 Found 488 hits with Last Name = 'mederski' and Initial = 'ww'
Found 488 hits with Last Name = 'mederski' and Initial = 'ww' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Type-2 angiotensin II receptor
(RAT) | BDBM50041700
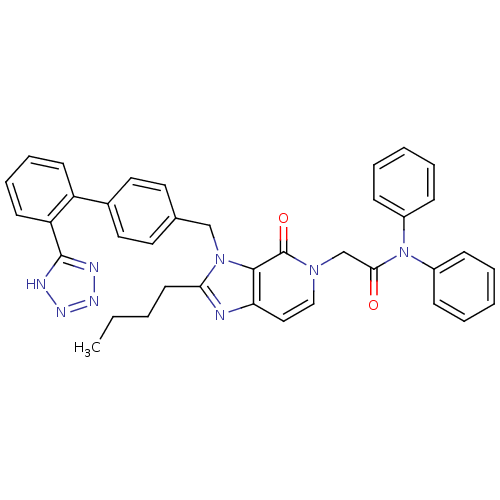
(2-{2-Butyl-4-oxo-3-[2'-(1H-tetrazol-5-yl)-biphenyl...)Show SMILES CCCCc1nc2ccn(CC(=O)N(c3ccccc3)c3ccccc3)c(=O)c2n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C38H34N8O2/c1-2-3-18-34-39-33-23-24-44(26-35(47)46(29-12-6-4-7-13-29)30-14-8-5-9-15-30)38(48)36(33)45(34)25-27-19-21-28(22-20-27)31-16-10-11-17-32(31)37-40-42-43-41-37/h4-17,19-24H,2-3,18,25-26H2,1H3,(H,40,41,42,43) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
E. Merck
Curated by ChEMBL
| Assay Description
Binding affinity against angiotensin II receptor type 2 (AT2) of rat adrenal medulla. |
J Med Chem 37: 1632-45 (1994)
BindingDB Entry DOI: 10.7270/Q2RR1X9M |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(RAT) | BDBM50070888

(CHEMBL298725 | Sodium; (Z)-2-benzo[1,2,5]thiadiazo...)Show SMILES COc1ccc(cc1F)C(=O)C(\Cc1cc(OC)c(OC)c(OC)c1)=C(/C([O-])=O)c1ccc2nsnc2c1 Show InChI InChI=1S/C27H23FN2O7S/c1-34-21-8-6-16(12-18(21)28)25(31)17(9-14-10-22(35-2)26(37-4)23(11-14)36-3)24(27(32)33)15-5-7-19-20(13-15)30-38-29-19/h5-8,10-13H,9H2,1-4H3,(H,32,33)/p-1/b24-17- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.0320 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA
Curated by ChEMBL
| Assay Description
In vitro ability to inhibit specific [125I]ET1 binding to rat aorta membranes Endothelin A receptor |
Bioorg Med Chem Lett 8: 1771-6 (1999)
BindingDB Entry DOI: 10.7270/Q2TM7BM4 |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(RAT) | BDBM50070884
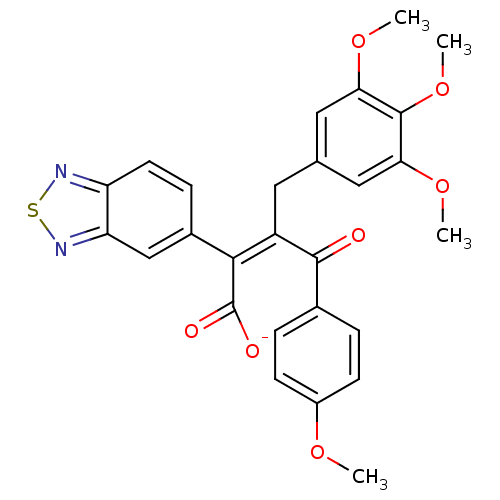
(CHEMBL48907 | Sodium; (Z)-2-benzo[1,2,5]thiadiazol...)Show SMILES COc1ccc(cc1)C(=O)C(\Cc1cc(OC)c(OC)c(OC)c1)=C(/C([O-])=O)c1ccc2nsnc2c1 Show InChI InChI=1S/C27H24N2O7S/c1-33-18-8-5-16(6-9-18)25(30)19(11-15-12-22(34-2)26(36-4)23(13-15)35-3)24(27(31)32)17-7-10-20-21(14-17)29-37-28-20/h5-10,12-14H,11H2,1-4H3,(H,31,32)/p-1/b24-19- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA
Curated by ChEMBL
| Assay Description
In vitro ability to inhibit specific [125I]ET1 binding to rat aorta membranes Endothelin A receptor |
Bioorg Med Chem Lett 8: 1771-6 (1999)
BindingDB Entry DOI: 10.7270/Q2TM7BM4 |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(RAT) | BDBM50069575
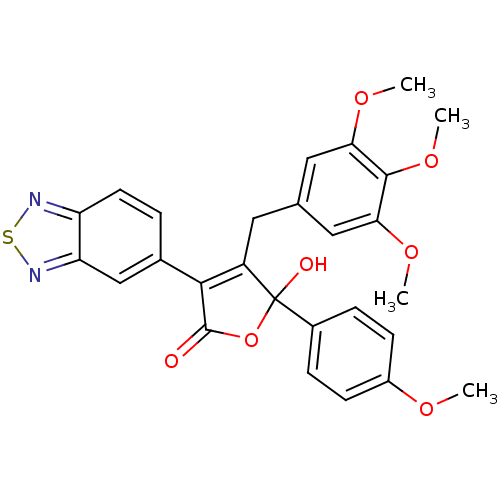
(3-Benzo[1,2,5]thiadiazol-5-yl-5-hydroxy-5-(4-metho...)Show SMILES COc1ccc(cc1)C1(O)OC(=O)C(=C1Cc1cc(OC)c(OC)c(OC)c1)c1ccc2nsnc2c1 |c:14| Show InChI InChI=1S/C27H24N2O7S/c1-32-18-8-6-17(7-9-18)27(31)19(11-15-12-22(33-2)25(35-4)23(13-15)34-3)24(26(30)36-27)16-5-10-20-21(14-16)29-37-28-20/h5-10,12-14,31H,11H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-ET-1 binding to rat aorta membrane Endothelin A receptor |
Bioorg Med Chem Lett 8: 17-22 (1999)
BindingDB Entry DOI: 10.7270/Q23B5Z9H |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(RAT) | BDBM50034267
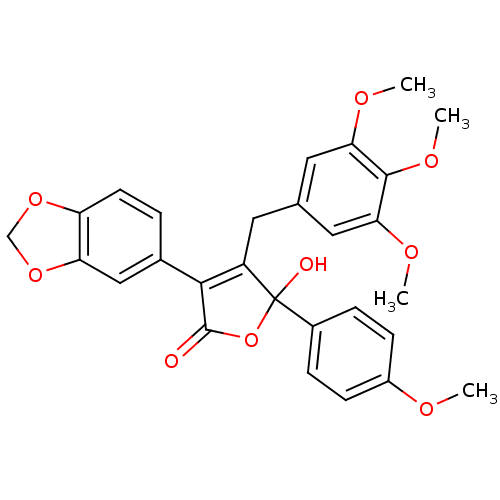
(3-(benzo[d][1,3]dioxol-5-yl)-5-hydroxy-5-(4-methox...)Show SMILES COc1ccc(cc1)C1(O)OC(=O)C(=C1Cc1cc(OC)c(OC)c(OC)c1)c1ccc2OCOc2c1 |c:14| Show InChI InChI=1S/C28H26O9/c1-31-19-8-6-18(7-9-19)28(30)20(11-16-12-23(32-2)26(34-4)24(13-16)33-3)25(27(29)37-28)17-5-10-21-22(14-17)36-15-35-21/h5-10,12-14,30H,11,15H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-ET-1 binding to rat aorta membrane Endothelin A receptor |
