

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bile salt export pump (Homo sapiens (Human)) | BDBM50339127 ((3S,6S,9S,12R,15S,18S,21S,24S,27R,30S,33S)-25,30-d...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of BSEP (unknown origin) expressed in HEK293 cells using [3H]taurocholic acid substrate | J Med Chem 57: 8503-16 (2014) Article DOI: 10.1021/jm500862r BindingDB Entry DOI: 10.7270/Q2HM5B1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier organic anion transporter family member 1B1 (Homo sapiens (Human)) | BDBM50339127 ((3S,6S,9S,12R,15S,18S,21S,24S,27R,30S,33S)-25,30-d...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of OATP1B1 (unknown origin) expressed in HEK293 cells using [3H]estradiol-17beta-glucuronide substrate | J Med Chem 57: 8503-16 (2014) Article DOI: 10.1021/jm500862r BindingDB Entry DOI: 10.7270/Q2HM5B1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50197073 (3-amino-7-((R)-3-((S)-1-aminoethyl)pyrrolidin-1-yl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant full-length C-terminal His6-tagged Escherichia coli DNA gyrase AB supercoiling activity using relaxed DNA as substrate prei... | J Med Chem 63: 7773-7816 (2020) Article DOI: 10.1021/acs.jmedchem.0c00347 BindingDB Entry DOI: 10.7270/Q2MW2MQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier organic anion transporter family member 1B3 (Homo sapiens (Human)) | BDBM50339127 ((3S,6S,9S,12R,15S,18S,21S,24S,27R,30S,33S)-25,30-d...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of OATP1B3 (unknown origin) expressed in HEK293 cells using [3H]estradiol-17beta-glucuronide substrate | J Med Chem 57: 8503-16 (2014) Article DOI: 10.1021/jm500862r BindingDB Entry DOI: 10.7270/Q2HM5B1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50542206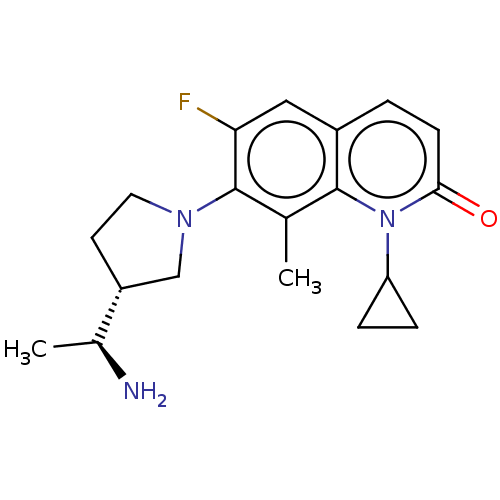 (CHEMBL4640061) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant full-length C-terminal His6-tagged Escherichia coli DNA gyrase AB supercoiling activity using relaxed DNA as substrate prei... | J Med Chem 63: 7773-7816 (2020) Article DOI: 10.1021/acs.jmedchem.0c00347 BindingDB Entry DOI: 10.7270/Q2MW2MQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM21690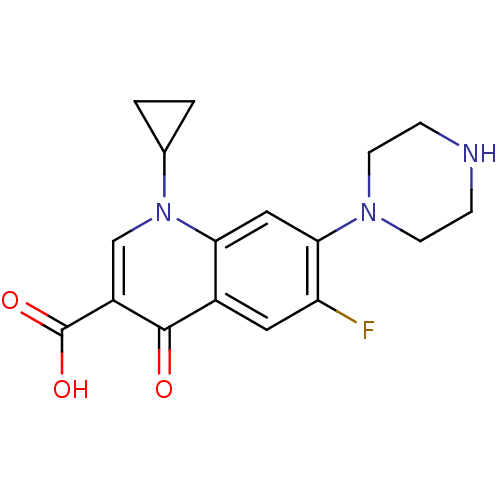 (1-cyclopropyl-6-fluoro-4-oxo-7-(piperazin-1-yl)-1,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant full-length C-terminal His6-tagged Escherichia coli DNA gyrase AB supercoiling activity using relaxed DNA as substrate prei... | J Med Chem 63: 7773-7816 (2020) Article DOI: 10.1021/acs.jmedchem.0c00347 BindingDB Entry DOI: 10.7270/Q2MW2MQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-dependent translocase ABCB1 (Homo sapiens (Human)) | BDBM50339127 ((3S,6S,9S,12R,15S,18S,21S,24S,27R,30S,33S)-25,30-d...