 Found 161 hits with Last Name = 'parikh' and Initial = 'vd'
Found 161 hits with Last Name = 'parikh' and Initial = 'vd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Stromelysin-1
(Homo sapiens (Human)) | BDBM50073839
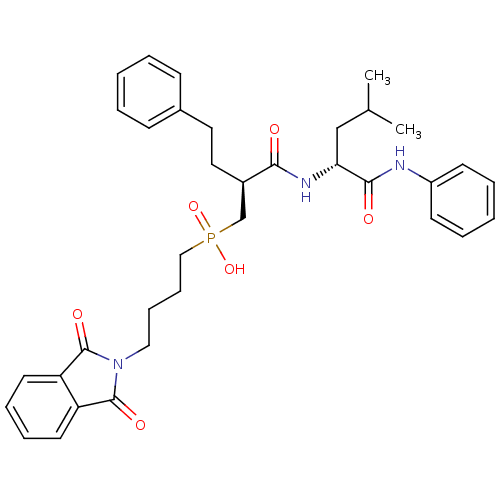
(CHEMBL283066 | [4-(1,3-Dioxo-1,3-dihydro-isoindol-...)Show SMILES CC(C)C[C@@H](NC(=O)[C@H](CCc1ccccc1)CP(O)(=O)CCCCN1C(=O)c2ccccc2C1=O)C(=O)Nc1ccccc1 Show InChI InChI=1S/C35H42N3O6P/c1-25(2)23-31(33(40)36-28-15-7-4-8-16-28)37-32(39)27(20-19-26-13-5-3-6-14-26)24-45(43,44)22-12-11-21-38-34(41)29-17-9-10-18-30(29)35(38)42/h3-10,13-18,25,27,31H,11-12,19-24H2,1-2H3,(H,36,40)(H,37,39)(H,43,44)/t27-,31-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Inhibition of recombinant matrix metalloprotease-3 (MMP-3) |
Bioorg Med Chem Lett 9: 127-32 (1999)
BindingDB Entry DOI: 10.7270/Q2JD4VZ4 |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50426340
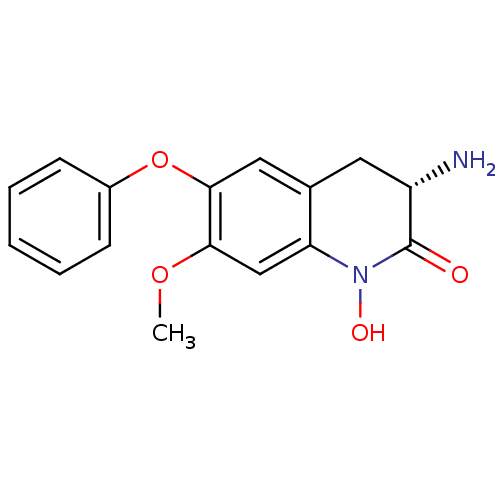
(CHEMBL2321943)Show SMILES COc1cc2N(O)C(=O)[C@@H](N)Cc2cc1Oc1ccccc1 |r| Show InChI InChI=1S/C16H16N2O4/c1-21-14-9-13-10(7-12(17)16(19)18(13)20)8-15(14)22-11-5-3-2-4-6-11/h2-6,8-9,12,20H,7,17H2,1H3/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Irreversible inhibition of human KAT2 using L-kynurenine as substrate measured every 5 mins over 16 hrs by SpectraMax plate reader analysis |
ACS Med Chem Lett 4: 37-40 (2013)
Article DOI: 10.1021/ml300237v
BindingDB Entry DOI: 10.7270/Q2KH0PNV |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50073823

(CHEMBL24871 | {4-[((S)-1-Acetyl-pyrrolidine-2-carb...)Show SMILES CC(C)C[C@@H](NC(=O)[C@H](CCc1ccccc1)CP(O)(=O)CCCCNC(=O)[C@@H]1CCCN1C(C)=O)C(=O)Nc1ccccc1 Show InChI InChI=1S/C34H49N4O6P/c1-25(2)23-30(33(41)36-29-15-8-5-9-16-29)37-32(40)28(19-18-27-13-6-4-7-14-27)24-45(43,44)22-11-10-20-35-34(42)31-17-12-21-38(31)26(3)39/h4-9,13-16,25,28,30-31H,10-12,17-24H2,1-3H3,(H,35,42)(H,36,41)(H,37,40)(H,43,44)/t28-,30-,31+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Inhibition of recombinant matrix metalloprotease-2 (MMP-2) |
Bioorg Med Chem Lett 9: 127-32 (1999)
BindingDB Entry DOI: 10.7270/Q2JD4VZ4 |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50426341
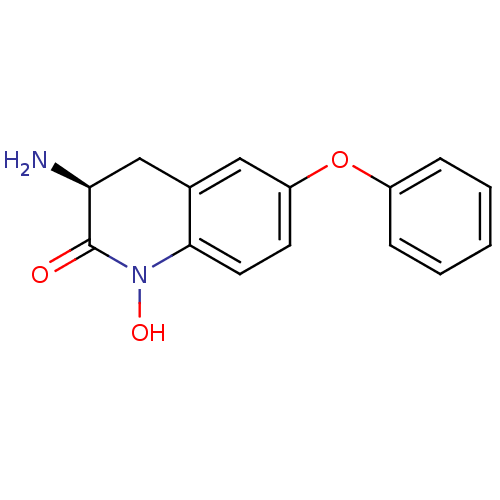
(CHEMBL2321944)Show InChI InChI=1S/C15H14N2O3/c16-13-9-10-8-12(20-11-4-2-1-3-5-11)6-7-14(10)17(19)15(13)18/h1-8,13,19H,9,16H2/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Irreversible inhibition of human KAT2 using L-kynurenine as substrate measured every 5 mins over 16 hrs by SpectraMax plate reader analysis |
ACS Med Chem Lett 4: 37-40 (2013)
Article DOI: 10.1021/ml300237v
BindingDB Entry DOI: 10.7270/Q2KH0PNV |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50386310
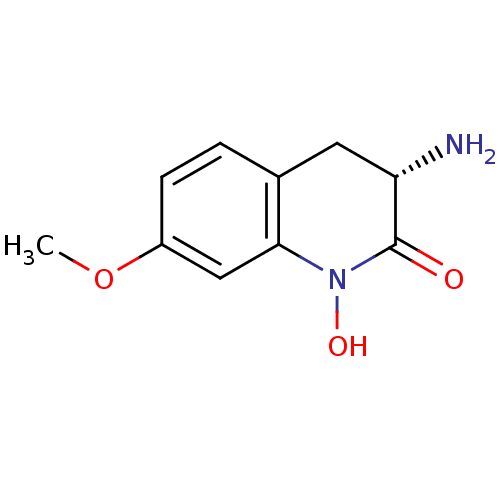
(CHEMBL2049092)Show InChI InChI=1S/C10H12N2O3/c1-15-7-3-2-6-4-8(11)10(13)12(14)9(6)5-7/h2-3,5,8,14H,4,11H2,1H3/t8-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Irreversible inhibition of human KAT2 using L-kynurenine as substrate measured every 5 mins over 16 hrs by SpectraMax plate reader analysis |
ACS Med Chem Lett 4: 37-40 (2013)
Article DOI: 10.1021/ml300237v
BindingDB Entry DOI: 10.7270/Q2KH0PNV |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50386292
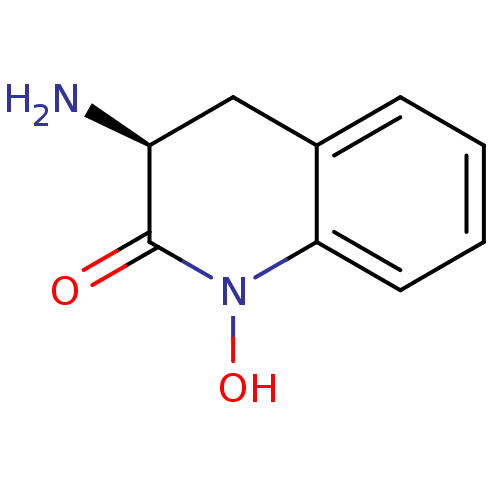
(CHEMBL2047851)Show InChI InChI=1S/C9H10N2O2/c10-7-5-6-3-1-2-4-8(6)11(13)9(7)12/h1-4,7,13H,5,10H2/t7-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Irreversible inhibition of human KAT2 using L-kynurenine as substrate measured every 5 mins over 16 hrs by SpectraMax plate reader analysis |
ACS Med Chem Lett 4: 37-40 (2013)
Article DOI: 10.1021/ml300237v
BindingDB Entry DOI: 10.7270/Q2KH0PNV |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50073839

