

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H4 receptor (Rattus norvegicus (rat)) | BDBM22566 (5-chloro-2-[(4-methylpiperazin-1-yl)carbonyl]-1H-i...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2.60 | -49.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The Ki values were calculated based on an experimentally determined appropriate Kd value according to Cheng and Prusoff. | J Pharmacol Exp Ther 309: 404-13 (2004) Article DOI: 10.1124/jpet.103.061754 BindingDB Entry DOI: 10.7270/Q2VX0DTD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM249277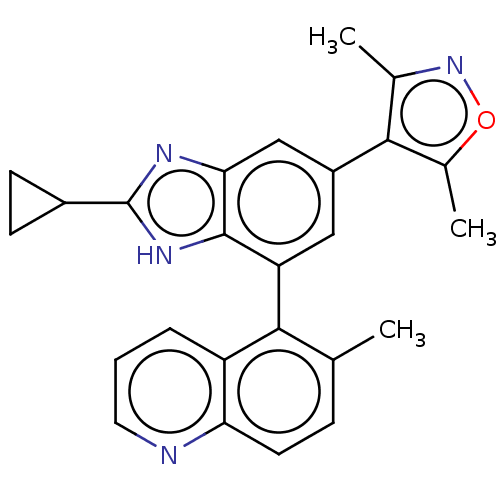 (US10017501, Compound 1020-18 | US9458145, 1020-18) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of Cy5-linked JQ1 probe binding to recombinant human N-terminal TEV-cleavable hexa-histidine tagged BRD4 bromodomain2 after 60 mins by HTR... | Bioorg Med Chem 27: 457-469 (2019) Article DOI: 10.1016/j.bmc.2018.11.020 BindingDB Entry DOI: 10.7270/Q2HD7ZZ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM22566 (5-chloro-2-[(4-methylpiperazin-1-yl)carbonyl]-1H-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 4.10 | -47.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The Ki values were calculated based on an experimentally determined appropriate Kd value according to Cheng and Prusoff. | J Pharmacol Exp Ther 309: 404-13 (2004) Article DOI: 10.1124/jpet.103.061754 BindingDB Entry DOI: 10.7270/Q2VX0DTD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Mus musculus (mouse)) | BDBM22566 (5-chloro-2-[(4-methylpiperazin-1-yl)carbonyl]-1H-i...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 4.60 | -47.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The Ki values were calculated based on an experimentally determined appropriate Kd value according to Cheng and Prusoff. | J Pharmacol Exp Ther 309: 404-13 (2004) Article DOI: 10.1124/jpet.103.061754 BindingDB Entry DOI: 10.7270/Q2VX0DTD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM249277 (US10017501, Compound 1020-18 | US9458145, 1020-18) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of Cy5-linked JQ1 probe binding to recombinant human N-terminal TEV-cleavable hexa-histidine tagged BRD4 bromodomain1 after 60 mins by HTR... | Bioorg Med Chem 27: 457-469 (2019) Article DOI: 10.1016/j.bmc.2018.11.020 BindingDB Entry DOI: 10.7270/Q2HD7ZZ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM249277 (US10017501, Compound 1020-18 | US9458145, 1020-18) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of tetra-acetylated Histone H4 peptide binding to recombinant human N-terminal TEV-cleavable hexa-histidine tagged BRD4 bromodomain1 after... | Bioorg Med Chem 27: 457-469 (2019) Article DOI: 10.1016/j.bmc.2018.11.020 BindingDB Entry DOI: 