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of MDR1 (unknown origin) expressed in MDA T0.3 cells using rhodamine 123 substrate | J Med Chem 57: 8503-16 (2014) Article DOI: 10.1021/jm500862r BindingDB Entry DOI: 10.7270/Q2HM5B1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier organic anion transporter family member 1B1 (Homo sapiens (Human)) | BDBM50339127 ((3S,6S,9S,12R,15S,18S,21S,24S,27R,30S,33S)-25,30-d...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of OATP1B1 (unknown origin) expressed in CHO cells using 8-fluorescein-cAMP substrate by fluorescent photometry | J Med Chem 57: 8503-16 (2014) Article DOI: 10.1021/jm500862r BindingDB Entry DOI: 10.7270/Q2HM5B1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier organic anion transporter family member 1B3 (Homo sapiens (Human)) | BDBM50030493 (CHEMBL3344501 | US9566312, Compound 2.17.4) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 880 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of OATP1B3 (unknown origin) expressed in HEK293 cells using [3H]estradiol-17beta-glucuronide substrate | J Med Chem 57: 8503-16 (2014) Article DOI: 10.1021/jm500862r BindingDB Entry DOI: 10.7270/Q2HM5B1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier organic anion transporter family member 1B1 (Homo sapiens (Human)) | BDBM50030555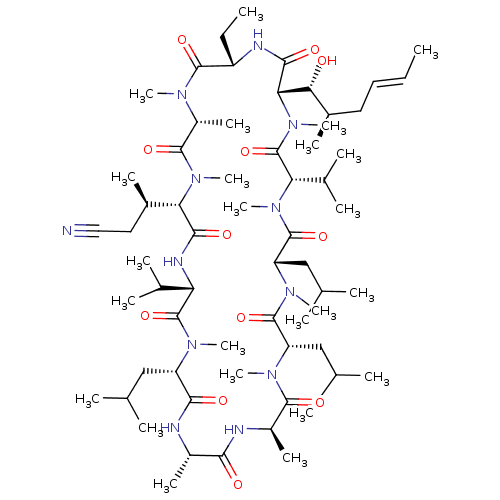 (CHEMBL3344493 | US9566312, Compound 2.10) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of OATP1B1 (unknown origin) expressed in CHO cells using 8-fluorescein-cAMP substrate by fluorescent photometry | J Med Chem 57: 8503-16 (2014) Article DOI: 10.1021/jm500862r BindingDB Entry DOI: 10.7270/Q2HM5B1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier organic anion transporter family member 1B1 (Homo sapiens (Human)) | BDBM50030493 (CHEMBL3344501 | US9566312, Compound 2.17.4) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of OATP1B1 (unknown origin) expressed in HEK293 cells using [3H]estradiol-17beta-glucuronide substrate | J Med Chem 57: 8503-16 (2014) Article DOI: 10.1021/jm500862r BindingDB Entry DOI: 10.7270/Q2HM5B1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50542207 (CHEMBL4634552) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 1.