

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM50036201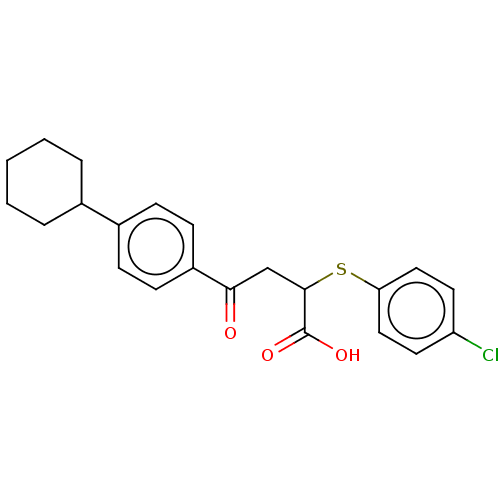 (CHEMBL3358688) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of F-bid from human recombinant N-terminal His-tagged Bcl-xL (1 to 209) expressed in Escherichia coli BL21(DE3) after 2 hrs by fluoresce... | Bioorg Med Chem Lett 24: 5836-9 (2014) Article DOI: 10.1016/j.bmcl.2014.09.073 BindingDB Entry DOI: 10.7270/Q2C82BWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM50036202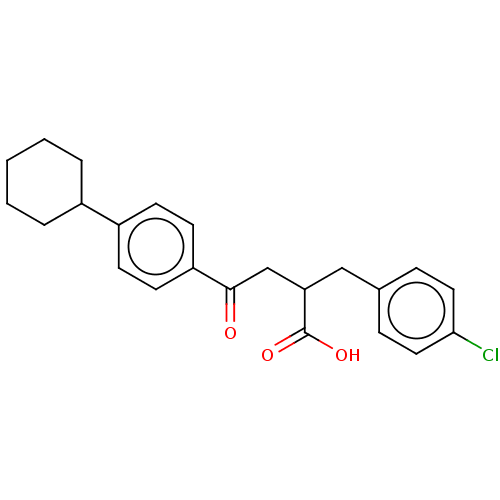 (CHEMBL3358689) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Reversible inhibition of human recombinant N-terminal His-tagged Bcl-xL (1 to 209) expressed in Escherichia coli BL21(DE3) after 30 to 120 mins by TR... | Bioorg Med Chem Lett 24: 5836-9 (2014) Article DOI: 10.1016/j.bmcl.2014.09.073 BindingDB Entry DOI: 10.7270/Q2C82BWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM50036201 (CHEMBL3358688) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of F-bid from human recombinant N-terminal His-tagged Mcl-1 (1 to 319) expressed in Escherichia coli BL21(DE3) after 120 mins by TR-FRET... | Bioorg Med Chem Lett 24: 5836-9 (2014) Article DOI: 10.1016/j.bmcl.2014.09.073 BindingDB Entry DOI: 10.7270/Q2C82BWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM50036201 (CHEMBL3358688) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of F-bid from human recombinant N-terminal His-tagged Bcl-xL (1 to 209) expressed in Escherichia coli BL21(DE3) after 120 mins by TR-FRE... | Bioorg Med Chem Lett 24: 5836-9 (2014) Article DOI: 10.1016/j.bmcl.2014.09.073 BindingDB Entry DOI: 10.7270/Q2C82BWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM50036201 (CHEMBL3358688) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Reversible inhibition of human recombinant N-terminal His-tagged Bcl-xL (1 to 209) expressed in Escherichia coli BL21(DE3) after 30 to 120 mins by TR... | Bioorg Med Chem Lett 24: 5836-9 (2014) Article DOI: 10.1016/j.bmcl.2014.09.073 BindingDB Entry DOI: 10.7270/Q2C82BWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM50036201 (CHEMBL3358688) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of F-bid from human recombinant N-terminal His-tagged Mcl-1 (1 to 319) expressed in Escherichia coli BL21(DE3) after 2 hrs by fluorescen... | Bioorg Med Chem Lett 24: 5836-9 (2014) Article DOI: 10.1016/j.bmcl.2014.09.073 BindingDB Entry DOI: 10.7270/Q2C82BWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM50036202 (CHEMBL3358689) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of F-bid from human recombinant N-terminal His-tagged Mcl-1 (1 to 319) expressed in Escherichia coli