 Found 2172 hits with Last Name = 'hom' and Initial = 't'
Found 2172 hits with Last Name = 'hom' and Initial = 't' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Beta-1/Beta-2/Beta-3 adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM50227815
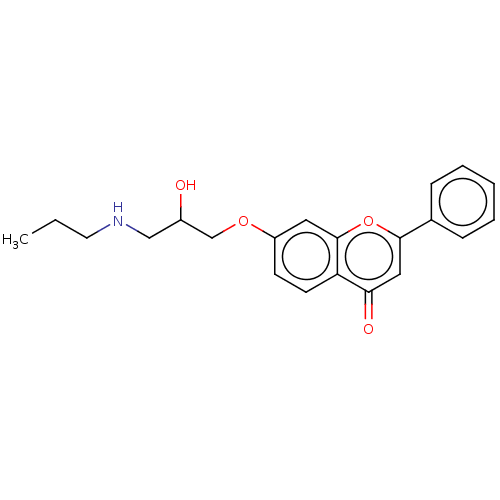
(Flavodilol)Show InChI InChI=1S/C21H23NO4/c1-2-10-22-13-16(23)14-25-17-8-9-18-19(24)12-20(26-21(18)11-17)15-6-4-3-5-7-15/h3-9,11-12,16,22-23H,2,10,13-14H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pennwalt Corporation
Curated by ChEMBL
| Assay Description
Inhibition constant from beta adrenergic receptor binding assay |
J Med Chem 32: 183-92 (1989)
BindingDB Entry DOI: 10.7270/Q2474D33 |
More data for this
Ligand-Target Pair | |
Beta-1/Beta-2/Beta-3 adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM50227820
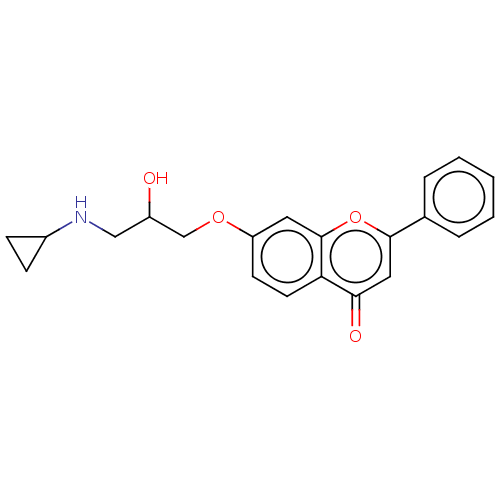
(CHEMBL57544)Show InChI InChI=1S/C21H21NO4/c23-16(12-22-15-6-7-15)13-25-17-8-9-18-19(24)11-20(26-21(18)10-17)14-4-2-1-3-5-14/h1-5,8-11,15-16,22-23H,6-7,12-13H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pennwalt Corporation
Curated by ChEMBL
| Assay Description
Inhibition constant from beta adrenergic receptor binding assay |
J Med Chem 32: 183-92 (1989)
BindingDB Entry DOI: 10.7270/Q2474D33 |
More data for this
Ligand-Target Pair | |
Beta-1/Beta-2/Beta-3 adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM25761

(Anapriline | Avlocardyl | CHEMBL27 | PROPANOLOL(-)...)Show InChI InChI=1S/C16H21NO2/c1-12(2)17-10-14(18)11-19-16-9-5-7-13-6-3-4-8-15(13)16/h3-9,12,14,17-18H,10-11H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pennwalt Corporation
Curated by ChEMBL
| Assay Description
Inhibition constant from beta adrenergic receptor binding assay |
J Med Chem 32: 183-92 (1989)
BindingDB Entry DOI: 10.7270/Q2474D33 |
More data for this
Ligand-Target Pair | |
Beta-1/Beta-2/Beta-3 adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM50227814
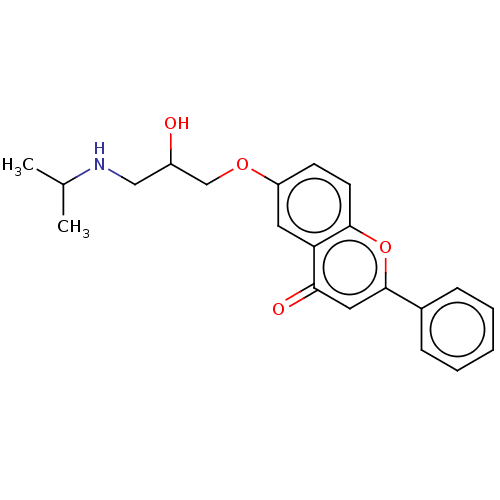
(CHEMBL291999)Show InChI InChI=1S/C21H23NO4/c1-14(2)22-12-16(23)13-25-17-8-9-20-18(10-17)19(24)11-21(26-20)15-6-4-3-5-7-15/h3-11,14,16,22-23H,12-13H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pennwalt Corporation
Curated by ChEMBL
| Assay Description
Inhibition constant from beta adrenergic receptor binding assay |
J Med Chem 32: 183-92 (1989)
BindingDB Entry DOI: 10.7270/Q2474D33 |
More data for this
Ligand-Target Pair | |
Beta-1/Beta-2/Beta-3 adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM50227817
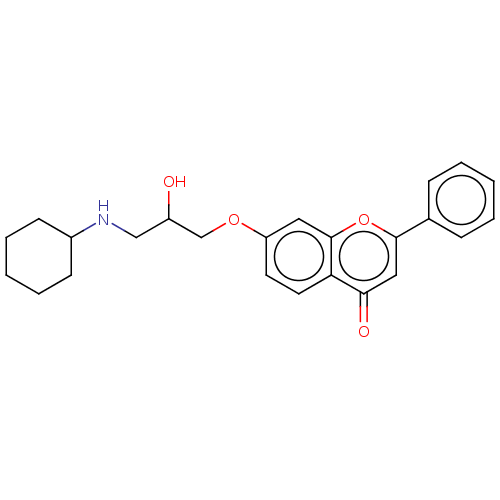
(CHEMBL57611)Show SMILES OC(CNC1CCCCC1)COc1ccc2c(c1)oc(cc2=O)-c1ccccc1 Show InChI InChI=1S/C24H27NO4/c26-19(15-25-18-9-5-2-6-10-18)16-28-20-11-12-21-22(27)14-23(29-24(21)13-20)17-7-3-1-4-8-17/h1,3-4,7-8,11-14,18-19,25-26H,2,5-6,9-10,15-16H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pennwalt Corporation
Curated by ChEMBL
| Assay Description
Inhibition constant obtained from beta adrenergic receptor binding assay |
J Med Chem 32: 183-92 (1989)
BindingDB Entry DOI: 10.7270/Q2474D33 |
More data for this
Ligand-Target Pair | |
Beta-1/Beta-2/Beta-3 adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM50227816
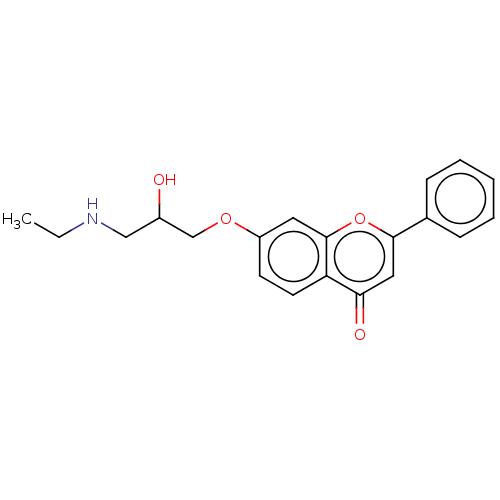
(CHEMBL57659)Show InChI InChI=1S/C20H21NO4/c1-2-21-12-15(22)13-24-16-8-9-17-18(23)11-19(25-20(17)10-16)14-6-4-3-5-7-14/h3-11,15,21-22H,2,12-13H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pennwalt Corporation
Curated by ChEMBL
| Assay Description
Inhibition constant from beta adrenergic receptor binding assay |
J Med Chem 32: 183-92 (1989)
BindingDB Entry DOI: 10.7270/Q2474D33 |
More data for this
Ligand-Target Pair | |
Beta-1/Beta-2/Beta-3 adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM50227819
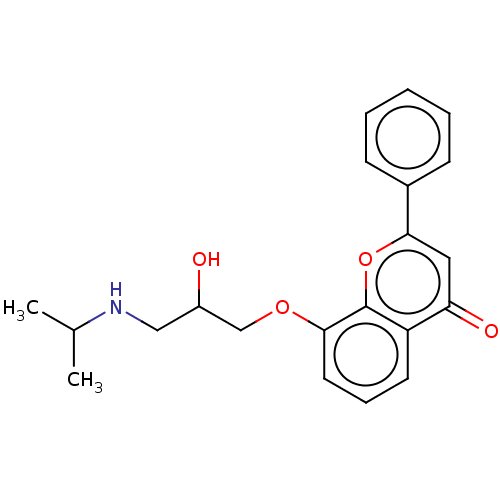
(CHEMBL273690)Show InChI InChI=1S/C21H23NO4/c1-14(2)22-12-16(23)13-25-19-10-6-9-17-18(24)11-20(26-21(17)19)15-7-4-3-5-8-15/h3-11,14,16,22-23H,12-13H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pennwalt Corporation
Curated by ChEMBL
| Assay Description
Inhibition constant from beta adrenergic receptor binding assay |
J Med Chem 32: 183-92 (1989)
BindingDB Entry DOI: 10.7270/Q2474D33 |
More data for this
Ligand-Target Pair | |
Beta-1/Beta-2/Beta-3 adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM50227818
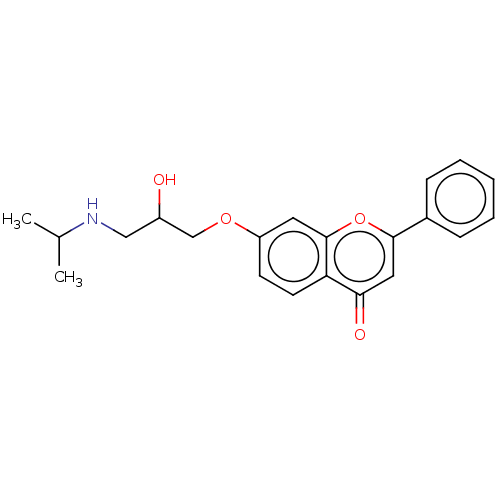
(CHEMBL56023)Show InChI InChI=1S/C21H23NO4/c1-14(2)22-12-16(23)13-25-17-8-9-18-19(24)11-20(26-21(18)10-17)15-6-4-3-5-7-15/h3-11,14,16,22-23H,12-13H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pennwalt Corporation
Curated by ChEMBL
| Assay Description
Inhibition constant from beta adrenergic receptor binding assay |
J Med Chem 32: 183-92 (1989)
BindingDB Entry DOI: 10.7270/Q2474D33 |
More data for this
Ligand-Target Pair | |
Beta-1/Beta-2/Beta-3 adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM50227813

