 Found 366 hits with Last Name = 'ishihara' and Initial = 't'
Found 366 hits with Last Name = 'ishihara' and Initial = 't' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50019290
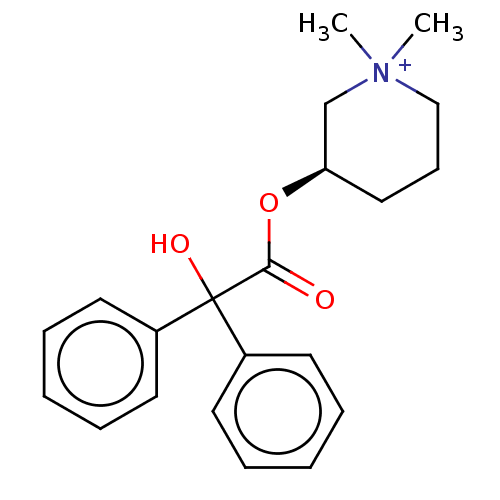
(CHEMBL3289390)Show SMILES [Br-].C[N+]1(C)CCC[C@H](C1)OC(=O)C(O)(c1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C21H26NO3.BrH/c1-22(2)15-9-14-19(16-22)25-20(23)21(24,17-10-5-3-6-11-17)18-12-7-4-8-13-18;/h3-8,10-13,19,24H,9,14-16H2,1-2H3;1H/q+1;/p-1/t19-;/m1./s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Keio University
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human M2R expressed in CHOK1 cells after 2 hrs by scintillation counting analysis |
Bioorg Med Chem 22: 3488-97 (2014)
Article DOI: 10.1016/j.bmc.2014.04.029
BindingDB Entry DOI: 10.7270/Q2VD7111 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50377964
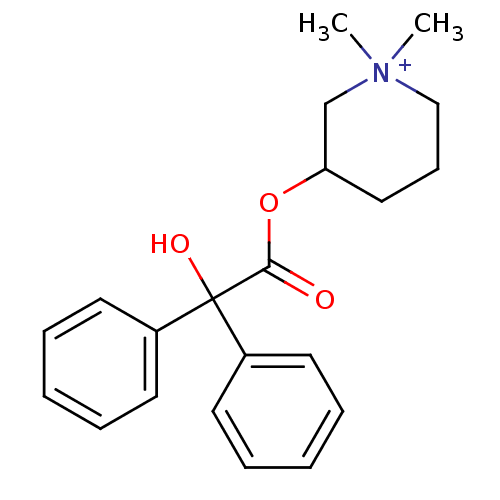
(Cantil | Glycophenylate | MEPENZOLATE BROMIDE | Me...)Show SMILES C[N+]1(C)CCCC(C1)OC(=O)C(O)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C21H26NO3/c1-22(2)15-9-14-19(16-22)25-20(23)21(24,17-10-5-3-6-11-17)18-12-7-4-8-13-18/h3-8,10-13,19,24H,9,14-16H2,1-2H3/q+1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Keio University
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human M2R expressed in CHOK1 cells after 2 hrs by scintillation counting analysis |
Bioorg Med Chem 22: 3488-97 (2014)
Article DOI: 10.1016/j.bmc.2014.04.029
BindingDB Entry DOI: 10.7270/Q2VD7111 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50019290

(CHEMBL3289390)Show SMILES [Br-].C[N+]1(C)CCC[C@H](C1)OC(=O)C(O)(c1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C21H26NO3.BrH/c1-22(2)15-9-14-19(16-22)25-20(23)21(24,17-10-5-3-6-11-17)18-12-7-4-8-13-18;/h3-8,10-13,19,24H,9,14-16H2,1-2H3;1H/q+1;/p-1/t19-;/m1./s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Keio University
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human M3R expressed in CHOK1 cells after 2 hrs by scintillation counting analysis |
Bioorg Med Chem 22: 3488-97 (2014)
Article DOI: 10.1016/j.bmc.2014.04.029
BindingDB Entry DOI: 10.7270/Q2VD7111 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50019289

(CHEMBL3289391)Show SMILES [Br-].C[N+]1(C)CCC[C@@H](C1)OC(=O)C(O)(c1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C21H26NO3.BrH/c1-22(2)15-9-14-19(16-22)25-20(23)21(24,17-10-5-3-6-11-17)18-12-7-4-8-13-18;/h3-8,10-13,19,24H,9,14-16H2,1-2H3;1H/q+1;/p-1/t19-;/m0./s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Keio University
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human M2R expressed in CHOK1 cells after 2 hrs by scintillation counting analysis |
Bioorg Med Chem 22: 3488-97 (2014)
Article DOI: 10.1016/j.bmc.2014.04.029
BindingDB Entry DOI: 10.7270/Q2VD7111 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50377964

(Cantil | Glycophenylate | MEPENZOLATE BROMIDE | Me...)Show SMILES C[N+]1(C)CCCC(C1)OC(=O)C(O)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C21H26NO3/c1-22(2)15-9-14-19(16-22)25-20(23)21(24,17-10-5-3-6-11-17)18-12-7-4-8-13-18/h3-8,10-13,19,24H,9,14-16H2,1-2H3/q+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
PC cid
PC sid
UniChem
Similars
| DrugBank
Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Keio University
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human M3R expressed in CHOK1 cells after 2 hrs by scintillation counting analysis |
Bioorg Med Chem 22: 3488-97 (2014)
Article DOI: 10.1016/j.bmc.2014.04.029
BindingDB Entry DOI: 10.7270/Q2VD7111 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50358251
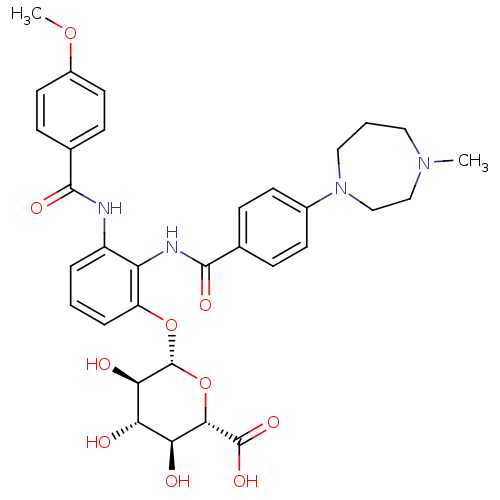
(CHEMBL1922344)Show SMILES COc1ccc(cc1)C(=O)Nc1cccc(O[C@@H]2O[C@@H]([C@@H](O)[C@H](O)[C@H]2O)C(O)=O)c1NC(=O)c1ccc(cc1)N1CCCN(C)CC1 |r| Show InChI InChI=1S/C33H38N4O10/c1-36-15-4-16-37(18-17-36)21-11-7-19(8-12-21)31(42)35-25-23(34-30(41)20-9-13-22(45-2)14-10-20)5-3-6-24(25)46-33-28(40)26(38)27(39)29(47-33)32(43)44/h3,5-14,26-29,33,38-40H,4,15-18H2,1-2H3,(H,34,41)(H,35,42)(H,43,44)/t26-,27-,28+,29-,33+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10A using chromogenic substrate S2222 by dixon plot analysis |
J Med Chem 54: 8051-65 (2011)
Article DOI: 10.1021/jm200868m
BindingDB Entry DOI: 10.7270/Q2D50ND9 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50019289

(CHEMBL3289391)Show SMILES [Br-].C[N+]1(C)CCC[C@@H](C1)OC(=O)C(O)(c1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C21H26NO3.BrH/c1-22(2)15-9-14-19(16-22)25-20(23)21(24,17-10-5-3-6-11-17)18-12-7-4-8-13-18;/h3-8,10-13,19,24H,9,14-16H2,1-2H3;1H/q+1;/p-1/t19-;/m0./s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Keio University
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human M3R expressed in CHOK1 cells after 2 hrs by scintillation counting analysis |
Bioorg Med Chem 22: 3488-97 (2014)
Article DOI: 10.1016/j.bmc.2014.04.029
BindingDB Entry DOI: 10.7270/Q2VD7111 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50358252
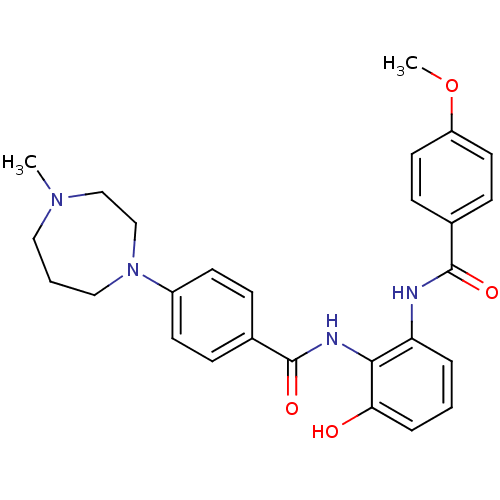