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant full-length C-terminal His6-tagged Escherichia coli DNA gyrase AB supercoiling activity using relaxed DNA as substrate prei... | J Med Chem 63: 7773-7816 (2020) Article DOI: 10.1021/acs.jmedchem.0c00347 BindingDB Entry DOI: 10.7270/Q2MW2MQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier organic anion transporter family member 1B1 (Homo sapiens (Human)) | BDBM50030538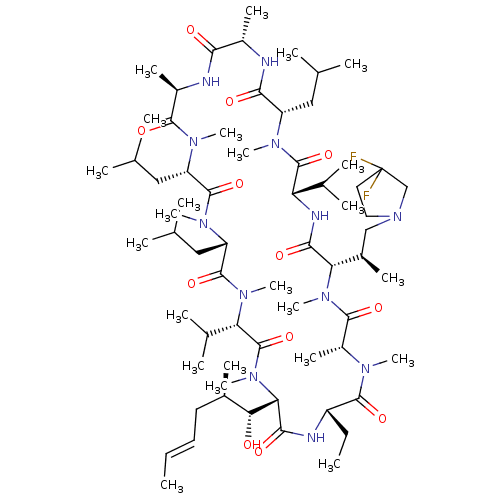 (CHEMBL3344497 | US9566312, Compound 2.5.18) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of OATP1B1 (unknown origin) expressed in CHO cells using 8-fluorescein-cAMP substrate by fluorescent photometry | J Med Chem 57: 8503-16 (2014) Article DOI: 10.1021/jm500862r BindingDB Entry DOI: 10.7270/Q2HM5B1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile salt export pump (Homo sapiens (Human)) | BDBM50030493 (CHEMBL3344501 | US9566312, Compound 2.17.4) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of BSEP (unknown origin) expressed in HEK293 cells using [3H]taurocholic acid substrate | J Med Chem 57: 8503-16 (2014) Article DOI: 10.1021/jm500862r BindingDB Entry DOI: 10.7270/Q2HM5B1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier organic anion transporter family member 1B1 (Homo sapiens (Human)) | BDBM50030541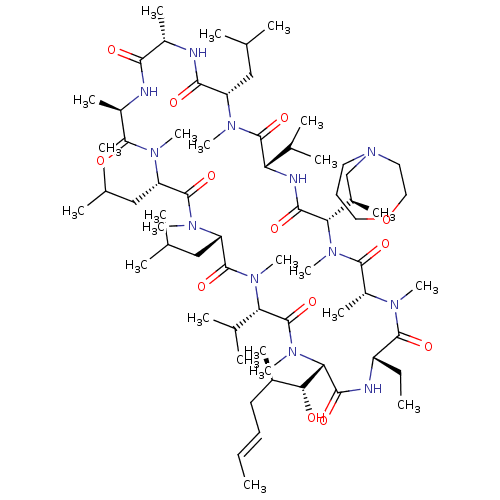 (CHEMBL3344496 | US9566312, Compound 2.5.21) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of OATP1B1 (unknown origin) expressed in CHO cells using 8-fluorescein-cAMP substrate by fluorescent photometry | J Med Chem 57: 8503-16 (2014) Article DOI: 10.1021/jm500862r BindingDB Entry DOI: 10.7270/Q2HM5B1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier organic anion transporter family member 1B1 (Homo sapiens (Human)) | BDBM50030536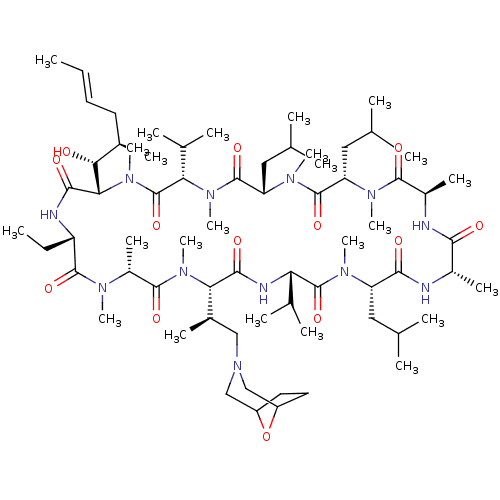 (CHEMBL3344498 | US9566312, Compound 2.5.3) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of OATP1B1 (unknown origin) expressed in CHO cells using 8-fluorescein-cAMP substrate by fluorescent photometry | J Med Chem 57: 8503-16 (2014) Article DOI: 10.1021/jm500862r BindingDB Entry DOI: 10.7270/Q2HM5B1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier organic anion transporter family member 1B1 (Homo sapiens (Human)) | BDBM50030535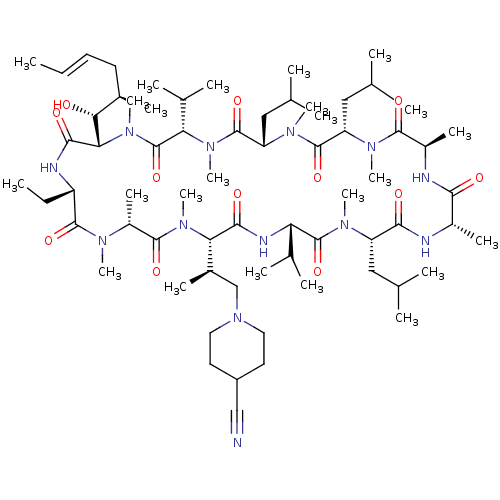 (CHEMBL3344499 | US9566312, Compound 2.5.26) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of OATP1B1 (unknown origin) expressed in CHO cells using 8-fluorescein-cAMP substrate by fluorescent photometry | J Med Chem 57: 8503-16 (2014) Article DOI: 10.1021/jm500862r BindingDB Entry DOI: 10.7270/Q2HM5B1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier organic anion transporter family member 1B1 (Homo sapiens (Human)) | BDBM50030534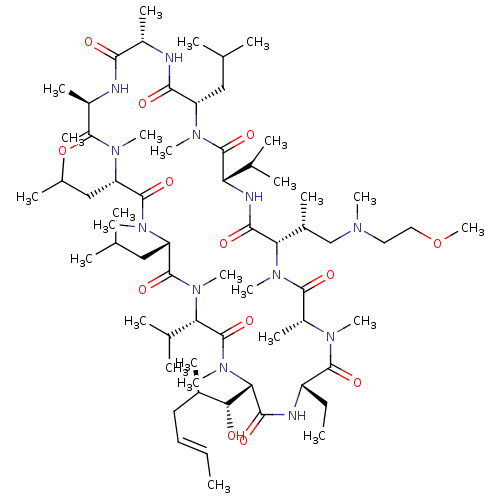 (CHEMBL3344500 | US9566312, Compound 2.5.22) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of OATP1B1 (unknown origin) expressed in CHO cells using 8-fluorescein-cAMP substrate by fluorescent photometry | J Med Chem 57: 8503-16 (2014) Article DOI: 10.1021/jm500862r BindingDB Entry DOI: 10.7270/Q2HM5B1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Broad substrate specificity ATP-binding cassette transporter ABCG2 (Homo sapiens (Human)) | BDBM50339127 ((3S,6S,9S,12R,15S,18S,21S,24S,27R,30S,33S)-25,30-d...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of BCRP (unknown origin) expressed in T8 cells using Bodipy FL-prazosin substrate | J Med Chem 57: 8503-16 (2014) Article DOI: 10.1021/jm500862r BindingDB Entry DOI: 10.7270/Q2HM5B1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50566539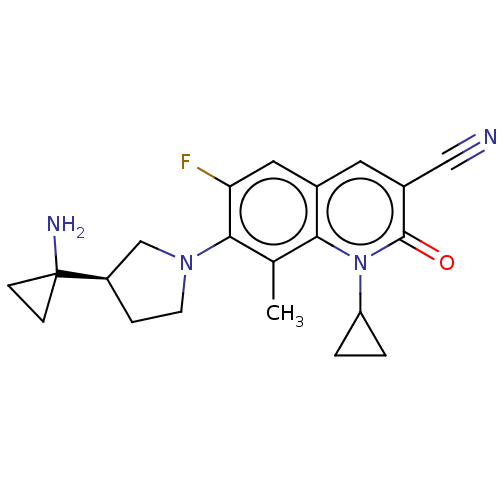 (CHEMBL4860033) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-dofetilide from human ERG after 60 mins by scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00375 BindingDB Entry DOI: 10.7270/Q2KH0S2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier organic anion transporter family member 1B1 (Homo sapiens (Human)) | BDBM50030554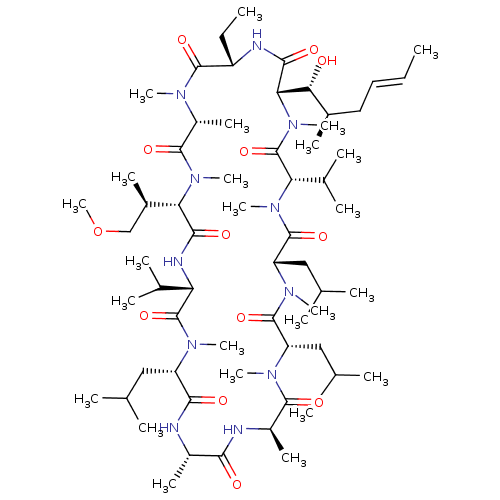 (CHEMBL3344494 | US9566312, Compound 2.1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of OATP1B1 (unknown origin) expressed in CHO cells using 8-fluorescein-cAMP substrate by fluorescent photometry | J Med Chem 57: 8503-16 (2014) Article DOI: 10.1021/jm500862r BindingDB Entry DOI: 10.7270/Q2HM5B1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50542205 (CHEMBL4643875) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.91E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant full-length C-terminal His6-tagged Escherichia coli DNA gyrase AB supercoiling activity using relaxed DNA as substrate prei... | J Med Chem 63: 7773-7816 (2020) Article