(CHEMBL293974)Show SMILES CC(C)(C)NCC(O)COc1ccc2c(c1)oc(cc2=O)-c1ccccc1 Show InChI InChI=1S/C22H25NO4/c1-22(2,3)23-13-16(24)14-26-17-9-10-18-19(25)12-20(27-21(18)11-17)15-7-5-4-6-8-15/h4-12,16,23-24H,13-14H2,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pennwalt Corporation
Curated by ChEMBL
| Assay Description
Inhibition constant from beta adrenergic receptor binding assay |
J Med Chem 32: 183-92 (1989)
BindingDB Entry DOI: 10.7270/Q2474D33 |
More data for this
Ligand-Target Pair | |
Beta-1/Beta-2/Beta-3 adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM50227821
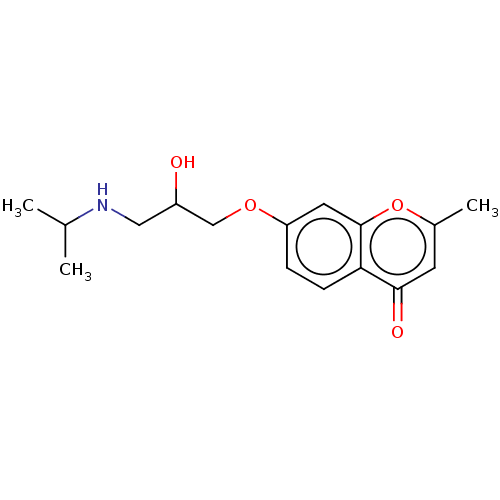
(CHEMBL56492)Show InChI InChI=1S/C16H21NO4/c1-10(2)17-8-12(18)9-20-13-4-5-14-15(19)6-11(3)21-16(14)7-13/h4-7,10,12,17-18H,8-9H2,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pennwalt Corporation
Curated by ChEMBL
| Assay Description
Inhibition constant from beta adrenergic receptor binding assay |
J Med Chem 32: 183-92 (1989)
BindingDB Entry DOI: 10.7270/Q2474D33 |
More data for this
Ligand-Target Pair | |
Baculoviral IAP repeat-containing protein 2
(Homo sapiens (Human)) | BDBM50450049

(CHEMBL4166057)Show SMILES C[C@@H]1CN(CC(=O)N2CC(C)(C)c3nc(CO)c(Cc4ccc(F)cc4)cc23)[C@@H](CN2[C@H](C)COC[C@H]2C)CN1 |r| Show InChI InChI=1S/C31H44FN5O3/c1-20-13-35(26(12-33-20)14-36-21(2)17-40-18-22(36)3)15-29(39)37-19-31(4,5)30-28(37)11-24(27(16-38)34-30)10-23-6-8-25(32)9-7-23/h6-9,11,20-22,26,33,38H,10,12-19H2,1-5H3/t20-,21-,22-,26-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Induction of intracellular cIAP1 degradation in human MDA-MB-231 cells after 2 hrs |
J Med Chem 61: 7314-7329 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00900
BindingDB Entry DOI: 10.7270/Q2TT4THH |
More data for this
Ligand-Target Pair | |
Baculoviral IAP repeat-containing protein 2
(Homo sapiens (Human)) | BDBM50450044

(CHEMBL4167141)Show SMILES C[C@@H]1CN(CC(=O)N2CC(C)(C)c3[nH]c(=O)c(Cc4ccc(F)cc4)cc23)[C@@H](CN2[C@H](C)COC[C@H]2C)CN1 |r| Show InChI InChI=1S/C30H42FN5O3/c1-19-13-34(25(12-32-19)14-35-20(2)16-39-17-21(35)3)15-27(37)36-18-30(4,5)28-26(36)11-23(29(38)33-28)10-22-6-8-24(31)9-7-22/h6-9,11,19-21,25,32H,10,12-18H2,1-5H3,(H,33,38)/t19-,20-,21-,25-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Induction of intracellular cIAP1 degradation in human MDA-MB-231 cells after 2 hrs |
J Med Chem 61: 7314-7329 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00900
BindingDB Entry DOI: 10.7270/Q2TT4THH |
More data for this
Ligand-Target Pair | |
Baculoviral IAP repeat-containing protein 2
(Homo sapiens (Human)) | BDBM50450038
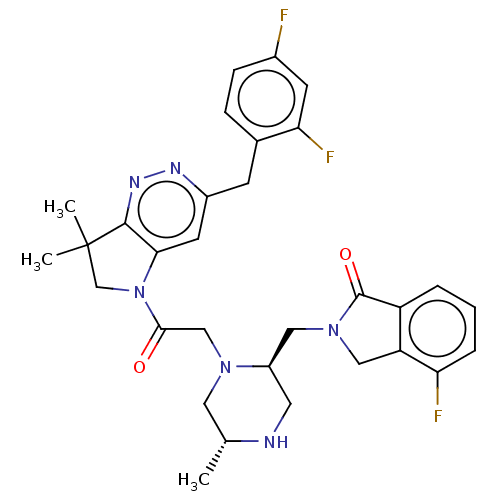
(CHEMBL4171490)Show SMILES C[C@@H]1CN(CC(=O)N2CC(C)(C)c3nnc(Cc4ccc(F)cc4F)cc23)[C@@H](CN2Cc3c(cccc3F)C2=O)CN1 |r| Show InChI InChI=1S/C31H33F3N6O2/c1-18-13-38(22(12-35-18)14-39-15-24-23(30(39)42)5-4-6-25(24)33)16-28(41)40-17-31(2,3)29-27(40)11-21(36-37-29)9-19-7-8-20(32)10-26(19)34/h4-8,10-11,18,22,35H,9,12-17H2,1-3H3/t18-,22-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Induction of intracellular cIAP1 degradation in human MDA-MB-231 cells after 2 hrs |
J Med Chem 61: 7314-7329 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00900
BindingDB Entry DOI: 10.7270/Q2TT4THH |
More data for this
Ligand-Target Pair | |
Baculoviral IAP repeat-containing protein 2
(Homo sapiens (Human)) | BDBM50450047