(CHEMBL1922235)Show SMILES COc1ccc(cc1)C(=O)Nc1cccc(O)c1NC(=O)c1ccc(cc1)N1CCCN(C)CC1 Show InChI InChI=1S/C27H30N4O4/c1-30-15-4-16-31(18-17-30)21-11-7-19(8-12-21)27(34)29-25-23(5-3-6-24(25)32)28-26(33)20-9-13-22(35-2)14-10-20/h3,5-14,32H,4,15-18H2,1-2H3,(H,28,33)(H,29,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10A using chromogenic substrate S2222 by dixon plot analysis |
J Med Chem 54: 8051-65 (2011)
Article DOI: 10.1021/jm200868m
BindingDB Entry DOI: 10.7270/Q2D50ND9 |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50358252

(CHEMBL1922235)Show SMILES COc1ccc(cc1)C(=O)Nc1cccc(O)c1NC(=O)c1ccc(cc1)N1CCCN(C)CC1 Show InChI InChI=1S/C27H30N4O4/c1-30-15-4-16-31(18-17-30)21-11-7-19(8-12-21)27(34)29-25-23(5-3-6-24(25)32)28-26(33)20-9-13-22(35-2)14-10-20/h3,5-14,32H,4,15-18H2,1-2H3,(H,28,33)(H,29,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of human plasma kallikrein using chromogenic substrate S2302 by dixon plot analysis |
J Med Chem 54: 8051-65 (2011)
Article DOI: 10.1021/jm200868m
BindingDB Entry DOI: 10.7270/Q2D50ND9 |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50358251

(CHEMBL1922344)Show SMILES COc1ccc(cc1)C(=O)Nc1cccc(O[C@@H]2O[C@@H]([C@@H](O)[C@H](O)[C@H]2O)C(O)=O)c1NC(=O)c1ccc(cc1)N1CCCN(C)CC1 |r| Show InChI InChI=1S/C33H38N4O10/c1-36-15-4-16-37(18-17-36)21-11-7-19(8-12-21)31(42)35-25-23(34-30(41)20-9-13-22(45-2)14-10-20)5-3-6-24(25)46-33-28(40)26(38)27(39)29(47-33)32(43)44/h3,5-14,26-29,33,38-40H,4,15-18H2,1-2H3,(H,34,41)(H,35,42)(H,43,44)/t26-,27-,28+,29-,33+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of human plasma kallikrein using chromogenic substrate S2302 by dixon plot analysis |
J Med Chem 54: 8051-65 (2011)
Article DOI: 10.1021/jm200868m
BindingDB Entry DOI: 10.7270/Q2D50ND9 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50358251

(CHEMBL1922344)Show SMILES COc1ccc(cc1)C(=O)Nc1cccc(O[C@@H]2O[C@@H]([C@@H](O)[C@H](O)[C@H]2O)C(O)=O)c1NC(=O)c1ccc(cc1)N1CCCN(C)CC1 |r| Show InChI InChI=1S/C33H38N4O10/c1-36-15-4-16-37(18-17-36)21-11-7-19(8-12-21)31(42)35-25-23(34-30(41)20-9-13-22(45-2)14-10-20)5-3-6-24(25)46-33-28(40)26(38)27(39)29(47-33)32(43)44/h3,5-14,26-29,33,38-40H,4,15-18H2,1-2H3,(H,34,41)(H,35,42)(H,43,44)/t26-,27-,28+,29-,33+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin using chromogenic substrate S2238 by dixon plot analysis |
J Med Chem 54: 8051-65 (2011)
Article DOI: 10.1021/jm200868m
BindingDB Entry DOI: 10.7270/Q2D50ND9 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50358252

(CHEMBL1922235)Show SMILES COc1ccc(cc1)C(=O)Nc1cccc(O)c1NC(=O)c1ccc(cc1)N1CCCN(C)CC1 Show InChI InChI=1S/C27H30N4O4/c1-30-15-4-16-31(18-17-30)21-11-7-19(8-12-21)27(34)29-25-23(5-3-6-24(25)32)28-26(33)20-9-13-22(35-2)14-10-20/h3,5-14,32H,4,15-18H2,1-2H3,(H,28,33)(H,29,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin using chromogenic substrate S2238 by dixon plot analysis |
J Med Chem 54: 8051-65 (2011)
Article DOI: 10.1021/jm200868m
BindingDB Entry DOI: 10.7270/Q2D50ND9 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50042002
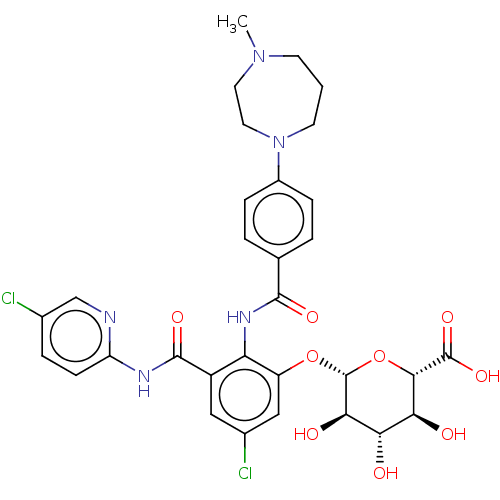
(CHEMBL3359576)Show SMILES CN1CCCN(CC1)c1ccc(cc1)C(=O)Nc1c(O[C@@H]2O[C@@H]([C@@H](O)[C@H](O)[C@H]2O)C(O)=O)cc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 |r| Show InChI InChI=1S/C31H33Cl2N5O9/c1-37-9-2-10-38(12-11-37)19-6-3-16(4-7-19)28(42)36-23-20(29(43)35-22-8-5-17(32)15-34-22)13-18(33)14-21(23)46-31-26(41)24(39)25(40)27(47-31)30(44)45/h3-8,13-15,24-27,31,39-41H,2,9-12H2,1H3,(H,36,42)(H,44,45)(H,34,35,43)/t24-,25-,26+,27-,31+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a using S-2222 chromogenic substrate assessed as hydrolysis by microplate reader |
Bioorg Med Chem 23: 277-89 (2015)
Article DOI: 10.1016/j.bmc.2014.11.042
BindingDB Entry DOI: 10.7270/Q2P55Q4X |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12657
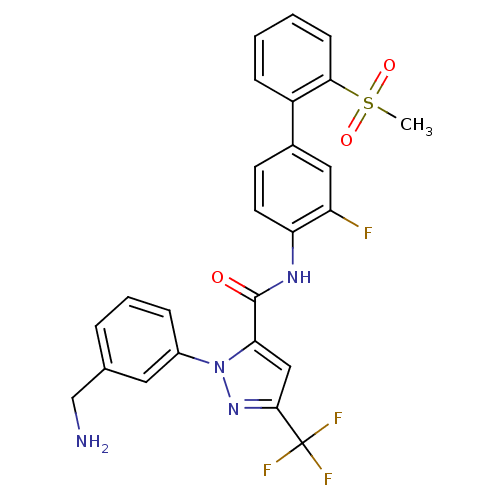
(1-[3-(Aminomethyl)phenyl]-N-[3-fluoro-2-(methylsul...)Show SMILES CS(=O)(=O)c1ccccc1-c1ccc(NC(=O)c2cc(nn2-c2cccc(CN)c2)C(F)(F)F)c(F)c1 Show InChI InChI=1S/C25H20F4N4O3S/c1-37(35,36)22-8-3-2-7-18(22)16-9-10-20(19(26)12-16)31-24(34)21-13-23(25(27,28)29)32-33(21)17-6-4-5-15(11-17)14-30/h2-13H,14,30H2,1H3,(H,31,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a using S-2222 chromogenic substrate assessed as hydrolysis by microplate reader |
Bioorg Med Chem 23: 277-89 (2015)
Article DOI: 10.1016/j.bmc.2014.11.042
BindingDB Entry DOI: 10.7270/Q2P55Q4X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bombesin receptor subtype-3
(Homo sapiens (Human)) | BDBM428851
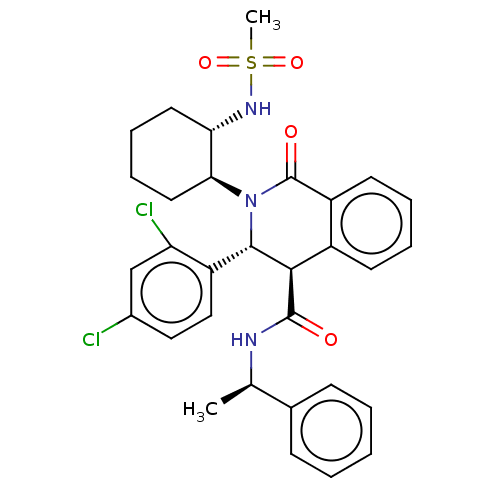
(US10532048, Example 236)Show SMILES C[C@@H](NC(=O)[C@H]1[C@@H](N([C@H]2CCCC[C@@H]2NS(C)(=O)=O)C(=O)c2ccccc12)c1ccc(Cl)cc1Cl)c1ccccc1 |r| Show InChI InChI=1S/C31H33Cl2N3O4S/c1-19(20-10-4-3-5-11-20)34-30(37)28-22-12-6-7-13-23(22)31(38)36(29(28)24-17-16-21(32)18-25(24)33)27-15-9-8-14-26(27)35-41(2,39)40/h3-7,10-13,16-19,26-29,35H,8-9,14-15H2,1-2H3,(H,34,37)/t19-,26+,27+,28-,29+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Seldar Pharma Inc.