(CHEMBL283066 | [4-(1,3-Dioxo-1,3-dihydro-isoindol-...)Show SMILES CC(C)C[C@@H](NC(=O)[C@H](CCc1ccccc1)CP(O)(=O)CCCCN1C(=O)c2ccccc2C1=O)C(=O)Nc1ccccc1 Show InChI InChI=1S/C35H42N3O6P/c1-25(2)23-31(33(40)36-28-15-7-4-8-16-28)37-32(39)27(20-19-26-13-5-3-6-14-26)24-45(43,44)22-12-11-21-38-34(41)29-17-9-10-18-30(29)35(38)42/h3-10,13-18,25,27,31H,11-12,19-24H2,1-2H3,(H,36,40)(H,37,39)(H,43,44)/t27-,31-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Inhibition of recombinant matrix metalloprotease-2 (MMP-2) |
Bioorg Med Chem Lett 9: 127-32 (1999)
BindingDB Entry DOI: 10.7270/Q2JD4VZ4 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50073823

(CHEMBL24871 | {4-[((S)-1-Acetyl-pyrrolidine-2-carb...)Show SMILES CC(C)C[C@@H](NC(=O)[C@H](CCc1ccccc1)CP(O)(=O)CCCCNC(=O)[C@@H]1CCCN1C(C)=O)C(=O)Nc1ccccc1 Show InChI InChI=1S/C34H49N4O6P/c1-25(2)23-30(33(41)36-29-15-8-5-9-16-29)37-32(40)28(19-18-27-13-6-4-7-14-27)24-45(43,44)22-11-10-20-35-34(42)31-17-12-21-38(31)26(3)39/h4-9,13-16,25,28,30-31H,10-12,17-24H2,1-3H3,(H,35,42)(H,36,41)(H,37,40)(H,43,44)/t28-,30-,31+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Inhibition of recombinant matrix metalloprotease-3 (MMP-3) |
Bioorg Med Chem Lett 9: 127-32 (1999)
BindingDB Entry DOI: 10.7270/Q2JD4VZ4 |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM107727
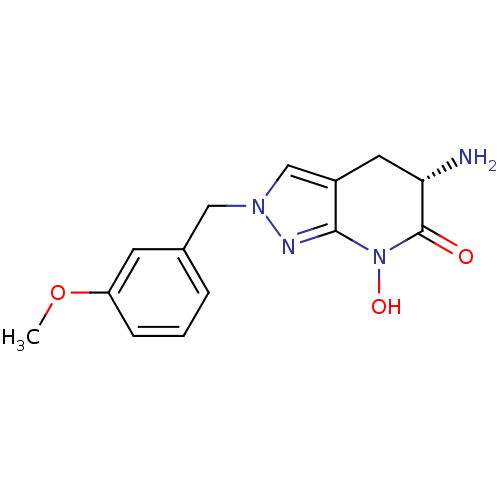
(US8933095, 11)Show SMILES COc1cccc(Cn2cc3C[C@H](N)C(=O)N(O)c3n2)c1 |r| Show InChI InChI=1S/C14H16N4O3/c1-21-11-4-2-3-9(5-11)7-17-8-10-6-12(15)14(19)18(20)13(10)16-17/h2-5,8,12,20H,6-7,15H2,1H3/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 8.93 | n/a | n/a | n/a | n/a | n/a | 37 |
Pfizer Inc.
US Patent
| Assay Description
Formation of kynurenic acid (KYNA) is indirectly assessed by a decrease in light absorbance at 370 nm (OD370) as the L-kynurenine (KYN) substrate is ... |
US Patent US8933095 (2015)
BindingDB Entry DOI: 10.7270/Q2J9653C |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM107727