DOI: 10.1021/acs.jmedchem.0c00347 BindingDB Entry DOI: 10.7270/Q2MW2MQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier organic anion transporter family member 1B1 (Homo sapiens (Human)) | BDBM50030533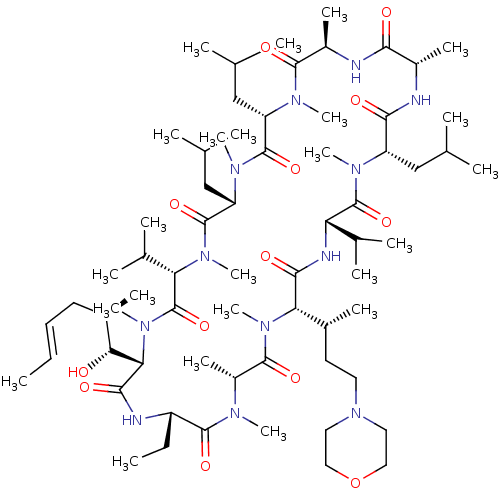 (CHEMBL3344502) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of OATP1B1 (unknown origin) expressed in CHO cells using 8-fluorescein-cAMP substrate by fluorescent photometry | J Med Chem 57: 8503-16 (2014) Article DOI: 10.1021/jm500862r BindingDB Entry DOI: 10.7270/Q2HM5B1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier organic anion transporter family member 1B1 (Homo sapiens (Human)) | BDBM50030542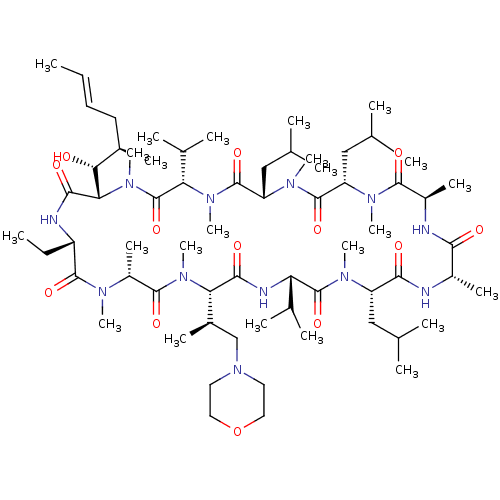 (CHEMBL3344495 | US9566312, Compound 2.5.2) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of OATP1B1 (unknown origin) expressed in CHO cells using 8-fluorescein-cAMP substrate by fluorescent photometry | J Med Chem 57: 8503-16 (2014) Article DOI: 10.1021/jm500862r BindingDB Entry DOI: 10.7270/Q2HM5B1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier organic anion transporter family member 1B1 (Homo sapiens (Human)) | BDBM50030493 (CHEMBL3344501 | US9566312, Compound 2.17.4) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of OATP1B1 (unknown origin) expressed in CHO cells using 8-fluorescein-cAMP substrate by fluorescent photometry | J Med Chem 57: 8503-16 (2014) Article DOI: 10.1021/jm500862r BindingDB Entry DOI: 10.7270/Q2HM5B1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-dependent translocase ABCB1 (Homo sapiens (Human)) | BDBM50030493 (CHEMBL3344501 | US9566312, Compound 2.17.4) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of MDR1 (unknown origin) expressed in MDA T0.3 cells using rhodamine 123 substrate | J Med Chem 57: 8503-16 (2014) Article DOI: 10.1021/jm500862r BindingDB Entry DOI: 10.7270/Q2HM5B1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier organic anion transporter family member 1B1 (Homo sapiens (Human)) | BDBM50030494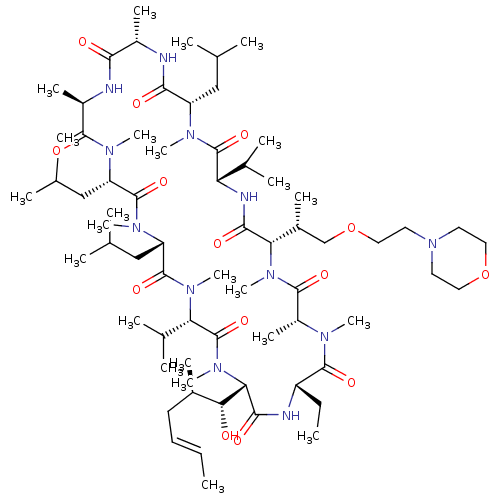 (CHEMBL3344503) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of OATP1B1 (unknown origin) expressed in CHO cells using 8-fluorescein-cAMP substrate by fluorescent photometry | J Med Chem 57: 8503-16 (2014) Article DOI: 10.1021/jm500862r BindingDB Entry DOI: 10.7270/Q2HM5B1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50566543 (CHEMBL4864037) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-dofetilide