10.7270/Q2HD7ZZ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50380682 (CHEMBL2017291 | I-BET151 (16)) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of tetra-acetylated Histone H4 peptide binding to recombinant human N-terminal TEV-cleavable hexa-histidine tagged BRD4 bromodomain1 after... | Bioorg Med Chem 27: 457-469 (2019) Article DOI: 10.1016/j.bmc.2018.11.020 BindingDB Entry DOI: 10.7270/Q2HD7ZZ6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50365463 (CHEMBL1232461) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of Cy5-linked JQ1 probe binding to recombinant human N-terminal TEV-cleavable hexa-histidine tagged BRD4 bromodomain2 after 60 mins by HTR... | Bioorg Med Chem 27: 457-469 (2019) Article DOI: 10.1016/j.bmc.2018.11.020 BindingDB Entry DOI: 10.7270/Q2HD7ZZ6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50365463 (CHEMBL1232461) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 163 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of tetra-acetylated Histone H4 peptide binding to recombinant human N-terminal TEV-cleavable hexa-histidine tagged BRD4 bromodomain1 after... | Bioorg Med Chem 27: 457-469 (2019) Article DOI: 10.1016/j.bmc.2018.11.020 BindingDB Entry DOI: 10.7270/Q2HD7ZZ6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50365463 (CHEMBL1232461) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of Cy5-linked JQ1 probe binding to recombinant human N-terminal TEV-cleavable hexa-histidine tagged BRD4 bromodomain1 after 60 mins by HTR... | Bioorg Med Chem 27: 457-469 (2019) Article DOI: 10.1016/j.bmc.2018.11.020 BindingDB Entry DOI: 10.7270/Q2HD7ZZ6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50380682 (CHEMBL2017291 | I-BET151 (16)) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 385 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of Cy5-linked JQ1 probe binding to recombinant human N-terminal TEV-cleavable hexa-histidine tagged BRD4 bromodomain2 after 60 mins by HTR... | Bioorg Med Chem 27: 457-469 (2019) Article DOI: 10.1016/j.bmc.2018.11.020 BindingDB Entry DOI: 10.7270/Q2HD7ZZ6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histamine H1 receptor (Cavia porcellus (domestic guinea pig)) | BDBM22566 (5-chloro-2-[(4-methylpiperazin-1-yl)carbonyl]-1H-i...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+3 | >-34.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The Ki values were calculated based on an experimentally determined appropriate Kd value according to Cheng and Prusoff. | J Pharmacol Exp Ther 309: 404-13 (2004) Article DOI: 10.1124/jpet.103.061754 BindingDB Entry DOI: 10.7270/Q2VX0DTD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Cavia porcellus (domestic guinea pig)) | BDBM22566 (5-chloro-2-[(4-methylpiperazin-1-yl)carbonyl]-1H-i...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+3 | >-34.