(CHEMBL4169478)Show SMILES C[C@@H]1CN(CC(=O)N2CC(C)(C)c3c2cc(Cc2ccc(F)cc2)c(=O)n3C)[C@@H](CN2[C@H](C)COC[C@H]2C)CN1 |r| Show InChI InChI=1S/C31H44FN5O3/c1-20-14-35(26(13-33-20)15-36-21(2)17-40-18-22(36)3)16-28(38)37-19-31(4,5)29-27(37)12-24(30(39)34(29)6)11-23-7-9-25(32)10-8-23/h7-10,12,20-22,26,33H,11,13-19H2,1-6H3/t20-,21-,22-,26-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Induction of intracellular cIAP1 degradation in human MDA-MB-231 cells after 2 hrs |
J Med Chem 61: 7314-7329 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00900
BindingDB Entry DOI: 10.7270/Q2TT4THH |
More data for this
Ligand-Target Pair | |
Baculoviral IAP repeat-containing protein 2
(Homo sapiens (Human)) | BDBM50450043
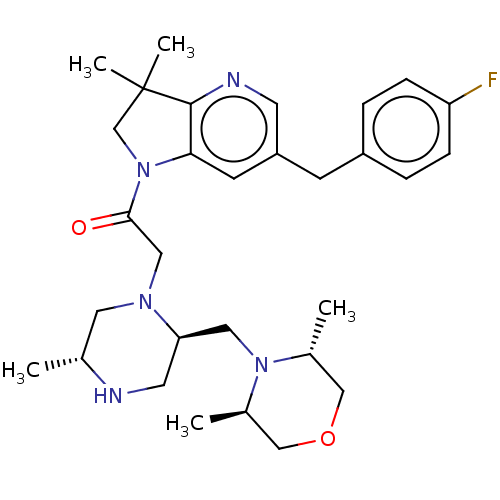
(CHEMBL4166607)Show SMILES C[C@@H]1CN(CC(=O)N2CC(C)(C)c3ncc(Cc4ccc(F)cc4)cc23)[C@@H](CN2[C@H](C)COC[C@H]2C)CN1 |r| Show InChI InChI=1S/C30H42FN5O2/c1-20-14-34(26(13-32-20)15-35-21(2)17-38-18-22(35)3)16-28(37)36-19-30(4,5)29-27(36)11-24(12-33-29)10-23-6-8-25(31)9-7-23/h6-9,11-12,20-22,26,32H,10,13-19H2,1-5H3/t20-,21-,22-,26-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Induction of intracellular cIAP1 degradation in human MDA-MB-231 cells after 2 hrs |
J Med Chem 61: 7314-7329 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00900
BindingDB Entry DOI: 10.7270/Q2TT4THH |
More data for this
Ligand-Target Pair | |
Baculoviral IAP repeat-containing protein 2
(Homo sapiens (Human)) | BDBM50450037
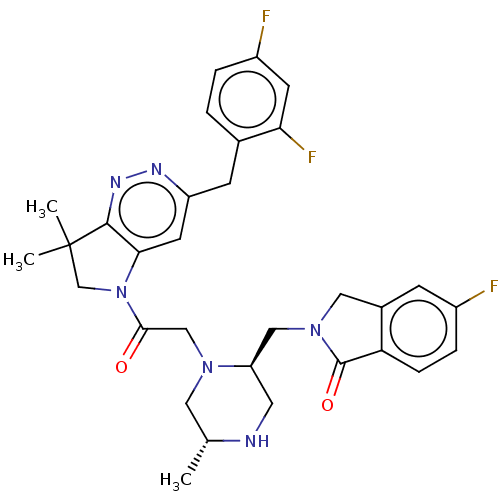
(CHEMBL4164271)Show SMILES C[C@@H]1CN(CC(=O)N2CC(C)(C)c3nnc(Cc4ccc(F)cc4F)cc23)[C@@H](CN2Cc3cc(F)ccc3C2=O)CN1 |r| Show InChI InChI=1S/C31H33F3N6O2/c1-18-13-38(24(12-35-18)15-39-14-20-8-21(32)6-7-25(20)30(39)42)16-28(41)40-17-31(2,3)29-27(40)11-23(36-37-29)9-19-4-5-22(33)10-26(19)34/h4-8,10-11,18,24,35H,9,12-17H2,1-3H3/t18-,24-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Induction of intracellular cIAP1 degradation in human MDA-MB-231 cells after 2 hrs |
J Med Chem 61: 7314-7329 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00900
BindingDB Entry DOI: 10.7270/Q2TT4THH |
More data for this
Ligand-Target Pair | |
Baculoviral IAP repeat-containing protein 2
(Homo sapiens (Human)) | BDBM50450046
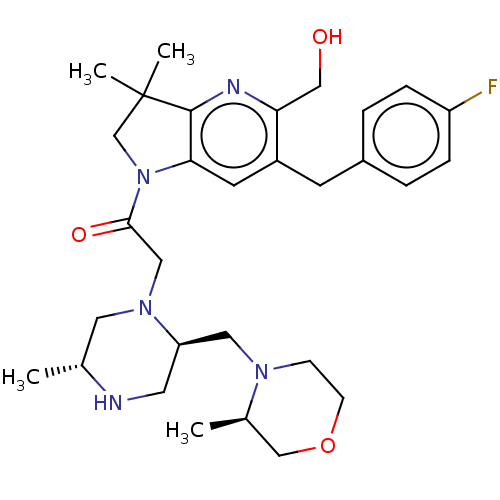
(CHEMBL4173974)Show SMILES C[C@@H]1CN(CC(=O)N2CC(C)(C)c3nc(CO)c(Cc4ccc(F)cc4)cc23)[C@@H](CN2CCOC[C@H]2C)CN1 |r| Show InChI InChI=1S/C30H42FN5O3/c1-20-14-35(25(13-32-20)15-34-9-10-39-18-21(34)2)16-28(38)36-19-30(3,4)29-27(36)12-23(26(17-37)33-29)11-22-5-7-24(31)8-6-22/h5-8,12,20-21,25,32,37H,9-11,13-19H2,1-4H3/t20-,21-,25-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Induction of intracellular cIAP1 degradation in human MDA-MB-231 cells after 2 hrs |
J Med Chem 61: 7314-7329 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00900
BindingDB Entry DOI: 10.7270/Q2TT4THH |
More data for this
Ligand-Target Pair | |
Baculoviral IAP repeat-containing protein 2
(Homo sapiens (Human)) | BDBM50239425
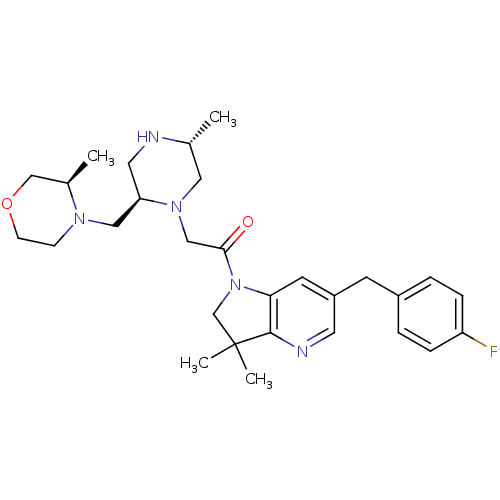
(CHEMBL4064619)Show SMILES C[C@@H]1CN(CC(=O)N2CC(C)(C)c3ncc(Cc4ccc(F)cc4)cc23)[C@@H](CN2CCOC[C@H]2C)CN1 |r| Show InChI InChI=1S/C29H40FN5O2/c1-20-15-34(25(14-31-20)16-33-9-10-37-18-21(33)2)17-27(36)35-19-29(3,4)28-26(35)12-23(13-32-28)11-22-5-7-24(30)8-6-22/h5-8,12-13,20-21,25,31H,9-11,14-19H2,1-4H3/t20-,21-,25-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Induction of intracellular cIAP1 degradation in human MDA-MB-231 cells after 2 hrs |
J Med Chem 61: 7314-7329 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00900
BindingDB Entry DOI: 10.7270/Q2TT4THH |
More data for this
Ligand-Target Pair | |
Baculoviral IAP repeat-containing protein 2
(Homo sapiens (Human)) | BDBM50450039