from human ERG after 60 mins by scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00375 BindingDB Entry DOI: 10.7270/Q2KH0S2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50566538 (CHEMBL4875315) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-dofetilide from human ERG after 60 mins by scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00375 BindingDB Entry DOI: 10.7270/Q2KH0S2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50566541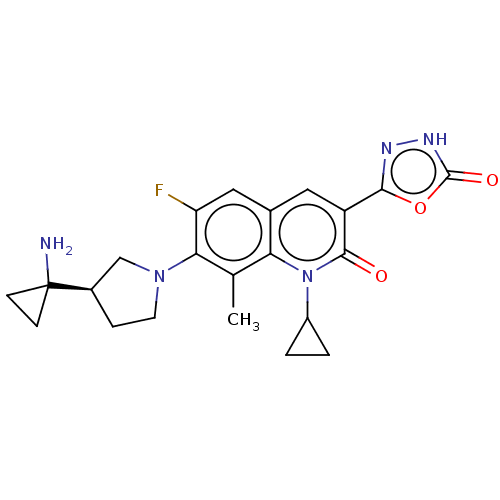 (CHEMBL4867915) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-dofetilide from human ERG after 60 mins by scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00375 BindingDB Entry DOI: 10.7270/Q2KH0S2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional coenzyme A synthase (Homo sapiens) | BDBM50263238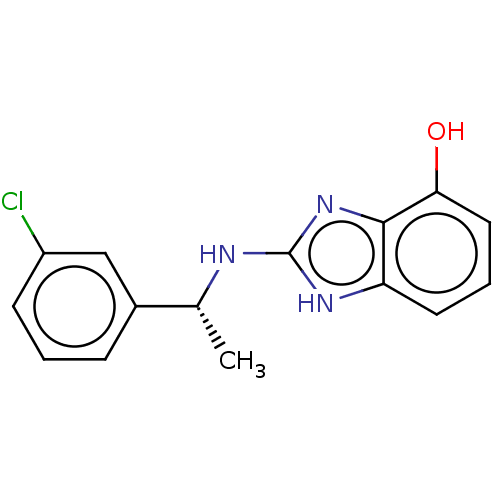 (CHEMBL4102651) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research , 5300 Chiron Way , Emeryville , California 94608 , United States. Curated by ChEMBL | Assay Description Inhibition of human PPAT | J Med Chem 61: 3309-3324 (2018) Article DOI: 10.1021/acs.jmedchem.7b01691 BindingDB Entry DOI: 10.7270/Q2S46VFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50566542 (CHEMBL4878164) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-dofetilide from human ERG after 60 mins by scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00375 BindingDB Entry DOI: 10.7270/Q2KH0S2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50566546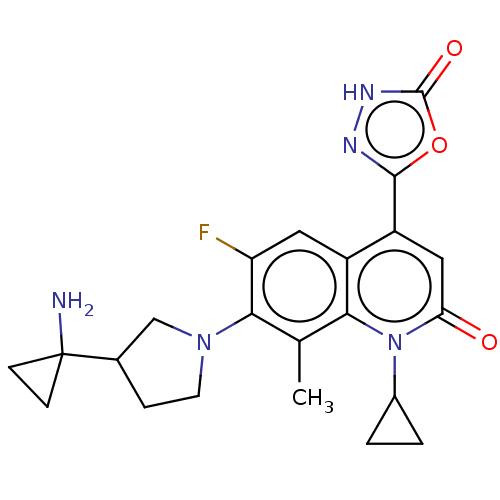 (CHEMBL4875871) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-dofetilide from human ERG after 60 mins by scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00375 BindingDB Entry DOI: 10.7270/Q2KH0S2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50566544 (CHEMBL4852170) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-dofetilide from human ERG after 60 mins by scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00375 BindingDB Entry DOI: 10.7270/Q2KH0S2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-binding cassette sub-family C member 2 (Homo sapiens (Human)) | BDBM50339127 ((3S,6S,9S,12R,15S,18S,21S,24S,27R,30S,33S)-25,30-d...