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The Ki values were calculated based on an experimentally determined appropriate Kd value according to Cheng and Prusoff. | J Pharmacol Exp Ther 309: 404-13 (2004) Article DOI: 10.1124/jpet.103.061754 BindingDB Entry DOI: 10.7270/Q2VX0DTD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM22566 (5-chloro-2-[(4-methylpiperazin-1-yl)carbonyl]-1H-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 4.70E+3 | -30.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The Ki values were calculated based on an experimentally determined appropriate Kd value according to Cheng and Prusoff. | J Pharmacol Exp Ther 309: 404-13 (2004) Article DOI: 10.1124/jpet.103.061754 BindingDB Entry DOI: 10.7270/Q2VX0DTD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM22566 (5-chloro-2-[(4-methylpiperazin-1-yl)carbonyl]-1H-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 5.13E+3 | -30.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The Ki values were calculated based on an experimentally determined appropriate Kd value according to Cheng and Prusoff. | J Pharmacol Exp Ther 309: 404-13 (2004) Article DOI: 10.1124/jpet.103.061754 BindingDB Entry DOI: 10.7270/Q2VX0DTD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Mus musculus (mouse)) | BDBM22566 (5-chloro-2-[(4-methylpiperazin-1-yl)carbonyl]-1H-i...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | >-28.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The Ki values were calculated based on an experimentally determined appropriate Kd value according to Cheng and Prusoff. | J Pharmacol Exp Ther 309: 404-13 (2004) Article DOI: 10.1124/jpet.103.061754 BindingDB Entry DOI: 10.7270/Q2VX0DTD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM22566 (5-chloro-2-[(4-methylpiperazin-1-yl)carbonyl]-1H-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | >-28.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The Ki values were calculated based on an experimentally determined appropriate Kd value according to Cheng and Prusoff. | J Pharmacol Exp Ther 309: 404-13 (2004) Article DOI: 10.1124/jpet.103.061754 BindingDB Entry DOI: 10.7270/Q2VX0DTD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50538456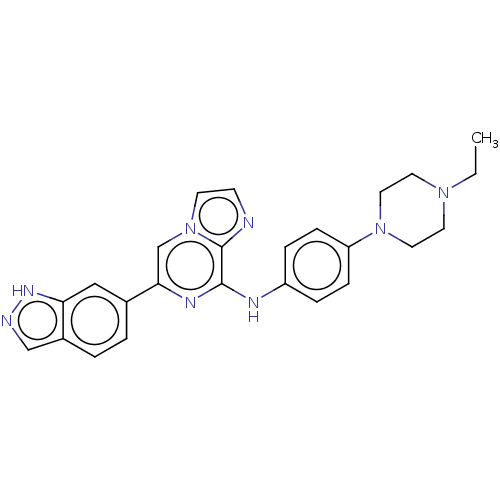 (CHEMBL4649800) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Curated by ChEMBL | Assay Description Inhibition of SYK (unknown origin) using XL665-labeled peptide as substrate in presence of ATP measured after 30 mins by TR-FRET assay | ACS Med Chem Lett 11: 506-513 (2020) Article DOI: 10.1021/acsmedchemlett.9b00621 BindingDB Entry DOI: 10.7270/Q2ZG6WR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50538457 (CHEMBL4638089) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Curated