(US8933095, 11)Show SMILES COc1cccc(Cn2cc3C[C@H](N)C(=O)N(O)c3n2)c1 |r| Show InChI InChI=1S/C14H16N4O3/c1-21-11-4-2-3-9(5-11)7-17-8-10-6-12(15)14(19)18(20)13(10)16-17/h2-5,8,12,20H,6-7,15H2,1H3/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 8.93 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
US Patent
| Assay Description
Formation of kynurenic acid (KYNA) is indirectly assessed by a decrease in light absorbance at 370 nm (OD370) as the L-kynurenine (KYN) substrate is ... |
US Patent US8598200 (2013)
BindingDB Entry DOI: 10.7270/Q2G73CBJ |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM107721
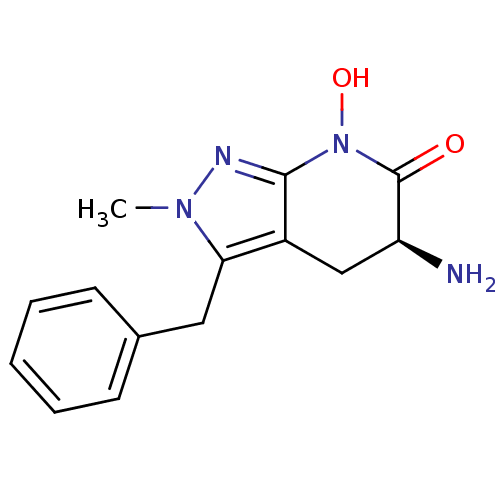
(US8933095, 3)Show InChI InChI=1S/C14H16N4O2/c1-17-12(7-9-5-3-2-4-6-9)10-8-11(15)14(19)18(20)13(10)16-17/h2-6,11,20H,7-8,15H2,1H3/t11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 11.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
US Patent
| Assay Description
Formation of kynurenic acid (KYNA) is indirectly assessed by a decrease in light absorbance at 370 nm (OD370) as the L-kynurenine (KYN) substrate is ... |
US Patent US8598200 (2013)
BindingDB Entry DOI: 10.7270/Q2G73CBJ |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM107721

(US8933095, 3)Show InChI InChI=1S/C14H16N4O2/c1-17-12(7-9-5-3-2-4-6-9)10-8-11(15)14(19)18(20)13(10)16-17/h2-6,11,20H,7-8,15H2,1H3/t11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 11.5 | n/a | n/a | n/a | n/a | n/a | 37 |
Pfizer Inc.
US Patent
| Assay Description
Formation of kynurenic acid (KYNA) is indirectly assessed by a decrease in light absorbance at 370 nm (OD370) as the L-kynurenine (KYN) substrate is ... |
US Patent US8933095 (2015)
BindingDB Entry DOI: 10.7270/Q2J9653C |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50073830

((4-Benzyl-benzyl)-[2-((R)-2,2-dimethyl-1-methylcar...)Show SMILES CNC(=O)[C@H](NC(=O)C(CCc1ccccc1)CP(O)(=O)Cc1ccc(Cc2ccccc2)cc1)C(C)(C)C Show InChI InChI=1S/C32H41N2O4P/c1-32(2,3)29(31(36)33-4)34-30(35)28(20-19-24-11-7-5-8-12-24)23-39(37,38)22-27-17-15-26(16-18-27)21-25-13-9-6-10-14-25/h5-18,28-29H,19-23H2,1-4H3,(H,33,36)(H,34,35)(H,37,38)/t28?,29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Matrix metalloproteinase-13 (MMP-13) |
Bioorg Med Chem Lett 9: 127-32 (1999)
BindingDB Entry DOI: 10.7270/Q2JD4VZ4 |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50386310

(CHEMBL2049092)Show InChI InChI=1S/C10H12N2O3/c1-15-7-3-2-6-4-8(11)10(13)12(14)9(6)5-7/h2-3,5,8,14H,4,11H2,1H3/t8-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human KAT2 using L-kynurenine as substrate measured after 15-20 hrs by SpectraMax plate reader analysis |
ACS Med Chem Lett 4: 37-40 (2013)
Article DOI: 10.1021/ml300237v
BindingDB Entry DOI: 10.7270/Q2KH0PNV |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50386310

(CHEMBL2049092)Show InChI InChI=1S/C10H12N2O3/c1-15-7-3-2-6-4-8(11)10(13)12(14)9(6)5-7/h2-3,5,8,14H,4,11H2,1H3/t8-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant KAT2 assessed as conversion of L-kynurenine into kynurenic acid after 15 to 20 hrs |
ACS Med Chem Lett 3: 187-192 (2012)
Article DOI: 10.1021/ml200204m
BindingDB Entry DOI: 10.7270/Q2MC9135 |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM107747
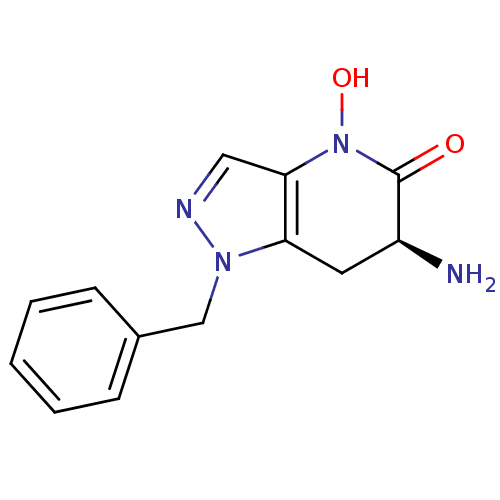
(CHEMBL2347115 | US8933095, 4)Show InChI InChI=1S/C13H14N4O2/c14-10-6-11-12(17(19)13(10)18)7-15-16(11)8-9-4-2-1-3-5-9/h1-5,7,10,19H,6,8,14H2/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 22.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
US Patent
| Assay Description
Formation of kynurenic acid (KYNA) is indirectly assessed by a decrease in light absorbance at 370 nm (OD370) as the L-kynurenine (KYN) substrate is ... |
US Patent US8598200 (2013)
BindingDB Entry DOI: 10.7270/Q2G73CBJ |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM107747

(CHEMBL2347115 | US8933095, 4)Show InChI InChI=1S/C13H14N4O2/c14-10-6-11-12(17(19)13(10)18)7-15-16(11)8-9-4-2-1-3-5-9/h1-5,7,10,19H,6,8,14H2/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 22.5 | n/a | n/a | n/a | n/a | n/a | 37 |
Pfizer Inc.
US Patent
| Assay Description
Formation of kynurenic acid (KYNA) is indirectly assessed by a decrease in light absorbance at 370 nm (OD370) as the L-kynurenine (KYN) substrate is ... |
US Patent US8933095 (2015)
BindingDB Entry DOI: 10.7270/Q2J9653C |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50386292

(CHEMBL2047851)Show InChI InChI=1S/C9H10N2O2/c10-7-5-6-3-1-2-4-8(6)11(13)9(7)12/h1-4,7,13H,5,10H2/t7-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human KAT2 using L-kynurenine as substrate measured after 15-20 hrs by SpectraMax plate reader analysis |
ACS Med Chem Lett 4: 37-40 (2013)
Article DOI: 10.1021/ml300237v
BindingDB Entry DOI: 10.7270/Q2KH0PNV |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50426341

(CHEMBL2321944)Show InChI InChI=1S/C15H14N2O3/c16-13-9-10-8-12(20-11-4-2-1-3-5-11)6-7-14(10)17(19)15(13)18/h1-8,13,19H,9,16H2/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human KAT2 using L-kynurenine as substrate measured after 15-20 hrs by SpectraMax plate reader analysis |
ACS Med Chem Lett 4: 37-40 (2013)
Article DOI: 10.1021/ml300237v
BindingDB Entry DOI: 10.7270/Q2KH0PNV |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50386292

