

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50122692 (CHEMBL282336 | N-[2'-(3,4-Dimethyl-isoxazol-5-ylsu...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human endothelin A receptor expressed in CHO cells | J Med Chem 46: 125-37 (2002) Article DOI: 10.1021/jm020289q BindingDB Entry DOI: 10.7270/Q2348M3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50122706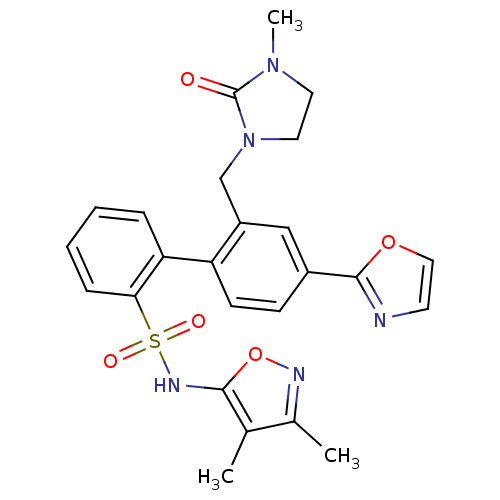 (2'-(3-Methyl-2-oxo-imidazolidin-1-ylmethyl)-4'-oxa...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human endothelin A receptor expressed in CHO cells | J Med Chem 46: 125-37 (2002) Article DOI: 10.1021/jm020289q BindingDB Entry DOI: 10.7270/Q2348M3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50122693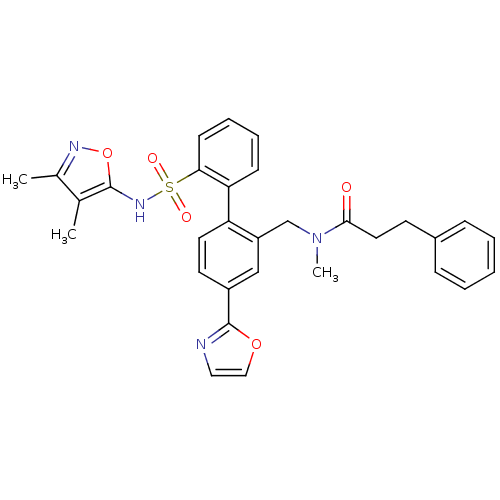 (CHEMBL29346 | N-[2'-(3,4-Dimethyl-isoxazol-5-ylsul...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human endothelin A receptor expressed in CHO cells | J Med Chem 46: 125-37 (2002) Article DOI: 10.1021/jm020289q BindingDB Entry DOI: 10.7270/Q2348M3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50122686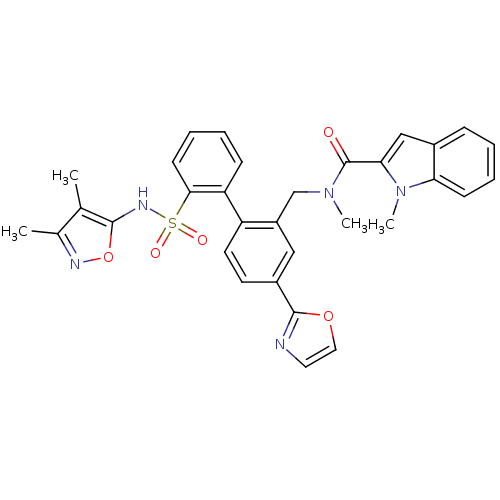 (1-Methyl-1H-indole-2-carboxylic acid [2'-(3,4-dime...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human endothelin A receptor expressed in CHO cells | J Med Chem 46: 125-37 (2002) Article DOI: 10.1021/jm020289q BindingDB Entry DOI: 10.7270/Q2348M3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50122694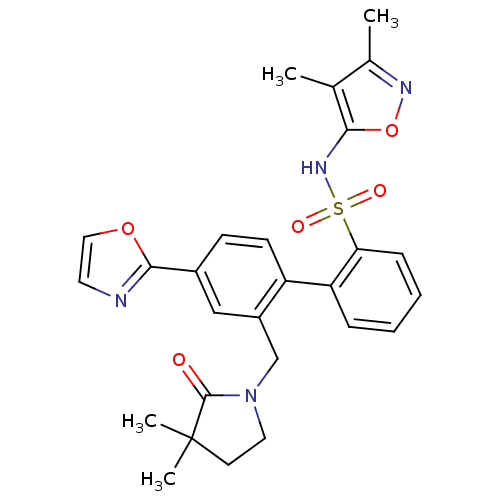 (2'-(3,3-Dimethyl-2-oxo-pyrrolidin-1-ylmethyl)-4'-o...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human endothelin A receptor expressed in CHO cells | J Med Chem 46: 125-37 (2002) Article DOI: 10.1021/jm020289q BindingDB Entry DOI: 10.7270/Q2348M3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50122715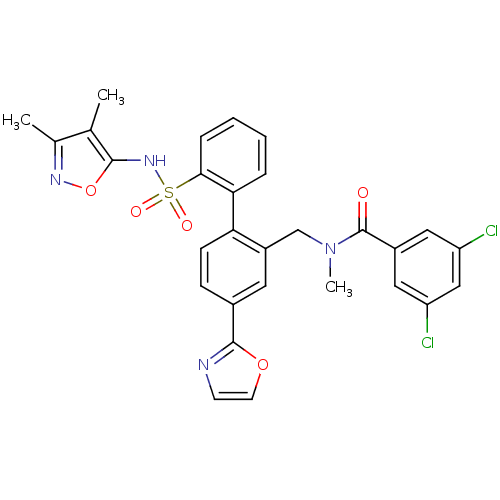 (3,5-Dichloro-N-[2'-(3,4-dimethyl-isoxazol-5-ylsulf...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human endothelin A receptor expressed in CHO cells | J Med Chem 46: 125-37 (2002) Article DOI: 10.1021/jm020289q BindingDB Entry DOI: 10.7270/Q2348M3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50122676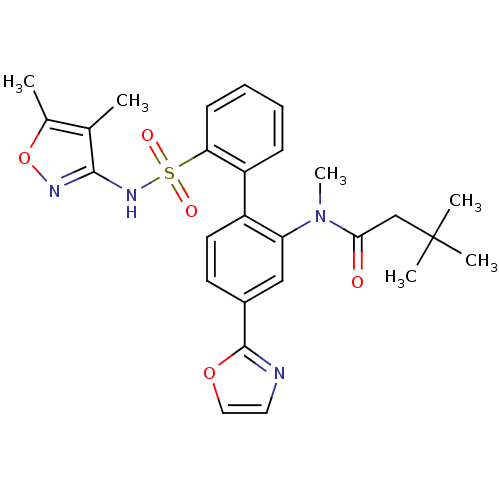 (CHEMBL274489 | N-[2'-(4,5-Dimethyl-isoxazol-3-ylsu...