by ChEMBL | Assay Description Inhibition of SYK (unknown origin) using XL665-labeled peptide as substrate in presence of ATP measured after 30 mins by TR-FRET assay | ACS Med Chem Lett 11: 506-513 (2020) Article DOI: 10.1021/acsmedchemlett.9b00621 BindingDB Entry DOI: 10.7270/Q2ZG6WR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50538455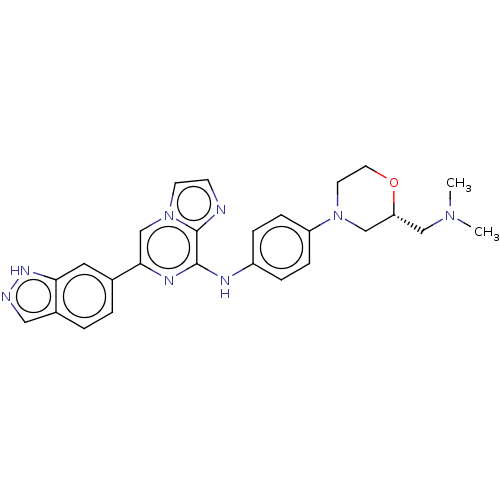 (CHEMBL4637943) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Curated by ChEMBL | Assay Description Inhibition of SYK (unknown origin) using XL665-labeled peptide as substrate in presence of ATP measured after 30 mins by TR-FRET assay | ACS Med Chem Lett 11: 506-513 (2020) Article DOI: 10.1021/acsmedchemlett.9b00621 BindingDB Entry DOI: 10.7270/Q2ZG6WR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50538454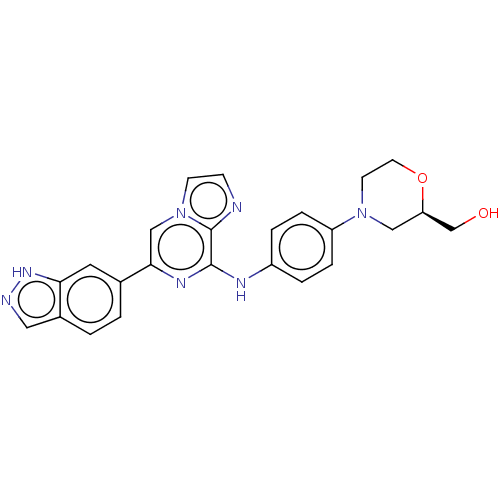 (CHEMBL4643354) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Curated by ChEMBL | Assay Description Inhibition of SYK (unknown origin) using XL665-labeled peptide as substrate in presence of ATP measured after 30 mins by TR-FRET assay | ACS Med Chem Lett 11: 506-513 (2020) Article DOI: 10.1021/acsmedchemlett.9b00621 BindingDB Entry DOI: 10.7270/Q2ZG6WR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50538453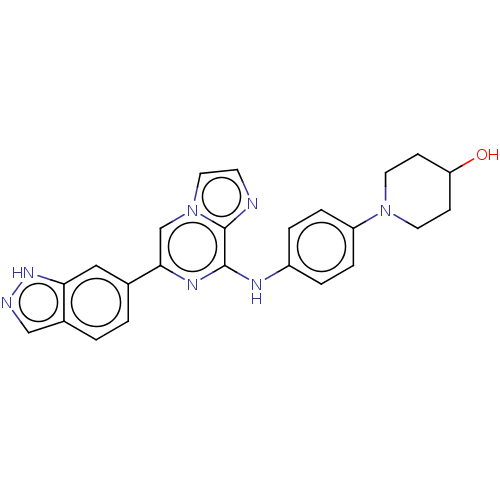 (CHEMBL4647927) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Curated by ChEMBL | Assay Description Inhibition of SYK (unknown origin) using XL665-labeled peptide as substrate in presence of ATP measured after 30 mins by TR-FRET assay | ACS Med Chem Lett 11: 506-513 (2020) Article DOI: 10.1021/acsmedchemlett.9b00621 BindingDB Entry DOI: 10.7270/Q2ZG6WR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50538469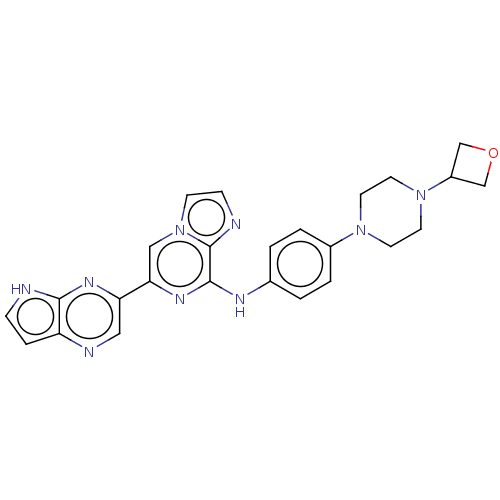 (CHEMBL4643695) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Curated by ChEMBL | Assay Description Inhibition of SYK (unknown origin) using XL665-labeled peptide as substrate in presence of ATP measured after 30 mins by TR-FRET assay | ACS Med Chem Lett 11: 506-513 (2020) Article DOI: 10.1021/acsmedchemlett.9b00621 BindingDB Entry DOI: 10.7270/Q2ZG6WR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50538460 (CHEMBL4634466) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Curated by ChEMBL | Assay Description Inhibition of SYK (unknown origin) using XL665-labeled peptide as substrate in presence of ATP measured after 30 mins by TR-FRET assay | ACS Med Chem Lett 11: 506-513 (2020) Article DOI: 10.1021/acsmedchemlett.9b00621 BindingDB Entry DOI: 10.7270/Q2ZG6WR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50538461 (CHEMBL4637326) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Curated by ChEMBL | Assay Description Inhibition of SYK (unknown origin) using XL665-labeled