BL21(DE3) after 120 mins by TR-FRET... | Bioorg Med Chem Lett 24: 5836-9 (2014) Article DOI: 10.1016/j.bmcl.2014.09.073 BindingDB Entry DOI: 10.7270/Q2C82BWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 2 (Homo sapiens (Human)) | BDBM50279272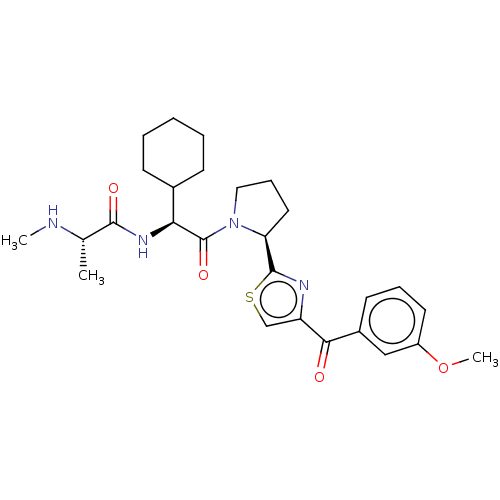 (CHEMBL4164385) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Concentration required to protect the cell against HIV-1 strain IIIB viral cytopathogenicity by 50% in MT-4 cells | ACS Med Chem Lett 8: 1042-1047 (2017) Article DOI: 10.1021/acsmedchemlett.7b00247 BindingDB Entry DOI: 10.7270/Q2XG9TNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 3 (Homo sapiens (Human)) | BDBM50279272 (CHEMBL4164385) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibitory concentration against SO561945 (HIV 1 mutant RT) viral viral infection of MT-4 cells | ACS Med Chem Lett 8: 1042-1047 (2017) Article DOI: 10.1021/acsmedchemlett.7b00247 BindingDB Entry DOI: 10.7270/Q2XG9TNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50367855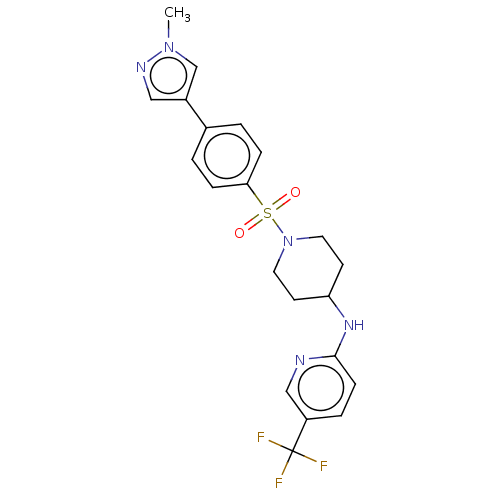 (CHEMBL4175929) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of GST-tagged human CDK2/CCNA2 (04 to 103 residues) pre-incubated for 5 mins before addition of histone H1 substrate and [gamma-33P]ATP an... | J Med Chem 61: 7710-7728 (2018) Article DOI: 10.1021/acs.jmedchem.8b00683 BindingDB Entry DOI: 10.7270/Q2HX1G6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 13/Cyclin-K (Homo sapiens (Human)) | BDBM50367676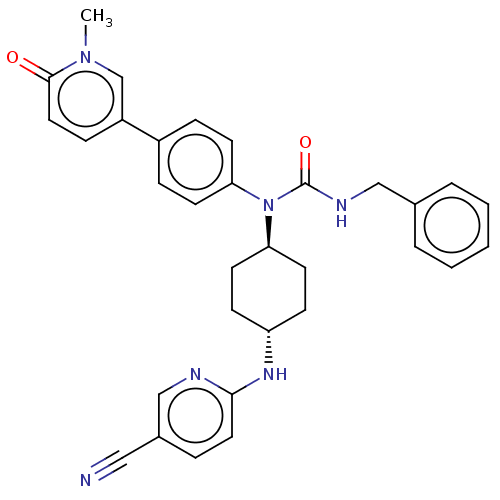 (CHEMBL4160662) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of N-terminal FLAG-tagged human full-length CDK13 (1 to 1512 residues)/N-terminal His-tagged CycK (1 to 580 residues) expressed in Sf9 cel... | J Med Chem 61: 7710-7728 (2018) Article DOI: 10.1021/acs.jmedchem.8b00683 BindingDB Entry DOI: 10.7270/Q2HX1G6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50367737 (CHEMBL4173631) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of GST-tagged human CDK2/CCNA2 (04 to 103 residues) pre-incubated for 5 mins before addition of histone H1 substrate and [gamma-33P]ATP an... | J Med Chem 61: 7710-7728 (2018) Article DOI: 10.1021/acs.jmedchem.8b00683 BindingDB Entry DOI: 10.7270/Q2HX1G6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 12 (Homo sapiens) | BDBM50367857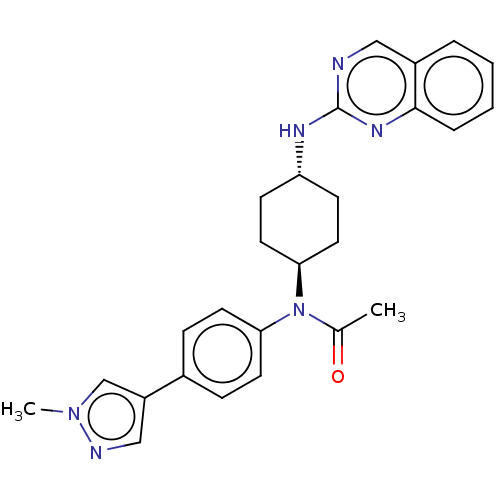 (CHEMBL4159417) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of N-terminal FLAG-tagged human full-length CDK12 (1 to 1490 residues)/N-terminal His-tagged CycK (1 to 580 residues) expressed in Sf9 cel... | J Med Chem 61: 7710-7728 (2018) Article DOI: 10.1021/acs.jmedchem.8b00683 BindingDB Entry DOI: 