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human endothelin A receptor expressed in CHO cells | J Med Chem 46: 125-37 (2002) Article DOI: 10.1021/jm020289q BindingDB Entry DOI: 10.7270/Q2348M3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50122712 (CHEMBL440780 | N-[2'-(3,4-Dimethyl-isoxazol-5-ylsu...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human endothelin A receptor expressed in CHO cells | J Med Chem 46: 125-37 (2002) Article DOI: 10.1021/jm020289q BindingDB Entry DOI: 10.7270/Q2348M3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50124984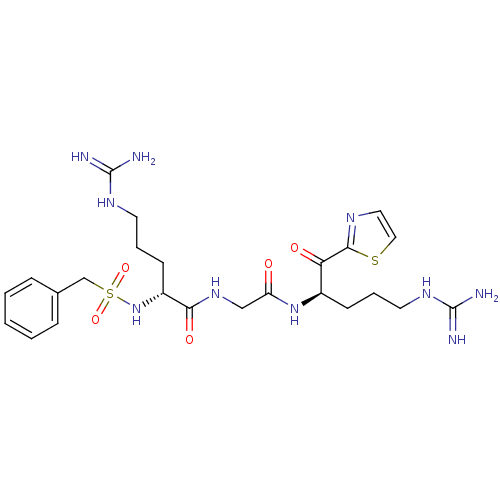 ((R)-5-Guanidino-2-phenylmethanesulfonylamino-penta...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc. Curated by ChEMBL | Assay Description In vitro binding affinity towards factor Xa | Bioorg Med Chem Lett 13: 723-8 (2003) BindingDB Entry DOI: 10.7270/Q2Z037JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12751 (1-(3-carbamimidoylphenyl)-3-methyl-N-[4-(2-sulfamo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Tested for binding affinity against human Coagulation factor Xa (trypsin-like serine protease) | Bioorg Med Chem Lett 12: 1651-5 (2002) BindingDB Entry DOI: 10.7270/Q2VT1RFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50122700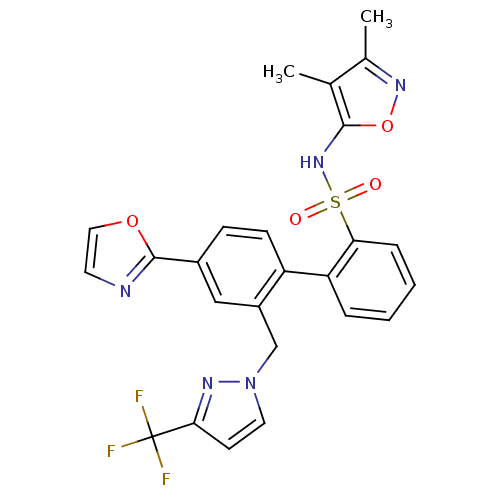 (4'-Oxazol-2-yl-2'-(3-trifluoromethyl-pyrazol-1-ylm...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human endothelin A receptor expressed in CHO cells | J Med Chem 46: 125-37 (2002) Article DOI: 10.1021/jm020289q BindingDB Entry DOI: 10.7270/Q2348M3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50122697 (2'-[(Methyl-phenyl-amino)-methyl]-4'-oxazol-2-yl-b...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human endothelin A receptor expressed in CHO cells | J Med Chem 46: 125-37 (2002) Article DOI: 10.1021/jm020289q BindingDB Entry DOI: 10.7270/Q2348M3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50122707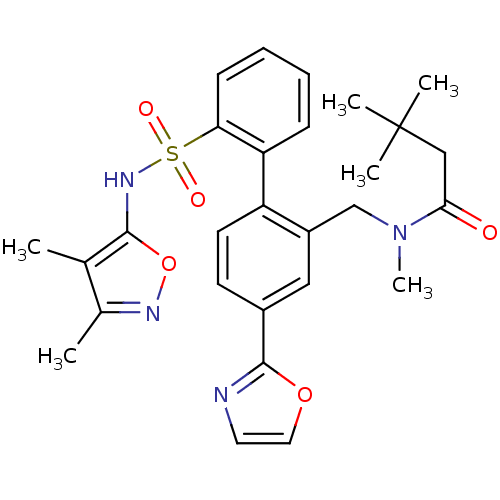 (CHEMBL281659 | N-[2'-(3,4-Dimethyl-isoxazol-5-ylsu...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human endothelin A receptor expressed in CHO cells | J Med Chem 46: 125-37 (2002) Article DOI: 10.1021/jm020289q BindingDB Entry DOI: 10.7270/Q2348M3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50122681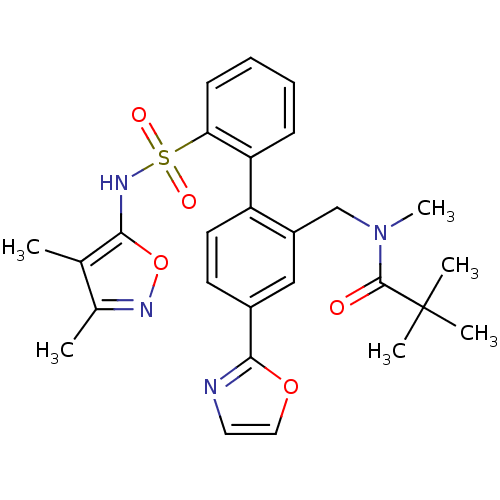 (CHEMBL27855 | N-[2'-(3,4-Dimethyl-isoxazol-5-ylsul...