US Patent
| Assay Description
A BB2 receptor binding test was carried out using a membrane sample prepared from a human prostate cancer-derived PC-3 cell. The PC-3 cell was cultur... |
US Patent US10532048 (2020)
BindingDB Entry DOI: 10.7270/Q22R3V1R |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50042001
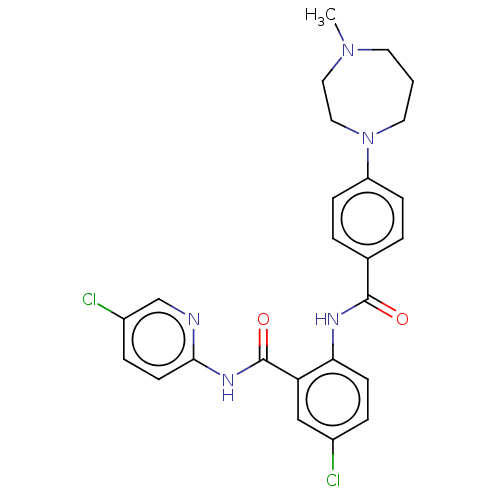
(CHEMBL3359575)Show SMILES Cl.CN1CCCN(CC1)c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C25H25Cl2N5O2.ClH/c1-31-11-2-12-32(14-13-31)20-7-3-17(4-8-20)24(33)29-22-9-5-18(26)15-21(22)25(34)30-23-10-6-19(27)16-28-23;/h3-10,15-16H,2,11-14H2,1H3,(H,29,33)(H,28,30,34);1H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a using S-2222 chromogenic substrate assessed as hydrolysis by microplate reader |
Bioorg Med Chem 23: 277-89 (2015)
Article DOI: 10.1016/j.bmc.2014.11.042
BindingDB Entry DOI: 10.7270/Q2P55Q4X |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50035155
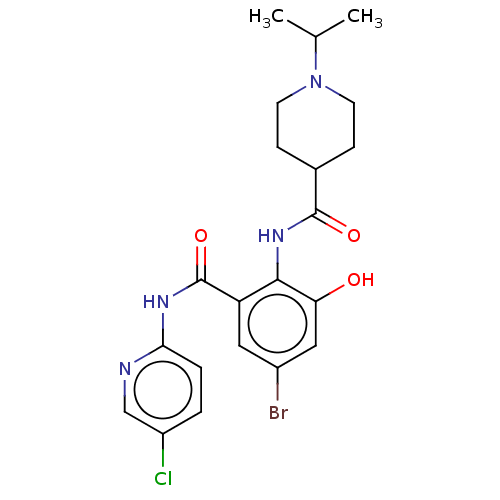
(CHEMBL3354239)Show SMILES CC(C)N1CCC(CC1)C(=O)Nc1c(O)cc(Br)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C21H24BrClN4O3/c1-12(2)27-7-5-13(6-8-27)20(29)26-19-16(9-14(22)10-17(19)28)21(30)25-18-4-3-15(23)11-24-18/h3-4,9-13,28H,5-8H2,1-2H3,(H,26,29)(H,24,25,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of purified factor 10a (unknown origin) using chromogenic substrate S-2222 by spectrophotometry |
Bioorg Med Chem 22: 6324-32 (2014)
Article DOI: 10.1016/j.bmc.2014.09.059
BindingDB Entry DOI: 10.7270/Q23X888S |
More data for this
Ligand-Target Pair | |
Bombesin receptor subtype-3
(Homo sapiens (Human)) | BDBM428855
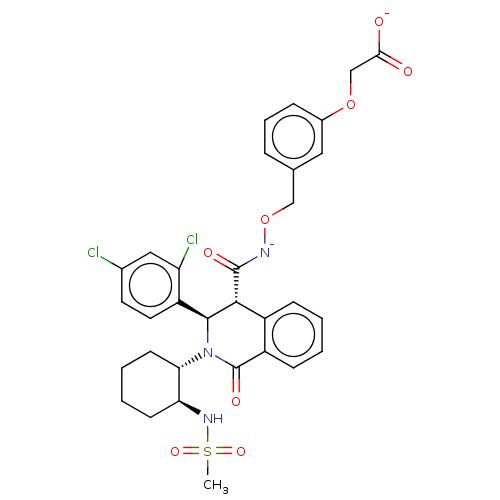
(US10532048, Example 631)Show SMILES CS(=O)(=O)N[C@H]1CCCC[C@@H]1N1[C@H]([C@H](C(=O)[N-]OCc2cccc(OCC([O-])=O)c2)c2ccccc2C1=O)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C32H33Cl2N3O8S/c1-46(42,43)36-26-11-4-5-12-27(26)37-30(24-14-13-20(33)16-25(24)34)29(22-9-2-3-10-23(22)32(37)41)31(40)35-45-17-19-7-6-8-21(15-19)44-18-28(38)39/h2-3,6-10,13-16,26-27,29-30,36H,4-5,11-12,17-18H2,1H3,(H2,35,38,39,40)/p-2/t26-,27-,29+,30-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Seldar Pharma Inc.
US Patent
| Assay Description
A BB2 receptor binding test was carried out using a membrane sample prepared from a human prostate cancer-derived PC-3 cell. The PC-3 cell was cultur... |
US Patent US10532048 (2020)
BindingDB Entry DOI: 10.7270/Q22R3V1R |
More data for this
Ligand-Target Pair | |
Bombesin receptor subtype-3
(Homo sapiens (Human)) | BDBM428852

(US10532048, Example 542)Show SMILES CS(=O)(=O)N[C@H]1CCCC[C@@H]1N1[C@H]([C@H](C(=O)[N-]OCc2cc(cs2)C([O-])=O)c2ccccc2C1=O)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C29H29Cl2N3O7S2/c1-43(39,40)33-23-8-4-5-9-24(23)34-26(21-11-10-17(30)13-22(21)31)25(19-6-2-3-7-20(19)28(34)36)27(35)32-41-14-18-12-16(15-42-18)29(37)38/h2-3,6-7,10-13,15,23-26,33H,4-5,8-9,14H2,1H3,(H2,32,35,37,38)/p-2/t23-,24-,25+,26-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Seldar Pharma Inc.
US Patent
| Assay Description
A BB2 receptor binding test was carried out using a membrane sample prepared from a human prostate cancer-derived PC-3 cell. The PC-3 cell was cultur... |
US Patent US10532048 (2020)
BindingDB Entry DOI: 10.7270/Q22R3V1R |
More data for this
Ligand-Target Pair | |
Bombesin receptor subtype-3
(Homo sapiens (Human)) | BDBM428853
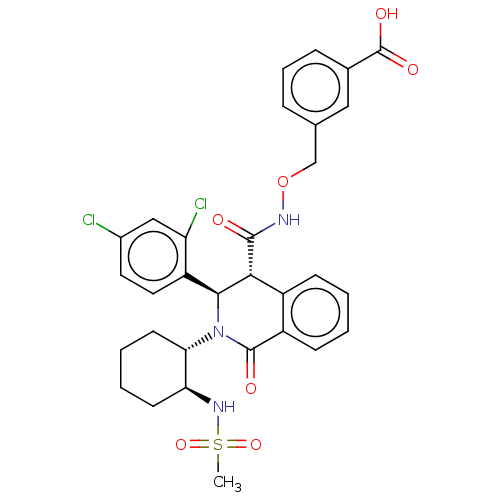
(US10532048, Example 560)Show SMILES CS(=O)(=O)N[C@H]1CCCC[C@@H]1N1[C@H]([C@H](C(=O)NOCc2cccc(c2)C(O)=O)c2ccccc2C1=O)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C31H31Cl2N3O7S/c1-44(41,42)35-25-11-4-5-12-26(25)36-28(23-14-13-20(32)16-24(23)33)27(21-9-2-3-10-22(21)30(36)38)29(37)34-43-17-18-7-6-8-19(15-18)31(39)40/h2-3,6-10,13-16,25-28,35H,4-5,11-12,17H2,1H3,(H,34,37)(H,39,40)/t25-,26-,27+,28-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Seldar Pharma Inc.