(CHEMBL2047851)Show InChI InChI=1S/C9H10N2O2/c10-7-5-6-3-1-2-4-8(6)11(13)9(7)12/h1-4,7,13H,5,10H2/t7-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant KAT2 assessed as conversion of L-kynurenine into kynurenic acid after 15 to 20 hrs |
ACS Med Chem Lett 3: 187-192 (2012)
Article DOI: 10.1021/ml200204m
BindingDB Entry DOI: 10.7270/Q2MC9135 |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM107739

(US8933095, 23)Show SMILES CCn1nc2N(O)C(=O)[C@@H](N)Cc2c1Cc1ccccc1 |r| Show InChI InChI=1S/C15H18N4O2/c1-2-18-13(8-10-6-4-3-5-7-10)11-9-12(16)15(20)19(21)14(11)17-18/h3-7,12,21H,2,8-9,16H2,1H3/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 24.3 | n/a | n/a | n/a | n/a | n/a | 37 |
Pfizer Inc.
US Patent
| Assay Description
Formation of kynurenic acid (KYNA) is indirectly assessed by a decrease in light absorbance at 370 nm (OD370) as the L-kynurenine (KYN) substrate is ... |
US Patent US8933095 (2015)
BindingDB Entry DOI: 10.7270/Q2J9653C |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM107739

(US8933095, 23)Show SMILES CCn1nc2N(O)C(=O)[C@@H](N)Cc2c1Cc1ccccc1 |r| Show InChI InChI=1S/C15H18N4O2/c1-2-18-13(8-10-6-4-3-5-7-10)11-9-12(16)15(20)19(21)14(11)17-18/h3-7,12,21H,2,8-9,16H2,1H3/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 24.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
US Patent
| Assay Description
Formation of kynurenic acid (KYNA) is indirectly assessed by a decrease in light absorbance at 370 nm (OD370) as the L-kynurenine (KYN) substrate is ... |
US Patent US8598200 (2013)
BindingDB Entry DOI: 10.7270/Q2G73CBJ |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM107730
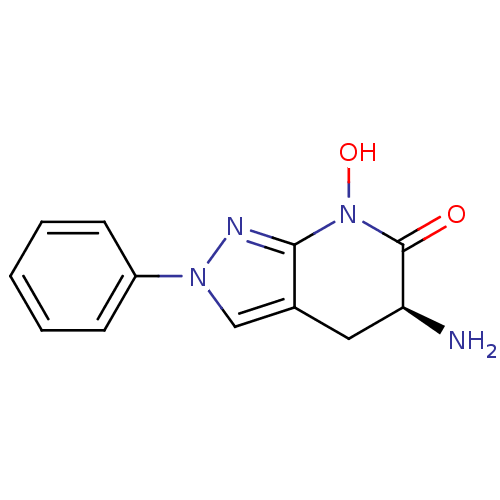
(CHEMBL2347108 | US8933095, 14)Show InChI InChI=1S/C12H12N4O2/c13-10-6-8-7-15(9-4-2-1-3-5-9)14-11(8)16(18)12(10)17/h1-5,7,10,18H,6,13H2/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 24.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
US Patent
| Assay Description
Formation of kynurenic acid (KYNA) is indirectly assessed by a decrease in light absorbance at 370 nm (OD370) as the L-kynurenine (KYN) substrate is ... |
US Patent US8598200 (2013)
BindingDB Entry DOI: 10.7270/Q2G73CBJ |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM107730

(CHEMBL2347108 | US8933095, 14)Show InChI InChI=1S/C12H12N4O2/c13-10-6-8-7-15(9-4-2-1-3-5-9)14-11(8)16(18)12(10)17/h1-5,7,10,18H,6,13H2/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 24.3 | n/a | n/a | n/a | n/a | n/a | 37 |
Pfizer Inc.
US Patent
| Assay Description
Formation of kynurenic acid (KYNA) is indirectly assessed by a decrease in light absorbance at 370 nm (OD370) as the L-kynurenine (KYN) substrate is ... |
US Patent US8933095 (2015)
BindingDB Entry DOI: 10.7270/Q2J9653C |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM107731

(US8933095, 15)Show SMILES COc1ccc(Cn2cc3C[C@H](N)C(=O)N(O)c3n2)cc1 |r| Show InChI InChI=1S/C14H16N4O3/c1-21-11-4-2-9(3-5-11)7-17-8-10-6-12(15)14(19)18(20)13(10)16-17/h2-5,8,12,20H,6-7,15H2,1H3/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 28.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
US Patent
| Assay Description
Formation of kynurenic acid (KYNA) is indirectly assessed by a decrease in light absorbance at 370 nm (OD370) as the L-kynurenine (KYN) substrate is ... |
US Patent US8598200 (2013)
BindingDB Entry DOI: 10.7270/Q2G73CBJ |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM107731