Bioorg Med Chem Lett 8: 17-22 (1999)
BindingDB Entry DOI: 10.7270/Q23B5Z9H |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50285171
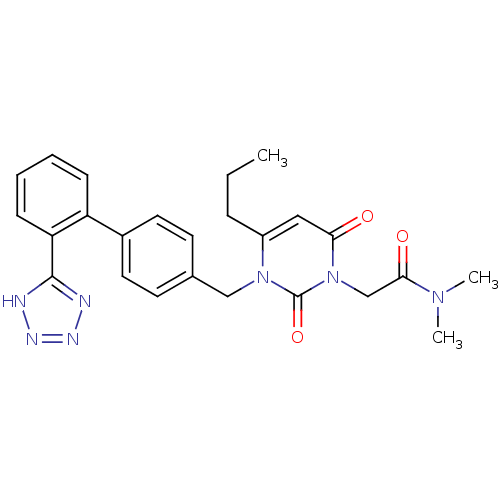
(2-{2,6-Dioxo-4-propyl-3-[2'-(1H-tetrazol-5-yl)-bip...)Show SMILES CCCc1cc(=O)n(CC(=O)N(C)C)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C25H27N7O3/c1-4-7-19-14-22(33)32(16-23(34)30(2)3)25(35)31(19)15-17-10-12-18(13-11-17)20-8-5-6-9-21(20)24-26-28-29-27-24/h5-6,8-14H,4,7,15-16H2,1-3H3,(H,26,27,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for binding affinity against angiotensin II receptor in rat adrenal cortex |
Bioorg Med Chem Lett 5: 2071-2076 (1995)
Article DOI: 10.1016/0960-894X(95)00370-9
BindingDB Entry DOI: 10.7270/Q2SF2W4J |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50285833
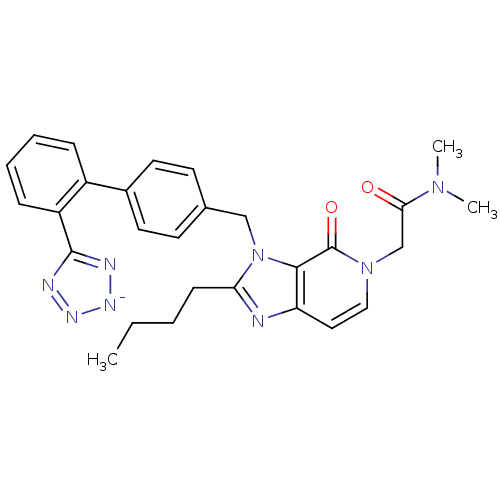
(1N,1N-dimethyl-2-(2-butyl-4-oxo-3-{4-[2-(1H-1,2,3,...)Show SMILES CCCCc1nc2ccn(CC(=O)N(C)C)c(=O)c2n1Cc1ccc(cc1)-c1ccccc1-c1nn[n-]n1 Show InChI InChI=1S/C28H29N8O2/c1-4-5-10-24-29-23-15-16-35(18-25(37)34(2)3)28(38)26(23)36(24)17-19-11-13-20(14-12-19)21-8-6-7-9-22(21)27-30-32-33-31-27/h6-9,11-16H,4-5,10,17-18H2,1-3H3/q-1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity determined against angiotensin II AT1 receptor in rat adrenal cortex preparation |
Bioorg Med Chem Lett 5: 2665-2670 (1995)
Article DOI: 10.1016/0960-894X(95)00477-B
BindingDB Entry DOI: 10.7270/Q2ZW1KVW |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(RAT) | BDBM50070886
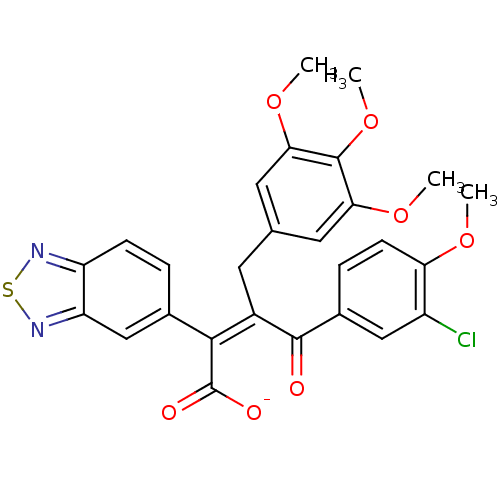
(CHEMBL51234 | Sodium; (Z)-2-benzo[1,2,5]thiadiazol...)Show SMILES COc1ccc(cc1Cl)C(=O)C(\Cc1cc(OC)c(OC)c(OC)c1)=C(/C([O-])=O)c1ccc2nsnc2c1 Show InChI InChI=1S/C27H23ClN2O7S/c1-34-21-8-6-16(12-18(21)28)25(31)17(9-14-10-22(35-2)26(37-4)23(11-14)36-3)24(27(32)33)15-5-7-19-20(13-15)30-38-29-19/h5-8,10-13H,9H2,1-4H3,(H,32,33)/p-1/b24-17- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA
Curated by ChEMBL
| Assay Description
In vitro ability to inhibit specific [125I]ET1 binding to rat aorta membranes Endothelin A receptor |
Bioorg Med Chem Lett 8: 1771-6 (1999)
BindingDB Entry DOI: 10.7270/Q2TM7BM4 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50041701

(2-{2-Butyl-4-oxo-3-[2'-(1H-tetrazol-5-yl)-biphenyl...)Show SMILES CCCCc1nc2ccn(CC(N)=O)c(=O)c2n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C26H26N8O2/c1-2-3-8-23-28-21-13-14-33(16-22(27)35)26(36)24(21)34(23)15-17-9-11-18(12-10-17)19-6-4-5-7-20(19)25-29-31-32-30-25/h4-7,9-14H,2-3,8,15-16H2,1H3,(H2,27,35)(H,29,30,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
E. Merck
Curated by ChEMBL
| Assay Description
Binding affinity against AT1 receptor in the presence of 0.01% BSA |
J Med Chem 37: 1632-45 (1994)
BindingDB Entry DOI: 10.7270/Q2RR1X9M |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(RAT) | BDBM50034263
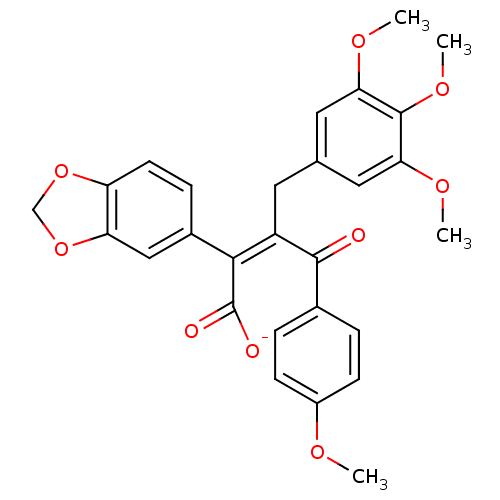
(CHEMBL25438 | PD-156707 | Sodium; (Z)-2-benzo[1,3]...)Show SMILES COc1ccc(cc1)C(=O)C(\Cc1cc(OC)c(OC)c(OC)c1)=C(/C([O-])=O)c1ccc2OCOc2c1 Show InChI InChI=1S/C28H26O9/c1-32-19-8-5-17(6-9-19)26(29)20(11-16-12-23(33-2)27(35-4)24(13-16)34-3)25(28(30)31)18-7-10-21-22(14-18)37-15-36-21/h5-10,12-14H,11,15H2,1-4H3,(H,30,31)/p-1/b25-20- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA
Curated by ChEMBL
| Assay Description
In vitro ability to inhibit specific [125I]ET1 binding to rat aorta membranes Endothelin A receptor |
Bioorg Med Chem Lett 8: 1771-6 (1999)
BindingDB Entry DOI: 10.7270/Q2TM7BM4 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50041688

(CHEMBL43500 | {2-Butyl-4-oxo-3-[2'-(1H-tetrazol-5-...)Show SMILES CCCCc1nc2ccn(CC(=O)OC)c(=O)c2n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C27H27N7O3/c1-3-4-9-23-28-22-14-15-33(17-24(35)37-2)27(36)25(22)34(23)16-18-10-12-19(13-11-18)20-7-5-6-8-21(20)26-29-31-32-30-26/h5-8,10-15H,3-4,9,16-17H2,1-2H3,(H,29,30,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
E. Merck
Curated by ChEMBL
| Assay Description
Binding affinity against AT1 receptor of rat adrenal cortical membranes |
J Med Chem 37: 1632-45 (1994)
BindingDB Entry DOI: 10.7270/Q2RR1X9M |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(RAT) | BDBM50070881
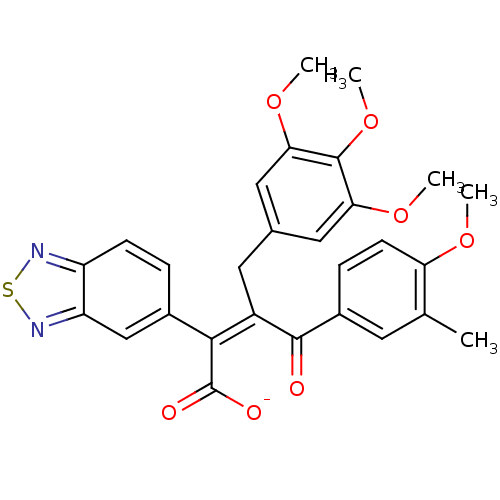
(CHEMBL295483 | Sodium; (Z)-2-benzo[1,2,5]thiadiazo...)Show SMILES COc1ccc(cc1C)C(=O)C(\Cc1cc(OC)c(OC)c(OC)c1)=C(/C([O-])=O)c1ccc2nsnc2c1 Show InChI InChI=1S/C28H26N2O7S/c1-15-10-18(7-9-22(15)34-2)26(31)19(11-16-12-23(35-3)27(37-5)24(13-16)36-4)25(28(32)33)17-6-8-20-21(14-17)30-38-29-20/h6-10,12-14H,11H2,1-5H3,(H,32,33)/p-1/b25-19- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA
Curated by ChEMBL
| Assay Description
In vitro ability to inhibit specific [125I]ET1 binding to rat aorta membranes Endothelin A receptor |
Bioorg Med Chem Lett 8: 1771-6 (1999)
BindingDB Entry DOI: 10.7270/Q2TM7BM4 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50285164
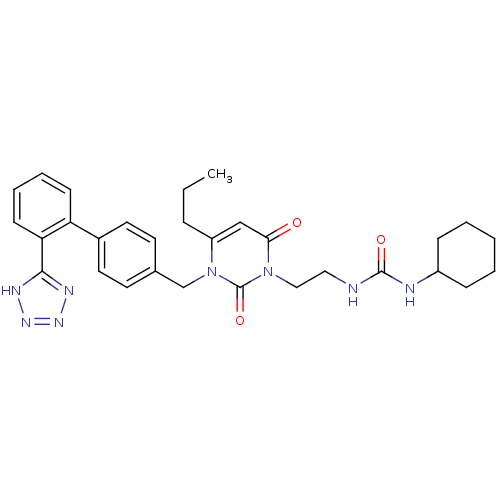
(1-Cyclohexyl-3-(2-{2,6-dioxo-4-propyl-3-[2'-(1H-te...)Show SMILES CCCc1cc(=O)n(CCNC(=O)NC2CCCCC2)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C30H36N8O3/c1-2-8-24-19-27(39)37(18-17-31-29(40)32-23-9-4-3-5-10-23)30(41)38(24)20-21-13-15-22(16-14-21)25-11-6-7-12-26(25)28-33-35-36-34-28/h6-7,11-16,19,23H,2-5,8-10,17-18,20H2,1H3,(H2,31,32,40)(H,33,34,35,36) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for binding affinity against angiotensin II receptor in rat adrenal cortex |
Bioorg Med Chem Lett 5: 2071-2076 (1995)
Article DOI: 10.1016/0960-894X(95)00370-9
BindingDB Entry DOI: 10.7270/Q2SF2W4J |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(RAT) | BDBM50051007
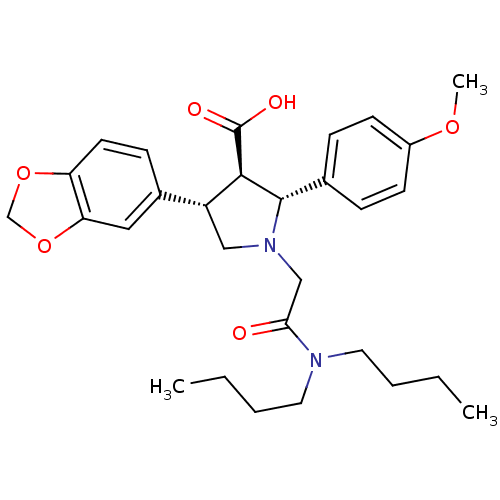
((2R,3R,4S)-4-Benzo[1,3]dioxol-5-yl-1-dibutylcarbam...)Show SMILES CCCCN(CCCC)C(=O)CN1C[C@@H]([C@H]([C@@H]1c1ccc(OC)cc1)C(O)=O)c1ccc2OCOc2c1 Show InChI InChI=1S/C29H38N2O6/c1-4-6-14-30(15-7-5-2)26(32)18-31-17-23(21-10-13-24-25(16-21)37-19-36-24)27(29(33)34)28(31)20-8-11-22(35-3)12-9-20/h8-13,16,23,27-28H,4-7,14-15,17-19H2,1-3H3,(H,33,34)/t23-,27-,28+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA
Curated by ChEMBL
| Assay Description
In vitro ability to inhibit specific [125I]ET1 binding to rat aorta membranes Endothelin A receptor |
Bioorg Med Chem Lett 8: 1771-6 (1999)
BindingDB Entry DOI: 10.7270/Q2TM7BM4 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50406795
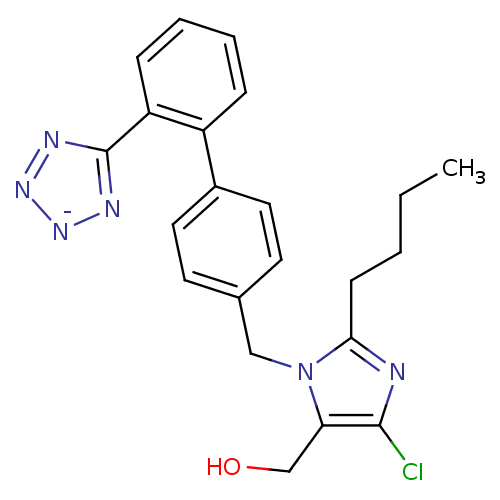