US Patent
| Assay Description
A BB2 receptor binding test was carried out using a membrane sample prepared from a human prostate cancer-derived PC-3 cell. The PC-3 cell was cultur... |
US Patent US10532048 (2020)
BindingDB Entry DOI: 10.7270/Q22R3V1R |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50035154
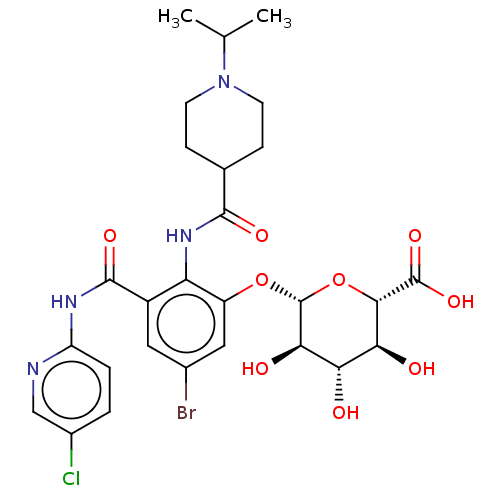
(CHEMBL3354240)Show SMILES CC(C)N1CCC(CC1)C(=O)Nc1c(O[C@@H]2O[C@@H]([C@@H](O)[C@H](O)[C@H]2O)C(O)=O)cc(Br)cc1C(=O)Nc1ccc(Cl)cn1 |r| Show InChI InChI=1S/C27H32BrClN4O9/c1-12(2)33-7-5-13(6-8-33)24(37)32-19-16(25(38)31-18-4-3-15(29)11-30-18)9-14(28)10-17(19)41-27-22(36)20(34)21(35)23(42-27)26(39)40/h3-4,9-13,20-23,27,34-36H,5-8H2,1-2H3,(H,32,37)(H,39,40)(H,30,31,38)/t20-,21-,22+,23-,27+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of purified factor 10a (unknown origin) using chromogenic substrate S-2222 by spectrophotometry |
Bioorg Med Chem 22: 6324-32 (2014)
Article DOI: 10.1016/j.bmc.2014.09.059
BindingDB Entry DOI: 10.7270/Q23X888S |
More data for this
Ligand-Target Pair | |
Bombesin receptor subtype-3
(Homo sapiens (Human)) | BDBM428854
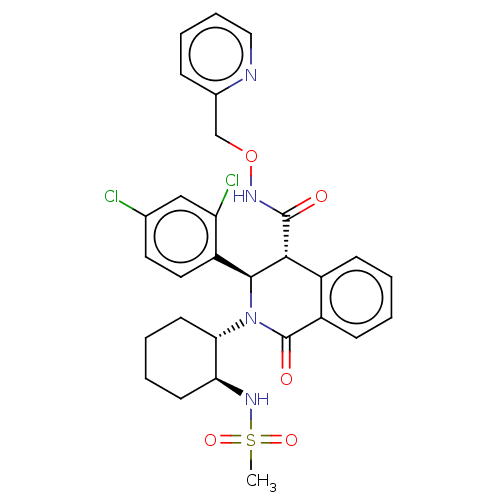
(US10532048, Example 589)Show SMILES CS(=O)(=O)N[C@H]1CCCC[C@@H]1N1[C@H]([C@H](C(=O)NOCc2ccccn2)c2ccccc2C1=O)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C29H30Cl2N4O5S/c1-41(38,39)34-24-11-4-5-12-25(24)35-27(22-14-13-18(30)16-23(22)31)26(20-9-2-3-10-21(20)29(35)37)28(36)33-40-17-19-8-6-7-15-32-19/h2-3,6-10,13-16,24-27,34H,4-5,11-12,17H2,1H3,(H,33,36)/t24-,25-,26+,27-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Seldar Pharma Inc.
US Patent
| Assay Description
A BB2 receptor binding test was carried out using a membrane sample prepared from a human prostate cancer-derived PC-3 cell. The PC-3 cell was cultur... |
US Patent US10532048 (2020)
BindingDB Entry DOI: 10.7270/Q22R3V1R |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50035158

(CHEMBL3354236)Show SMILES CC(C)N1CCC(CC1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C21H24Cl2N4O2/c1-13(2)27-9-7-14(8-10-27)20(28)25-18-5-3-15(22)11-17(18)21(29)26-19-6-4-16(23)12-24-19/h3-6,11-14H,7-10H2,1-2H3,(H,25,28)(H,24,26,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of purified factor 10a (unknown origin) using chromogenic substrate S-2222 by spectrophotometry |
Bioorg Med Chem 22: 6324-32 (2014)
Article DOI: 10.1016/j.bmc.2014.09.059
BindingDB Entry DOI: 10.7270/Q23X888S |
More data for this
Ligand-Target Pair | |
Bombesin receptor subtype-3
(Homo sapiens (Human)) | BDBM428856
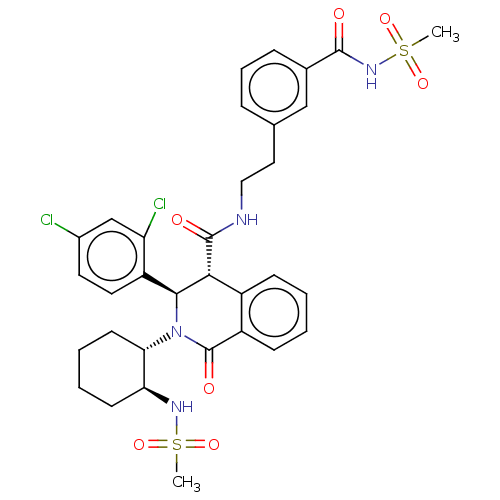
(US10532048, Example 700)Show SMILES CS(=O)(=O)N[C@H]1CCCC[C@@H]1N1[C@H]([C@H](C(=O)NCCc2cccc(c2)C(=O)NS(C)(=O)=O)c2ccccc2C1=O)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C33H36Cl2N4O7S2/c1-47(43,44)37-27-12-5-6-13-28(27)39-30(25-15-14-22(34)19-26(25)35)29(23-10-3-4-11-24(23)33(39)42)32(41)36-17-16-20-8-7-9-21(18-20)31(40)38-48(2,45)46/h3-4,7-11,14-15,18-19,27-30,37H,5-6,12-13,16-17H2,1-2H3,(H,36,41)(H,38,40)/t27-,28-,29+,30-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Seldar Pharma Inc.
US Patent
| Assay Description
A BB2 receptor binding test was carried out using a membrane sample prepared from a human prostate cancer-derived PC-3 cell. The PC-3 cell was cultur... |
US Patent US10532048 (2020)
BindingDB Entry DOI: 10.7270/Q22R3V1R |
More data for this
Ligand-Target Pair | |
Bombesin receptor subtype-3
(Homo sapiens (Human)) | BDBM428859
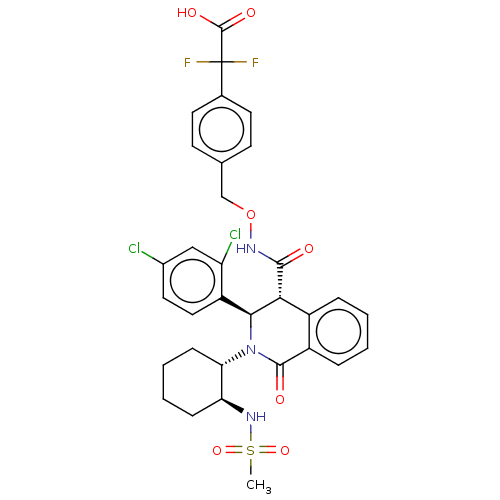
(US10532048, Example 712)Show SMILES CS(=O)(=O)N[C@H]1CCCC[C@@H]1N1[C@H]([C@H](C(=O)NOCc2ccc(cc2)C(F)(F)C(O)=O)c2ccccc2C1=O)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C32H31Cl2F2N3O7S/c1-47(44,45)38-25-8-4-5-9-26(25)39-28(23-15-14-20(33)16-24(23)34)27(21-6-2-3-7-22(21)30(39)41)29(40)37-46-17-18-10-12-19(13-11-18)32(35,36)31(42)43/h2-3,6-7,10-16,25-28,38H,4-5,8-9,17H2,1H3,(H,37,40)(H,42,43)/t25-,26-,27+,28-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Seldar Pharma Inc.
US Patent
| Assay Description
A BB2 receptor binding test was carried out using a membrane sample prepared from a human prostate cancer-derived PC-3 cell. The PC-3 cell was cultur... |
US Patent US10532048 (2020)
BindingDB Entry DOI: 10.7270/Q22R3V1R |
More data for this
Ligand-Target Pair | |
Bombesin receptor subtype-3
(Homo sapiens (Human)) | BDBM428860
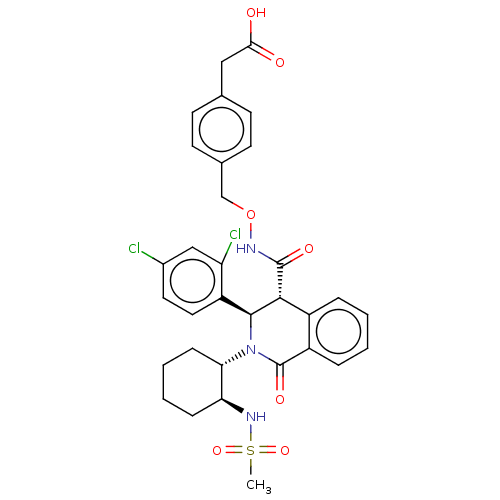
(US10532048, Example 856)Show SMILES CS(=O)(=O)N[C@H]1CCCC[C@@H]1N1[C@H]([C@H](C(=O)NOCc2ccc(CC(O)=O)cc2)c2ccccc2C1=O)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C32H33Cl2N3O7S/c1-45(42,43)36-26-8-4-5-9-27(26)37-30(24-15-14-21(33)17-25(24)34)29(22-6-2-3-7-23(22)32(37)41)31(40)35-44-18-20-12-10-19(11-13-20)16-28(38)39/h2-3,6-7,10-15,17,26-27,29-30,36H,4-5,8-9,16,18H2,1H3,(H,35,40)(H,38,39)/t26-,27-,29+,30-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Seldar Pharma Inc.