peptide as substrate in presence of ATP measured after 30 mins by TR-FRET assay | ACS Med Chem Lett 11: 506-513 (2020) Article DOI: 10.1021/acsmedchemlett.9b00621 BindingDB Entry DOI: 10.7270/Q2ZG6WR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50538451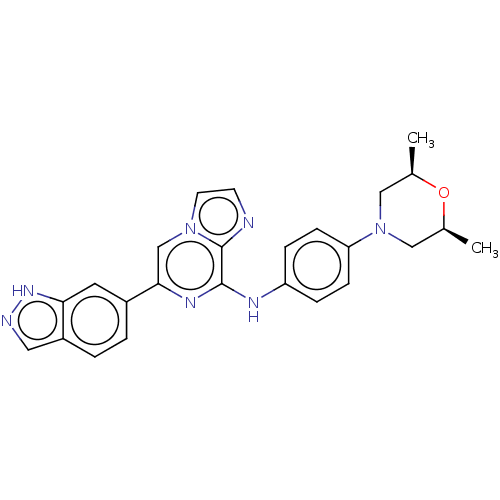 (CHEMBL4642016) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Curated by ChEMBL | Assay Description Inhibition of SYK (unknown origin) using XL665-labeled peptide as substrate in presence of ATP measured after 30 mins by TR-FRET assay | ACS Med Chem Lett 11: 506-513 (2020) Article DOI: 10.1021/acsmedchemlett.9b00621 BindingDB Entry DOI: 10.7270/Q2ZG6WR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50538445 (CHEMBL4634723) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Curated by ChEMBL | Assay Description Inhibition of SYK (unknown origin) using XL665-labeled peptide as substrate in presence of ATP measured after 30 mins by TR-FRET assay | ACS Med Chem Lett 11: 506-513 (2020) Article DOI: 10.1021/acsmedchemlett.9b00621 BindingDB Entry DOI: 10.7270/Q2ZG6WR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50538449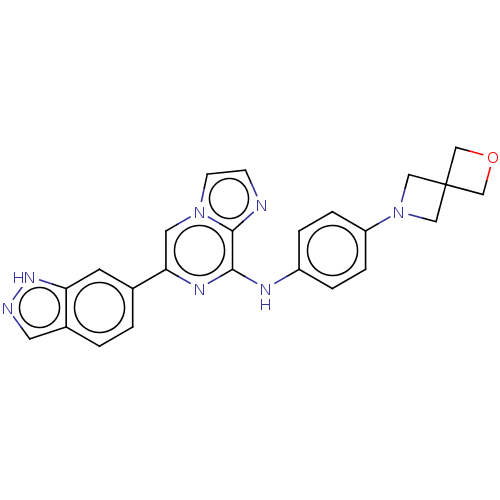 (CHEMBL4633338) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Curated by ChEMBL | Assay Description Inhibition of SYK (unknown origin) using XL665-labeled peptide as substrate in presence of ATP measured after 30 mins by TR-FRET assay | ACS Med Chem Lett 11: 506-513 (2020) Article DOI: 10.1021/acsmedchemlett.9b00621 BindingDB Entry DOI: 10.7270/Q2ZG6WR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50538444 (CHEMBL4640287) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Curated by ChEMBL | Assay Description Inhibition of SYK (unknown origin) using XL665-labeled peptide as substrate in presence of ATP measured after 30 mins by TR-FRET assay | ACS Med Chem Lett 11: 506-513 (2020) Article DOI: 10.1021/acsmedchemlett.9b00621 BindingDB Entry DOI: 10.7270/Q2ZG6WR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM212271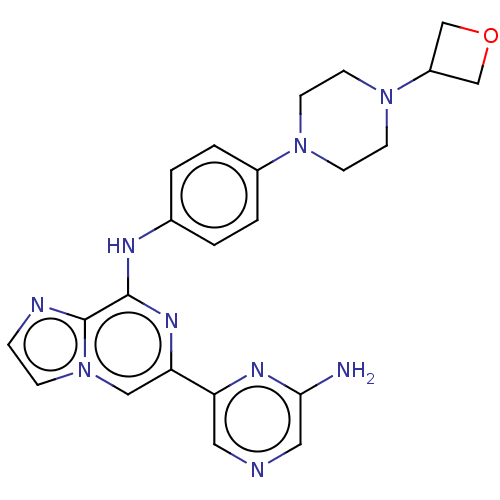 (6-(6-aminopyrazin-2-yl)-N-(4-(4-(oxetan-3-yl)piper...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Curated by ChEMBL | Assay Description Inhibition of SYK (unknown origin) using XL665-labeled peptide as substrate in presence of ATP measured after 30 mins by TR-FRET assay | ACS Med Chem Lett 11: 506-513 (2020) Article DOI: 10.1021/acsmedchemlett.9b00621 BindingDB Entry DOI: 10.7270/Q2ZG6WR1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50538459 (CHEMBL4644977) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Curated by ChEMBL | Assay Description Inhibition of SYK (unknown origin) using XL665-labeled peptide as substrate in presence of ATP measured after 30 mins by TR-FRET assay | ACS Med Chem