(Cozaar | LOSARTAN POTASSIUM)Show SMILES CCCCc1nc(Cl)c(CO)n1Cc1ccc(cc1)-c1ccccc1-c1nn[n-]n1 Show InChI InChI=1S/C22H22ClN6O/c1-2-3-8-20-24-21(23)19(14-30)29(20)13-15-9-11-16(12-10-15)17-6-4-5-7-18(17)22-25-27-28-26-22/h4-7,9-12,30H,2-3,8,13-14H2,1H3/q-1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
E. Merck
Curated by ChEMBL
| Assay Description
Binding affinity against AT1 receptor in the presence of 0.01% BSA |
J Med Chem 37: 1632-45 (1994)
BindingDB Entry DOI: 10.7270/Q2RR1X9M |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50041689

(5-Benzyl-2-butyl-3-[2'-(1H-tetrazol-5-yl)-biphenyl...)Show SMILES CCCCc1nc2ccn(Cc3ccccc3)c(=O)c2n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C31H29N7O/c1-2-3-13-28-32-27-18-19-37(20-22-9-5-4-6-10-22)31(39)29(27)38(28)21-23-14-16-24(17-15-23)25-11-7-8-12-26(25)30-33-35-36-34-30/h4-12,14-19H,2-3,13,20-21H2,1H3,(H,33,34,35,36) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
E. Merck
Curated by ChEMBL
| Assay Description
Binding affinity against AT1 receptor of rat adrenal cortical membranes |
J Med Chem 37: 1632-45 (1994)
BindingDB Entry DOI: 10.7270/Q2RR1X9M |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(RABBIT) | BDBM50282613
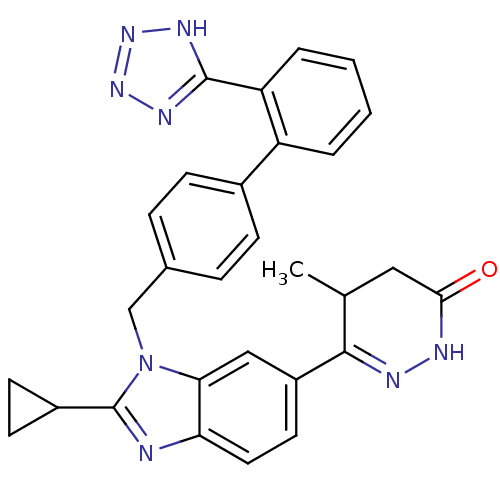
(6-{2-Cyclopropyl-3-[2'-(1H-tetrazol-5-yl)-biphenyl...)Show SMILES CC1CC(=O)NN=C1c1ccc2nc(C3CC3)n(Cc3ccc(cc3)-c3ccccc3-c3nnn[nH]3)c2c1 |c:6| Show InChI InChI=1S/C29H26N8O/c1-17-14-26(38)31-32-27(17)21-12-13-24-25(15-21)37(29(30-24)20-10-11-20)16-18-6-8-19(9-7-18)22-4-2-3-5-23(22)28-33-35-36-34-28/h2-9,12-13,15,17,20H,10-11,14,16H2,1H3,(H,31,38)(H,33,34,35,36) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against Angiotensin II receptor, type 1 induced contractions in isolated rabbit aortic rings |
Bioorg Med Chem Lett 4: 1297-1302 (1994)
Article DOI: 10.1016/S0960-894X(01)80348-6
BindingDB Entry DOI: 10.7270/Q2G44QRD |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(RABBIT) | BDBM50403242
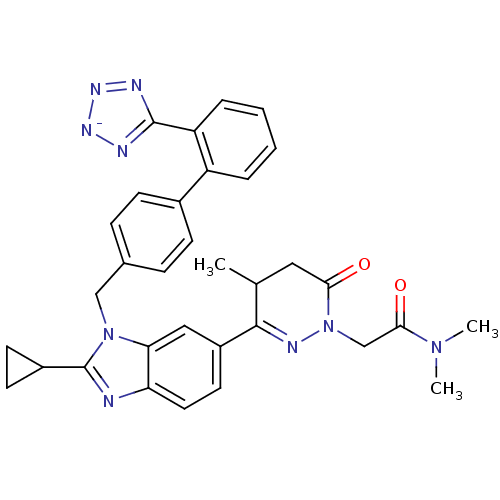
(CHEMBL2079790)Show SMILES CC1CC(=O)N(CC(=O)N(C)C)N=C1c1ccc2nc(C3CC3)n(Cc3ccc(cc3)-c3ccccc3-c3nn[n-]n3)c2c1 |c:12| Show InChI InChI=1S/C33H32N9O2/c1-20-16-29(43)42(19-30(44)40(2)3)37-31(20)24-14-15-27-28(17-24)41(33(34-27)23-12-13-23)18-21-8-10-22(11-9-21)25-6-4-5-7-26(25)32-35-38-39-36-32/h4-11,14-15,17,20,23H,12-13,16,18-19H2,1-3H3/q-1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against Angiotensin II receptor, type 1 induced contractions in isolated rabbit aortic rings |
Bioorg Med Chem Lett 4: 1297-1302 (1994)
Article DOI: 10.1016/S0960-894X(01)80348-6
BindingDB Entry DOI: 10.7270/Q2G44QRD |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(RABBIT) | BDBM50403245
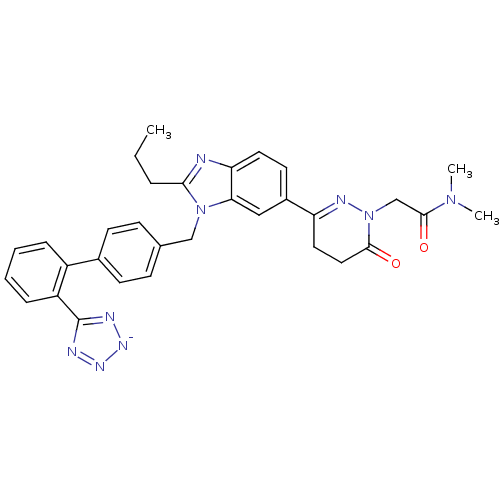
(CHEMBL2079788)Show SMILES CCCc1nc2ccc(cc2n1Cc1ccc(cc1)-c1ccccc1-c1nn[n-]n1)C1=NN(CC(=O)N(C)C)C(=O)CC1 |t:35| Show InChI InChI=1S/C32H32N9O2/c1-4-7-29-33-27-15-14-23(26-16-17-30(42)41(36-26)20-31(43)39(2)3)18-28(27)40(29)19-21-10-12-22(13-11-21)24-8-5-6-9-25(24)32-34-37-38-35-32/h5-6,8-15,18H,4,7,16-17,19-20H2,1-3H3/q-1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against Angiotensin II receptor, type 1 induced contractions in isolated rabbit aortic rings |
Bioorg Med Chem Lett 4: 1297-1302 (1994)
Article DOI: 10.1016/S0960-894X(01)80348-6
BindingDB Entry DOI: 10.7270/Q2G44QRD |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(RABBIT) | BDBM50403247