US Patent
| Assay Description
A BB2 receptor binding test was carried out using a membrane sample prepared from a human prostate cancer-derived PC-3 cell. The PC-3 cell was cultur... |
US Patent US10532048 (2020)
BindingDB Entry DOI: 10.7270/Q22R3V1R |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50041996
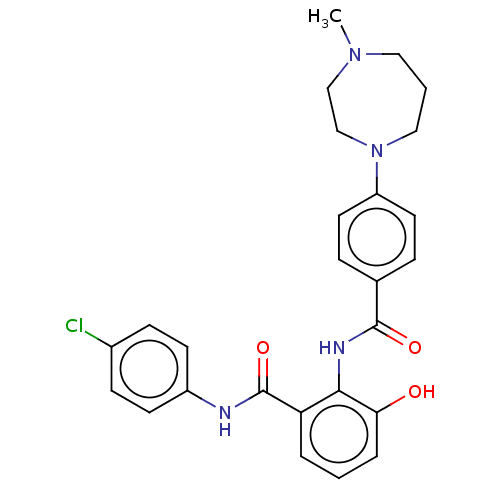
(CHEMBL3359570)Show SMILES Cl.CN1CCCN(CC1)c1ccc(cc1)C(=O)Nc1c(O)cccc1C(=O)Nc1ccc(Cl)cc1 Show InChI InChI=1S/C26H27ClN4O3/c1-30-14-3-15-31(17-16-30)21-12-6-18(7-13-21)25(33)29-24-22(4-2-5-23(24)32)26(34)28-20-10-8-19(27)9-11-20/h2,4-13,32H,3,14-17H2,1H3,(H,28,34)(H,29,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a using S-2222 chromogenic substrate assessed as hydrolysis by microplate reader |
Bioorg Med Chem 23: 277-89 (2015)
Article DOI: 10.1016/j.bmc.2014.11.042
BindingDB Entry DOI: 10.7270/Q2P55Q4X |
More data for this
Ligand-Target Pair | |
Bombesin receptor subtype-3
(Homo sapiens (Human)) | BDBM428857
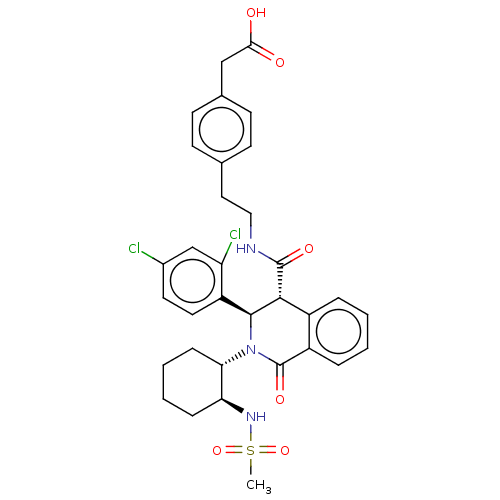
(US10532048, Example 701)Show SMILES CS(=O)(=O)N[C@H]1CCCC[C@@H]1N1[C@H]([C@H](C(=O)NCCc2ccc(CC(O)=O)cc2)c2ccccc2C1=O)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C33H35Cl2N3O6S/c1-45(43,44)37-27-8-4-5-9-28(27)38-31(25-15-14-22(34)19-26(25)35)30(23-6-2-3-7-24(23)33(38)42)32(41)36-17-16-20-10-12-21(13-11-20)18-29(39)40/h2-3,6-7,10-15,19,27-28,30-31,37H,4-5,8-9,16-18H2,1H3,(H,36,41)(H,39,40)/t27-,28-,30+,31-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Seldar Pharma Inc.
US Patent
| Assay Description
A BB2 receptor binding test was carried out using a membrane sample prepared from a human prostate cancer-derived PC-3 cell. The PC-3 cell was cultur... |
US Patent US10532048 (2020)
BindingDB Entry DOI: 10.7270/Q22R3V1R |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50209900
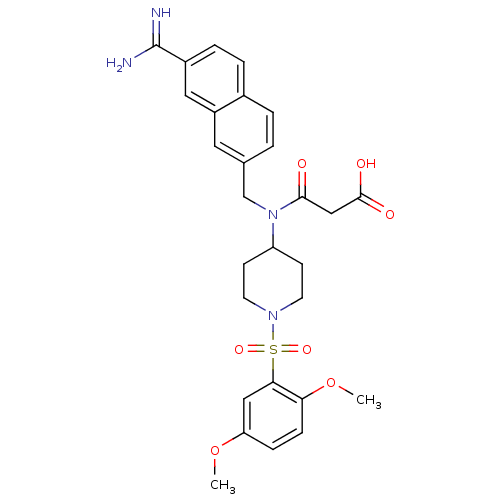
(3-(({7-[amino(imino)methyl]-2-naphthyl}methyl)-{1-...)Show SMILES COc1ccc(OC)c(c1)S(=O)(=O)N1CCC(CC1)N(Cc1ccc2ccc(cc2c1)C(N)=N)C(=O)CC(O)=O Show InChI InChI=1S/C28H32N4O7S/c1-38-23-7-8-24(39-2)25(15-23)40(36,37)31-11-9-22(10-12-31)32(26(33)16-27(34)35)17-18-3-4-19-5-6-20(28(29)30)14-21(19)13-18/h3-8,13-15,22H,9-12,16-17H2,1-2H3,(H3,29,30)(H,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem 15: 4175-92 (2007)
Article DOI: 10.1016/j.bmc.2007.03.066
BindingDB Entry DOI: 10.7270/Q27H1J90 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50035157
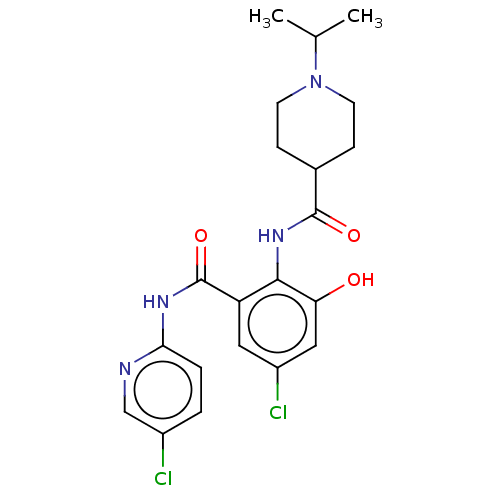
(CHEMBL3354237)Show SMILES CC(C)N1CCC(CC1)C(=O)Nc1c(O)cc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C21H24Cl2N4O3/c1-12(2)27-7-5-13(6-8-27)20(29)26-19-16(9-15(23)10-17(19)28)21(30)25-18-4-3-14(22)11-24-18/h3-4,9-13,28H,5-8H2,1-2H3,(H,26,29)(H,24,25,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of purified factor 10a (unknown origin) using chromogenic substrate S-2222 by spectrophotometry |
Bioorg Med Chem 22: 6324-32 (2014)
Article DOI: 10.1016/j.bmc.2014.09.059
BindingDB Entry DOI: 10.7270/Q23X888S |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50042000
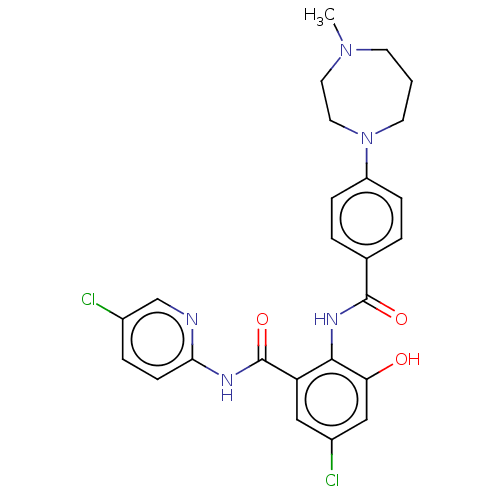
(CHEMBL3359574)Show SMILES Cl.CN1CCCN(CC1)c1ccc(cc1)C(=O)Nc1c(O)cc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C25H25Cl2N5O3/c1-31-9-2-10-32(12-11-31)19-6-3-16(4-7-19)24(34)30-23-20(13-18(27)14-21(23)33)25(35)29-22-8-5-17(26)15-28-22/h3-8,13-15,33H,2,9-12H2,1H3,(H,30,34)(H,28,29,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a using S-2222 chromogenic substrate assessed as hydrolysis by microplate reader |
Bioorg Med Chem 23: 277-89 (2015)
Article DOI: 10.1016/j.bmc.2014.11.042
BindingDB Entry DOI: 10.7270/Q2P55Q4X |
More data for this
Ligand-Target Pair | |
Bombesin receptor subtype-3
(Homo sapiens (Human)) | BDBM428858
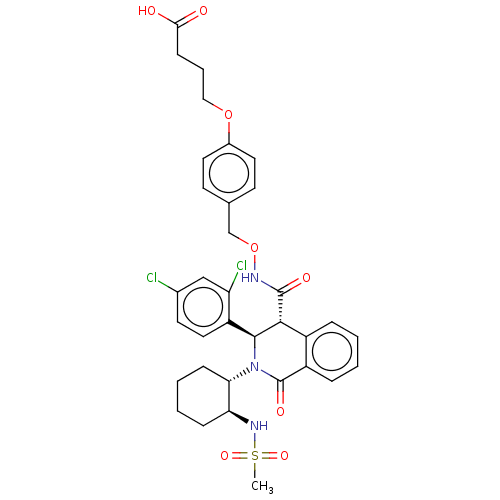
(US10532048, Example 709)Show SMILES CS(=O)(=O)N[C@H]1CCCC[C@@H]1N1[C@H]([C@H](C(=O)NOCc2ccc(OCCCC(O)=O)cc2)c2ccccc2C1=O)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C34H37Cl2N3O8S/c1-48(44,45)38-28-9-4-5-10-29(28)39-32(26-17-14-22(35)19-27(26)36)31(24-7-2-3-8-25(24)34(39)43)33(42)37-47-20-21-12-15-23(16-13-21)46-18-6-11-30(40)41/h2-3,7-8,12-17,19,28-29,31-32,38H,4-6,9-11,18,20H2,1H3,(H,37,42)(H,40,41)/t28-,29-,31+,32-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Seldar Pharma Inc.