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human endothelin A receptor expressed in CHO cells | J Med Chem 46: 125-37 (2002) Article DOI: 10.1021/jm020289q BindingDB Entry DOI: 10.7270/Q2348M3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50454329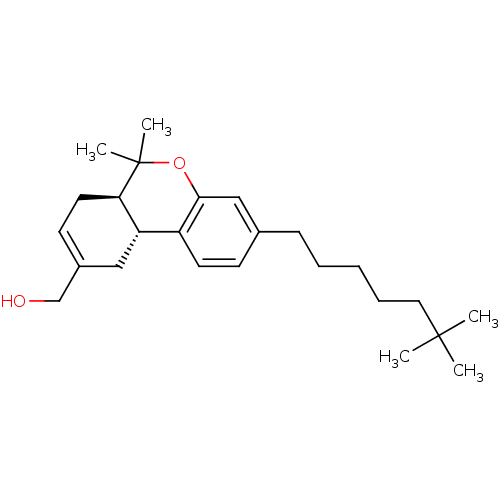 (CHEMBL2112647) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clemson University Curated by ChEMBL | Assay Description Ability to bind with Cannabinoid receptor 2 using [H]CP 55,940 as radioligand from cloned human receptor preparation | J Med Chem 39: 3875-7 (1996) Article DOI: 10.1021/jm960394y BindingDB Entry DOI: 10.7270/Q2R49PVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50330773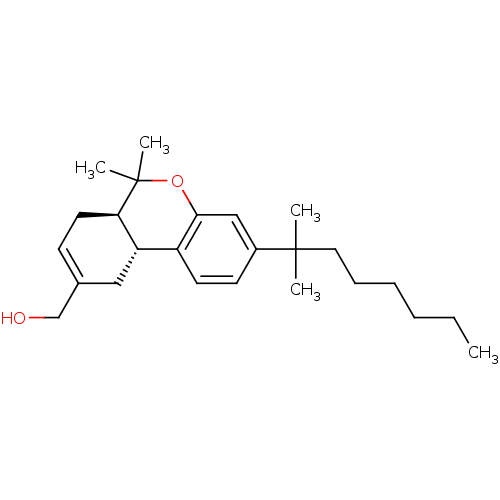 (((6aR,10aR)-6,6-dimethyl-3-(2-methyloctan-2-yl)-6a...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clemson University Curated by ChEMBL | Assay Description Binding affinity to CB2 receptor | Bioorg Med Chem 18: 7809-15 (2010) Article DOI: 10.1016/j.bmc.2010.09.061 BindingDB Entry DOI: 10.7270/Q2ZK5HP7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50156455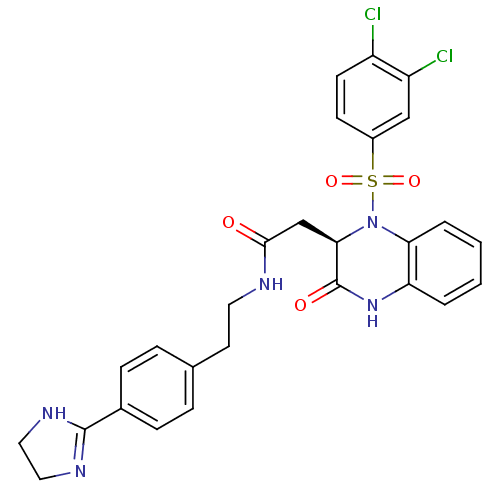 ((R)-N-(4-(4,5-dihydro-1H-imidazol-2-yl)phenethyl)-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [3H]DAK from human bradykinin B1 receptor expressed in CHO-D-/aequorin cells by rapid filtration technique | Bioorg Med Chem Lett 18: 4477-81 (2008) Article DOI: 10.1016/j.bmcl.2008.07.055 BindingDB Entry DOI: 10.7270/Q2F76CCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50122713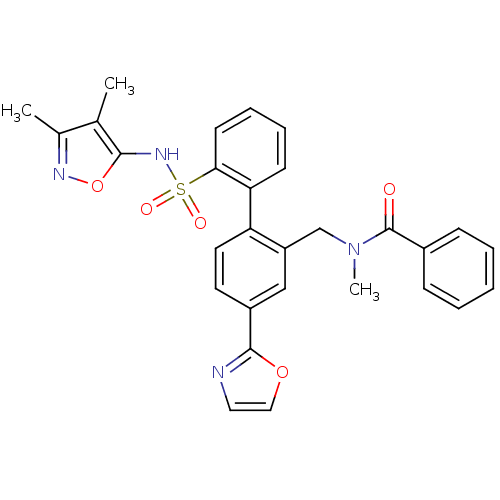 (CHEMBL282359 | N-[2'-(3,4-Dimethyl-isoxazol-5-ylsu...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human endothelin A receptor expressed in CHO cells | J Med Chem 46: 125-37 (2002) Article DOI: 10.1021/jm020289q BindingDB Entry DOI: 10.7270/Q2348M3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50122698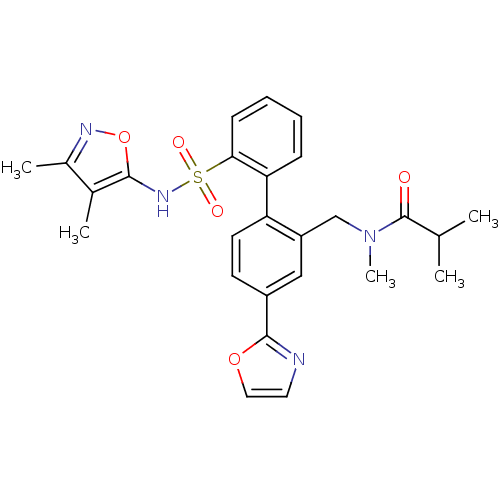 (CHEMBL28863 | N-[2'-(3,4-Dimethyl-isoxazol-5-ylsul...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human endothelin A receptor expressed in CHO cells | J Med Chem 46: 125-37 (2002) Article DOI: 10.1021/jm020289q BindingDB Entry DOI: 10.7270/Q2348M3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50193861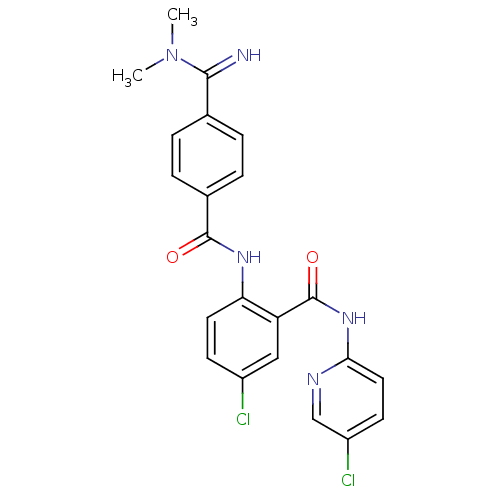 (5-chloro-N-(5-chloro-pyridin-2-yl)-2-[4-(N,N-dimet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Inhibition of Factor 10a (unknown origin) | Bioorg Med Chem Lett 19: 2179-85 (2009) Article DOI: 10.1016/j.bmcl.2009.02.111 BindingDB Entry DOI: 10.7270/Q22Z15F5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50122684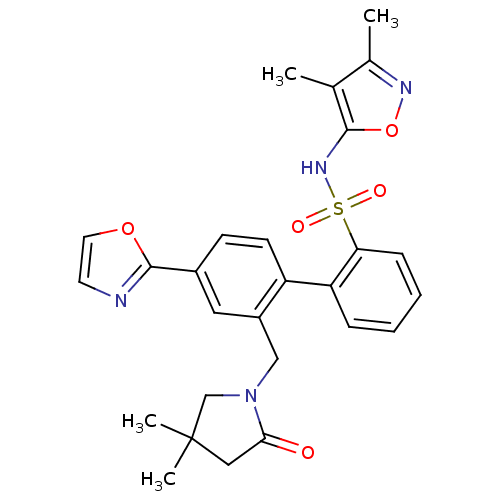 (2'-(4,4-Dimethyl-2-oxo-pyrrolidin-1-ylmethyl)-4'-o...