10.7270/Q2HX1G6F | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cyclin-T1/Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of GST-tagged human CDK9/CycT1 (04 to 110 residues) assessed as reduction in ATP-dependent ULight-4E-BP1 (Thr37/Thr46) substrate peptide p... | J Med Chem 61: 7710-7728 (2018) Article DOI: 10.1021/acs.jmedchem.8b00683 BindingDB Entry DOI: 10.7270/Q2HX1G6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-C (Homo sapiens (Human)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of GST-tagged human CDK8/CycC (04 to 109 residues) using kinase tracer 236 probe incubated for 60 mins by TR-FRET assay | J Med Chem 61: 7710-7728 (2018) Article DOI: 10.1021/acs.jmedchem.8b00683 BindingDB Entry DOI: 10.7270/Q2HX1G6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50367857 (CHEMBL4159417) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of GST-tagged human CDK2/CCNA2 (04 to 103 residues) pre-incubated for 5 mins before addition of histone H1 substrate and [gamma-33P]ATP an... | J Med Chem 61: 7710-7728 (2018) Article DOI: 10.1021/acs.jmedchem.8b00683 BindingDB Entry DOI: 10.7270/Q2HX1G6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50367955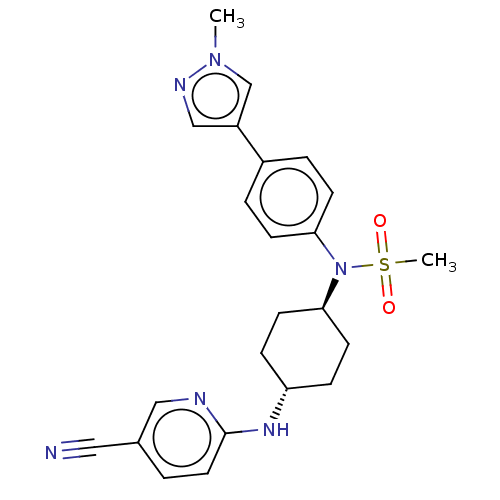 (CHEMBL4174716) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of GST-tagged human CDK2/CCNA2 (04 to 103 residues) pre-incubated for 5 mins before addition of histone H1 substrate and [gamma-33P]ATP an... | J Med Chem 61: 7710-7728 (2018) Article DOI: 10.1021/acs.jmedchem.8b00683 BindingDB Entry DOI: 10.7270/Q2HX1G6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50367873 (CHEMBL4169139) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of GST-tagged human CDK2/CCNA2 (04 to 103 residues) pre-incubated for 5 mins before addition of histone H1 substrate and [gamma-33P]ATP an... | J Med Chem 61: 7710-7728 (2018) Article DOI: 10.1021/acs.jmedchem.8b00683 BindingDB Entry DOI: 10.7270/Q2HX1G6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM50279265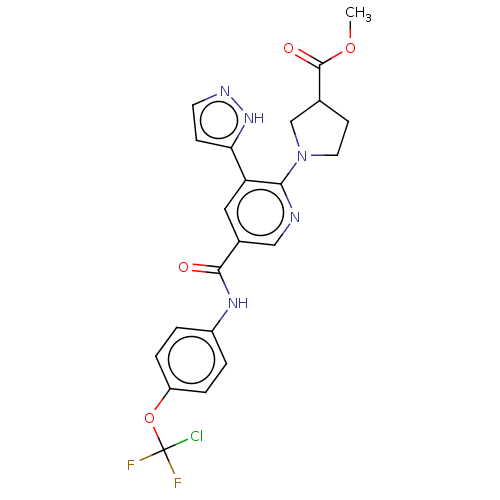 (CHEMBL4162041) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human His-tagged full length ABL1 allosteric site expressed in baculovirus expression system by TR-FRET assay | ACS Med Chem Lett 8: 1042-1047 (2017) Article DOI: 10.1021/acsmedchemlett.7b00247 BindingDB Entry DOI: 10.7270/Q2XG9TNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase KRas (Homo sapiens (Human)) | BDBM50242325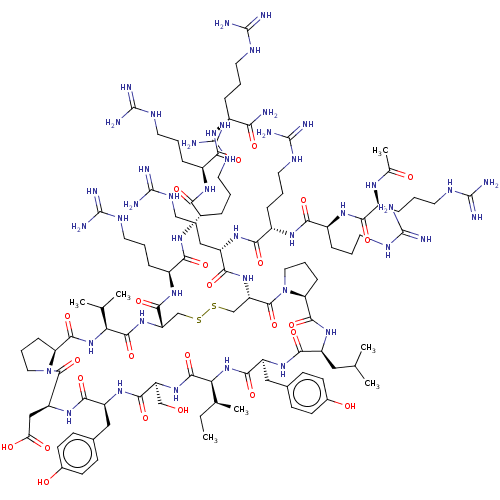 (CHEMBL4072295) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Inhibition of biotinylated wild-type K-Ras (unknown origin) assessed as inhibition of human SOS1 (564 to 1049 residues)-mediated