US Patent
| Assay Description
A BB2 receptor binding test was carried out using a membrane sample prepared from a human prostate cancer-derived PC-3 cell. The PC-3 cell was cultur... |
US Patent US10532048 (2020)
BindingDB Entry DOI: 10.7270/Q22R3V1R |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50209891

(CHEMBL390266 | [(((E)-3-{3-[amino(imino)methyl]phe...)Show SMILES COc1ccc(OC)c(c1)S(=O)(=O)N1CCC(CC1)N(C\C=C\c1cccc(c1)C(N)=N)S(=O)(=O)CC(O)=O Show InChI InChI=1S/C25H32N4O8S2/c1-36-21-8-9-22(37-2)23(16-21)39(34,35)28-13-10-20(11-14-28)29(38(32,33)17-24(30)31)12-4-6-18-5-3-7-19(15-18)25(26)27/h3-9,15-16,20H,10-14,17H2,1-2H3,(H3,26,27)(H,30,31)/b6-4+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem 15: 4175-92 (2007)
Article DOI: 10.1016/j.bmc.2007.03.066
BindingDB Entry DOI: 10.7270/Q27H1J90 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50209893
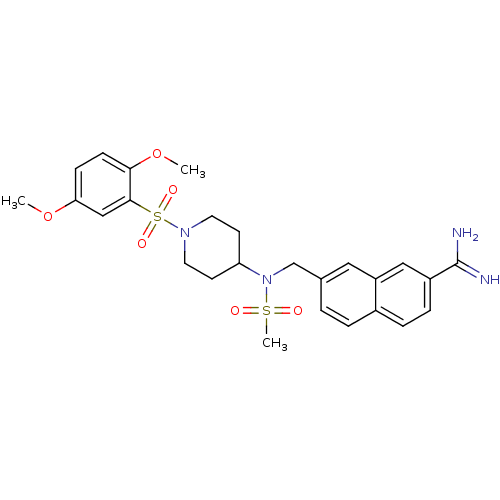
(7-({[1-(2,5-dimethoxy-benzenesulfonyl)-piperidin-4...)Show SMILES COc1ccc(OC)c(c1)S(=O)(=O)N1CCC(CC1)N(Cc1ccc2ccc(cc2c1)C(N)=N)S(C)(=O)=O Show InChI InChI=1S/C26H32N4O6S2/c1-35-23-8-9-24(36-2)25(16-23)38(33,34)29-12-10-22(11-13-29)30(37(3,31)32)17-18-4-5-19-6-7-20(26(27)28)15-21(19)14-18/h4-9,14-16,22H,10-13,17H2,1-3H3,(H3,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem 15: 4175-92 (2007)
Article DOI: 10.1016/j.bmc.2007.03.066
BindingDB Entry DOI: 10.7270/Q27H1J90 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50209895
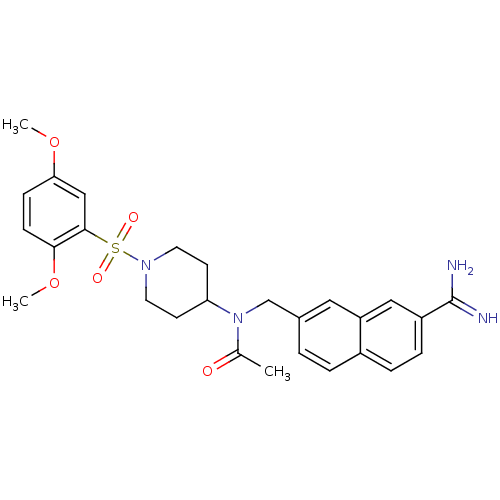
(CHEMBL244221 | N-({7-[amino(imino)methyl]-2-naphth...)Show SMILES COc1ccc(OC)c(c1)S(=O)(=O)N1CCC(CC1)N(Cc1ccc2ccc(cc2c1)C(N)=N)C(C)=O Show InChI InChI=1S/C27H32N4O5S/c1-18(32)31(17-19-4-5-20-6-7-21(27(28)29)15-22(20)14-19)23-10-12-30(13-11-23)37(33,34)26-16-24(35-2)8-9-25(26)36-3/h4-9,14-16,23H,10-13,17H2,1-3H3,(H3,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem 15: 4175-92 (2007)
Article DOI: 10.1016/j.bmc.2007.03.066
BindingDB Entry DOI: 10.7270/Q27H1J90 |
More data for this
Ligand-Target Pair | |
Bombesin receptor subtype-3
(Homo sapiens (Human)) | BDBM428841
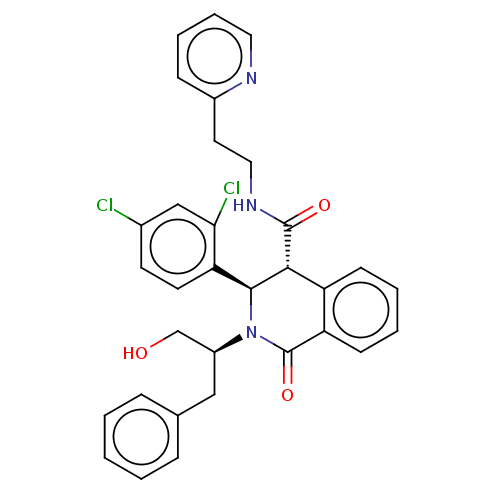
(US10532048, Example 61)Show SMILES OC[C@H](Cc1ccccc1)N1[C@H]([C@H](C(=O)NCCc2ccccn2)c2ccccc2C1=O)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C32H29Cl2N3O3/c33-22-13-14-27(28(34)19-22)30-29(31(39)36-17-15-23-10-6-7-16-35-23)25-11-4-5-12-26(25)32(40)37(30)24(20-38)18-21-8-2-1-3-9-21/h1-14,16,19,24,29-30,38H,15,17-18,20H2,(H,36,39)/t24-,29+,30-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 12.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Seldar Pharma Inc.