(CHEMBL4160872)Show SMILES C[C@@H]1CN(CC(=O)N2CC(C)(C)c3nnc(Cc4ccc(F)cc4F)cc23)[C@@H](CN2Cc3ccccc3C2=O)CN1 |r| Show InChI InChI=1S/C31H34F2N6O2/c1-19-14-37(24(13-34-19)16-38-15-21-6-4-5-7-25(21)30(38)41)17-28(40)39-18-31(2,3)29-27(39)12-23(35-36-29)10-20-8-9-22(32)11-26(20)33/h4-9,11-12,19,24,34H,10,13-18H2,1-3H3/t19-,24-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Induction of intracellular cIAP1 degradation in human MDA-MB-231 cells after 2 hrs |
J Med Chem 61: 7314-7329 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00900
BindingDB Entry DOI: 10.7270/Q2TT4THH |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50238110
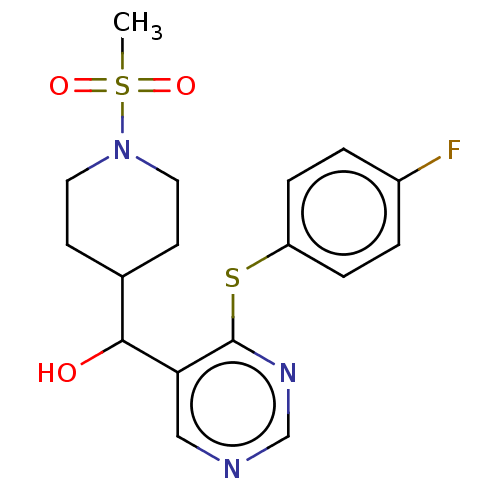
(CHEMBL4088766)Show SMILES CS(=O)(=O)N1CCC(CC1)C(O)c1cncnc1Sc1ccc(F)cc1 Show InChI InChI=1S/C17H20FN3O3S2/c1-26(23,24)21-8-6-12(7-9-21)16(22)15-10-19-11-20-17(15)25-14-4-2-13(18)3-5-14/h2-5,10-12,16,22H,6-9H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B2 expressed in HEK293A cells using deoxycorticosterone as substrate pretreated for 1 hr followed by substrate addition meas... |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Baculoviral IAP repeat-containing protein 2
(Homo sapiens (Human)) | BDBM50441356
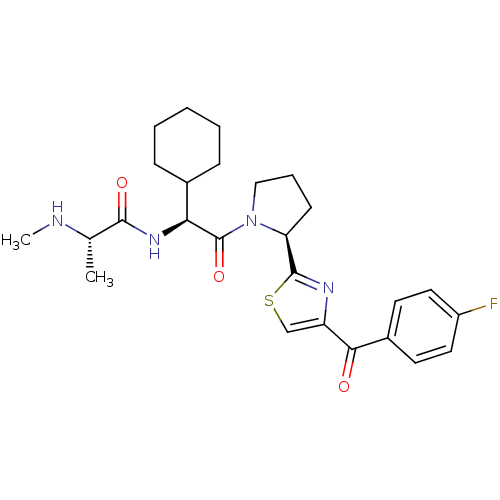
(CHEMBL2431768)Show SMILES CN[C@@H](C)C(=O)N[C@@H](C1CCCCC1)C(=O)N1CCC[C@H]1c1nc(cs1)C(=O)c1ccc(F)cc1 |r| Show InChI InChI=1S/C26H33FN4O3S/c1-16(28-2)24(33)30-22(17-7-4-3-5-8-17)26(34)31-14-6-9-21(31)25-29-20(15-35-25)23(32)18-10-12-19(27)13-11-18/h10-13,15-17,21-22,28H,3-9,14H2,1-2H3,(H,30,33)/t16-,21-,22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Induction of intracellular cIAP1 degradation in human MDA-MB-231 cells after 2 hrs |
J Med Chem 61: 7314-7329 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00900
BindingDB Entry DOI: 10.7270/Q2TT4THH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Baculoviral IAP repeat-containing protein 2
(Homo sapiens (Human)) | BDBM50450054

(CHEMBL4159232)Show SMILES C[C@@H]1CN(CC(=O)N2CC(C)(C)c3ncc(Cc4ccc(F)cc4O)cc23)[C@@H](CN2[C@H](C)COC[C@H]2C)CN1 |r| Show InChI InChI=1S/C30H42FN5O3/c1-19-13-34(25(12-32-19)14-35-20(2)16-39-17-21(35)3)15-28(38)36-18-30(4,5)29-26(36)9-22(11-33-29)8-23-6-7-24(31)10-27(23)37/h6-7,9-11,19-21,25,32,37H,8,12-18H2,1-5H3/t19-,20-,21-,25-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Induction of intracellular cIAP1 degradation in human MDA-MB-231 cells after 2 hrs |
J Med Chem 61: 7314-7329 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00900
BindingDB Entry DOI: 10.7270/Q2TT4THH |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B1, mitochondrial
(Homo sapiens (Human)) | BDBM50238110