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human MRP2 expressed in Sf9 cells inside out vesicles using CDCF substrate | J Med Chem 57: 8503-16 (2014) Article DOI: 10.1021/jm500862r BindingDB Entry DOI: 10.7270/Q2HM5B1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50566545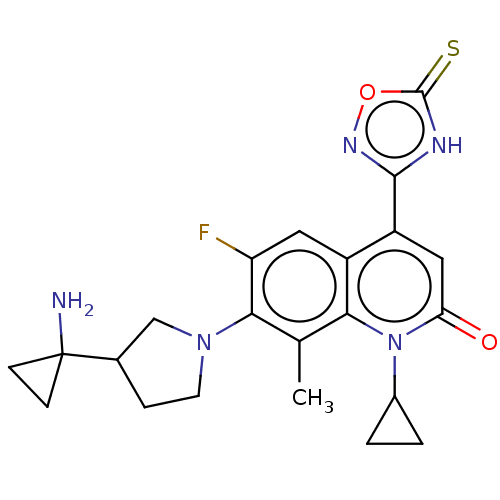 (CHEMBL4861495) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-dofetilide from human ERG after 60 mins by scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00375 BindingDB Entry DOI: 10.7270/Q2KH0S2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Broad substrate specificity ATP-binding cassette transporter ABCG2 (Homo sapiens (Human)) | BDBM50030493 (CHEMBL3344501 | US9566312, Compound 2.17.4) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of BCRP (unknown origin) expressed in T8 cells using Bodipy FL-prazosin substrate | J Med Chem 57: 8503-16 (2014) Article DOI: 10.1021/jm500862r BindingDB Entry DOI: 10.7270/Q2HM5B1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50566510 (CHEMBL4860105) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human ERG expressed in CHO-K1 cells by Qpatch clamp method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00375 BindingDB Entry DOI: 10.7270/Q2KH0S2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50566540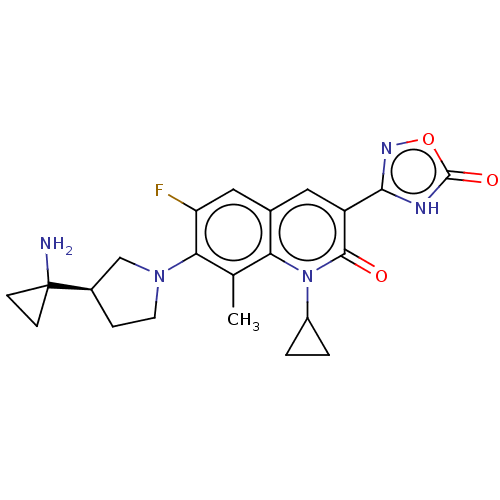 (CHEMBL4877922) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-dofetilide from human ERG after 60 mins by scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00375 BindingDB Entry DOI: 10.7270/Q2KH0S2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50566547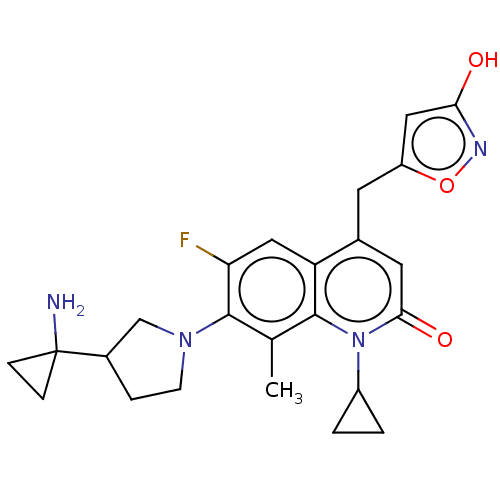 (CHEMBL4869998) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-dofetilide from human ERG after 60 mins by scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00375 BindingDB Entry DOI: 10.7270/Q2KH0S2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50566548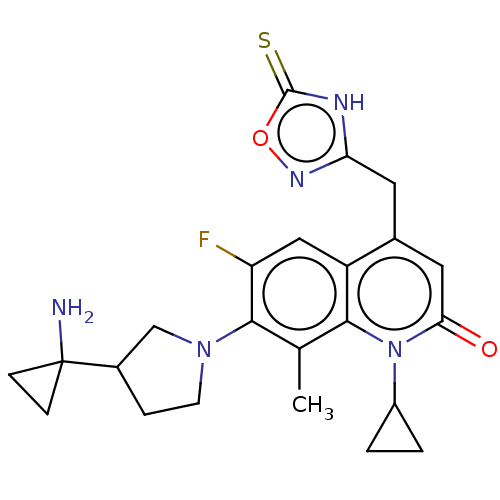 (CHEMBL4861063) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-dofetilide from human ERG after 60 mins by scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00375 BindingDB Entry DOI: 10.7270/Q2KH0S2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50566549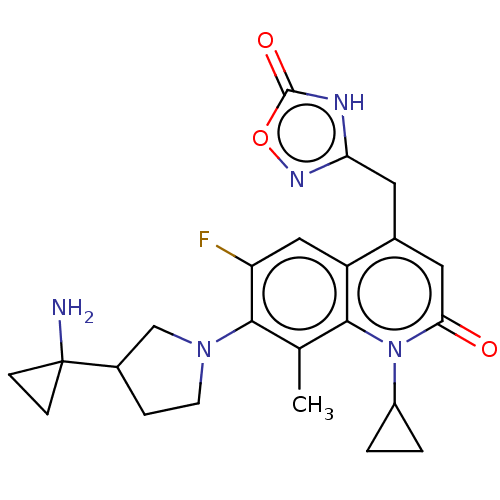 (CHEMBL4856534) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-dofetilide from human ERG after 60 mins by scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00375 BindingDB Entry DOI: 10.7270/Q2KH0S2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50566513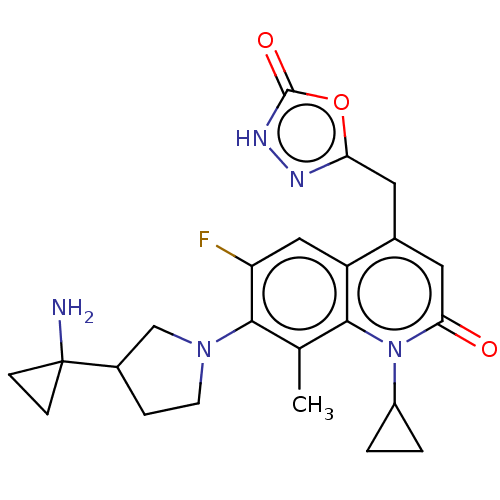 (CHEMBL4869164) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-dofetilide from human ERG after 60 mins by scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00375 BindingDB Entry DOI: 10.7270/Q2KH0S2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50566514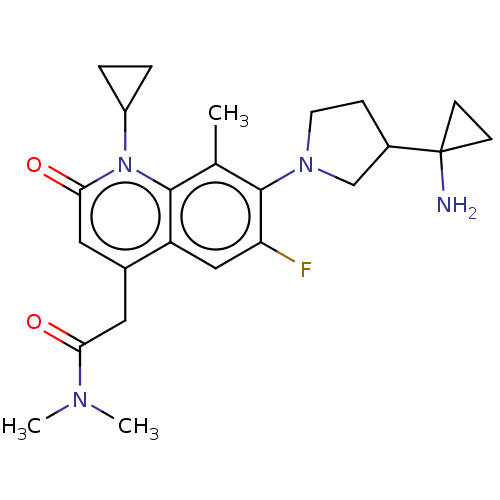 (CHEMBL4865462) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-dofetilide from human ERG after 60 mins by scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00375 BindingDB Entry DOI: 10.7270/Q2KH0S2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50566517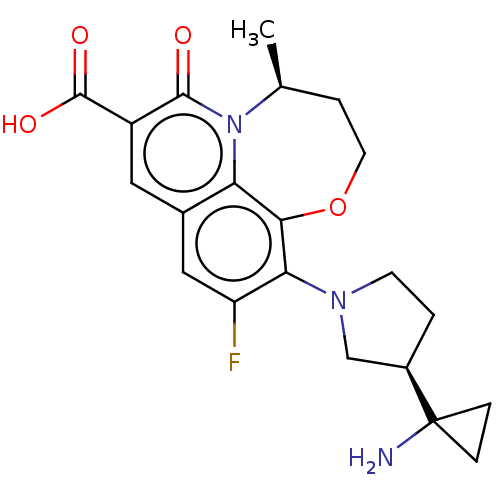 (CHEMBL4873798) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human ERG expressed in CHO-K1 cells by Qpatch clamp method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00375 BindingDB Entry DOI: 10.7270/Q2KH0S2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50566519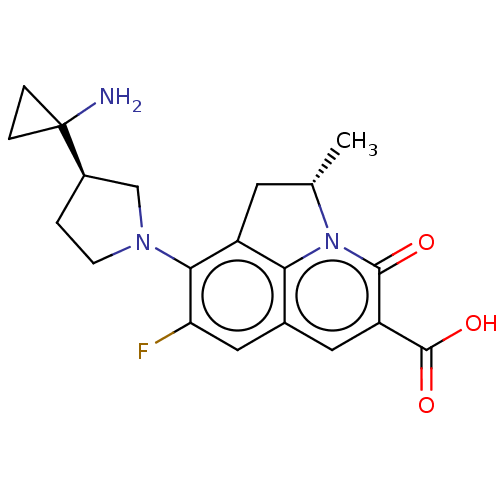 (CHEMBL4868731) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human ERG expressed in CHO-K1 cells by Qpatch clamp method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00375 BindingDB Entry DOI: 10.7270/Q2KH0S2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50566535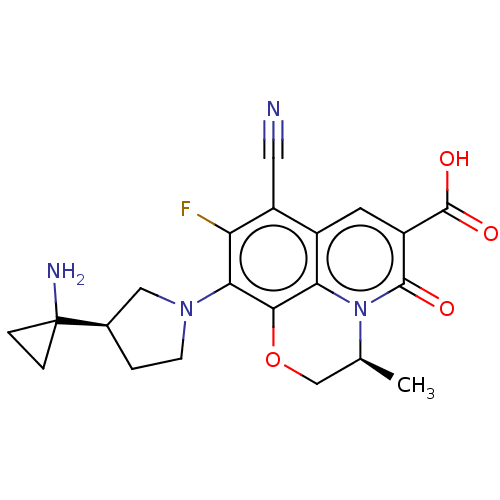 (CHEMBL4860370) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human ERG expressed in CHO-K1 cells by Qpatch clamp method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00375 BindingDB Entry DOI: 10.7270/Q2KH0S2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C8 (Homo sapiens (Human)) | BDBM50030493 (CHEMBL3344501 | US9566312, Compound 2.17.4) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of CYP2C8 (unknown origin) | J Med Chem 57: 8503-16 (2014) Article DOI: 10.1021/jm500862r BindingDB Entry DOI: 10.7270/Q2HM5B1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50030493 (CHEMBL3344501 | US9566312, Compound 2.17.4) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of CYP2D6 (unknown origin) | J Med Chem 57: 8503-16 (2014) Article DOI: 10.1021/jm500862r BindingDB Entry DOI: 10.7270/Q2HM5B1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50030493 (CHEMBL3344501 | US9566312, Compound 2.17.4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) | J Med Chem 57: 8503-16 (2014) Article DOI: 10.1021/jm500862r BindingDB Entry DOI: 10.7270/Q2HM5B1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 433 total ) | Next | Last >> |