BODIPY-GDP-GTP excha... | Bioorg Med Chem Lett 27: 2757-2761 (2017) Article DOI: 10.1016/j.bmcl.2017.04.063 BindingDB Entry DOI: 10.7270/Q2FF3VSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 12 (Homo sapiens) | BDBM50367957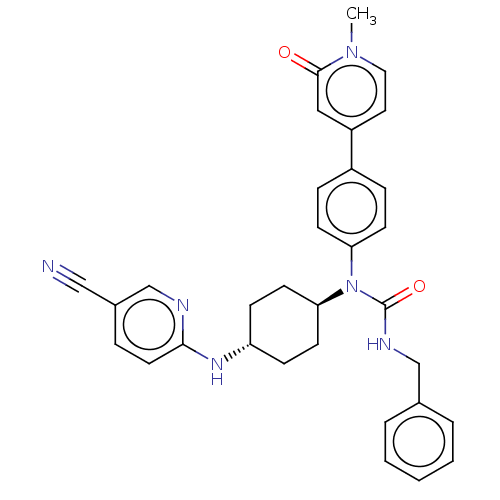 (CHEMBL4174715) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of N-terminal FLAG-tagged human full-length CDK12 (1 to 1490 residues)/N-terminal His-tagged CycK (1 to 580 residues) expressed in Sf9 cel... | J Med Chem 61: 7710-7728 (2018) Article DOI: 10.1021/acs.jmedchem.8b00683 BindingDB Entry DOI: 10.7270/Q2HX1G6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-K/Cyclin-dependent kinase 12 (Homo sapiens (Human)) | BDBM50367676 (CHEMBL4160662) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of N-terminal FLAG-tagged human full-length CDK12 (1 to 1490 residues)/N-terminal His-tagged CycK (1 to 580 residues) expressed in Sf9 cel... | J Med Chem 61: 7710-7728 (2018) Article DOI: 10.1021/acs.jmedchem.8b00683 BindingDB Entry DOI: 10.7270/Q2HX1G6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 12 (Homo sapiens) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of N-terminal FLAG-tagged human full-length CDK12 (1 to 1490 residues)/N-terminal His-tagged CycK (1 to 580 residues) expressed in Sf9 cel... | J Med Chem 61: 7710-7728 (2018) Article DOI: 10.1021/acs.jmedchem.8b00683 BindingDB Entry DOI: 10.7270/Q2HX1G6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 13/Cyclin-K (Homo sapiens (Human)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of N-terminal FLAG-tagged human full-length CDK13 (1 to 1512 residues)/N-terminal His-tagged CycK (1 to 580 residues) expressed in Sf9 cel... | J Med Chem 61: 7710-7728 (2018) Article DOI: 10.1021/acs.jmedchem.8b00683 BindingDB Entry DOI: 10.7270/Q2HX1G6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 12 (Homo sapiens) | BDBM50367685 (CHEMBL4172363) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of N-terminal FLAG-tagged human full-length CDK12 (1 to 1490 residues)/N-terminal His-tagged CycK (1 to 580 residues) expressed in Sf9 cel... | J Med Chem 61: 7710-7728 (2018) Article DOI: 10.1021/acs.jmedchem.8b00683 BindingDB Entry DOI: 10.7270/Q2HX1G6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 12 (Homo sapiens) | BDBM50367707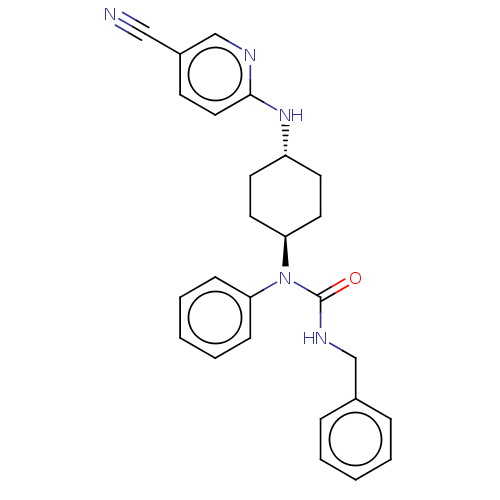 (CHEMBL4167916) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of N-terminal FLAG-tagged human full-length CDK12 (1 to 1490 residues)/N-terminal His-tagged CycK (1 to 580 residues) expressed in Sf9 cel... | J Med Chem 61: 7710-7728 (2018) Article DOI: 10.1021/acs.jmedchem.8b00683 BindingDB Entry DOI: 