(CHEMBL4088766)Show SMILES CS(=O)(=O)N1CCC(CC1)C(O)c1cncnc1Sc1ccc(F)cc1 Show InChI InChI=1S/C17H20FN3O3S2/c1-26(23,24)21-8-6-12(7-9-21)16(22)15-10-19-11-20-17(15)25-14-4-2-13(18)3-5-14/h2-5,10-12,16,22H,6-9H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B1 expressed in HEK293A cells |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Baculoviral IAP repeat-containing protein 2
(Homo sapiens (Human)) | BDBM50450042
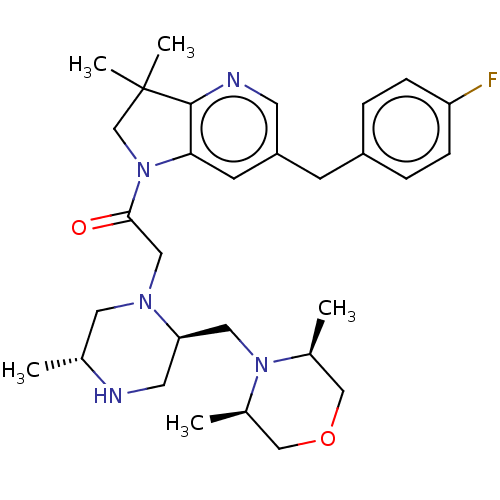
(CHEMBL4168197)Show SMILES C[C@@H]1CN(CC(=O)N2CC(C)(C)c3ncc(Cc4ccc(F)cc4)cc23)[C@@H](CN2[C@@H](C)COC[C@H]2C)CN1 |r| Show InChI InChI=1S/C30H42FN5O2/c1-20-14-34(26(13-32-20)15-35-21(2)17-38-18-22(35)3)16-28(37)36-19-30(4,5)29-27(36)11-24(12-33-29)10-23-6-8-25(31)9-7-23/h6-9,11-12,20-22,26,32H,10,13-19H2,1-5H3/t20-,21-,22+,26-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Induction of intracellular cIAP1 degradation in human MDA-MB-231 cells after 2 hrs |
J Med Chem 61: 7314-7329 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00900
BindingDB Entry DOI: 10.7270/Q2TT4THH |
More data for this
Ligand-Target Pair | |
Baculoviral IAP repeat-containing protein 2
(Homo sapiens (Human)) | BDBM50450048
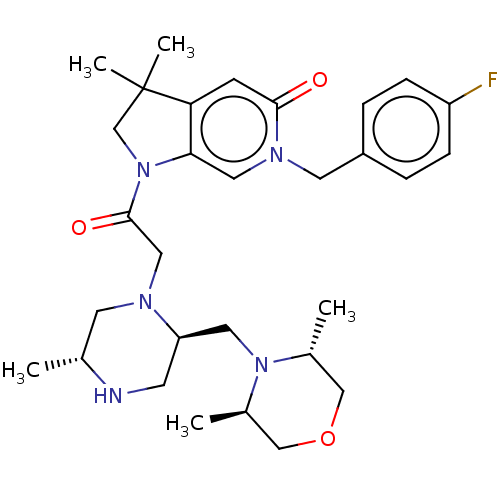
(CHEMBL4177336)Show SMILES C[C@@H]1CN(CC(=O)N2CC(C)(C)c3cc(=O)n(Cc4ccc(F)cc4)cc23)[C@@H](CN2[C@H](C)COC[C@H]2C)CN1 |r| Show InChI InChI=1S/C30H42FN5O3/c1-20-12-33(25(11-32-20)14-35-21(2)17-39-18-22(35)3)16-29(38)36-19-30(4,5)26-10-28(37)34(15-27(26)36)13-23-6-8-24(31)9-7-23/h6-10,15,20-22,25,32H,11-14,16-19H2,1-5H3/t20-,21-,22-,25-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Induction of intracellular cIAP1 degradation in human MDA-MB-231 cells after 2 hrs |
J Med Chem 61: 7314-7329 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00900
BindingDB Entry DOI: 10.7270/Q2TT4THH |
More data for this
Ligand-Target Pair | |
Baculoviral IAP repeat-containing protein 2
(Homo sapiens (Human)) | BDBM50450051
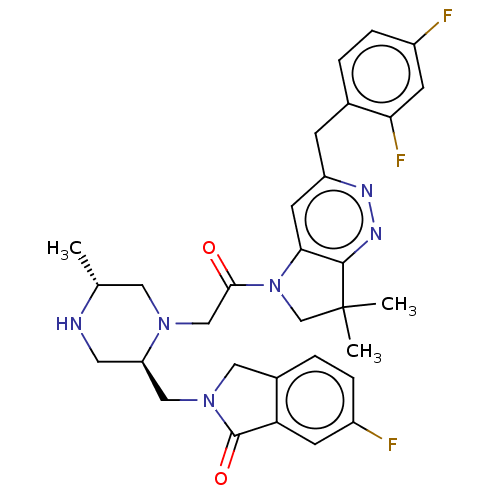
(CHEMBL4174922)Show SMILES C[C@@H]1CN(CC(=O)N2CC(C)(C)c3nnc(Cc4ccc(F)cc4F)cc23)[C@@H](CN2Cc3ccc(F)cc3C2=O)CN1 |r| Show InChI InChI=1S/C31H33F3N6O2/c1-18-13-38(24(12-35-18)15-39-14-20-5-7-21(32)9-25(20)30(39)42)16-28(41)40-17-31(2,3)29-27(40)11-23(36-37-29)8-19-4-6-22(33)10-26(19)34/h4-7,9-11,18,24,35H,8,12-17H2,1-3H3/t18-,24-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Induction of intracellular cIAP1 degradation in human MDA-MB-231 cells after 2 hrs |
J Med Chem 61: 7314-7329 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00900
BindingDB Entry DOI: 10.7270/Q2TT4THH |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase XIAP
(Homo sapiens (Human)) | BDBM50450047

(CHEMBL4169478)Show SMILES C[C@@H]1CN(CC(=O)N2CC(C)(C)c3c2cc(Cc2ccc(F)cc2)c(=O)n3C)[C@@H](CN2[C@H](C)COC[C@H]2C)CN1 |r| Show InChI InChI=1S/C31H44FN5O3/c1-20-14-35(26(13-33-20)15-36-21(2)17-40-18-22(36)3)16-28(38)37-19-31(4,5)29-27(37)12-24(30(39)34(29)6)11-23-7-9-25(32)10-8-23/h7-10,12,20-22,26,33H,11,13-19H2,1-6H3/t20-,21-,22-,26-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of full length FLAG-tagged XIAP (unknown origin) interaction with full length untagged caspase-9 expressed in HEK293 cells after 2 hrs by ... |
J Med Chem 61: 7314-7329 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00900
BindingDB Entry DOI: 10.7270/Q2TT4THH |
More data for this
Ligand-Target Pair | |
Baculoviral IAP repeat-containing protein 2
(Homo sapiens (Human)) | BDBM50450050

(CHEMBL4167717)Show SMILES C[C@@H]1CN(CC(=O)N2CC(C)(C)c3nnc(Cc4ccc(F)cc4F)cc23)[C@@H](CN2Cc3cc(ccc3C2=O)C#N)CN1 |r| Show InChI InChI=1S/C32H33F2N7O2/c1-19-14-39(25(13-36-19)16-40-15-22-8-20(12-35)4-7-26(22)31(40)43)17-29(42)41-18-32(2,3)30-28(41)11-24(37-38-30)9-21-5-6-23(33)10-27(21)34/h4-8,10-11,19,25,36H,9,13-18H2,1-3H3/t19-,25-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Induction of intracellular cIAP1 degradation in human MDA-MB-231 cells after 2 hrs |
J Med Chem 61: 7314-7329 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00900
BindingDB Entry DOI: 10.7270/Q2TT4THH |
More data for this
Ligand-Target Pair | |
Baculoviral IAP repeat-containing protein 2
(Homo sapiens (Human)) | BDBM50450053
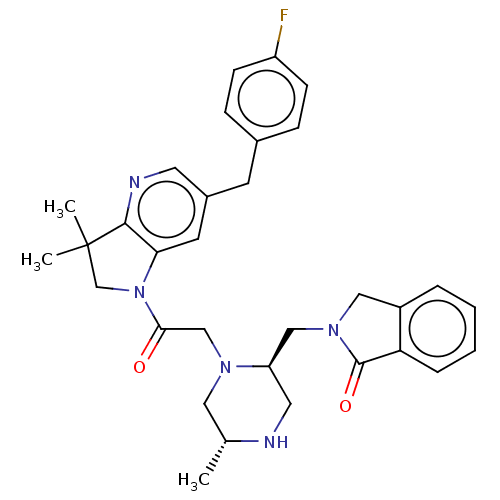
(CHEMBL4175992)Show SMILES C[C@@H]1CN(CC(=O)N2CC(C)(C)c3ncc(Cc4ccc(F)cc4)cc23)[C@@H](CN2Cc3ccccc3C2=O)CN1 |r| Show InChI InChI=1S/C32H36FN5O2/c1-21-16-36(26(15-34-21)18-37-17-24-6-4-5-7-27(24)31(37)40)19-29(39)38-20-32(2,3)30-28(38)13-23(14-35-30)12-22-8-10-25(33)11-9-22/h4-11,13-14,21,26,34H,12,15-20H2,1-3H3/t21-,26-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Induction of intracellular cIAP1 degradation in human MDA-MB-231 cells after 2 hrs |
J Med Chem 61: 7314-7329 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00900
BindingDB Entry DOI: 10.7270/Q2TT4THH |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase XIAP
(Homo sapiens (Human)) | BDBM50450049