Lett 11: 506-513 (2020) Article DOI: 10.1021/acsmedchemlett.9b00621 BindingDB Entry DOI: 10.7270/Q2ZG6WR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50538468 (CHEMBL4640138) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Curated by ChEMBL | Assay Description Inhibition of SYK (unknown origin) using XL665-labeled peptide as substrate in presence of ATP measured after 30 mins by TR-FRET assay | ACS Med Chem Lett 11: 506-513 (2020) Article DOI: 10.1021/acsmedchemlett.9b00621 BindingDB Entry DOI: 10.7270/Q2ZG6WR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50538473 (CHEMBL4633180) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Curated by ChEMBL | Assay Description Inhibition of SYK (unknown origin) using XL665-labeled peptide as substrate in presence of ATP measured after 30 mins by TR-FRET assay | ACS Med Chem Lett 11: 506-513 (2020) Article DOI: 10.1021/acsmedchemlett.9b00621 BindingDB Entry DOI: 10.7270/Q2ZG6WR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50538472 (CHEMBL4636843) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Curated by ChEMBL | Assay Description Inhibition of SYK (unknown origin) using XL665-labeled peptide as substrate in presence of ATP measured after 30 mins by TR-FRET assay | ACS Med Chem Lett 11: 506-513 (2020) Article DOI: 10.1021/acsmedchemlett.9b00621 BindingDB Entry DOI: 10.7270/Q2ZG6WR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50538448 (CHEMBL4643774) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Curated by ChEMBL | Assay Description Inhibition of SYK (unknown origin) using XL665-labeled peptide as substrate in presence of ATP measured after 30 mins by TR-FRET assay | ACS Med Chem Lett 11: 506-513 (2020) Article DOI: 10.1021/acsmedchemlett.9b00621 BindingDB Entry DOI: 10.7270/Q2ZG6WR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50538458 (CHEMBL4634721) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Curated by ChEMBL | Assay Description Inhibition of SYK (unknown origin) using XL665-labeled peptide as substrate in presence of ATP measured after 30 mins by TR-FRET assay | ACS Med Chem Lett 11: 506-513 (2020) Article DOI: 10.1021/acsmedchemlett.9b00621 BindingDB Entry DOI: 10.7270/Q2ZG6WR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50538450 (CHEMBL4637020) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Curated by ChEMBL | Assay Description Inhibition of SYK (unknown origin) using XL665-labeled peptide as substrate in presence of ATP measured after 30 mins by TR-FRET assay | ACS Med Chem Lett 11: 506-513 (2020) Article DOI: 10.1021/acsmedchemlett.9b00621 BindingDB Entry DOI: 10.7270/Q2ZG6WR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50538452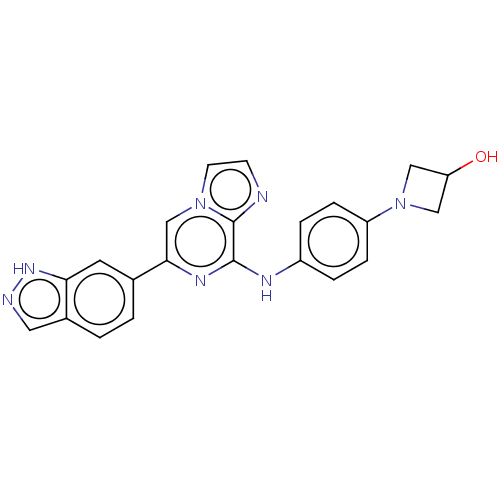 (CHEMBL4633153) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Curated