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human endothelin A receptor expressed in CHO cells | J Med Chem 46: 125-37 (2002) Article DOI: 10.1021/jm020289q BindingDB Entry DOI: 10.7270/Q2348M3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50362366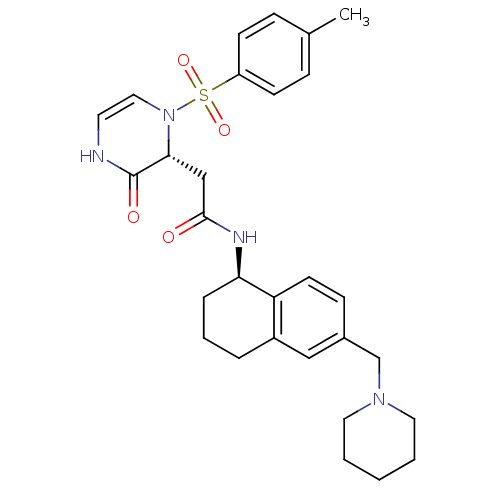 (CHEMBL1939755) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-DAK from human bradykinin B1 receptor expressed in CHO-D-/aequorin cells membrane after 90 mins by scintillation counting | Bioorg Med Chem Lett 22: 1061-7 (2012) Article DOI: 10.1016/j.bmcl.2011.11.112 BindingDB Entry DOI: 10.7270/Q22J6CB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4 (Rattus norvegicus (Rat)) | BDBM50049757 (()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.0470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]cytisine from alpha4beta2 nAChR in rat brain membrane | Bioorg Med Chem Lett 19: 1682-5 (2009) Article DOI: 10.1016/j.bmcl.2009.01.099 BindingDB Entry DOI: 10.7270/Q2HD7VJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4 (Rattus norvegicus (Rat)) | BDBM50062641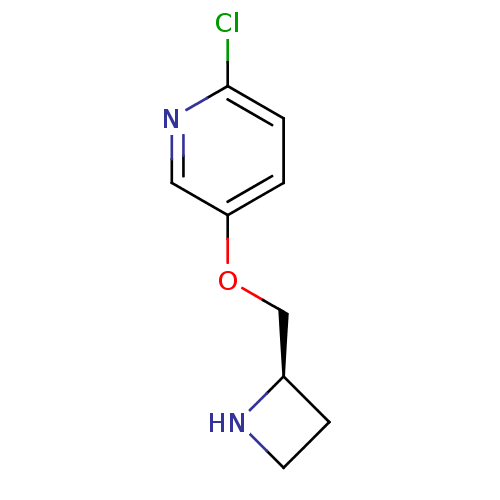 (5-((R)-1-Azetidin-2-ylmethoxy)-2-chloro-pyridine |...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]cytisine from alpha4beta2 nAChR in rat brain membrane | Bioorg Med Chem Lett 19: 1682-5 (2009) Article DOI: 10.1016/j.bmcl.2009.01.099 BindingDB Entry DOI: 10.7270/Q2HD7VJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50122696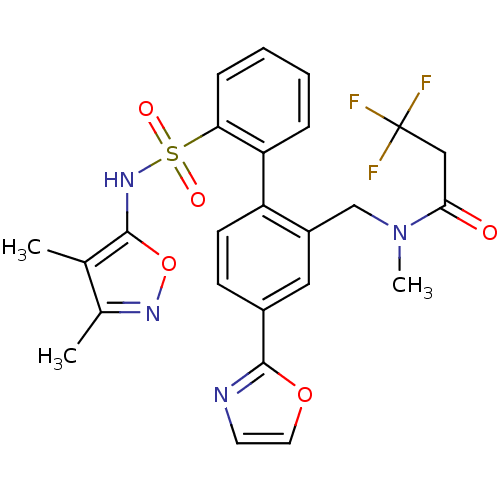 (CHEMBL281549 | N-[2'-(3,4-Dimethyl-isoxazol-5-ylsu...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human endothelin A receptor expressed in CHO cells | J Med Chem 46: 125-37 (2002) Article DOI: 10.1021/jm020289q BindingDB Entry DOI: 10.7270/Q2348M3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50485494 (CHEMBL2063089) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus (isolate BK) genotype 1b NS3/4a protease D168V mutant expressed in Escherichia coli by time-resolved fluorescence ana... | ACS Med Chem Lett 3: 332-6 (2012) Article DOI: 10.1021/ml300017p BindingDB Entry DOI: 10.7270/Q2KH0R6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50573891 (A-1282576 | A-1282576.0 | A-12825760 | ABT-493 | G...