(CHEMBL4166057)Show SMILES C[C@@H]1CN(CC(=O)N2CC(C)(C)c3nc(CO)c(Cc4ccc(F)cc4)cc23)[C@@H](CN2[C@H](C)COC[C@H]2C)CN1 |r| Show InChI InChI=1S/C31H44FN5O3/c1-20-13-35(26(12-33-20)14-36-21(2)17-40-18-22(36)3)15-29(39)37-19-31(4,5)30-28(37)11-24(27(16-38)34-30)10-23-6-8-25(32)9-7-23/h6-9,11,20-22,26,33,38H,10,12-19H2,1-5H3/t20-,21-,22-,26-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of full length FLAG-tagged XIAP (unknown origin) interaction with full length untagged caspase-9 expressed in HEK293 cells after 2 hrs by ... |
J Med Chem 61: 7314-7329 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00900
BindingDB Entry DOI: 10.7270/Q2TT4THH |
More data for this
Ligand-Target Pair | |
Baculoviral IAP repeat-containing protein 2
(Homo sapiens (Human)) | BDBM50450058
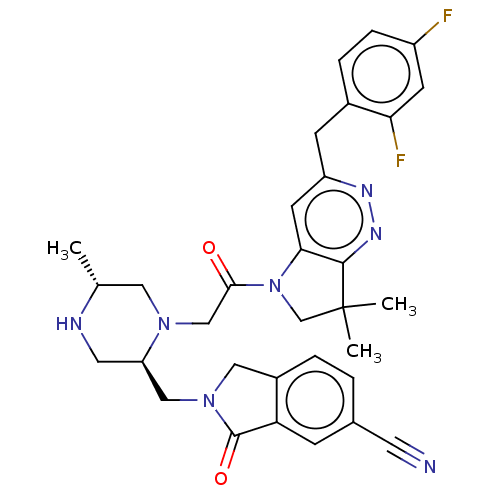
(CHEMBL4159806)Show SMILES C[C@@H]1CN(CC(=O)N2CC(C)(C)c3nnc(Cc4ccc(F)cc4F)cc23)[C@@H](CN2Cc3ccc(cc3C2=O)C#N)CN1 |r| Show InChI InChI=1S/C32H33F2N7O2/c1-19-14-39(25(13-36-19)16-40-15-22-5-4-20(12-35)8-26(22)31(40)43)17-29(42)41-18-32(2,3)30-28(41)11-24(37-38-30)9-21-6-7-23(33)10-27(21)34/h4-8,10-11,19,25,36H,9,13-18H2,1-3H3/t19-,25-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Induction of intracellular cIAP1 degradation in human MDA-MB-231 cells after 2 hrs |
J Med Chem 61: 7314-7329 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00900
BindingDB Entry DOI: 10.7270/Q2TT4THH |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1
(Homo sapiens (Human)) | BDBM10112
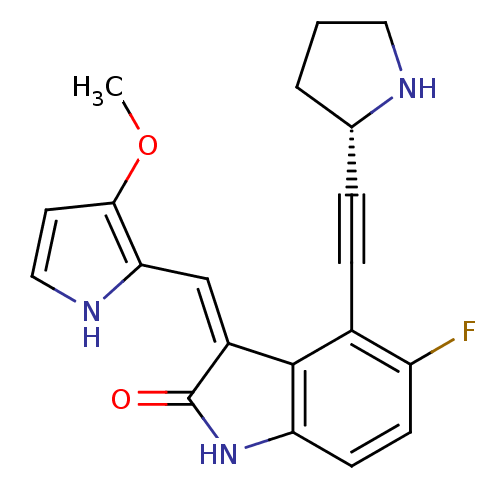
((3Z)-5-fluoro-3-[(3-methoxy-1H-pyrrol-2-yl)methyli...)Show SMILES COc1cc[nH]c1\C=C1/C(=O)Nc2ccc(F)c(C#C[C@@H]3CCCN3)c12 |r| Show InChI InChI=1S/C20H18FN3O2/c1-26-18-8-10-23-17(18)11-14-19-13(5-4-12-3-2-9-22-12)15(21)6-7-16(19)24-20(14)25/h6-8,10-12,22-23H,2-3,9H2,1H3,(H,24,25)/b14-11-/t12-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Hoffmann-La Roche Inc.
| Assay Description
Kinase assays were performed using a recombinant human cyclin E-CDK2 complex. The enzyme was assayed with substrate in the presence of 1uM ATP/[gamma... |
Bioorg Med Chem Lett 14: 913-7 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.009
BindingDB Entry DOI: 10.7270/Q20V8B1V |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B1, mitochondrial
(Homo sapiens (Human)) | BDBM50238115
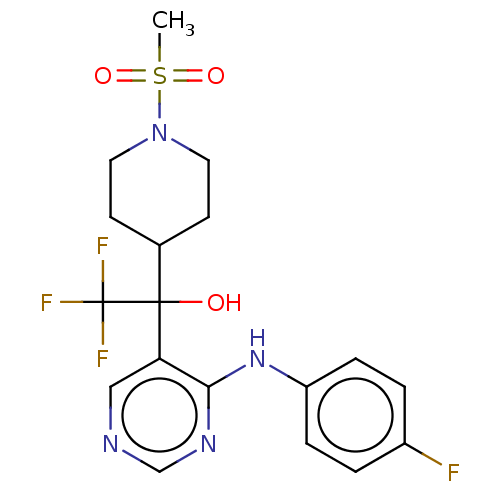
(CHEMBL4082403)Show SMILES CS(=O)(=O)N1CCC(CC1)C(O)(c1cncnc1Nc1ccc(F)cc1)C(F)(F)F Show InChI InChI=1S/C18H20F4N4O3S/c1-30(28,29)26-8-6-12(7-9-26)17(27,18(20,21)22)15-10-23-11-24-16(15)25-14-4-2-13(19)3-5-14/h2-5,10-12,27H,6-9H2,1H3,(H,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B1 expressed in HEK293A cells |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B1, mitochondrial
(Homo sapiens (Human)) | BDBM50238115

(CHEMBL4082403)Show SMILES CS(=O)(=O)N1CCC(CC1)C(O)(c1cncnc1Nc1ccc(F)cc1)C(F)(F)F Show InChI InChI=1S/C18H20F4N4O3S/c1-30(28,29)26-8-6-12(7-9-26)17(27,18(20,21)22)15-10-23-11-24-16(15)25-14-4-2-13(19)3-5-14/h2-5,10-12,27H,6-9H2,1H3,(H,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the activity of Angiotensin I converting enzyme by 50% |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase KAT6A [507-778]
(Homo sapiens) | BDBM50518833
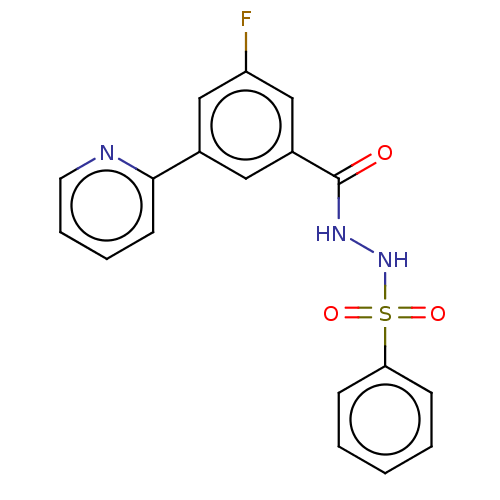
(CHEMBL4528993 | US10829446, Compound 45)Show SMILES Fc1cc(cc(c1)-c1ccccn1)C(=O)NNS(=O)(=O)c1ccccc1 Show InChI InChI=1S/C18H14FN3O3S/c19-15-11-13(17-8-4-5-9-20-17)10-14(12-15)18(23)21-22-26(24,25)16-6-2-1-3-7-16/h1-12,22H,(H,21,23) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Cancer Therapeutics CRC
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal NusA-fused His6-tagged human KAT6A (507 to 778 residues) expressed in Escherichia coli BL21 (DE3) cells using 0.4 uM acetyl ... |
J Med Chem 62: 7146-7159 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00665
BindingDB Entry DOI: 10.7270/Q26D5XCM |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50238108
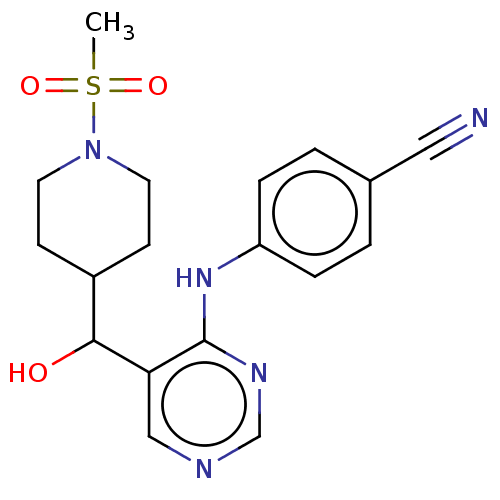
(CHEMBL4070230)Show SMILES CS(=O)(=O)N1CCC(CC1)C(O)c1cncnc1Nc1ccc(cc1)C#N Show InChI InChI=1S/C18H21N5O3S/c1-27(25,26)23-8-6-14(7-9-23)17(24)16-11-20-12-21-18(16)22-15-4-2-13(10-19)3-5-15/h2-5,11-12,14,17,24H,6-9H2,1H3,(H,20,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the activity of Angiotensin I converting enzyme by 50% |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50238103

(CHEMBL4070323)Show SMILES CC(O)(C1CCN(CC1)S(C)(=O)=O)c1cncnc1Nc1ccc(F)cc1 Show InChI InChI=1S/C18H23FN4O3S/c1-18(24,13-7-9-23(10-8-13)27(2,25)26)16-11-20-12-21-17(16)22-15-5-3-14(19)4-6-15/h3-6,11-13,24H,7-10H2,1-2H3,(H,20,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the activity of Angiotensin I converting enzyme by 50% |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50456355