US Patent
| Assay Description
A BB2 receptor binding test was carried out using a membrane sample prepared from a human prostate cancer-derived PC-3 cell. The PC-3 cell was cultur... |
US Patent US10532048 (2020)
BindingDB Entry DOI: 10.7270/Q22R3V1R |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX15
(Rattus norvegicus) | BDBM50011938
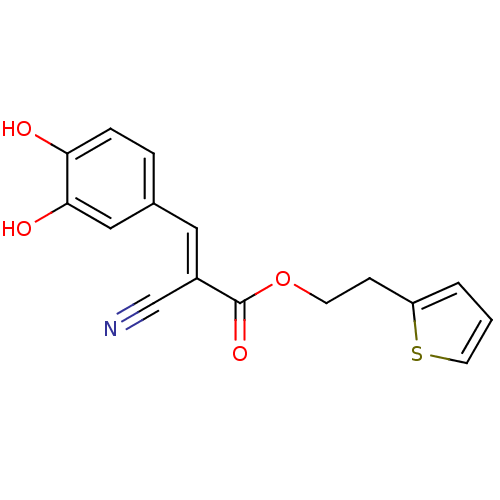
(2-Cyano-3-(3,4-dihydroxy-phenyl)-acrylic acid 2-th...)Show InChI InChI=1S/C16H13NO4S/c17-10-12(8-11-3-4-14(18)15(19)9-11)16(20)21-6-5-13-2-1-7-22-13/h1-4,7-9,18-19H,5-6H2/b12-8+ | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against 12-lipoxygenase in rat platelet rich plasma |
J Med Chem 34: 1503-5 (1991)
BindingDB Entry DOI: 10.7270/Q22Z14G6 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50209904
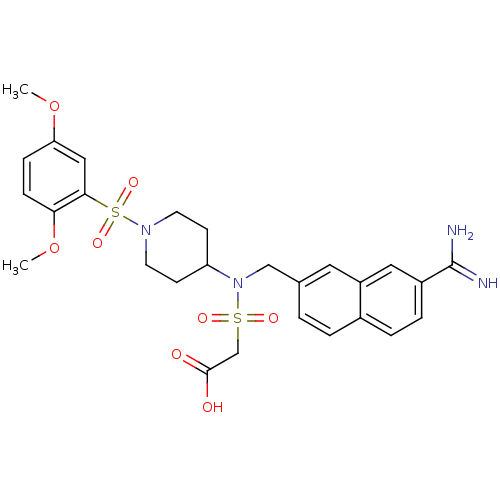
(CHEMBL244436 | [(({7-[amino(imino)methyl]-2-naphth...)Show SMILES COc1ccc(OC)c(c1)S(=O)(=O)N1CCC(CC1)N(Cc1ccc2ccc(cc2c1)C(N)=N)S(=O)(=O)CC(O)=O Show InChI InChI=1S/C27H32N4O8S2/c1-38-23-7-8-24(39-2)25(15-23)41(36,37)30-11-9-22(10-12-30)31(40(34,35)17-26(32)33)16-18-3-4-19-5-6-20(27(28)29)14-21(19)13-18/h3-8,13-15,22H,9-12,16-17H2,1-2H3,(H3,28,29)(H,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem 15: 4175-92 (2007)
Article DOI: 10.1016/j.bmc.2007.03.066
BindingDB Entry DOI: 10.7270/Q27H1J90 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50209899
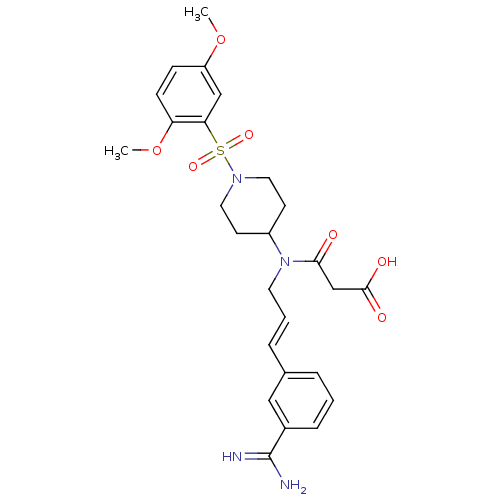
(3-(((E)-3-{3-[amino(imino)methyl]phenyl}prop-2-en-...)Show SMILES COc1ccc(OC)c(c1)S(=O)(=O)N1CCC(CC1)N(C\C=C\c1cccc(c1)C(N)=N)C(=O)CC(O)=O Show InChI InChI=1S/C26H32N4O7S/c1-36-21-8-9-22(37-2)23(16-21)38(34,35)29-13-10-20(11-14-29)30(24(31)17-25(32)33)12-4-6-18-5-3-7-19(15-18)26(27)28/h3-9,15-16,20H,10-14,17H2,1-2H3,(H3,27,28)(H,32,33)/b6-4+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem 15: 4175-92 (2007)
Article DOI: 10.1016/j.bmc.2007.03.066
BindingDB Entry DOI: 10.7270/Q27H1J90 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50035156
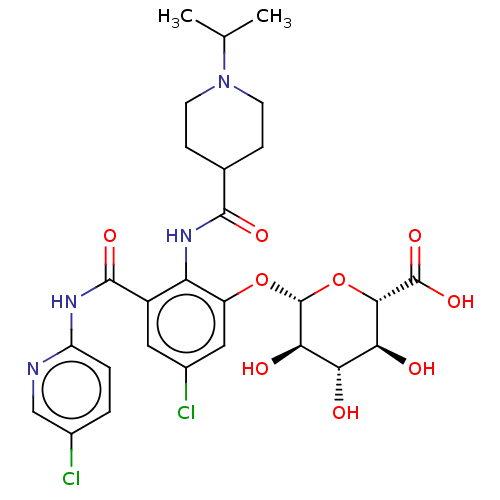
(CHEMBL3354238)Show SMILES CC(C)N1CCC(CC1)C(=O)Nc1c(O[C@@H]2O[C@@H]([C@@H](O)[C@H](O)[C@H]2O)C(O)=O)cc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 |r| Show InChI InChI=1S/C27H32Cl2N4O9/c1-12(2)33-7-5-13(6-8-33)24(37)32-19-16(25(38)31-18-4-3-14(28)11-30-18)9-15(29)10-17(19)41-27-22(36)20(34)21(35)23(42-27)26(39)40/h3-4,9-13,20-23,27,34-36H,5-8H2,1-2H3,(H,32,37)(H,39,40)(H,30,31,38)/t20-,21-,22+,23-,27+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of purified factor 10a (unknown origin) using chromogenic substrate S-2222 by spectrophotometry |
Bioorg Med Chem 22: 6324-32 (2014)
Article DOI: 10.1016/j.bmc.2014.09.059
BindingDB Entry DOI: 10.7270/Q23X888S |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX15
(Rattus norvegicus) | BDBM50009001

(5,6,7-Trihydroxyflavone | 5,6,7-trihydroxy-2-pheny...)Show InChI InChI=1S/C15H10O5/c16-9-6-11(8-4-2-1-3-5-8)20-12-7-10(17)14(18)15(19)13(9)12/h1-7,17-19H | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against 12-lipoxygenase in rat platelet rich plasma |
J Med Chem 34: 1503-5 (1991)
BindingDB Entry DOI: 10.7270/Q22Z14G6 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50041999
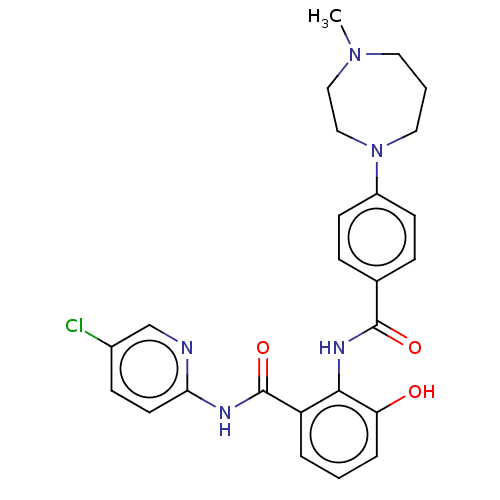
(CHEMBL3359573)Show SMILES Cl.CN1CCCN(CC1)c1ccc(cc1)C(=O)Nc1c(O)cccc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C25H26ClN5O3.ClH/c1-30-12-3-13-31(15-14-30)19-9-6-17(7-10-19)24(33)29-23-20(4-2-5-21(23)32)25(34)28-22-11-8-18(26)16-27-22;/h2,4-11,16,32H,3,12-15H2,1H3,(H,29,33)(H,27,28,34);1H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a using S-2222 chromogenic substrate assessed as hydrolysis by microplate reader |
Bioorg Med Chem 23: 277-89 (2015)
Article DOI: 10.1016/j.bmc.2014.11.042
BindingDB Entry DOI: 10.7270/Q2P55Q4X |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50058973
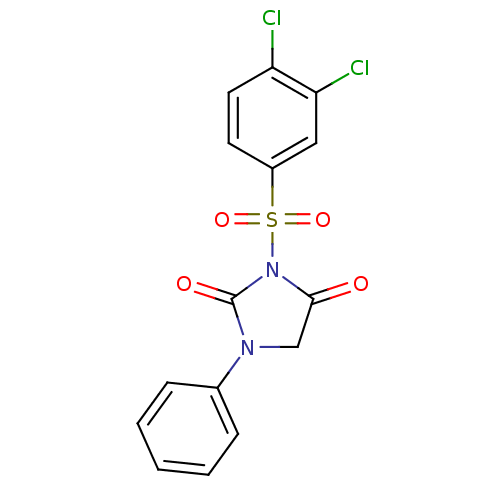
(3-(3,4-Dichloro-benzenesulfonyl)-1-phenyl-imidazol...)Show SMILES Clc1ccc(cc1Cl)S(=O)(=O)N1C(=O)CN(C1=O)c1ccccc1 Show InChI InChI=1S/C15H10Cl2N2O4S/c16-12-7-6-11(8-13(12)17)24(22,23)19-14(20)9-18(15(19)21)10-4-2-1-3-5-10/h1-8H,9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Suntory Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human heart chymase in vitro. |
J Med Chem 40: 2156-63 (1997)
Article DOI: 10.1021/jm960793t
BindingDB Entry DOI: 10.7270/Q2QJ7GDG |
More data for this
Ligand-Target Pair | |
Bombesin receptor subtype-3
(Homo sapiens (Human)) | BDBM428850
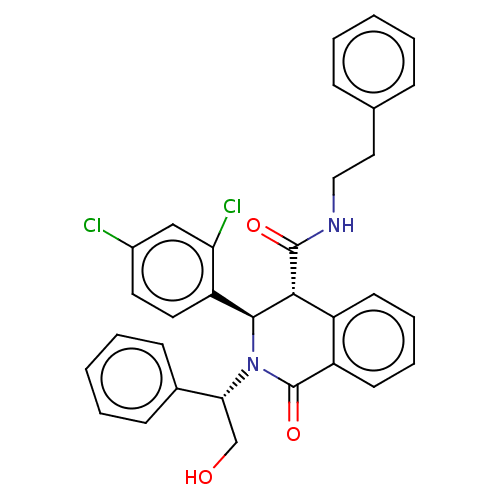
(US10532048, Example 62)Show SMILES OC[C@@H](N1[C@H]([C@H](C(=O)NCCc2ccccc2)c2ccccc2C1=O)c1ccc(Cl)cc1Cl)c1ccccc1 |r| Show InChI InChI=1S/C32H28Cl2N2O3/c33-23-15-16-26(27(34)19-23)30-29(31(38)35-18-17-21-9-3-1-4-10-21)24-13-7-8-14-25(24)32(39)36(30)28(20-37)22-11-5-2-6-12-22/h1-16,19,28-30,37H,17-18,20H2,(H,35,38)/t28-,29-,30+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 18.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Seldar Pharma Inc.
