ccc(cc1)-c1[nH]c2ccc(F)cc2c1CCC(=O)NC1CCNC1=O |w:21.24| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc.
US Patent
| |
US Patent US9725441 (2017)
BindingDB Entry DOI: 10.7270/Q2FJ2MXG |
More data for this
Ligand-Target Pair | |
Apolipoprotein L1
(Homo sapiens) | BDBM615132
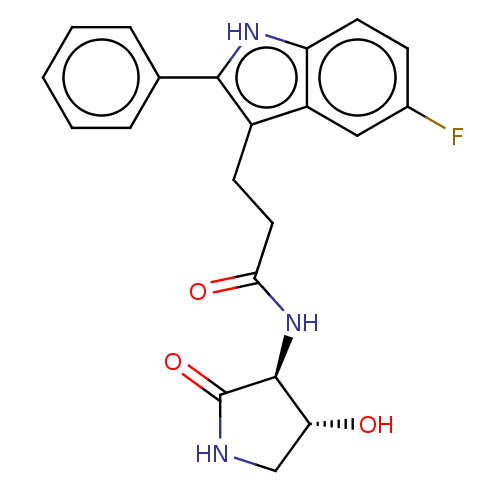
(US20230271945, Compound 51)Show SMILES O[C@@H]1CNC(=O)[C@H]1NC(=O)CCc1c([nH]c2ccc(F)cc12)-c1ccccc1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc.
US Patent
| |
US Patent US9725441 (2017)
BindingDB Entry DOI: 10.7270/Q2FJ2MXG |
More data for this
Ligand-Target Pair | |
Apolipoprotein L1
(Homo sapiens) | BDBM615132

(US20230271945, Compound 51)Show SMILES O[C@@H]1CNC(=O)[C@H]1NC(=O)CCc1c([nH]c2ccc(F)cc12)-c1ccccc1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc.
US Patent
| |
US Patent US9725441 (2017)
BindingDB Entry DOI: 10.7270/Q2FJ2MXG |
More data for this
Ligand-Target Pair | |
Apolipoprotein L1
(Homo sapiens) | BDBM615133
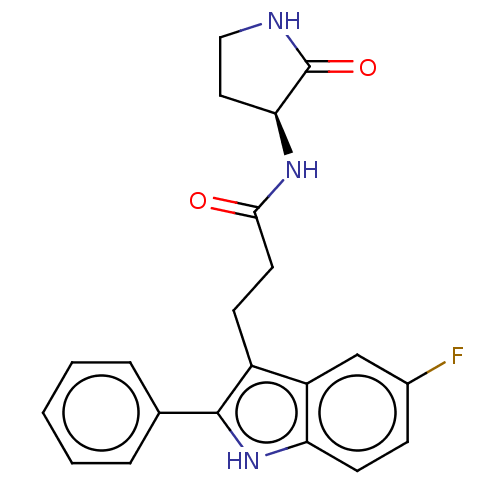
(US20230271945, Compound 52)Show SMILES Fc1ccc2[nH]c(c(CCC(=O)N[C@H]3CCNC3=O)c2c1)-c1ccccc1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc.
US Patent
| |
US Patent US9725441 (2017)
BindingDB Entry DOI: 10.7270/Q2FJ2MXG |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data