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HCV GT-1a NS3/4a protease using Ac-DE-D(Edans)-EE-Abu-c-[COO]-AS-K(Dabcy1)-NH2 preincubated for 1 hr followed by substrate addition | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00554 BindingDB Entry DOI: 10.7270/Q2377DH9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50122690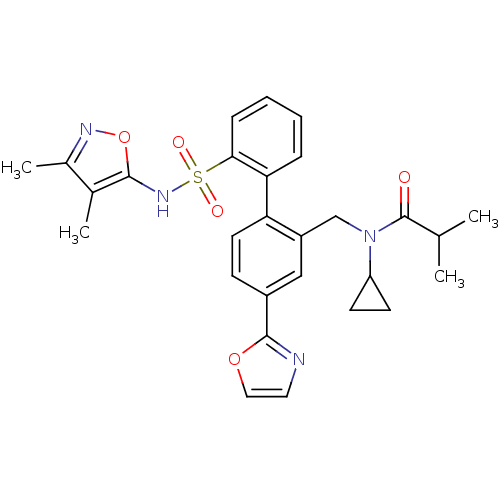 (CHEMBL28963 | N-Cyclopropyl-N-[2'-(3,4-dimethyl-is...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human endothelin A receptor expressed in CHO cells | J Med Chem 46: 125-37 (2002) Article DOI: 10.1021/jm020289q BindingDB Entry DOI: 10.7270/Q2348M3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM103838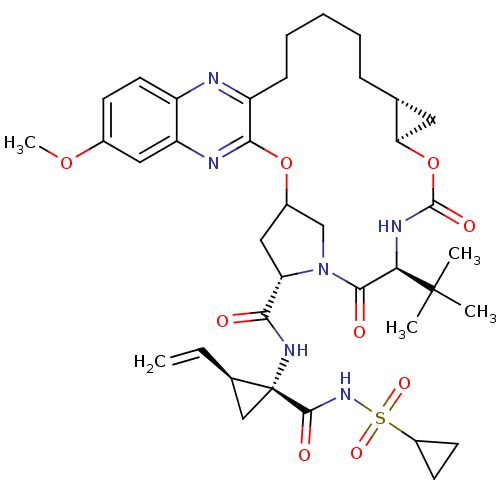 (MK-5172) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical School | Assay Description NS3/4A protease was preincubated with increasing concentration of drugs in protease reaction buffer for 1 hour. Inhibition assays were performed in ... | ACS Chem Biol 8: 1469-78 (2013) Article DOI: 10.1021/cb400100g BindingDB Entry DOI: 10.7270/Q2FQ9V7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50249120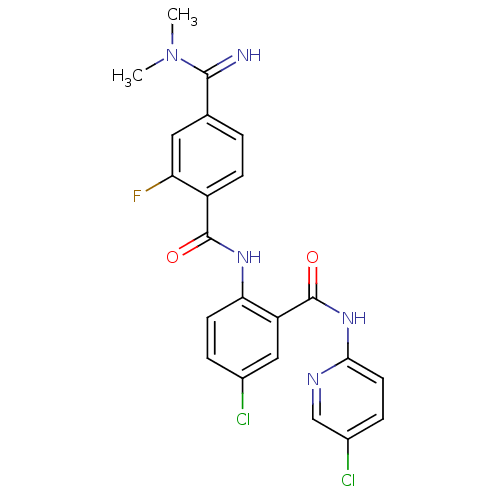 (CHEMBL472967 | N-(4-chloro-2-(5-chloropyridin-2-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Inhibition of Factor 10a (unknown origin) | Bioorg Med Chem Lett 19: 2179-85 (2009) Article DOI: 10.1016/j.bmcl.2009.02.111 BindingDB Entry DOI: 10.7270/Q22Z15F5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50485491 (CHEMBL2063088) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus (isolate BK) genotype 1b NS3/4a protease D168V mutant expressed in Escherichia coli by time-resolved fluorescence ana... | ACS Med Chem Lett 3: 332-6 (2012) Article DOI: 10.1021/ml300017p BindingDB Entry DOI: 10.7270/Q2KH0R6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM520 (1,3-thiazol-5-ylmethyl N-[(2S,3S,5S)-3-hydroxy-5-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 0.0620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Dissociation constant obtained by inhibition of Wild-type protease | J Med Chem 43: 3386-99 (2000) BindingDB Entry DOI: 10.7270/Q23J3DP4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM519 ((2S)-N-[(2S,3R)-4-[(3S,4aS,8aS)-3-(tert-butylcarba...