(US8933095, 15)Show SMILES COc1ccc(Cn2cc3C[C@H](N)C(=O)N(O)c3n2)cc1 |r| Show InChI InChI=1S/C14H16N4O3/c1-21-11-4-2-9(3-5-11)7-17-8-10-6-12(15)14(19)18(20)13(10)16-17/h2-5,8,12,20H,6-7,15H2,1H3/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 28.3 | n/a | n/a | n/a | n/a | n/a | 37 |
Pfizer Inc.
US Patent
| Assay Description
Formation of kynurenic acid (KYNA) is indirectly assessed by a decrease in light absorbance at 370 nm (OD370) as the L-kynurenine (KYN) substrate is ... |
US Patent US8933095 (2015)
BindingDB Entry DOI: 10.7270/Q2J9653C |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50386294
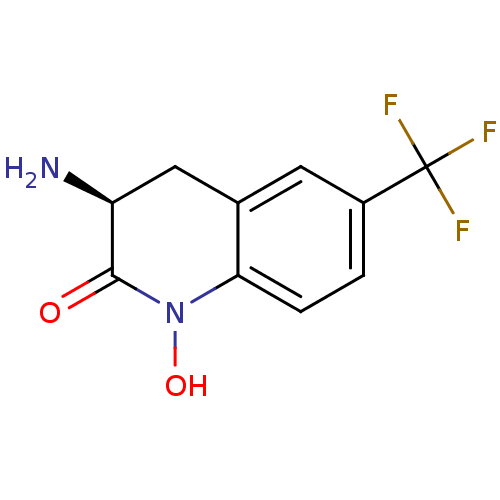
(CHEMBL2049095)Show InChI InChI=1S/C10H9F3N2O2/c11-10(12,13)6-1-2-8-5(3-6)4-7(14)9(16)15(8)17/h1-3,7,17H,4,14H2/t7-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant KAT2 assessed as conversion of L-kynurenine into kynurenic acid after 15 to 20 hrs |
ACS Med Chem Lett 3: 187-192 (2012)
Article DOI: 10.1021/ml200204m
BindingDB Entry DOI: 10.7270/Q2MC9135 |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50386308
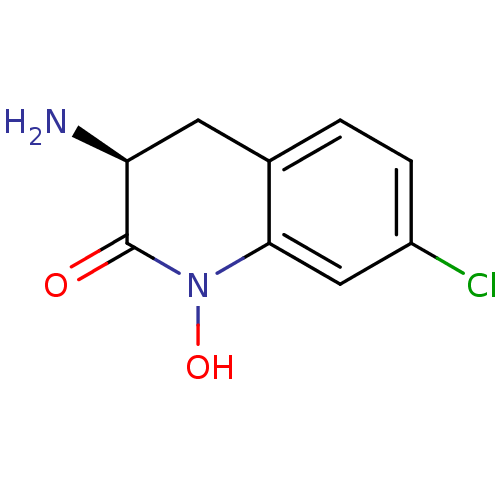
(CHEMBL2047861)Show InChI InChI=1S/C9H9ClN2O2/c10-6-2-1-5-3-7(11)9(13)12(14)8(5)4-6/h1-2,4,7,14H,3,11H2/t7-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant KAT2 assessed as conversion of L-kynurenine into kynurenic acid after 15 to 20 hrs |
ACS Med Chem Lett 3: 187-192 (2012)
Article DOI: 10.1021/ml200204m
BindingDB Entry DOI: 10.7270/Q2MC9135 |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50386312
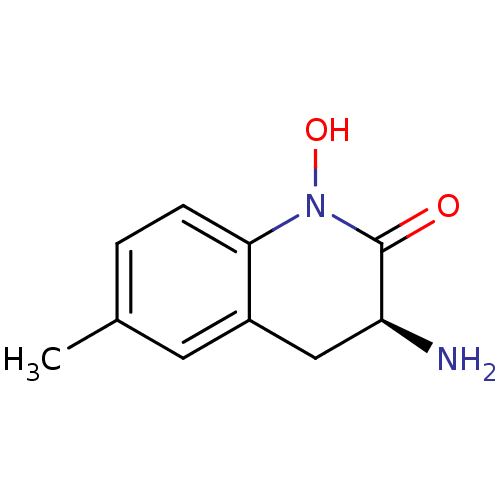
(CHEMBL2049094)Show InChI InChI=1S/C10H12N2O2/c1-6-2-3-9-7(4-6)5-8(11)10(13)12(9)14/h2-4,8,14H,5,11H2,1H3/t8-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant KAT2 assessed as conversion of L-kynurenine into kynurenic acid after 15 to 20 hrs |
ACS Med Chem Lett 3: 187-192 (2012)
Article DOI: 10.1021/ml200204m
BindingDB Entry DOI: 10.7270/Q2MC9135 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50073822
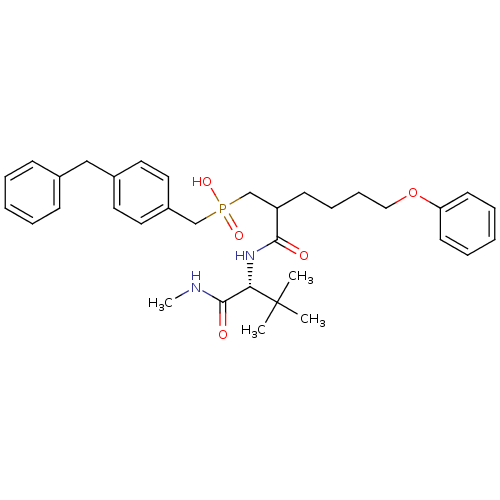
((4-Benzyl-benzyl)-[2-((R)-2,2-dimethyl-1-methylcar...)Show SMILES CNC(=O)[C@H](NC(=O)C(CCCCOc1ccccc1)CP(O)(=O)Cc1ccc(Cc2ccccc2)cc1)C(C)(C)C Show InChI InChI=1S/C34H45N2O5P/c1-34(2,3)31(33(38)35-4)36-32(37)29(15-11-12-22-41-30-16-9-6-10-17-30)25-42(39,40)24-28-20-18-27(19-21-28)23-26-13-7-5-8-14-26/h5-10,13-14,16-21,29,31H,11-12,15,22-25H2,1-4H3,(H,35,38)(H,36,37)(H,39,40)/t29?,31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Matrix metalloproteinase-13 (MMP-13) |
Bioorg Med Chem Lett 9: 127-32 (1999)
BindingDB Entry DOI: 10.7270/Q2JD4VZ4 |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM107745
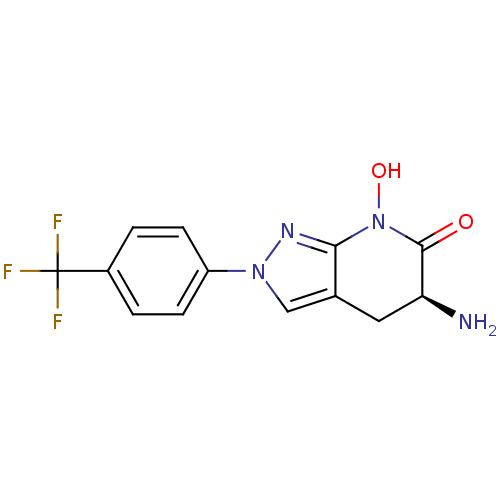
(US8933095, 29)Show SMILES N[C@H]1Cc2cn(nc2N(O)C1=O)-c1ccc(cc1)C(F)(F)F |r| Show InChI InChI=1S/C13H11F3N4O2/c14-13(15,16)8-1-3-9(4-2-8)19-6-7-5-10(17)12(21)20(22)11(7)18-19/h1-4,6,10,22H,5,17H2/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 31.6 | n/a | n/a | n/a | n/a | n/a | 37 |
Pfizer Inc.
US Patent
| Assay Description
Formation of kynurenic acid (KYNA) is indirectly assessed by a decrease in light absorbance at 370 nm (OD370) as the L-kynurenine (KYN) substrate is ... |
US Patent US8933095 (2015)
BindingDB Entry DOI: 10.7270/Q2J9653C |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM107745

