

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50067536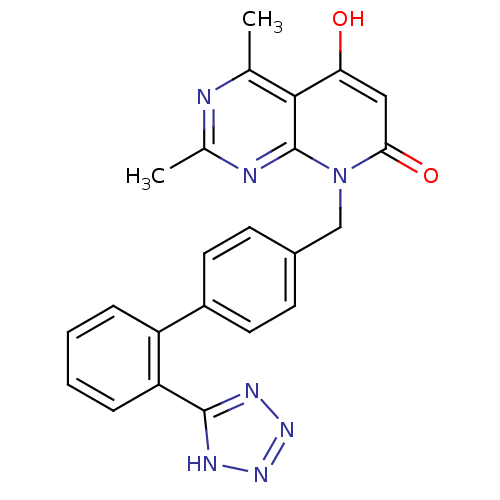 (5-Hydroxy-2,4-dimethyl-8-[2'-(1H-tetrazol-5-yl)-bi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Compound was evaluated for the in vitro inhibition of specific binding of [125I]-A II to Angiotensin II receptor, type 1 from rat adrenal membranes. | J Med Chem 41: 4251-60 (1998) Article DOI: 10.1021/jm970690q BindingDB Entry DOI: 10.7270/Q2FB55NK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50471877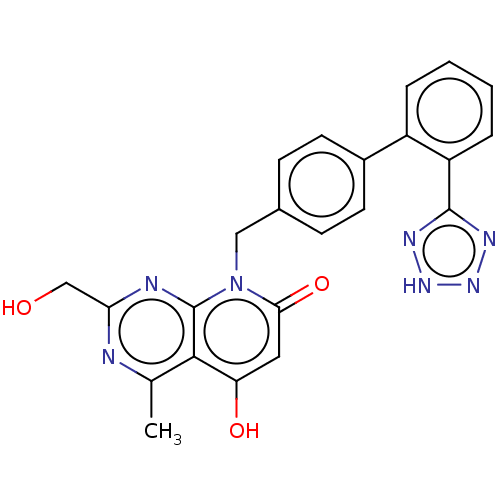 (CHEMBL135574) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Compound was evaluated for the in vitro inhibition of specific binding of [125I]-A II to Angiotensin II receptor, type 1 from rat adrenal membranes. | J Med Chem 41: 4251-60 (1998) Article DOI: 10.1021/jm970690q BindingDB Entry DOI: 10.7270/Q2FB55NK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50040439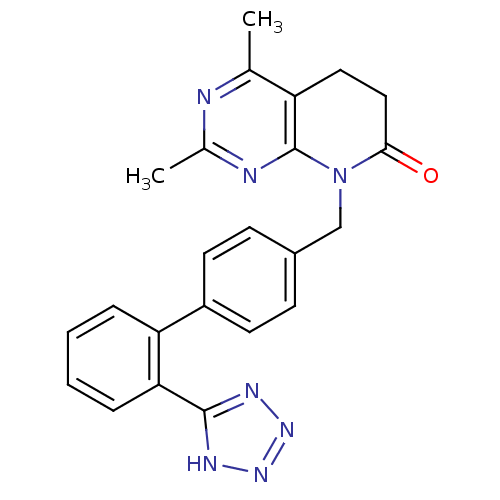 (2,4-Dimethyl-8-[2'-(1H-tetrazol-5-yl)-biphenyl-4-y...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Compound was evaluated for the in vitro inhibition of specific binding of [125I]-A II to Angiotensin II receptor, type 1 from rat adrenal membranes. | J Med Chem 41: 4251-60 (1998) Article DOI: 10.1021/jm970690q BindingDB Entry DOI: 10.7270/Q2FB55NK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50471878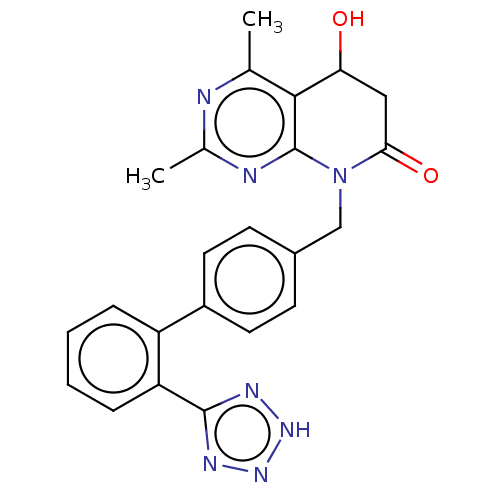 (CHEMBL337067) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Compound was evaluated for the in vitro inhibition of specific binding of [125I]-A II to Angiotensin II receptor, type 1 from rat adrenal membranes. | J Med Chem 41: 4251-60 (1998) Article DOI: 10.1021/jm970690q BindingDB Entry DOI: 10.7270/Q2FB55NK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50471879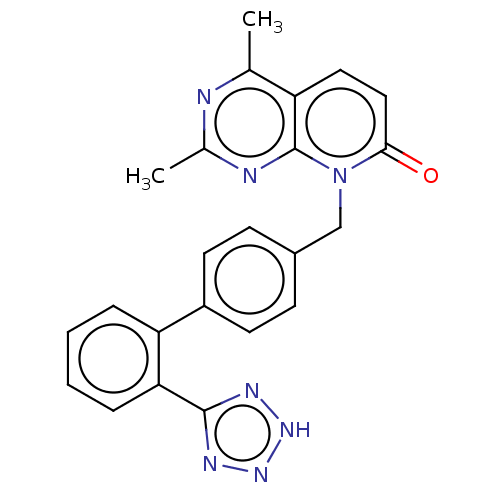 (CHEMBL135309) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Compound was evaluated for the in vitro inhibition of specific binding of [125I]-A II to Angiotensin II receptor, type 1 from rat adrenal membranes. | J Med Chem 41: 4251-60 (1998) Article DOI: 10.1021/jm970690q BindingDB Entry DOI: 10.7270/Q2FB55NK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50177595 ((2S)-5-amino-2-{[(2S)-1-{[(4R,7S,10S,13S,16R)-13-[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Antagonism of OT-induced response at OT receptor in rat uterine strips | Bioorg Med Chem Lett 16: 954-9 (2006) Article DOI: 10.1016/j.bmcl.2005.10.107 BindingDB Entry DOI: 10.7270/Q27945GR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50177593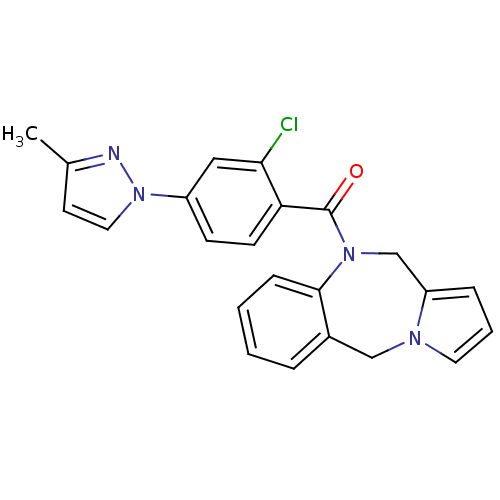 ((5H,11H-benzo[e]pyrrolo[1,2-a][1,4]diazepin-10-yl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]AVP from human V2 receptor transfected in LV2 cells | Bioorg Med Chem Lett 16: 954-9 (2006) Article DOI: 10.1016/j.bmcl.2005.10.107 BindingDB Entry DOI: 10.7270/Q27945GR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50177599 (CHEMBL202447 | [2-chloro-4-(3-methyl-pyrazol-1-yl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 91.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]AVP from human V2 receptor transfected in LV2 cells | Bioorg Med Chem Lett 16: 954-9 (2006) Article DOI: 10.1016/j.bmcl.2005.10.107 BindingDB Entry DOI: 10.7270/Q27945GR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50177591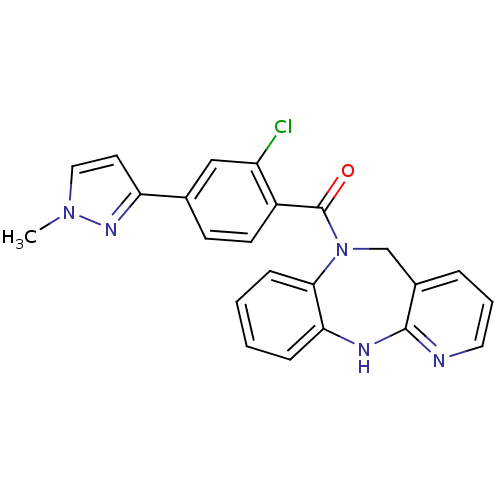 (CHEMBL203513 | [2-chloro-4-(1-methyl-1H-pyrazol-3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 183 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]AVP from human V2 receptor transfected in LV2 cells | Bioorg Med Chem Lett 16: 954-9 (2006) Article DOI: 10.1016/j.bmcl.2005.10.107 BindingDB Entry DOI: 10.7270/Q27945GR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50177597 (CHEMBL203739 | [2-bromo-4-(3-methyl-pyrazol-1-yl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 296 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]AVP from human V2 receptor transfected in LV2 cells | Bioorg Med Chem Lett 16: 954-9 (2006) Article DOI: 10.1016/j.bmcl.2005.10.107 BindingDB Entry DOI: 10.7270/Q27945GR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50177594 (CHEMBL381763 | [2-chloro-4-(5-methyl-pyrazol-1-yl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]AVP from human V2 receptor transfected in LV2 cells | Bioorg Med Chem Lett 16: 954-9 (2006) Article DOI: 10.1016/j.bmcl.2005.10.107 BindingDB Entry DOI: 10.7270/Q27945GR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50177598 ((5,11-dihydro-benzo[b]pyrido[2,3-e][1,4]diazepin-6...