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 0.0620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Dissociation constant obtained by inhibition of Wild-type protease | J Med Chem 43: 3386-99 (2000) BindingDB Entry DOI: 10.7270/Q23J3DP4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50122703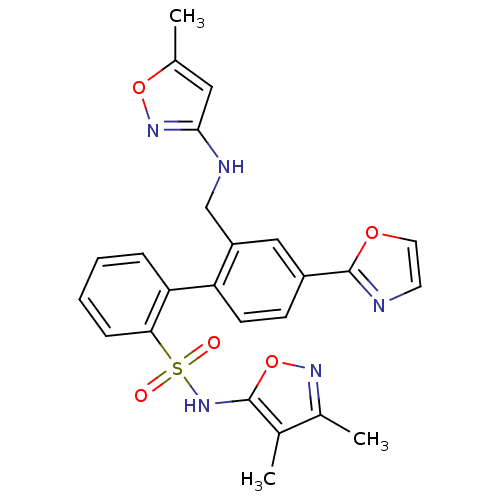 (2'-[(5-Methyl-isoxazol-3-ylamino)-methyl]-4'-oxazo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human endothelin A receptor expressed in CHO cells | J Med Chem 46: 125-37 (2002) Article DOI: 10.1021/jm020289q BindingDB Entry DOI: 10.7270/Q2348M3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apelin receptor (Homo sapiens (Human)) | BDBM50588316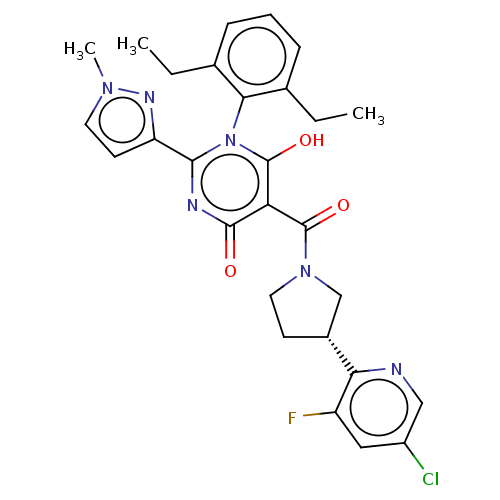 (CHEMBL5170657) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01504 BindingDB Entry DOI: 10.7270/Q21V5JX5 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apelin receptor (Homo sapiens (Human)) | BDBM50567169 (CHEMBL4873876) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.0740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]Apelin-13 from human APJ receptor stably expressed in human HEK293 cell membrane incubated for 120 mins by TopCount scintillation... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01878 BindingDB Entry DOI: 10.7270/Q20G3PXZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM19023 (1-(4-methoxyphenyl)-7-oxo-6-[4-(2-oxopiperidin-1-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Inhibition of Factor 10a (unknown origin) | Bioorg Med Chem Lett 19: 2179-85 (2009) Article DOI: 10.1016/j.bmcl.2009.02.111 BindingDB Entry DOI: 10.7270/Q22Z15F5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50600748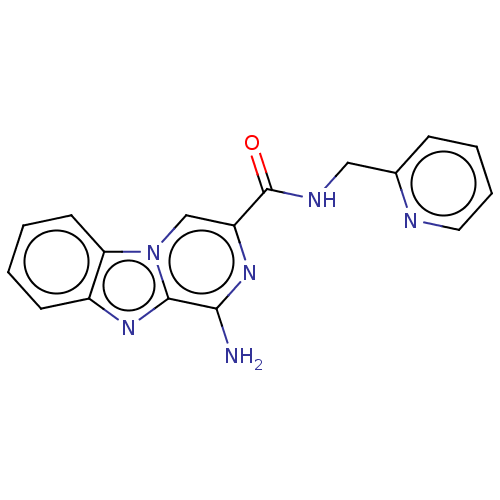 (CHEMBL5176737) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00101 BindingDB Entry DOI: 10.7270/Q2417231 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50600748 (CHEMBL5176737) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00101 BindingDB Entry DOI: 10.7270/Q2417231 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50110291 ((7-Chloro-quinolin-4-yl)-[4-(3-piperidin-1-yl-prop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description Affinity for displacement of [125I]-iodoproxyfan from human histamine H3 receptors stably expressed in CHO cells | J Med Chem 45: 1128-41 (2002) BindingDB Entry DOI: 10.7270/Q2H70F4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50212159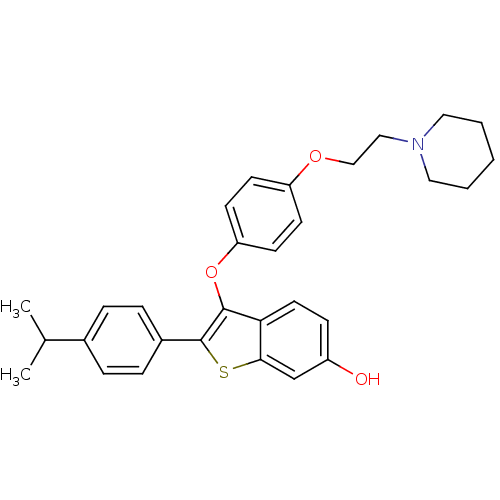 (2-(4-isopropylphenyl)-3-(4-(2-(piperidin-1-yl)etho...