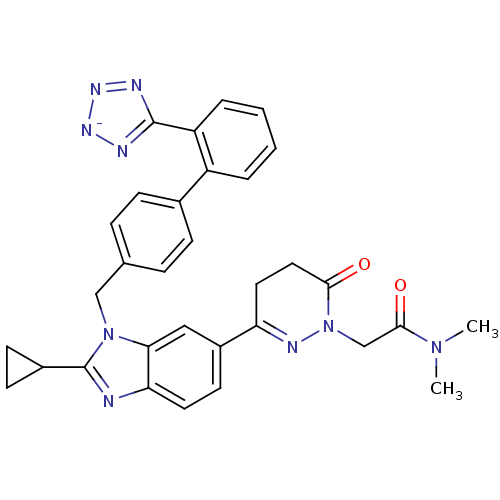
(CHEMBL2079791)Show SMILES CN(C)C(=O)CN1N=C(CCC1=O)c1ccc2nc(C3CC3)n(Cc3ccc(cc3)-c3ccccc3-c3nn[n-]n3)c2c1 |c:7| Show InChI InChI=1S/C32H30N9O2/c1-39(2)30(43)19-41-29(42)16-15-26(36-41)23-13-14-27-28(17-23)40(32(33-27)22-11-12-22)18-20-7-9-21(10-8-20)24-5-3-4-6-25(24)31-34-37-38-35-31/h3-10,13-14,17,22H,11-12,15-16,18-19H2,1-2H3/q-1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against Angiotensin II receptor, type 1 induced contractions in isolated rabbit aortic rings |
Bioorg Med Chem Lett 4: 1297-1302 (1994)
Article DOI: 10.1016/S0960-894X(01)80348-6
BindingDB Entry DOI: 10.7270/Q2G44QRD |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(RABBIT) | BDBM50403246

(CHEMBL2079787)Show SMILES CN(C)C(=O)CN1N=C(c2ccc3nc(C4CC4)n(Cc4ccc(cc4)-c4ccccc4-c4nn[n-]n4)c3c2)C(C)(C)CC1=O |t:7| Show InChI InChI=1S/C34H34N9O2/c1-34(2)18-29(44)43(20-30(45)41(3)4)38-31(34)24-15-16-27-28(17-24)42(33(35-27)23-13-14-23)19-21-9-11-22(12-10-21)25-7-5-6-8-26(25)32-36-39-40-37-32/h5-12,15-17,23H,13-14,18-20H2,1-4H3/q-1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against Angiotensin II receptor, type 1 induced contractions in isolated rabbit aortic rings |
Bioorg Med Chem Lett 4: 1297-1302 (1994)
Article DOI: 10.1016/S0960-894X(01)80348-6
BindingDB Entry DOI: 10.7270/Q2G44QRD |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50285165
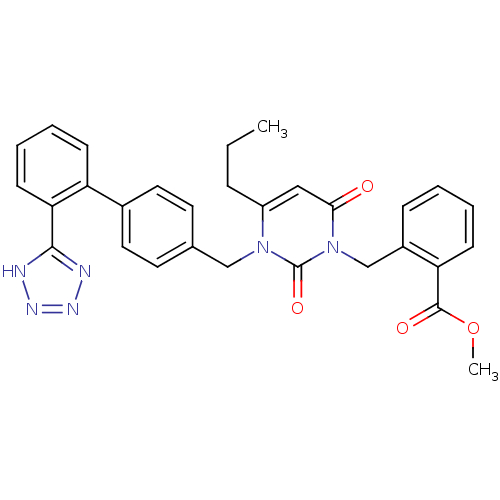
(2-{2,6-Dioxo-4-propyl-3-[2'-(1H-tetrazol-5-yl)-bip...)Show SMILES CCCc1cc(=O)n(Cc2ccccc2C(=O)OC)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C30H28N6O4/c1-3-8-23-17-27(37)36(19-22-9-4-5-11-25(22)29(38)40-2)30(39)35(23)18-20-13-15-21(16-14-20)24-10-6-7-12-26(24)28-31-33-34-32-28/h4-7,9-17H,3,8,18-19H2,1-2H3,(H,31,32,33,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for binding affinity against angiotensin II receptor in rat adrenal cortex |
Bioorg Med Chem Lett 5: 2071-2076 (1995)
Article DOI: 10.1016/0960-894X(95)00370-9
BindingDB Entry DOI: 10.7270/Q2SF2W4J |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50285163

(2-{4-Butyl-2,6-dioxo-3-[2'-(1H-tetrazol-5-yl)-biph...)Show SMILES CCCCc1cc(=O)n(Cc2ccccc2C(=O)OC)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C31H30N6O4/c1-3-4-10-24-18-28(38)37(20-23-9-5-6-12-26(23)30(39)41-2)31(40)36(24)19-21-14-16-22(17-15-21)25-11-7-8-13-27(25)29-32-34-35-33-29/h5-9,11-18H,3-4,10,19-20H2,1-2H3,(H,32,33,34,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for binding affinity against angiotensin II receptor in rat adrenal cortex |
Bioorg Med Chem Lett 5: 2071-2076 (1995)
Article DOI: 10.1016/0960-894X(95)00370-9
BindingDB Entry DOI: 10.7270/Q2SF2W4J |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50285818
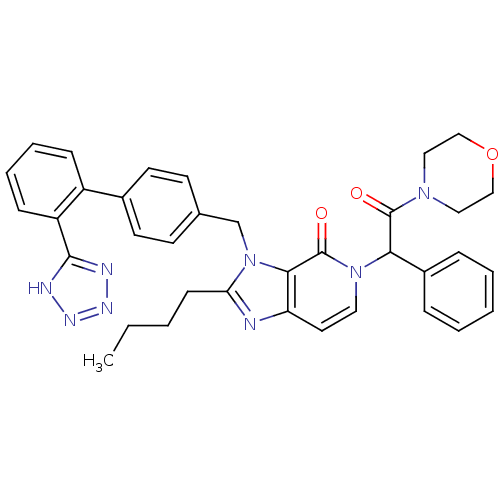
(2-Butyl-5-(2-morpholin-4-yl-2-oxo-1-phenyl-ethyl)-...)Show SMILES CCCCc1nc2ccn(C(C(=O)N3CCOCC3)c3ccccc3)c(=O)c2n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C36H36N8O3/c1-2-3-13-31-37-30-18-19-43(32(27-9-5-4-6-10-27)35(45)42-20-22-47-23-21-42)36(46)33(30)44(31)24-25-14-16-26(17-15-25)28-11-7-8-12-29(28)34-38-40-41-39-34/h4-12,14-19,32H,2-3,13,20-24H2,1H3,(H,38,39,40,41) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity determined against angiotensin II AT1 receptor in rat adrenal cortex preparation |