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 413 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]AVP from human V2 receptor transfected in LV2 cells | Bioorg Med Chem Lett 16: 954-9 (2006) Article DOI: 10.1016/j.bmcl.2005.10.107 BindingDB Entry DOI: 10.7270/Q27945GR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50177600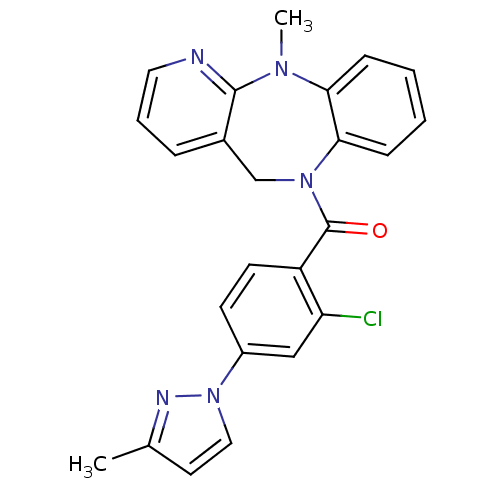 (CHEMBL205013 | [2-chloro-4-(3-methyl-pyrazol-1-yl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 475 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]AVP from human V2 receptor transfected in LV2 cells | Bioorg Med Chem Lett 16: 954-9 (2006) Article DOI: 10.1016/j.bmcl.2005.10.107 BindingDB Entry DOI: 10.7270/Q27945GR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50177592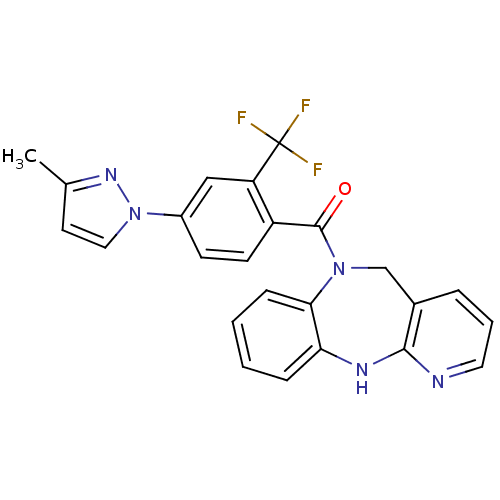 ((5,11-dihydro-benzo[b]pyrido[2,3-e][1,4]diazepin-6...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 493 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]AVP from human V2 receptor transfected in LV2 cells | Bioorg Med Chem Lett 16: 954-9 (2006) Article DOI: 10.1016/j.bmcl.2005.10.107 BindingDB Entry DOI: 10.7270/Q27945GR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50177593 ((5H,11H-benzo[e]pyrrolo[1,2-a][1,4]diazepin-10-yl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 778 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]AVP from human V1a receptor expressed in CHO cells | Bioorg Med Chem Lett 16: 954-9 (2006) Article DOI: 10.1016/j.bmcl.2005.10.107 BindingDB Entry DOI: 10.7270/Q27945GR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50177601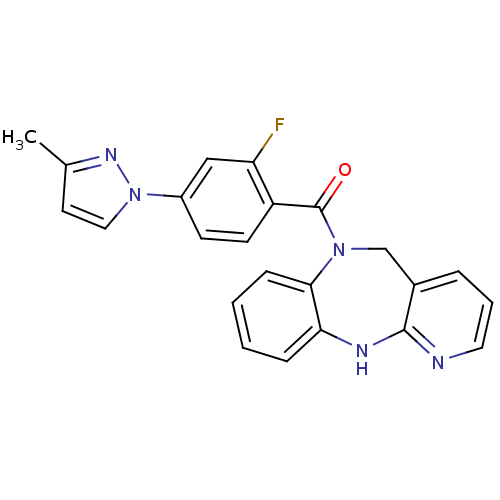 ((5,11-dihydro-benzo[b]pyrido[2,3-e][1,4]diazepin-6...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 933 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]AVP from human V2 receptor transfected in LV2 cells | Bioorg Med Chem Lett 16: 954-9 (2006) Article DOI: 10.1016/j.bmcl.2005.10.107 BindingDB Entry DOI: 10.7270/Q27945GR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50177592 ((5,11-dihydro-benzo[b]pyrido[2,3-e][1,4]diazepin-6...