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from human recombinant ERalpha | Bioorg Med Chem Lett 17: 3544-9 (2007) Article DOI: 10.1016/j.bmcl.2007.04.044 BindingDB Entry DOI: 10.7270/Q2ZS2W6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50110288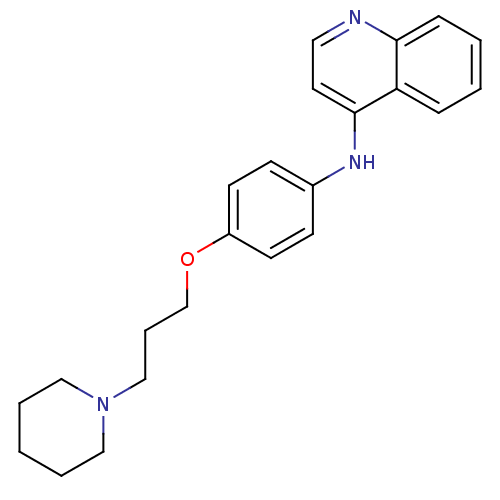 (CHEMBL15153 | N-(4-(3-(piperidin-1-yl)propoxy)phen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin Curated by ChEMBL | Assay Description Affinity for displacement of [125I]-iodoproxyfan from human histamine H3 receptors stably expressed in CHO cells | J Med Chem 45: 1128-41 (2002) BindingDB Entry DOI: 10.7270/Q2H70F4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium- and chloride-dependent glycine transporter 1 (Homo sapiens (Human)) | BDBM202260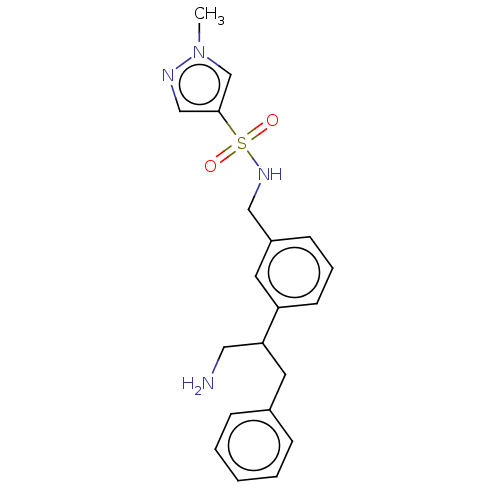 (US9238619, 18) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Deutschland GmbH & Co. KG; AbbVie Inc. US Patent | Assay Description Radioligand binding to human GlyT1c transporter-expressing membranes was determined as described in Mezler et al., Molecular Pharmacology 74:1705-171... | US Patent US9238619 (2016) BindingDB Entry DOI: 10.7270/Q2XS5T6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50246967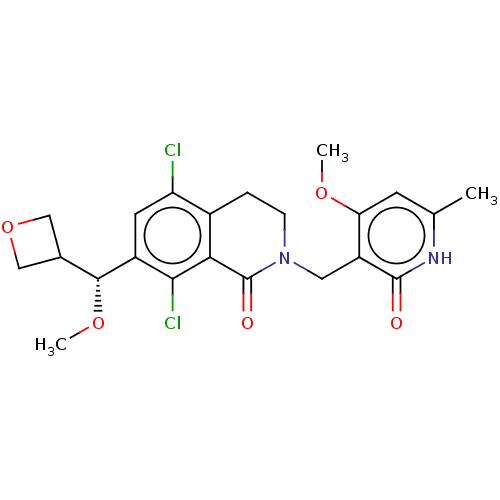 (CHEMBL4080228 | US10570121, Example 81) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | MCE PC cid PC sid PDB UniChem | PDB Article PubMed | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
WuXi AppTec Curated by ChEMBL | Assay Description Binding affinity to EZH2 (unknown origin) | J Med Chem 61: 650-665 (2018) Article DOI: 10.1021/acs.jmedchem.7b01375 BindingDB Entry DOI: 10.7270/Q2X069G8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sodium- and chloride-dependent glycine transporter 1 (Homo sapiens (Human)) | BDBM202266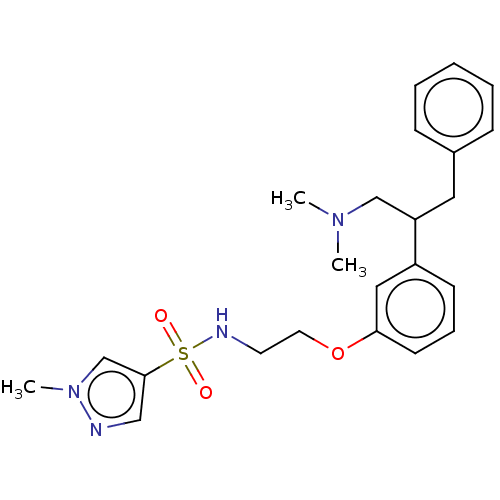 (US9238619, 24) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Deutschland GmbH & Co. KG; AbbVie Inc. US Patent | Assay Description Radioligand binding to human GlyT1c transporter-expressing membranes was determined as described in Mezler et al., Molecular Pharmacology 74:1705-171... | US Patent US9238619 (2016) BindingDB Entry DOI: 10.7270/Q2XS5T6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium- and chloride-dependent glycine transporter 1 (Homo sapiens (Human)) | BDBM202271 (US9238619, 29) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Deutschland