10.7270/Q2HX1G6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 12 (Homo sapiens) | BDBM50367845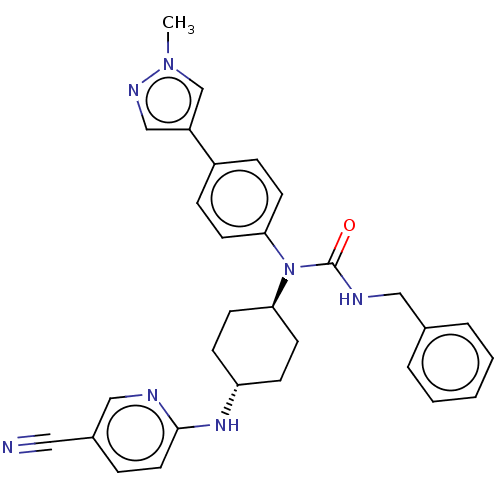 (CHEMBL4173254) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of N-terminal FLAG-tagged human full-length CDK12 (1 to 1490 residues)/N-terminal His-tagged CycK (1 to 580 residues) expressed in Sf9 cel... | J Med Chem 61: 7710-7728 (2018) Article DOI: 10.1021/acs.jmedchem.8b00683 BindingDB Entry DOI: 10.7270/Q2HX1G6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP (Homo sapiens (Human)) | BDBM50279272 (CHEMBL4164385) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human His-tagged XIAP (Asn252 to Thr356 residues) expressed in Escherichia coli by TR-FRET assay | ACS Med Chem Lett 8: 1042-1047 (2017) Article DOI: 10.1021/acsmedchemlett.7b00247 BindingDB Entry DOI: 10.7270/Q2XG9TNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase KRas (Homo sapiens (Human)) | BDBM50242347 (CHEMBL4082777) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Inhibition of biotinylated wild-type K-Ras (unknown origin) assessed as inhibition of human SOS1 (564 to 1049 residues)-mediated BODIPY-GDP-GTP excha... | Bioorg Med Chem Lett 27: 2757-2761 (2017) Article DOI: 10.1016/j.bmcl.2017.04.063 BindingDB Entry DOI: 10.7270/Q2FF3VSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 2 (Homo sapiens (Human)) | BDBM50279273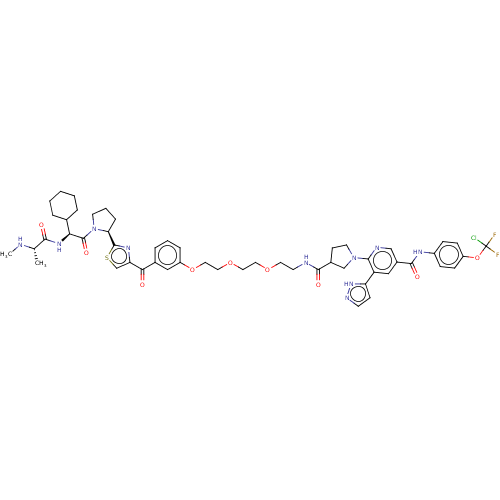 (CHEMBL4160980) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Concentration required to protect the cell against HIV-1 strain IIIB viral cytopathogenicity by 50% in MT-4 cells | ACS Med Chem Lett 8: 1042-1047 (2017) Article DOI: 10.1021/acsmedchemlett.7b00247 BindingDB Entry DOI: 10.7270/Q2XG9TNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM50448451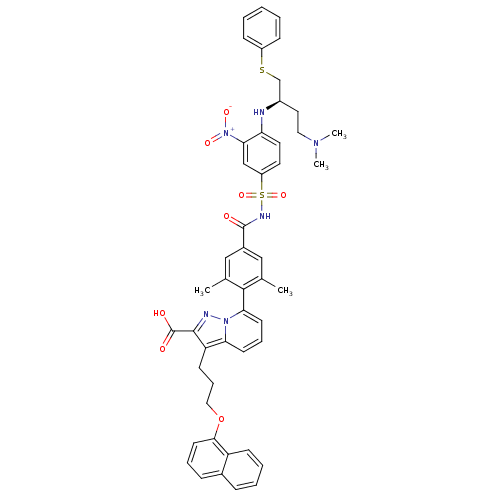 (CHEMBL3126120) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 88 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of FITC-Bid from GST-tagged human Mcl-1 expressed in Escherichia coli after 2 hrs by TR-FRET assay | J Med Chem 56: 9635-45 (2014) Article DOI: 10.1021/jm401170c BindingDB Entry DOI: 10.7270/Q2MS3V86 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP (Homo sapiens (Human)) | BDBM50279274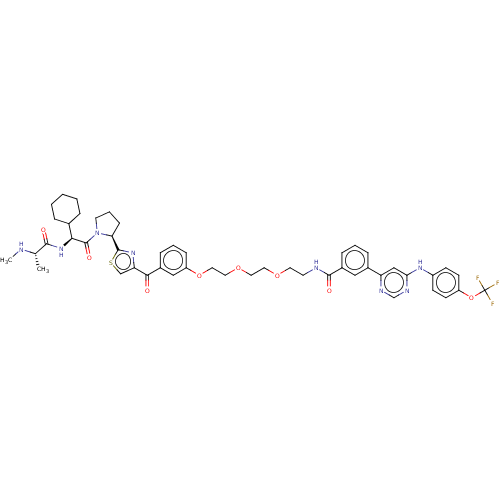 (CHEMBL4172268) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Concentration required to protect the cell against HIV-1 strain IIIB viral cytopathogenicity by 50% in MT-4 cells | ACS Med Chem Lett 8: 1042-1047 (2017) Article DOI: 10.1021/acsmedchemlett.7b00247 BindingDB Entry DOI: 10.7270/Q2XG9TNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 12 (Homo sapiens) | BDBM50367684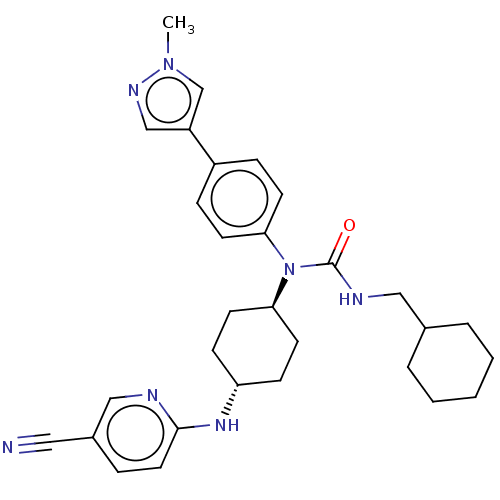 (CHEMBL4162567) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 99 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of N-terminal FLAG-tagged human full-length CDK12 (1 to 1490 residues)/N-terminal His-tagged CycK (1 to 580 residues) expressed in Sf9 cel... | J Med Chem 61: 7710-7728 (2018) Article DOI: 10.1021/acs.jmedchem.8b00683 BindingDB Entry DOI: 10.7270/Q2HX1G6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase KRas (Homo sapiens (Human)) | BDBM50242340 (CHEMBL4070657) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 105 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Inhibition of biotinylated wild-type K-Ras (unknown origin) assessed as inhibition of human SOS1 (564 to 1049 residues)-mediated BODIPY-GDP-GTP excha... | Bioorg Med Chem Lett 27: 2757-2761 (2017) Article DOI: 10.1016/j.bmcl.2017.04.063 BindingDB Entry DOI: 10.7270/Q2FF3VSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 12 (Homo sapiens) | BDBM50367682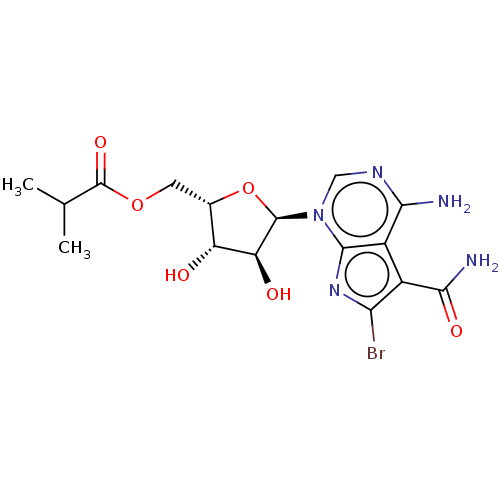 (CHEMBL4162128) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of N-terminal FLAG-tagged human full-length CDK12 (1 to 1490 residues)/N-terminal His-tagged CycK (1 to 580 residues) expressed in Sf9 cel... | J Med Chem 61: 7710-7728 (2018) Article DOI: 10.1021/acs.jmedchem.8b00683 BindingDB Entry DOI: 10.7270/Q2HX1G6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 12 (Homo sapiens) | BDBM50367955 (CHEMBL4174716) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of N-terminal FLAG-tagged human full-length CDK12 (1 to 1490 residues)/N-terminal His-tagged CycK (1 to 580 residues) expressed in Sf9 cel... | J Med Chem 61: 7710-7728 (2018) Article DOI: 10.1021/acs.jmedchem.8b00683 BindingDB Entry DOI: 10.7270/Q2HX1G6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP (Homo sapiens (Human)) | BDBM50279273 (CHEMBL4160980) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human His-tagged XIAP (Asn252 to Thr356 residues) expressed in Escherichia coli by TR-FRET assay | ACS Med Chem Lett 8: 1042-1047 (2017) Article DOI: 10.1021/acsmedchemlett.7b00247 BindingDB Entry DOI: 10.7270/Q2XG9TNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| CDK-activating kinase assembly factor MAT1/Cyclin-H/Cyclin-dependent kinase 7 (Homo sapiens (Human)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of GST-tagged human GST-tagged CDK7/CycH/MAT1 (04 to 108 residues) using kinase tracer 236 probe incubated for 60 mins by TR-FRET assay | J Med Chem 61: 7710-7728 (2018) Article DOI: 10.1021/acs.jmedchem.8b00683 BindingDB Entry DOI: 10.7270/Q2HX1G6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 12 (Homo sapiens) | BDBM50367919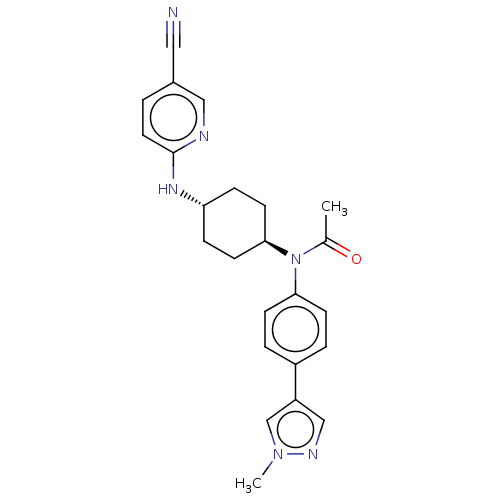 (CHEMBL4159594) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of N-terminal FLAG-tagged human full-length CDK12 (1 to 1490 residues)/N-terminal His-tagged CycK (1 to 580 residues) expressed in Sf9 cel... | J Med Chem 61: 7710-7728 (2018) Article DOI: 10.1021/acs.jmedchem.8b00683 BindingDB Entry DOI: 10.7270/Q2HX1G6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 3 (Homo sapiens (Human)) | BDBM50279273 (CHEMBL4160980) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human His-tagged cIAP2 (Gln238 to Ser349 residues) expressed in Escherichia coli by TR-FRET assay | ACS Med Chem Lett 8: 1042-1047 (2017) Article DOI: 10.1021/acsmedchemlett.7b00247 BindingDB Entry DOI: 10.7270/Q2XG9TNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase KRas (Homo sapiens (Human)) | BDBM50242326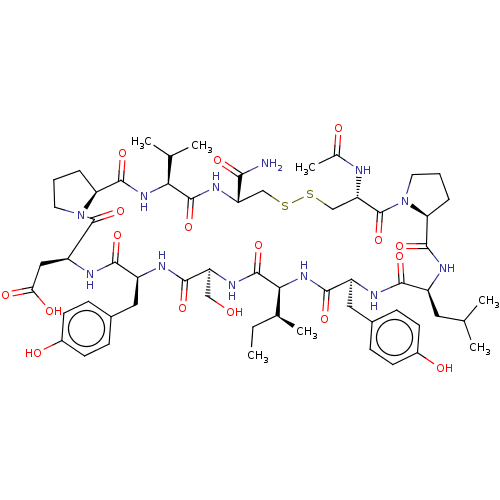 (CHEMBL4100766) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 142 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Inhibition of biotinylated wild-type K-Ras (unknown origin) assessed as inhibition of human SOS1 (564 to 1049 residues)-mediated BODIPY-GDP-GTP excha... | Bioorg Med Chem Lett 27: 2757-2761 (2017) Article DOI: 10.1016/j.bmcl.2017.04.063 BindingDB Entry DOI: 10.7270/Q2FF3VSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 12 (Homo sapiens) | BDBM50367840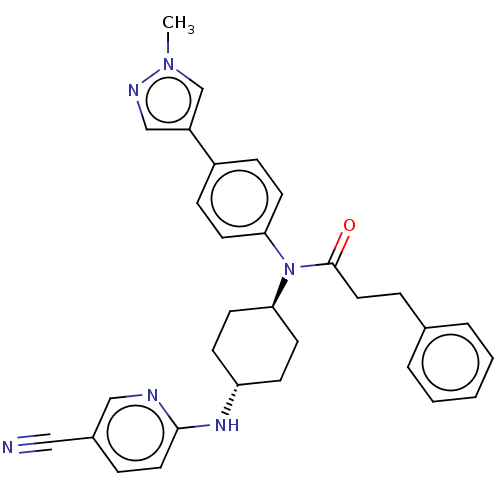 (CHEMBL4162954) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of N-terminal FLAG-tagged human full-length CDK12 (1 to 1490 residues)/N-terminal His-tagged CycK (1 to 580 residues) expressed in Sf9 cel... | J Med Chem 61: 7710-7728 (2018) Article DOI: 10.1021/acs.jmedchem.8b00683 BindingDB Entry DOI: 10.7270/Q2HX1G6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 12 (Homo sapiens) | BDBM50367676 (CHEMBL4160662) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 195 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of CDK12 in human SK-BR-3 cells assessed as reduction in RNA polymerase 2 Ser2 phosphorylation incubated for 4 hrs by | J Med Chem 61: 7710-7728 (2018) Article DOI: 10.1021/acs.jmedchem.8b00683 BindingDB Entry DOI: 10.7270/Q2HX1G6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 12 (Homo sapiens) | BDBM50367856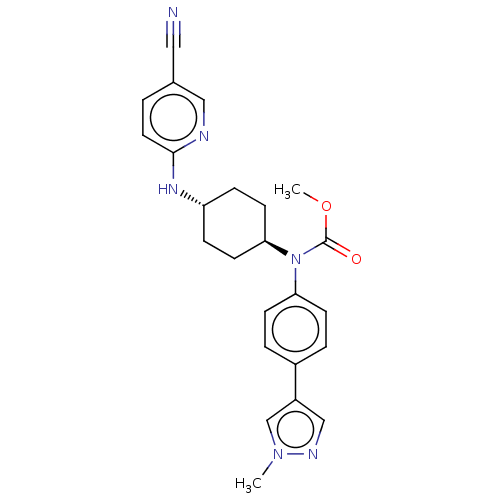 (CHEMBL4166407) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of N-terminal FLAG-tagged human full-length CDK12 (1 to 1490 residues)/N-terminal His-tagged CycK (1 to 580 residues) expressed in Sf9 cel... | J Med Chem 61: 7710-7728 (2018) Article DOI: 10.1021/acs.jmedchem.8b00683 BindingDB Entry DOI: 10.7270/Q2HX1G6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase KRas (Homo