by ChEMBL | Assay Description Inhibition of SYK (unknown origin) using XL665-labeled peptide as substrate in presence of ATP measured after 30 mins by TR-FRET assay | ACS Med Chem Lett 11: 506-513 (2020) Article DOI: 10.1021/acsmedchemlett.9b00621 BindingDB Entry DOI: 10.7270/Q2ZG6WR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50538467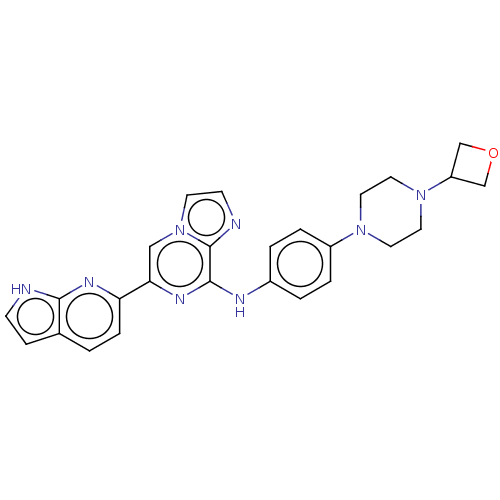 (CHEMBL4641358) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Curated by ChEMBL | Assay Description Inhibition of SYK (unknown origin) using XL665-labeled peptide as substrate in presence of ATP measured after 30 mins by TR-FRET assay | ACS Med Chem Lett 11: 506-513 (2020) Article DOI: 10.1021/acsmedchemlett.9b00621 BindingDB Entry DOI: 10.7270/Q2ZG6WR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50538465 (CHEMBL4645149) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Curated by ChEMBL | Assay Description Inhibition of SYK (unknown origin) using XL665-labeled peptide as substrate in presence of ATP measured after 30 mins by TR-FRET assay | ACS Med Chem Lett 11: 506-513 (2020) Article DOI: 10.1021/acsmedchemlett.9b00621 BindingDB Entry DOI: 10.7270/Q2ZG6WR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50538464 (CHEMBL4649107) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Curated by ChEMBL | Assay Description Inhibition of SYK (unknown origin) using XL665-labeled peptide as substrate in presence of ATP measured after 30 mins by TR-FRET assay | ACS Med Chem Lett 11: 506-513 (2020) Article DOI: 10.1021/acsmedchemlett.9b00621 BindingDB Entry DOI: 10.7270/Q2ZG6WR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50538446 (CHEMBL4642075) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Curated by ChEMBL | Assay Description Inhibition of SYK (unknown origin) using XL665-labeled peptide as substrate in presence of ATP measured after 30 mins by TR-FRET assay | ACS Med Chem Lett 11: 506-513 (2020) Article DOI: 10.1021/acsmedchemlett.9b00621 BindingDB Entry DOI: 10.7270/Q2ZG6WR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50538466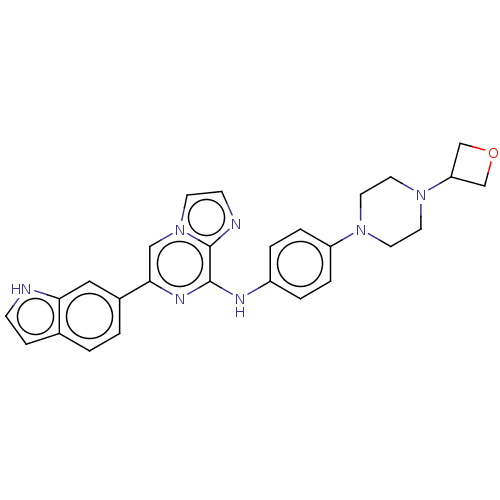 (CHEMBL4648053) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Curated by ChEMBL | Assay Description Inhibition of SYK (unknown origin) using XL665-labeled peptide as substrate in presence of ATP measured after 30 mins by TR-FRET assay | ACS Med Chem Lett 11: 506-513 (2020) Article DOI: 10.1021/acsmedchemlett.9b00621 BindingDB Entry DOI: 10.7270/Q2ZG6WR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50538462 (CHEMBL4635157) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Curated by ChEMBL | Assay Description Inhibition of SYK (unknown origin) using XL665-labeled peptide as substrate in presence of ATP measured after 30 mins by