(US8933095, 29)Show SMILES N[C@H]1Cc2cn(nc2N(O)C1=O)-c1ccc(cc1)C(F)(F)F |r| Show InChI InChI=1S/C13H11F3N4O2/c14-13(15,16)8-1-3-9(4-2-8)19-6-7-5-10(17)12(21)20(22)11(7)18-19/h1-4,6,10,22H,5,17H2/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 31.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
US Patent
| Assay Description
Formation of kynurenic acid (KYNA) is indirectly assessed by a decrease in light absorbance at 370 nm (OD370) as the L-kynurenine (KYN) substrate is ... |
US Patent US8598200 (2013)
BindingDB Entry DOI: 10.7270/Q2G73CBJ |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM107724
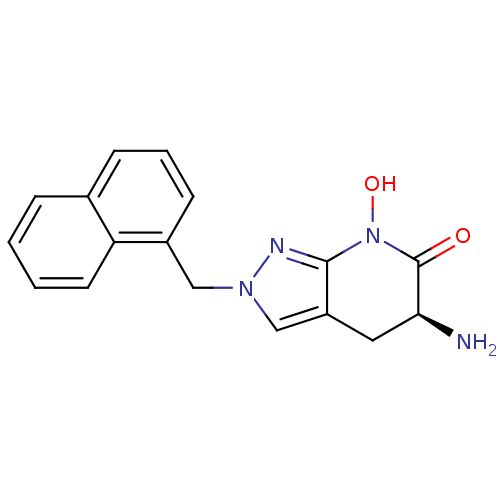
(US8933095, 8)Show SMILES N[C@H]1Cc2cn(Cc3cccc4ccccc34)nc2N(O)C1=O |r| Show InChI InChI=1S/C17H16N4O2/c18-15-8-13-10-20(19-16(13)21(23)17(15)22)9-12-6-3-5-11-4-1-2-7-14(11)12/h1-7,10,15,23H,8-9,18H2/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
US Patent
| Assay Description
Formation of kynurenic acid (KYNA) is indirectly assessed by a decrease in light absorbance at 370 nm (OD370) as the L-kynurenine (KYN) substrate is ... |
US Patent US8598200 (2013)
BindingDB Entry DOI: 10.7270/Q2G73CBJ |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50386311
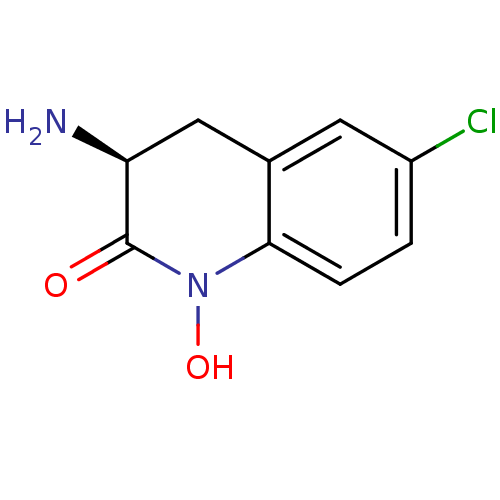
(CHEMBL2049093)Show InChI InChI=1S/C9H9ClN2O2/c10-6-1-2-8-5(3-6)4-7(11)9(13)12(8)14/h1-3,7,14H,4,11H2/t7-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant KAT2 assessed as conversion of L-kynurenine into kynurenic acid after 15 to 20 hrs |
ACS Med Chem Lett 3: 187-192 (2012)
Article DOI: 10.1021/ml200204m
BindingDB Entry DOI: 10.7270/Q2MC9135 |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM107724

(US8933095, 8)Show SMILES N[C@H]1Cc2cn(Cc3cccc4ccccc34)nc2N(O)C1=O |r| Show InChI InChI=1S/C17H16N4O2/c18-15-8-13-10-20(19-16(13)21(23)17(15)22)9-12-6-3-5-11-4-1-2-7-14(11)12/h1-7,10,15,23H,8-9,18H2/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | 37 |
Pfizer Inc.
US Patent
| Assay Description
Formation of kynurenic acid (KYNA) is indirectly assessed by a decrease in light absorbance at 370 nm (OD370) as the L-kynurenine (KYN) substrate is ... |
US Patent US8933095 (2015)
BindingDB Entry DOI: 10.7270/Q2J9653C |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50426340

(CHEMBL2321943)Show SMILES COc1cc2N(O)C(=O)[C@@H](N)Cc2cc1Oc1ccccc1 |r| Show InChI InChI=1S/C16H16N2O4/c1-21-14-9-13-10(7-12(17)16(19)18(13)20)8-15(14)22-11-5-3-2-4-6-11/h2-6,8-9,12,20H,7,17H2,1H3/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human KAT2 using L-kynurenine as substrate measured after 15-20 hrs by SpectraMax plate reader analysis |
ACS Med Chem Lett 4: 37-40 (2013)
Article DOI: 10.1021/ml300237v
BindingDB Entry DOI: 10.7270/Q2KH0PNV |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50386309
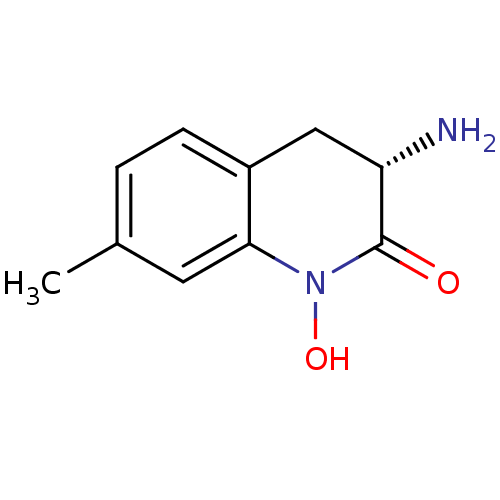
(CHEMBL2047862)Show InChI InChI=1S/C10H12N2O2/c1-6-2-3-7-5-8(11)10(13)12(14)9(7)4-6/h2-4,8,14H,5,11H2,1H3/t8-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant KAT2 assessed as conversion of L-kynurenine into kynurenic acid after 15 to 20 hrs |
ACS Med Chem Lett 3: 187-192 (2012)
Article DOI: 10.1021/ml200204m
BindingDB Entry DOI: 10.7270/Q2MC9135 |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM107743

(US8933095, 27)Show SMILES Cn1nc2N(O)C(=O)[C@@H](N)Cc2c1Cc1ccc(cc1)C(F)(F)F |r| Show InChI InChI=1S/C15H15F3N4O2/c1-21-12(6-8-2-4-9(5-3-8)15(16,17)18)10-7-11(19)14(23)22(24)13(10)20-21/h2-5,11,24H,6-7,19H2,1H3/t11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
US Patent
| Assay Description
Formation of kynurenic acid (KYNA) is indirectly assessed by a decrease in light absorbance at 370 nm (OD370) as the L-kynurenine (KYN) substrate is ... |
US Patent US8598200 (2013)
BindingDB Entry DOI: 10.7270/Q2G73CBJ |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM107743