GmbH & Co. KG; AbbVie Inc. US Patent | Assay Description Radioligand binding to human GlyT1c transporter-expressing membranes was determined as described in Mezler et al., Molecular Pharmacology 74:1705-171... | US Patent US9238619 (2016) BindingDB Entry DOI: 10.7270/Q2XS5T6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium- and chloride-dependent glycine transporter 1 (Homo sapiens (Human)) | BDBM202272 (US9238619, 30) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Deutschland GmbH & Co. KG; AbbVie Inc. US Patent | Assay Description Radioligand binding to human GlyT1c transporter-expressing membranes was determined as described in Mezler et al., Molecular Pharmacology 74:1705-171... | US Patent US9238619 (2016) BindingDB Entry DOI: 10.7270/Q2XS5T6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium- and chloride-dependent glycine transporter 1 (Homo sapiens (Human)) | BDBM202274 (US9238619, 32) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Deutschland GmbH & Co. KG; AbbVie Inc. US Patent | Assay Description Radioligand binding to human GlyT1c transporter-expressing membranes was determined as described in Mezler et al., Molecular Pharmacology 74:1705-171... | US Patent US9238619 (2016) BindingDB Entry DOI: 10.7270/Q2XS5T6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium- and chloride-dependent glycine transporter 1 (Homo sapiens (Human)) | BDBM202275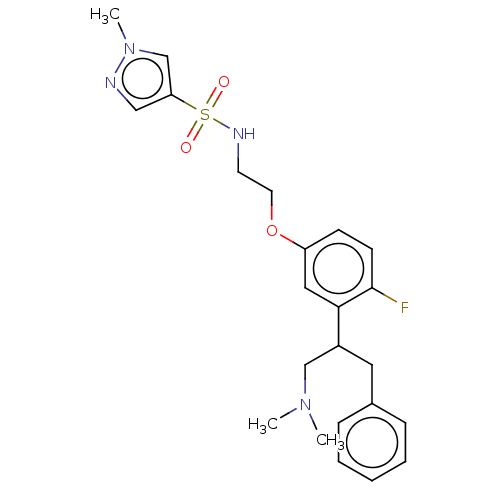 (US9238619, 33) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Deutschland GmbH & Co. KG; AbbVie Inc. US Patent | Assay Description Radioligand binding to human GlyT1c transporter-expressing membranes was determined as described in Mezler et al., Molecular Pharmacology 74:1705-171... | US Patent US9238619 (2016) BindingDB Entry DOI: 10.7270/Q2XS5T6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium- and chloride-dependent glycine transporter 1 (Homo sapiens (Human)) | BDBM202316 (US9238619, 52) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Deutschland GmbH & Co. KG; AbbVie Inc. US Patent | Assay Description Radioligand binding to human GlyT1c transporter-expressing membranes was determined as described in Mezler et al., Molecular Pharmacology 74:1705-171... | US Patent US9238619 (2016) BindingDB Entry DOI: 10.7270/Q2XS5T6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 42336 total ) | Next | Last >> |