(CHEMBL4207147 | US11001575, Example 554)Show SMILES COc1cccc(c1)[C@@H](C)NC(=O)CN1Cc2ccc(cc2C1=O)-c1nc(NC2CCOCC2)ncc1Cl |r| Show InChI InChI=1S/C28H30ClN5O4/c1-17(18-4-3-5-22(12-18)37-2)31-25(35)16-34-15-20-7-6-19(13-23(20)27(34)36)26-24(29)14-30-28(33-26)32-21-8-10-38-11-9-21/h3-7,12-14,17,21H,8-11,15-16H2,1-2H3,(H,31,35)(H,30,32,33)/t17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of full length human N-terminal MAHHHHHH-tagged ERK2 expressed in Escherichia coli BL21 (DE3) using ATF2-GFP as substrate after 30 mins by... |
J Med Chem 61: 4978-4992 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00421
BindingDB Entry DOI: 10.7270/Q2HT2RZZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Baculoviral IAP repeat-containing protein 2
(Homo sapiens (Human)) | BDBM50450057
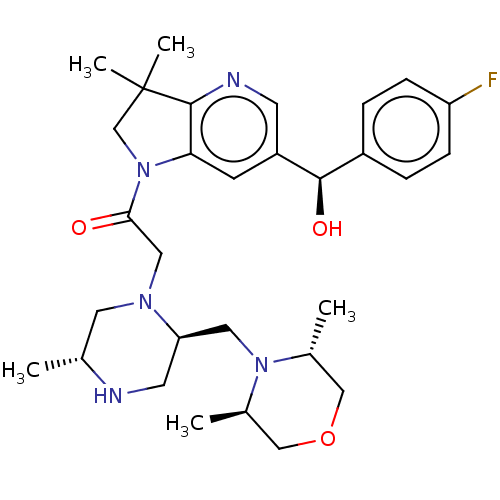
(CHEMBL4162607)Show SMILES C[C@@H]1CN(CC(=O)N2CC(C)(C)c3ncc(cc23)[C@H](O)c2ccc(F)cc2)[C@@H](CN2[C@H](C)COC[C@H]2C)CN1 |r| Show InChI InChI=1S/C30H42FN5O3/c1-19-13-34(25(12-32-19)14-35-20(2)16-39-17-21(35)3)15-27(37)36-18-30(4,5)29-26(36)10-23(11-33-29)28(38)22-6-8-24(31)9-7-22/h6-11,19-21,25,28,32,38H,12-18H2,1-5H3/t19-,20-,21-,25-,28-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Induction of intracellular cIAP1 degradation in human MDA-MB-231 cells after 2 hrs |
J Med Chem 61: 7314-7329 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00900
BindingDB Entry DOI: 10.7270/Q2TT4THH |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1/3
(Homo sapiens (Human)) | BDBM50456348
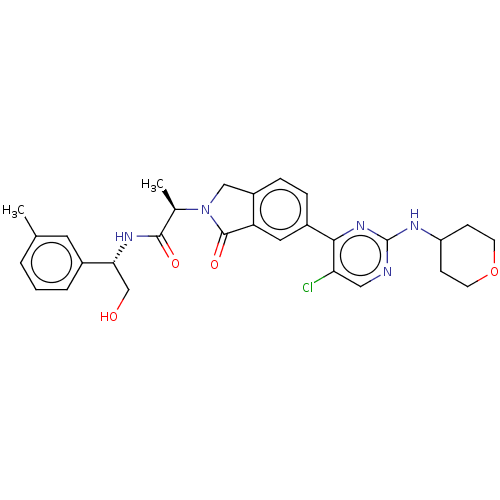
(CHEMBL4212211 | US11001575, Example 683)Show SMILES C[C@@H](N1Cc2ccc(cc2C1=O)-c1nc(NC2CCOCC2)ncc1Cl)C(=O)N[C@H](CO)c1cccc(C)c1 |r| Show InChI InChI=1S/C29H32ClN5O4/c1-17-4-3-5-19(12-17)25(16-36)33-27(37)18(2)35-15-21-7-6-20(13-23(21)28(35)38)26-24(30)14-31-29(34-26)32-22-8-10-39-11-9-22/h3-7,12-14,18,22,25,36H,8-11,15-16H2,1-2H3,(H,33,37)(H,31,32,34)/t18-,25-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of ERK1/2 phosphorylation in human A375 cells harboring BRAF V600E mutant after 4 hrs by ELISA |
J Med Chem 61: 4978-4992 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00421
BindingDB Entry DOI: 10.7270/Q2HT2RZZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Baculoviral IAP repeat-containing protein 2
(Homo sapiens (Human)) | BDBM50450052

(CHEMBL4168144)Show SMILES C[C@@H]1CN(CC(=O)N2CC(C)(C)c3nnc(Cc4ccc(F)cc4)cc23)[C@@H](CN2Cc3ccccc3C2=O)CN1 |r| Show InChI InChI=1S/C31H35FN6O2/c1-20-15-36(25(14-33-20)17-37-16-22-6-4-5-7-26(22)30(37)40)18-28(39)38-19-31(2,3)29-27(38)13-24(34-35-29)12-21-8-10-23(32)11-9-21/h4-11,13,20,25,33H,12,14-19H2,1-3H3/t20-,25-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Induction of intracellular cIAP1 degradation in human MDA-MB-231 cells after 2 hrs |
J Med Chem 61: 7314-7329 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00900
BindingDB Entry DOI: 10.7270/Q2TT4THH |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50456356

(CHEMBL4209691 | US11001575, Example 674)Show SMILES C[C@@H](N1Cc2ccc(cc2C1=O)-c1nc(NC2CCOCC2)ncc1Cl)C(=O)N[C@H](CO)c1ccccc1 |r| Show InChI InChI=1S/C28H30ClN5O4/c1-17(26(36)32-24(16-35)18-5-3-2-4-6-18)34-15-20-8-7-19(13-22(20)27(34)37)25-23(29)14-30-28(33-25)31-21-9-11-38-12-10-21/h2-8,13-14,17,21,24,35H,9-12,15-16H2,1H3,(H,32,36)(H,30,31,33)/t17-,24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of full length human N-terminal MAHHHHHH-tagged ERK2 expressed in Escherichia coli BL21 (DE3) using ATF2-GFP as substrate after 30 mins by... |
J Med Chem 61: 4978-4992 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00421
BindingDB Entry DOI: 10.7270/Q2HT2RZZ |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase XIAP
(Homo sapiens (Human)) | BDBM50450046

(CHEMBL4173974)Show SMILES C[C@@H]1CN(CC(=O)N2CC(C)(C)c3nc(CO)c(Cc4ccc(F)cc4)cc23)[C@@H](CN2CCOC[C@H]2C)CN1 |r| Show InChI InChI=1S/C30H42FN5O3/c1-20-14-35(25(13-32-20)15-34-9-10-39-18-21(34)2)16-28(38)36-19-30(3,4)29-27(36)12-23(26(17-37)33-29)11-22-5-7-24(31)8-6-22/h5-8,12,20-21,25,32,37H,9-11,13-19H2,1-4H3/t20-,21-,25-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of full length FLAG-tagged XIAP (unknown origin) interaction with full length untagged caspase-9 expressed in HEK293 cells after 2 hrs by ... |
J Med Chem 61: 7314-7329 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00900
BindingDB Entry DOI: 10.7270/Q2TT4THH |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase XIAP
(Homo sapiens (Human)) | BDBM50450043