TR-FRET assay | ACS Med Chem Lett 11: 506-513 (2020) Article DOI: 10.1021/acsmedchemlett.9b00621 BindingDB Entry DOI: 10.7270/Q2ZG6WR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50015448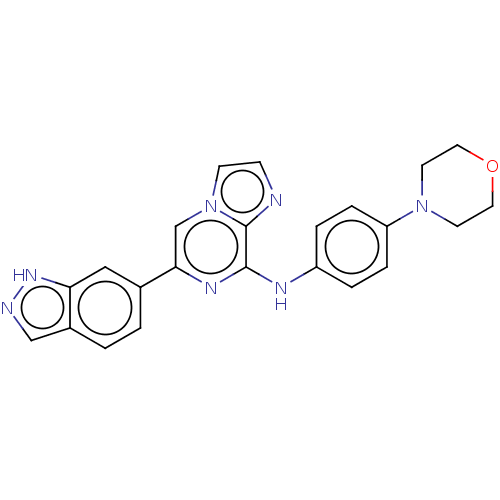 (CHEMBL3265032) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Curated by ChEMBL | Assay Description Inhibition of SYK (unknown origin) using XL665-labeled peptide as substrate in presence of ATP measured after 30 mins by TR-FRET assay | ACS Med Chem Lett 11: 506-513 (2020) Article DOI: 10.1021/acsmedchemlett.9b00621 BindingDB Entry DOI: 10.7270/Q2ZG6WR1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50015449 (CHEMBL3265033) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Curated by ChEMBL | Assay Description Inhibition of SYK (unknown origin) using XL665-labeled peptide as substrate in presence of ATP measured after 30 mins by TR-FRET assay | ACS Med Chem Lett 11: 506-513 (2020) Article DOI: 10.1021/acsmedchemlett.9b00621 BindingDB Entry DOI: 10.7270/Q2ZG6WR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50538470 (CHEMBL4645344) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Curated by ChEMBL | Assay Description Inhibition of SYK (unknown origin) using XL665-labeled peptide as substrate in presence of ATP measured after 30 mins by TR-FRET assay | ACS Med Chem Lett 11: 506-513 (2020) Article DOI: 10.1021/acsmedchemlett.9b00621 BindingDB Entry DOI: 10.7270/Q2ZG6WR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50538476 (CHEMBL4649400) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Curated by ChEMBL | Assay Description Inhibition of SYK (unknown origin) using XL665-labeled peptide as substrate in presence of ATP measured after 30 mins by TR-FRET assay | ACS Med Chem Lett 11: 506-513 (2020) Article DOI: 10.1021/acsmedchemlett.9b00621 BindingDB Entry DOI: 10.7270/Q2ZG6WR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50538463 (CHEMBL4647318) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Curated by ChEMBL | Assay Description Inhibition of SYK (unknown origin) using XL665-labeled peptide as substrate in presence of ATP measured after 30 mins by TR-FRET assay | ACS Med Chem Lett 11: 506-513 (2020) Article DOI: 10.1021/acsmedchemlett.9b00621 BindingDB Entry DOI: 10.7270/Q2ZG6WR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50538475 (CHEMBL4641986) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Curated by ChEMBL | Assay Description Inhibition of SYK (unknown origin) using XL665-labeled peptide as substrate in presence of ATP measured after 30 mins by TR-FRET assay | ACS Med Chem Lett 11: 506-513 (2020) Article DOI: 10.1021/acsmedchemlett.9b00621 BindingDB Entry DOI: 10.7270/Q2ZG6WR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 211 total ) | Next | Last >> |