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Antagonism of OT-induced response at OT receptor in rat uterine strips | Bioorg Med Chem Lett 16: 954-9 (2006) Article DOI: 10.1016/j.bmcl.2005.10.107 BindingDB Entry DOI: 10.7270/Q27945GR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50177591 (CHEMBL203513 | [2-chloro-4-(1-methyl-1H-pyrazol-3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]AVP from human V1a receptor expressed in CHO cells | Bioorg Med Chem Lett 16: 954-9 (2006) Article DOI: 10.1016/j.bmcl.2005.10.107 BindingDB Entry DOI: 10.7270/Q27945GR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50177592 ((5,11-dihydro-benzo[b]pyrido[2,3-e][1,4]diazepin-6...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]AVP from human V1a receptor expressed in CHO cells | Bioorg Med Chem Lett 16: 954-9 (2006) Article DOI: 10.1016/j.bmcl.2005.10.107 BindingDB Entry DOI: 10.7270/Q27945GR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (RAT) | BDBM50177592 ((5,11-dihydro-benzo[b]pyrido[2,3-e][1,4]diazepin-6...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Antagonism of AVP-induced response at V1a receptor in isolated rat tail arteries | Bioorg Med Chem Lett 16: 954-9 (2006) Article DOI: 10.1016/j.bmcl.2005.10.107 BindingDB Entry DOI: 10.7270/Q27945GR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50177592 ((5,11-dihydro-benzo[b]pyrido[2,3-e][1,4]diazepin-6...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.67 | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Binding to human V2 receptor expressed in LV2 cells by cAMP production | Bioorg Med Chem Lett 16: 954-9 (2006) Article DOI: 10.1016/j.bmcl.2005.10.107 BindingDB Entry DOI: 10.7270/Q27945GR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50177593 ((5H,11H-benzo[e]pyrrolo[1,2-a][1,4]diazepin-10-yl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | n/a | n/a | 0.740 | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Binding to human V2 receptor expressed in LV2 cells by cAMP production | Bioorg Med Chem Lett 16: 954-9 (2006) Article DOI: 10.1016/j.bmcl.2005.10.107 BindingDB Entry DOI: 10.7270/Q27945GR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||