(US8933095, 27)Show SMILES Cn1nc2N(O)C(=O)[C@@H](N)Cc2c1Cc1ccc(cc1)C(F)(F)F |r| Show InChI InChI=1S/C15H15F3N4O2/c1-21-12(6-8-2-4-9(5-3-8)15(16,17)18)10-7-11(19)14(23)22(24)13(10)20-21/h2-5,11,24H,6-7,19H2,1H3/t11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | 37 |
Pfizer Inc.
US Patent
| Assay Description
Formation of kynurenic acid (KYNA) is indirectly assessed by a decrease in light absorbance at 370 nm (OD370) as the L-kynurenine (KYN) substrate is ... |
US Patent US8933095 (2015)
BindingDB Entry DOI: 10.7270/Q2J9653C |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM107744
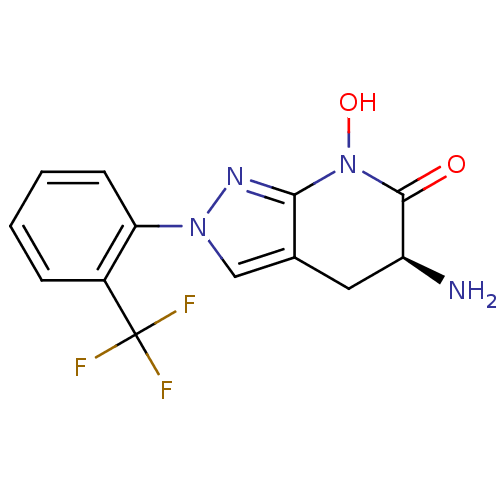
(US8933095, 28)Show SMILES N[C@H]1Cc2cn(nc2N(O)C1=O)-c1ccccc1C(F)(F)F |r| Show InChI InChI=1S/C13H11F3N4O2/c14-13(15,16)8-3-1-2-4-10(8)19-6-7-5-9(17)12(21)20(22)11(7)18-19/h1-4,6,9,22H,5,17H2/t9-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 38.8 | n/a | n/a | n/a | n/a | n/a | 37 |
Pfizer Inc.
US Patent
| Assay Description
Formation of kynurenic acid (KYNA) is indirectly assessed by a decrease in light absorbance at 370 nm (OD370) as the L-kynurenine (KYN) substrate is ... |
US Patent US8933095 (2015)
BindingDB Entry DOI: 10.7270/Q2J9653C |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM107744

(US8933095, 28)Show SMILES N[C@H]1Cc2cn(nc2N(O)C1=O)-c1ccccc1C(F)(F)F |r| Show InChI InChI=1S/C13H11F3N4O2/c14-13(15,16)8-3-1-2-4-10(8)19-6-7-5-9(17)12(21)20(22)11(7)18-19/h1-4,6,9,22H,5,17H2/t9-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 38.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
US Patent
| Assay Description
Formation of kynurenic acid (KYNA) is indirectly assessed by a decrease in light absorbance at 370 nm (OD370) as the L-kynurenine (KYN) substrate is ... |
US Patent US8598200 (2013)
BindingDB Entry DOI: 10.7270/Q2G73CBJ |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50386303
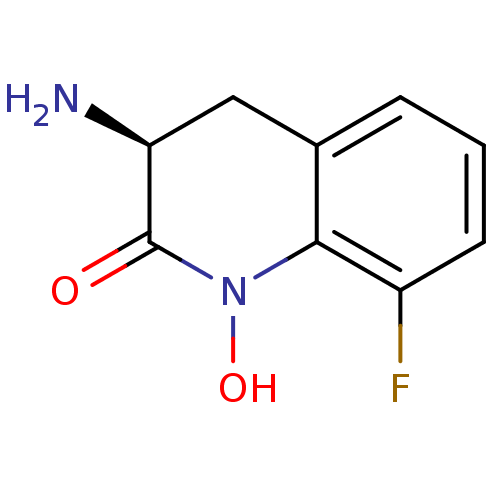
(CHEMBL2047856)Show InChI InChI=1S/C9H9FN2O2/c10-6-3-1-2-5-4-7(11)9(13)12(14)8(5)6/h1-3,7,14H,4,11H2/t7-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant KAT2 assessed as conversion of L-kynurenine into kynurenic acid after 15 to 20 hrs |
ACS Med Chem Lett 3: 187-192 (2012)
Article DOI: 10.1021/ml200204m
BindingDB Entry DOI: 10.7270/Q2MC9135 |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM107742

(US8933095, 26)Show SMILES Cn1nc2N(O)C(=O)[C@@H](N)Cc2c1Cc1ccccc1C(F)(F)F |r| Show InChI InChI=1S/C15H15F3N4O2/c1-21-12(6-8-4-2-3-5-10(8)15(16,17)18)9-7-11(19)14(23)22(24)13(9)20-21/h2-5,11,24H,6-7,19H2,1H3/t11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 40.7 | n/a | n/a | n/a | n/a | n/a | 37 |
Pfizer Inc.
US Patent
| Assay Description
Formation of kynurenic acid (KYNA) is indirectly assessed by a decrease in light absorbance at 370 nm (OD370) as the L-kynurenine (KYN) substrate is ... |
US Patent US8933095 (2015)
BindingDB Entry DOI: 10.7270/Q2J9653C |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM107742

(US8933095, 26)Show SMILES Cn1nc2N(O)C(=O)[C@@H](N)Cc2c1Cc1ccccc1C(F)(F)F |r| Show InChI InChI=1S/C15H15F3N4O2/c1-21-12(6-8-4-2-3-5-10(8)15(16,17)18)9-7-11(19)14(23)22(24)13(9)20-21/h2-5,11,24H,6-7,19H2,1H3/t11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 40.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
US Patent
| Assay Description
Formation of kynurenic acid (KYNA) is indirectly assessed by a decrease in light absorbance at 370 nm (OD370) as the L-kynurenine (KYN) substrate is ... |
US Patent US8598200 (2013)
BindingDB Entry DOI: 10.7270/Q2G73CBJ |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
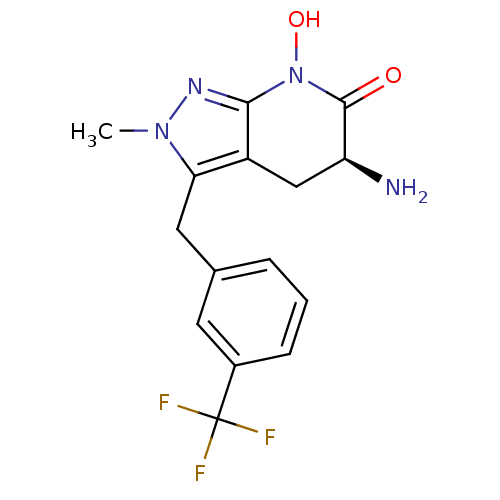
(Homo sapiens (Human)) | BDBM107741
(US8933095, 25)Show SMILES Cn1nc2N(O)C(=O)[C@@H](N)Cc2c1Cc1cccc(c1)C(F)(F)F |r| Show InChI InChI=1S/C15H15F3N4O2/c1-21-12(6-8-3-2-4-9(5-8)15(16,17)18)10-7-11(19)14(23)22(24)13(10)20-21/h2-5,11,24H,6-7,19H2,1H3/t11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 42.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
US Patent
| Assay Description
Formation of kynurenic acid (KYNA) is indirectly assessed by a decrease in light absorbance at 370 nm (OD370) as the L-kynurenine (KYN) substrate is ... |
US Patent US8598200 (2013)
BindingDB Entry DOI: 10.7270/Q2G73CBJ |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM107741