Bioorg Med Chem Lett 5: 2665-2670 (1995)
Article DOI: 10.1016/0960-894X(95)00477-B
BindingDB Entry DOI: 10.7270/Q2ZW1KVW |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50285832
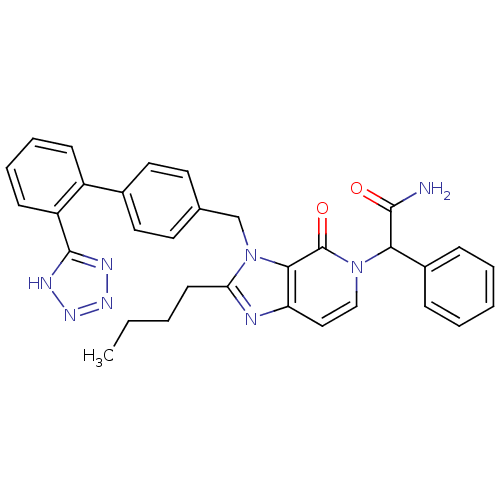
(2-{2-Butyl-4-oxo-3-[2'-(1H-tetrazol-5-yl)-biphenyl...)Show SMILES CCCCc1nc2ccn(C(C(N)=O)c3ccccc3)c(=O)c2n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C32H30N8O2/c1-2-3-13-27-34-26-18-19-39(28(30(33)41)23-9-5-4-6-10-23)32(42)29(26)40(27)20-21-14-16-22(17-15-21)24-11-7-8-12-25(24)31-35-37-38-36-31/h4-12,14-19,28H,2-3,13,20H2,1H3,(H2,33,41)(H,35,36,37,38) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity determined against angiotensin II AT1 receptor in rat adrenal cortex preparation |
Bioorg Med Chem Lett 5: 2665-2670 (1995)
Article DOI: 10.1016/0960-894X(95)00477-B
BindingDB Entry DOI: 10.7270/Q2ZW1KVW |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50041683
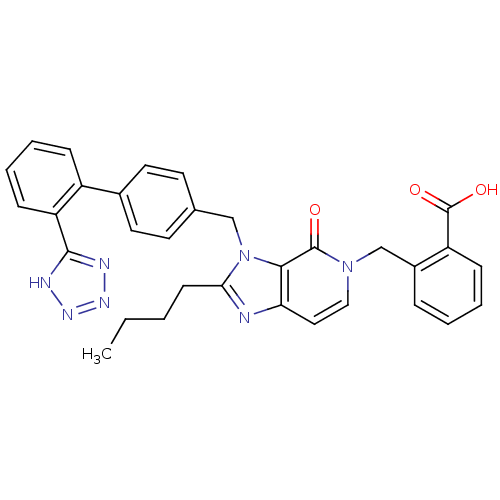
(2-{2-Butyl-4-oxo-3-[2'-(1H-tetrazol-5-yl)-biphenyl...)Show SMILES CCCCc1nc2ccn(Cc3ccccc3C(O)=O)c(=O)c2n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C32H29N7O3/c1-2-3-12-28-33-27-17-18-38(20-23-8-4-5-10-25(23)32(41)42)31(40)29(27)39(28)19-21-13-15-22(16-14-21)24-9-6-7-11-26(24)30-34-36-37-35-30/h4-11,13-18H,2-3,12,19-20H2,1H3,(H,41,42)(H,34,35,36,37) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
E. Merck
Curated by ChEMBL
| Assay Description
Binding affinity against AT1 receptor in the presence of 0.01% BSA |
J Med Chem 37: 1632-45 (1994)
BindingDB Entry DOI: 10.7270/Q2RR1X9M |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(RABBIT) | BDBM82258

(CAS_114798-26-4 | Losartan | NSC_3961)Show SMILES CCCCc1nc(Cl)c(CO)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C22H23ClN6O/c1-2-3-8-20-24-21(23)19(14-30)29(20)13-15-9-11-16(12-10-15)17-6-4-5-7-18(17)22-25-27-28-26-22/h4-7,9-12,30H,2-3,8,13-14H2,1H3,(H,25,26,27,28) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against Angiotensin II receptor, type 1 induced contractions in isolated rabbit aortic rings |
Bioorg Med Chem Lett 4: 1297-1302 (1994)
Article DOI: 10.1016/S0960-894X(01)80348-6
BindingDB Entry DOI: 10.7270/Q2G44QRD |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(RABBIT) | BDBM50403243

(CHEMBL2079789)Show SMILES CCc1nc2ccc(cc2n1Cc1ccc(cc1)-c1ccccc1-c1nn[n-]n1)C1=NN(CC(=O)N(C)C)C(=O)CC1 |t:34| Show InChI InChI=1S/C31H30N9O2/c1-4-28-32-26-14-13-22(25-15-16-29(41)40(35-25)19-30(42)38(2)3)17-27(26)39(28)18-20-9-11-21(12-10-20)23-7-5-6-8-24(23)31-33-36-37-34-31/h5-14,17H,4,15-16,18-19H2,1-3H3/q-1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against Angiotensin II receptor, type 1 induced contractions in isolated rabbit aortic rings |
Bioorg Med Chem Lett 4: 1297-1302 (1994)
Article DOI: 10.1016/S0960-894X(01)80348-6
BindingDB Entry DOI: 10.7270/Q2G44QRD |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(RAT) | BDBM50070882
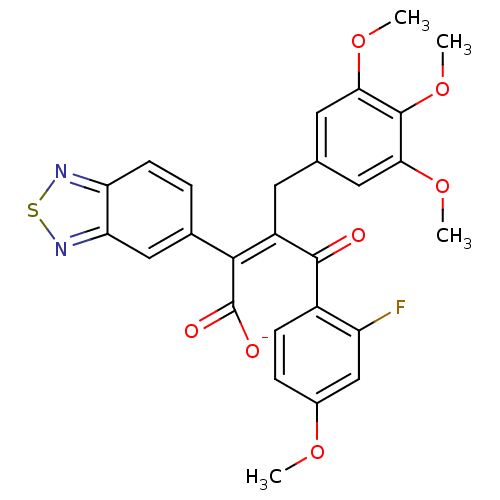
((Z)-2-Benzo[1,2,5]thiadiazol-5-yl-4-(2-fluoro-4-me...)Show SMILES COc1ccc(C(=O)C(\Cc2cc(OC)c(OC)c(OC)c2)=C(/C([O-])=O)c2ccc3nsnc3c2)c(F)c1 Show InChI InChI=1S/C27H23FN2O7S/c1-34-16-6-7-17(19(28)13-16)25(31)18(9-14-10-22(35-2)26(37-4)23(11-14)36-3)24(27(32)33)15-5-8-20-21(12-15)30-38-29-20/h5-8,10-13H,9H2,1-4H3,(H,32,33)/p-1/b24-18- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA
Curated by ChEMBL
| Assay Description
In vitro ability to inhibit specific [125I]ET1 binding to rat aorta membranes Endothelin A receptor |
Bioorg Med Chem Lett 8: 1771-6 (1999)
BindingDB Entry DOI: 10.7270/Q2TM7BM4 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(RABBIT) | BDBM50282616
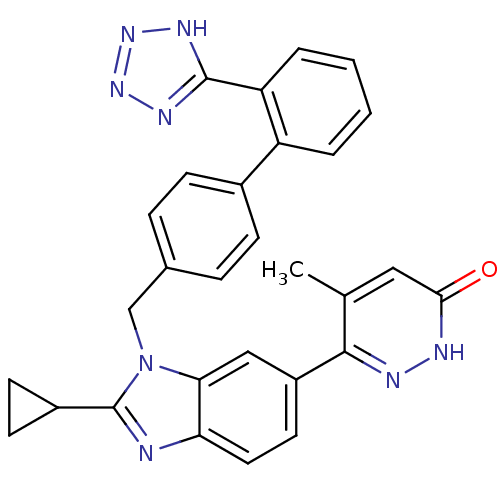
(6-{2-Cyclopropyl-3-[2'-(1H-tetrazol-5-yl)-biphenyl...)Show SMILES Cc1cc(=O)[nH]nc1-c1ccc2nc(C3CC3)n(Cc3ccc(cc3)-c3ccccc3-c3nnn[nH]3)c2c1 Show InChI InChI=1S/C29H24N8O/c1-17-14-26(38)31-32-27(17)21-12-13-24-25(15-21)37(29(30-24)20-10-11-20)16-18-6-8-19(9-7-18)22-4-2-3-5-23(22)28-33-35-36-34-28/h2-9,12-15,20H,10-11,16H2,1H3,(H,31,38)(H,33,34,35,36) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against Angiotensin II receptor, type 1 induced contractions in isolated rabbit aortic rings |
Bioorg Med Chem Lett 4: 1297-1302 (1994)
Article DOI: 10.1016/S0960-894X(01)80348-6
BindingDB Entry DOI: 10.7270/Q2G44QRD |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50285820
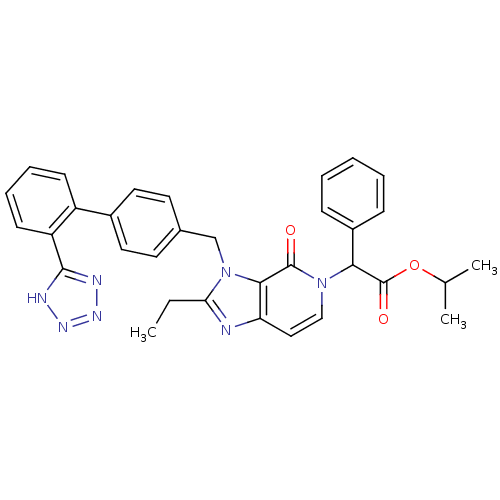
(CHEMBL88663 | {2-Ethyl-4-oxo-3-[2'-(1H-tetrazol-5-...)Show SMILES CCc1nc2ccn(C(C(=O)OC(C)C)c3ccccc3)c(=O)c2n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C33H31N7O3/c1-4-28-34-27-18-19-39(29(33(42)43-21(2)3)24-10-6-5-7-11-24)32(41)30(27)40(28)20-22-14-16-23(17-15-22)25-12-8-9-13-26(25)31-35-37-38-36-31/h5-19,21,29H,4,20H2,1-3H3,(H,35,36,37,38) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity determined against angiotensin II AT1 receptor in rat adrenal cortex preparation |
Bioorg Med Chem Lett 5: 2665-2670 (1995)
Article DOI: 10.1016/0960-894X(95)00477-B
BindingDB Entry DOI: 10.7270/Q2ZW1KVW |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50285829

(CHEMBL314603 | {4-Oxo-2-propyl-3-[2'-(1H-tetrazol-...)Show SMILES CCCc1nc2ccn(C(C(=O)OC(C)C)c3ccccc3)c(=O)c2n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C34H33N7O3/c1-4-10-29-35-28-19-20-40(30(34(43)44-22(2)3)25-11-6-5-7-12-25)33(42)31(28)41(29)21-23-15-17-24(18-16-23)26-13-8-9-14-27(26)32-36-38-39-37-32/h5-9,11-20,22,30H,4,10,21H2,1-3H3,(H,36,37,38,39) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity determined against angiotensin II AT1 receptor in rat adrenal cortex preparation |
Bioorg Med Chem Lett 5: 2665-2670 (1995)
Article DOI: 10.1016/0960-894X(95)00477-B
BindingDB Entry DOI: 10.7270/Q2ZW1KVW |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50285166
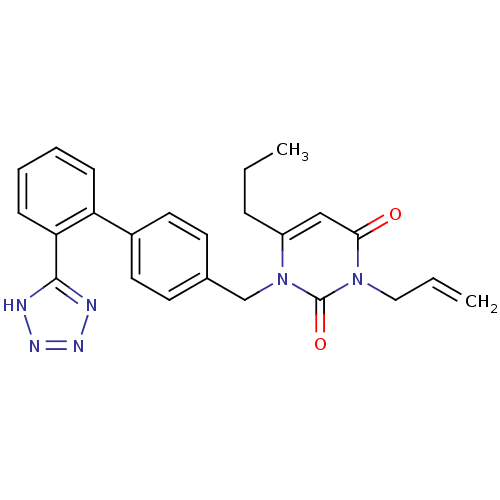
(3-Allyl-6-propyl-1-[2'-(1H-tetrazol-5-yl)-biphenyl...)Show SMILES CCCc1cc(=O)n(CC=C)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C24H24N6O2/c1-3-7-19-15-22(31)29(14-4-2)24(32)30(19)16-17-10-12-18(13-11-17)20-8-5-6-9-21(20)23-25-27-28-26-23/h4-6,8-13,15H,2-3,7,14,16H2,1H3,(H,25,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for binding affinity against angiotensin II receptor in rat adrenal cortex |
Bioorg Med Chem Lett 5: 2071-2076 (1995)
Article DOI: 10.1016/0960-894X(95)00370-9
BindingDB Entry DOI: 10.7270/Q2SF2W4J |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(RAT) | BDBM50070877
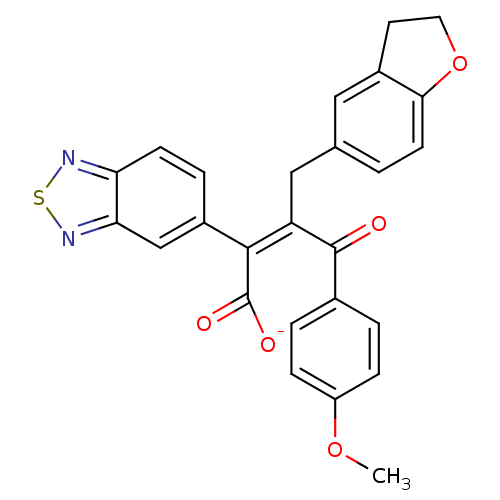