US Patent
| Assay Description
A BB2 receptor binding test was carried out using a membrane sample prepared from a human prostate cancer-derived PC-3 cell. The PC-3 cell was cultur... |
US Patent US10532048 (2020)
BindingDB Entry DOI: 10.7270/Q22R3V1R |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50058984

(3-(3,4-Dichloro-benzenesulfonyl)-1-(3,4-dimethyl-p...)Show SMILES Cc1ccc(cc1C)N1CC(=O)N(C1=O)S(=O)(=O)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C17H14Cl2N2O4S/c1-10-3-4-12(7-11(10)2)20-9-16(22)21(17(20)23)26(24,25)13-5-6-14(18)15(19)8-13/h3-8H,9H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Suntory Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human heart chymase in vitro. |
J Med Chem 40: 2156-63 (1997)
Article DOI: 10.1021/jm960793t
BindingDB Entry DOI: 10.7270/Q2QJ7GDG |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50058990
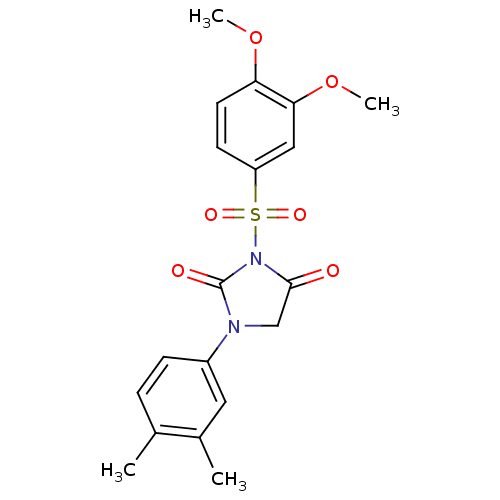
(3-(3,4-Dimethoxy-benzenesulfonyl)-1-(3,4-dimethyl-...)Show SMILES COc1ccc(cc1OC)S(=O)(=O)N1C(=O)CN(C1=O)c1ccc(C)c(C)c1 Show InChI InChI=1S/C19H20N2O6S/c1-12-5-6-14(9-13(12)2)20-11-18(22)21(19(20)23)28(24,25)15-7-8-16(26-3)17(10-15)27-4/h5-10H,11H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Suntory Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human heart chymase in vitro. |
J Med Chem 40: 2156-63 (1997)
Article DOI: 10.1021/jm960793t
BindingDB Entry DOI: 10.7270/Q2QJ7GDG |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50058996
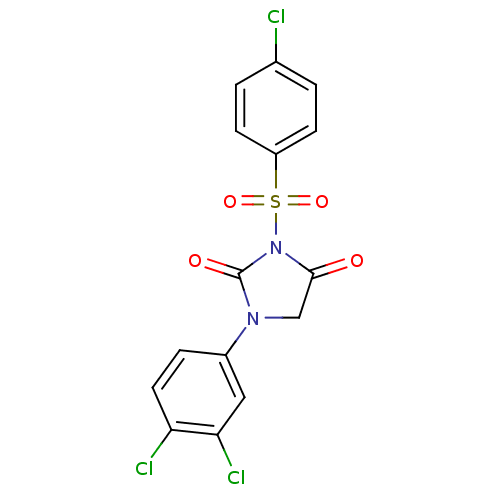
(3-(4-Chloro-benzenesulfonyl)-1-(3,4-dichloro-pheny...)Show SMILES Clc1ccc(cc1)S(=O)(=O)N1C(=O)CN(C1=O)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C15H9Cl3N2O4S/c16-9-1-4-11(5-2-9)25(23,24)20-14(21)8-19(15(20)22)10-3-6-12(17)13(18)7-10/h1-7H,8H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Suntory Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human heart chymase in vitro. |
J Med Chem 40: 2156-63 (1997)
Article DOI: 10.1021/jm960793t
BindingDB Entry DOI: 10.7270/Q2QJ7GDG |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50358251

(CHEMBL1922344)Show SMILES COc1ccc(cc1)C(=O)Nc1cccc(O[C@@H]2O[C@@H]([C@@H](O)[C@H](O)[C@H]2O)C(O)=O)c1NC(=O)c1ccc(cc1)N1CCCN(C)CC1 |r| Show InChI InChI=1S/C33H38N4O10/c1-36-15-4-16-37(18-17-36)21-11-7-19(8-12-21)31(42)35-25-23(34-30(41)20-9-13-22(45-2)14-10-20)5-3-6-24(25)46-33-28(40)26(38)27(39)29(47-33)32(43)44/h3,5-14,26-29,33,38-40H,4,15-18H2,1-2H3,(H,34,41)(H,35,42)(H,43,44)/t26-,27-,28+,29-,33+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 28.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10A using chromogenic substrate S2222 by fluorometric analysis |
J Med Chem 54: 8051-65 (2011)
Article DOI: 10.1021/jm200868m
BindingDB Entry DOI: 10.7270/Q2D50ND9 |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50059007
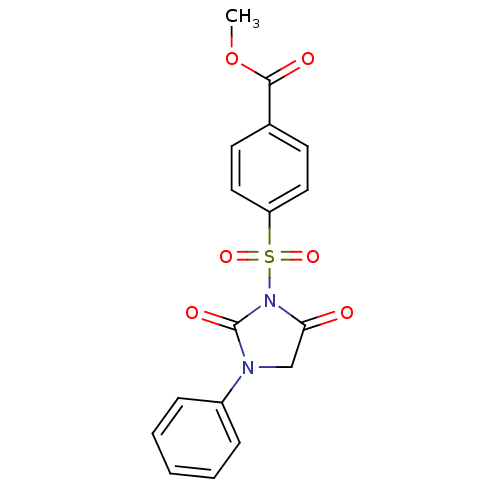
(4-(2,5-Dioxo-3-phenyl-imidazolidine-1-sulfonyl)-be...)Show SMILES COC(=O)c1ccc(cc1)S(=O)(=O)N1C(=O)CN(C1=O)c1ccccc1 Show InChI InChI=1S/C17H14N2O6S/c1-25-16(21)12-7-9-14(10-8-12)26(23,24)19-15(20)11-18(17(19)22)13-5-3-2-4-6-13/h2-10H,11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Suntory Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human heart chymase in vitro. |
J Med Chem 40: 2156-63 (1997)
Article DOI: 10.1021/jm960793t
BindingDB Entry DOI: 10.7270/Q2QJ7GDG |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50058964
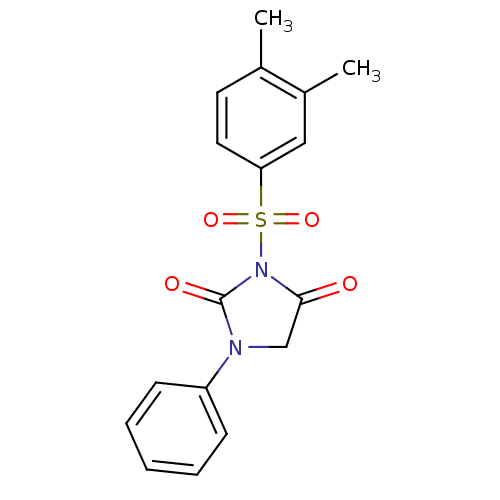
(3-(3,4-Dimethyl-benzenesulfonyl)-1-phenyl-imidazol...)Show SMILES Cc1ccc(cc1C)S(=O)(=O)N1C(=O)CN(C1=O)c1ccccc1 Show InChI InChI=1S/C17H16N2O4S/c1-12-8-9-15(10-13(12)2)24(22,23)19-16(20)11-18(17(19)21)14-6-4-3-5-7-14/h3-10H,11H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Suntory Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human heart chymase in vitro. |
J Med Chem 40: 2156-63 (1997)
Article DOI: 10.1021/jm960793t
BindingDB Entry DOI: 10.7270/Q2QJ7GDG |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data