(CHEMBL4166607)Show SMILES C[C@@H]1CN(CC(=O)N2CC(C)(C)c3ncc(Cc4ccc(F)cc4)cc23)[C@@H](CN2[C@H](C)COC[C@H]2C)CN1 |r| Show InChI InChI=1S/C30H42FN5O2/c1-20-14-34(26(13-32-20)15-35-21(2)17-38-18-22(35)3)16-28(37)36-19-30(4,5)29-27(36)11-24(12-33-29)10-23-6-8-25(31)9-7-23/h6-9,11-12,20-22,26,32H,10,13-19H2,1-5H3/t20-,21-,22-,26-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of full length FLAG-tagged XIAP (unknown origin) interaction with full length untagged caspase-9 expressed in HEK293 cells after 2 hrs by ... |
J Med Chem 61: 7314-7329 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00900
BindingDB Entry DOI: 10.7270/Q2TT4THH |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1
(Homo sapiens (Human)) | BDBM10109
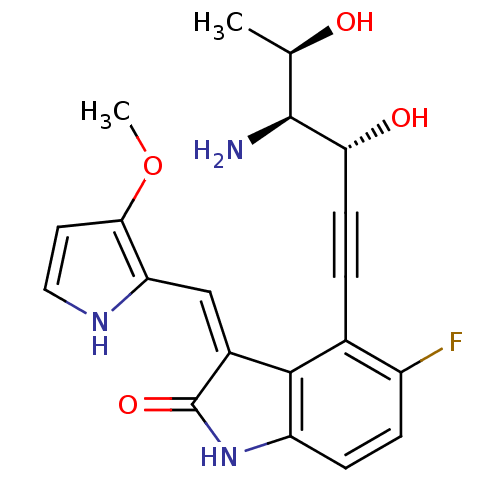
((3Z)-4-[(3R,4S,5R)-4-amino-3,5-dihydroxyhex-1-yn-1...)Show SMILES COc1cc[nH]c1\C=C1/C(=O)Nc2ccc(F)c(C#C[C@@H](O)[C@@H](N)[C@@H](C)O)c12 |r| Show InChI InChI=1S/C20H20FN3O4/c1-10(25)19(22)16(26)6-3-11-13(21)4-5-14-18(11)12(20(27)24-14)9-15-17(28-2)7-8-23-15/h4-5,7-10,16,19,23,25-26H,22H2,1-2H3,(H,24,27)/b12-9-/t10-,16-,19+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Hoffmann-La Roche Inc.
| Assay Description
Kinase assays were performed using a recombinant human cyclin E-CDK2 complex. The enzyme was assayed with substrate in the presence of 1uM ATP/[gamma... |
Bioorg Med Chem Lett 14: 913-7 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.009
BindingDB Entry DOI: 10.7270/Q20V8B1V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Platelet-activating factor receptor
(Homo sapiens (Human)) | BDBM50282045
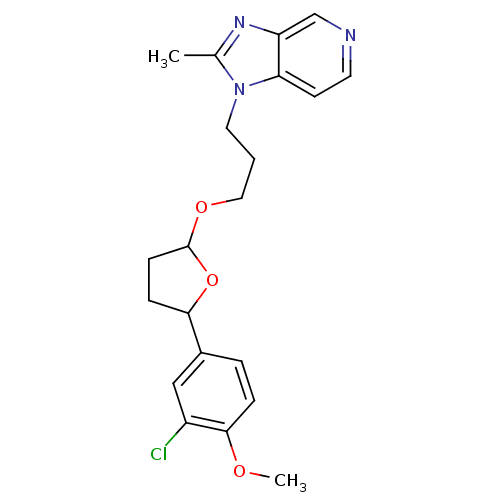
(1-{3-[5-(3-Chloro-4-methoxy-phenyl)-tetrahydro-fur...)Show SMILES COc1ccc(cc1Cl)C1CCC(OCCCn2c(C)nc3cnccc23)O1 Show InChI InChI=1S/C21H24ClN3O3/c1-14-24-17-13-23-9-8-18(17)25(14)10-3-11-27-21-7-6-19(28-21)15-4-5-20(26-2)16(22)12-15/h4-5,8-9,12-13,19,21H,3,6-7,10-11H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PAF receptor binding to washed human platelet membranes determined in vitro |
Bioorg Med Chem Lett 3: 1499-1504 (1993)
Article DOI: 10.1016/S0960-894X(00)80006-2
BindingDB Entry DOI: 10.7270/Q2CC115V |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50238109
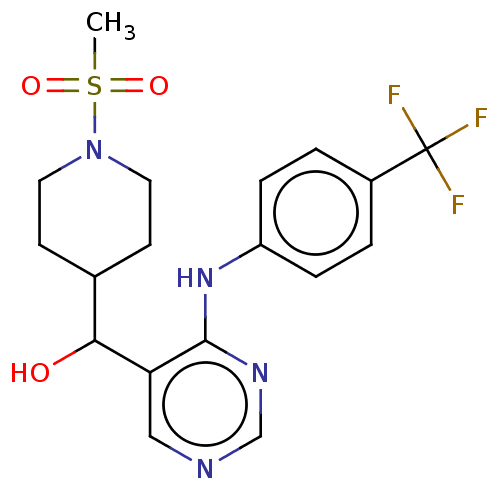
(CHEMBL4091167)Show SMILES CS(=O)(=O)N1CCC(CC1)C(O)c1cncnc1Nc1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C18H21F3N4O3S/c1-29(27,28)25-8-6-12(7-9-25)16(26)15-10-22-11-23-17(15)24-14-4-2-13(3-5-14)18(19,20)21/h2-5,10-12,16,26H,6-9H2,1H3,(H,22,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the activity of Angiotensin I converting enzyme by 50% |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50238105

(CHEMBL4099824)Show SMILES CS(=O)(=O)N1CCC(CC1)C(O)c1cncnc1Nc1ccc(F)cc1 Show InChI InChI=1S/C17H21FN4O3S/c1-26(24,25)22-8-6-12(7-9-22)16(23)15-10-19-11-20-17(15)21-14-4-2-13(18)3-5-14/h2-5,10-12,16,23H,6-9H2,1H3,(H,19,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the activity of Angiotensin I converting enzyme by 50% |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1
(Homo sapiens (Human)) | BDBM10099
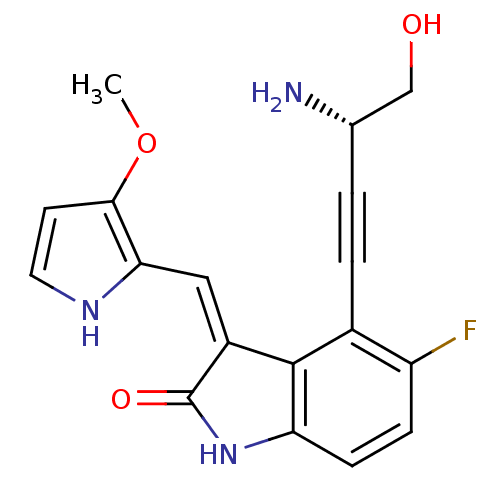
((3Z)-4-[(3S)-3-amino-4-hydroxybut-1-yn-1-yl]-5-flu...)Show SMILES COc1cc[nH]c1\C=C1/C(=O)Nc2ccc(F)c(C#C[C@H](N)CO)c12 |r| Show InChI InChI=1S/C18H16FN3O3/c1-25-16-6-7-21-15(16)8-12-17-11(3-2-10(20)9-23)13(19)4-5-14(17)22-18(12)24/h4-8,10,21,23H,9,20H2,1H3,(H,22,24)/b12-8-/t10-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Hoffmann-La Roche Inc.
| Assay Description
Kinase assays were performed using a recombinant human cyclin E-CDK2 complex. The enzyme was assayed with substrate in the presence of 1uM ATP/[gamma... |
Bioorg Med Chem Lett 14: 913-7 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.009
BindingDB Entry DOI: 10.7270/Q20V8B1V |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50456348

(CHEMBL4212211 | US11001575, Example 683)Show SMILES C[C@@H](N1Cc2ccc(cc2C1=O)-c1nc(NC2CCOCC2)ncc1Cl)C(=O)N[C@H](CO)c1cccc(C)c1 |r| Show InChI InChI=1S/C29H32ClN5O4/c1-17-4-3-5-19(12-17)25(16-36)33-27(37)18(2)35-15-21-7-6-20(13-23(21)28(35)38)26-24(30)14-31-29(34-26)32-22-8-10-39-11-9-22/h3-7,12-14,18,22,25,36H,8-11,15-16H2,1-2H3,(H,33,37)(H,31,32,34)/t18-,25-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of full length human N-terminal MAHHHHHH-tagged ERK2 expressed in Escherichia coli BL21 (DE3) using ATF2-GFP as substrate after 30 mins by... |
J Med Chem 61: 4978-4992 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00421
BindingDB Entry DOI: 10.7270/Q2HT2RZZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data