(US8933095, 25)Show SMILES Cn1nc2N(O)C(=O)[C@@H](N)Cc2c1Cc1cccc(c1)C(F)(F)F |r| Show InChI InChI=1S/C15H15F3N4O2/c1-21-12(6-8-3-2-4-9(5-8)15(16,17)18)10-7-11(19)14(23)22(24)13(10)20-21/h2-5,11,24H,6-7,19H2,1H3/t11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 42.2 | n/a | n/a | n/a | n/a | n/a | 37 |
Pfizer Inc.
US Patent
| Assay Description
Formation of kynurenic acid (KYNA) is indirectly assessed by a decrease in light absorbance at 370 nm (OD370) as the L-kynurenine (KYN) substrate is ... |
US Patent US8933095 (2015)
BindingDB Entry DOI: 10.7270/Q2J9653C |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM107722
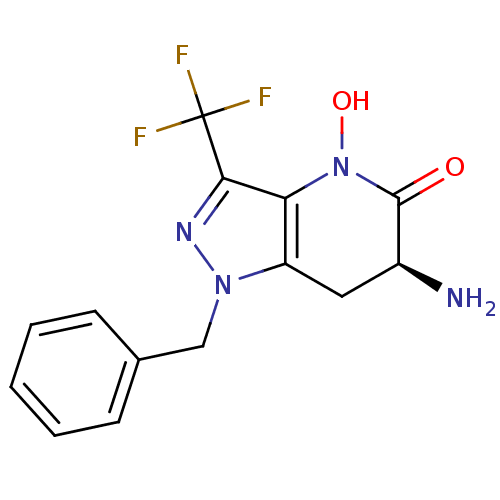
(CHEMBL2347114 | US8933095, 5)Show SMILES N[C@H]1Cc2c(N(O)C1=O)c(nn2Cc1ccccc1)C(F)(F)F |r| Show InChI InChI=1S/C14H13F3N4O2/c15-14(16,17)12-11-10(6-9(18)13(22)21(11)23)20(19-12)7-8-4-2-1-3-5-8/h1-5,9,23H,6-7,18H2/t9-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 42.7 | n/a | n/a | n/a | n/a | n/a | 37 |
Pfizer Inc.
US Patent
| Assay Description
Formation of kynurenic acid (KYNA) is indirectly assessed by a decrease in light absorbance at 370 nm (OD370) as the L-kynurenine (KYN) substrate is ... |
US Patent US8933095 (2015)
BindingDB Entry DOI: 10.7270/Q2J9653C |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM107723
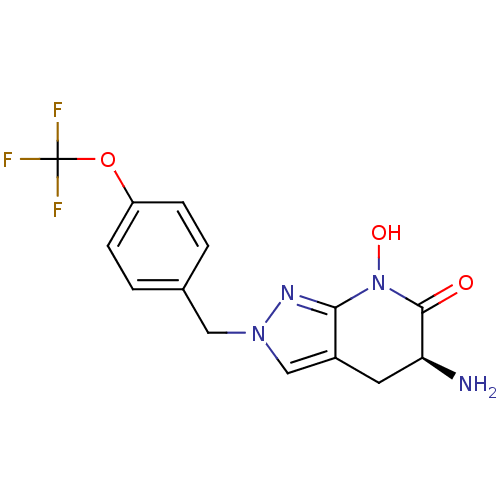
(US8933095, 6)Show SMILES N[C@H]1Cc2cn(Cc3ccc(OC(F)(F)F)cc3)nc2N(O)C1=O |r| Show InChI InChI=1S/C14H13F3N4O3/c15-14(16,17)24-10-3-1-8(2-4-10)6-20-7-9-5-11(18)13(22)21(23)12(9)19-20/h1-4,7,11,23H,5-6,18H2/t11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 43.6 | n/a | n/a | n/a | n/a | n/a | 37 |
Pfizer Inc.
US Patent
| Assay Description
Formation of kynurenic acid (KYNA) is indirectly assessed by a decrease in light absorbance at 370 nm (OD370) as the L-kynurenine (KYN) substrate is ... |
US Patent US8933095 (2015)
BindingDB Entry DOI: 10.7270/Q2J9653C |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM107723

(US8933095, 6)Show SMILES N[C@H]1Cc2cn(Cc3ccc(OC(F)(F)F)cc3)nc2N(O)C1=O |r| Show InChI InChI=1S/C14H13F3N4O3/c15-14(16,17)24-10-3-1-8(2-4-10)6-20-7-9-5-11(18)13(22)21(23)12(9)19-20/h1-4,7,11,23H,5-6,18H2/t11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 43.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
US Patent
| Assay Description
Formation of kynurenic acid (KYNA) is indirectly assessed by a decrease in light absorbance at 370 nm (OD370) as the L-kynurenine (KYN) substrate is ... |
US Patent US8598200 (2013)
BindingDB Entry DOI: 10.7270/Q2G73CBJ |
More data for this
Ligand-Target Pair | |
Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM107722

(CHEMBL2347114 | US8933095, 5)Show SMILES N[C@H]1Cc2c(N(O)C1=O)c(nn2Cc1ccccc1)C(F)(F)F |r| Show InChI InChI=1S/C14H13F3N4O2/c15-14(16,17)12-11-10(6-9(18)13(22)21(11)23)20(19-12)7-8-4-2-1-3-5-8/h1-5,9,23H,6-7,18H2/t9-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 43.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
US Patent
| Assay Description
Formation of kynurenic acid (KYNA) is indirectly assessed by a decrease in light absorbance at 370 nm (OD370) as the L-kynurenine (KYN) substrate is ... |
US Patent US8598200 (2013)
BindingDB Entry DOI: 10.7270/Q2G73CBJ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data