sapiens (Human)) | BDBM50242332 (CHEMBL4083770) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 216 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Inhibition of biotinylated wild-type K-Ras (unknown origin) assessed as inhibition of human SOS1 (564 to 1049 residues)-mediated BODIPY-GDP-GTP excha... | Bioorg Med Chem Lett 27: 2757-2761 (2017) Article DOI: 10.1016/j.bmcl.2017.04.063 BindingDB Entry DOI: 10.7270/Q2FF3VSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-T1/Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM50367682 (CHEMBL4162128) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of GST-tagged human CDK9/CycT1 (04 to 110 residues) assessed as reduction in ATP-dependent ULight-4E-BP1 (Thr37/Thr46) substrate peptide p... | J Med Chem 61: 7710-7728 (2018) Article DOI: 10.1021/acs.jmedchem.8b00683 BindingDB Entry DOI: 10.7270/Q2HX1G6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 2 (Homo sapiens (Human)) | BDBM50279274 (CHEMBL4172268) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibitory concentration against SO561945 (HIV 1 mutant RT) viral viral infection of MT-4 cells | ACS Med Chem Lett 8: 1042-1047 (2017) Article DOI: 10.1021/acsmedchemlett.7b00247 BindingDB Entry DOI: 10.7270/Q2XG9TNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 12 (Homo sapiens) | BDBM50367854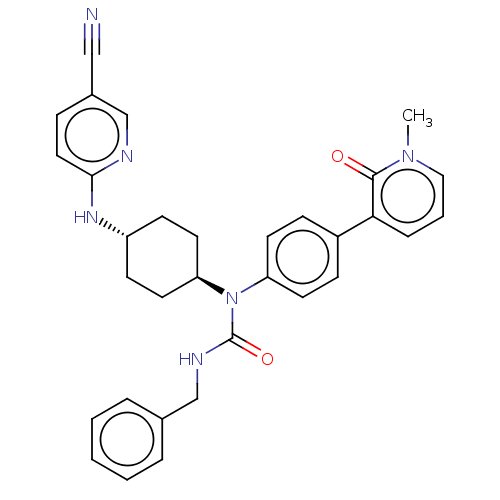 (CHEMBL4164483) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of N-terminal FLAG-tagged human full-length CDK12 (1 to 1490 residues)/N-terminal His-tagged CycK (1 to 580 residues) expressed in Sf9 cel... | J Med Chem 61: 7710-7728 (2018) Article DOI: 10.1021/acs.jmedchem.8b00683 BindingDB Entry DOI: 10.7270/Q2HX1G6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 12 (Homo sapiens) | BDBM50367682 (CHEMBL4162128) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 259 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of CDK12 in human SK-BR-3 cells assessed as reduction in RNA polymerase 2 Ser2 phosphorylation incubated for 4 hrs by | J Med Chem 61: 7710-7728 (2018) Article DOI: 10.1021/acs.jmedchem.8b00683 BindingDB Entry DOI: 10.7270/Q2HX1G6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 12 (Homo sapiens) | BDBM50367679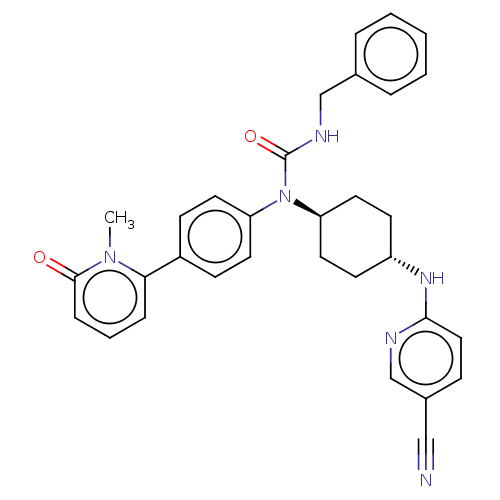 (CHEMBL4164064) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of N-terminal FLAG-tagged human full-length CDK12 (1 to 1490 residues)/N-terminal His-tagged CycK (1 to 580 residues) expressed in Sf9 cel... | J Med Chem 61: 7710-7728 (2018) Article DOI: 10.1021/acs.jmedchem.8b00683 BindingDB Entry DOI: 10.7270/Q2HX1G6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 517 total ) | Next | Last >> |