(CHEMBL262000 | Sodium; (Z)-2-benzo[1,2,5]thiadiazo...)Show SMILES COc1ccc(cc1)C(=O)C(\Cc1ccc2OCCc2c1)=C(/C([O-])=O)c1ccc2nsnc2c1 Show InChI InChI=1S/C26H20N2O5S/c1-32-19-6-3-16(4-7-19)25(29)20(13-15-2-9-23-17(12-15)10-11-33-23)24(26(30)31)18-5-8-21-22(14-18)28-34-27-21/h2-9,12,14H,10-11,13H2,1H3,(H,30,31)/p-1/b24-20- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA
Curated by ChEMBL
| Assay Description
In vitro ability to inhibit specific [125I]ET1 binding to rat aorta membranes Endothelin A receptor |
Bioorg Med Chem Lett 8: 1771-6 (1999)
BindingDB Entry DOI: 10.7270/Q2TM7BM4 |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(RAT) | BDBM50070871
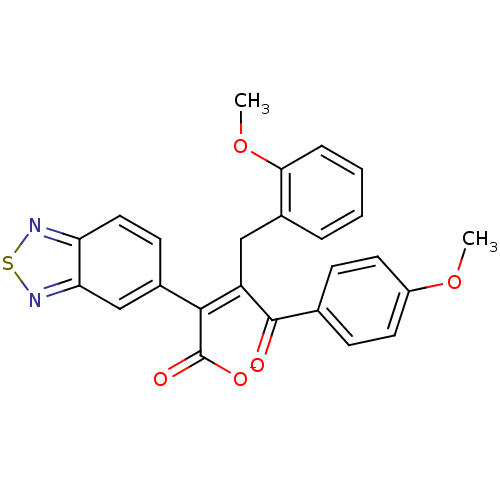
(CHEMBL431160 | Sodium; (Z)-2-benzo[1,2,5]thiadiazo...)Show SMILES COc1ccc(cc1)C(=O)C(\Cc1ccccc1OC)=C(/C([O-])=O)c1ccc2nsnc2c1 Show InChI InChI=1S/C25H20N2O5S/c1-31-18-10-7-15(8-11-18)24(28)19(13-16-5-3-4-6-22(16)32-2)23(25(29)30)17-9-12-20-21(14-17)27-33-26-20/h3-12,14H,13H2,1-2H3,(H,29,30)/p-1/b23-19- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA
Curated by ChEMBL
| Assay Description
In vitro ability to inhibit specific [125I]ET1 binding to rat aorta membranes Endothelin A receptor |
Bioorg Med Chem Lett 8: 1771-6 (1999)
BindingDB Entry DOI: 10.7270/Q2TM7BM4 |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(RAT) | BDBM50070890
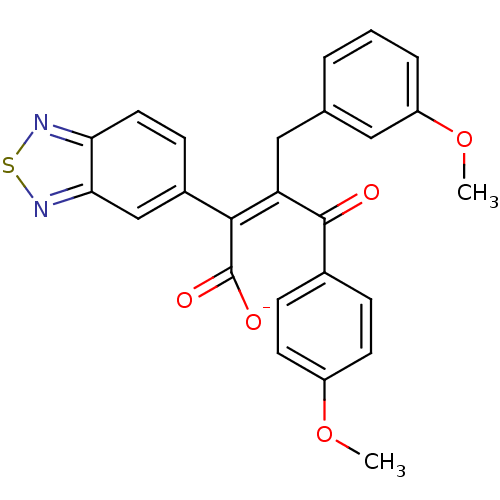
(CHEMBL51890 | Sodium; (Z)-2-benzo[1,2,5]thiadiazol...)Show SMILES COc1ccc(cc1)C(=O)C(\Cc1cccc(OC)c1)=C(/C([O-])=O)c1ccc2nsnc2c1 Show InChI InChI=1S/C25H20N2O5S/c1-31-18-9-6-16(7-10-18)24(28)20(13-15-4-3-5-19(12-15)32-2)23(25(29)30)17-8-11-21-22(14-17)27-33-26-21/h3-12,14H,13H2,1-2H3,(H,29,30)/p-1/b23-20- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA
Curated by ChEMBL
| Assay Description
In vitro ability to inhibit specific [125I]ET1 binding to rat aorta membranes Endothelin A receptor |
Bioorg Med Chem Lett 8: 1771-6 (1999)
BindingDB Entry DOI: 10.7270/Q2TM7BM4 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50285160
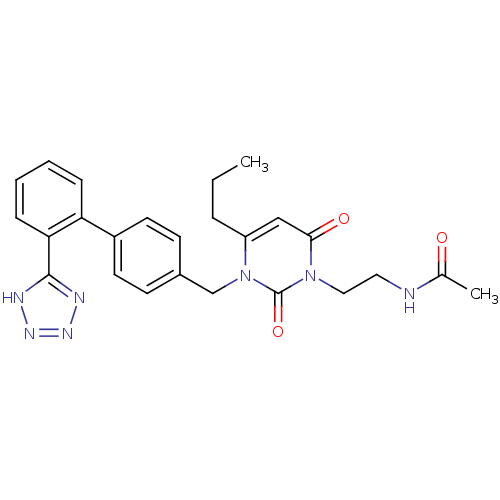
(CHEMBL292781 | N-(2-{2,6-Dioxo-4-propyl-3-[2'-(1H-...)Show SMILES CCCc1cc(=O)n(CCNC(C)=O)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C25H27N7O3/c1-3-6-20-15-23(34)31(14-13-26-17(2)33)25(35)32(20)16-18-9-11-19(12-10-18)21-7-4-5-8-22(21)24-27-29-30-28-24/h4-5,7-12,15H,3,6,13-14,16H2,1-2H3,(H,26,33)(H,27,28,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for binding affinity against angiotensin II receptor in rat adrenal cortex |
Bioorg Med Chem Lett 5: 2071-2076 (1995)
Article DOI: 10.1016/0960-894X(95)00370-9
BindingDB Entry DOI: 10.7270/Q2SF2W4J |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(Homo sapiens (Human)) | BDBM50075421
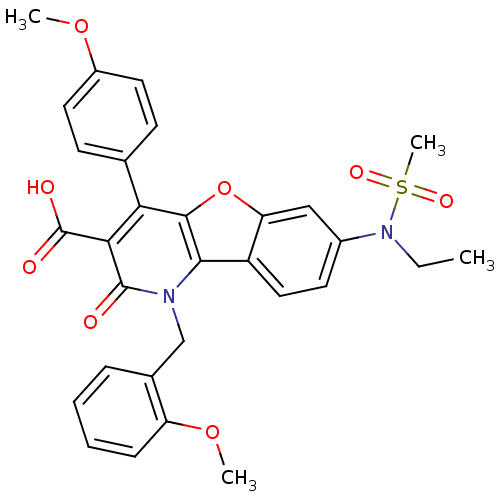
(7-(Ethyl-methanesulfonyl-amino)-1-(2-methoxy-benzy...)Show SMILES CCN(c1ccc2c(c1)oc1c(-c3ccc(OC)cc3)c(C(O)=O)c(=O)n(Cc3ccccc3OC)c21)S(C)(=O)=O Show InChI InChI=1S/C30H28N2O8S/c1-5-32(41(4,36)37)20-12-15-22-24(16-20)40-28-25(18-10-13-21(38-2)14-11-18)26(30(34)35)29(33)31(27(22)28)17-19-8-6-7-9-23(19)39-3/h6-16H,5,17H2,1-4H3,(H,34,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA
Curated by ChEMBL
| Assay Description
Ability to displace [125I]-Endothelin-1 from endothelin B receptor in porcine kidney membranes |
Bioorg Med Chem Lett 9: 619-22 (1999)
BindingDB Entry DOI: 10.7270/Q2DB811P |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50285834
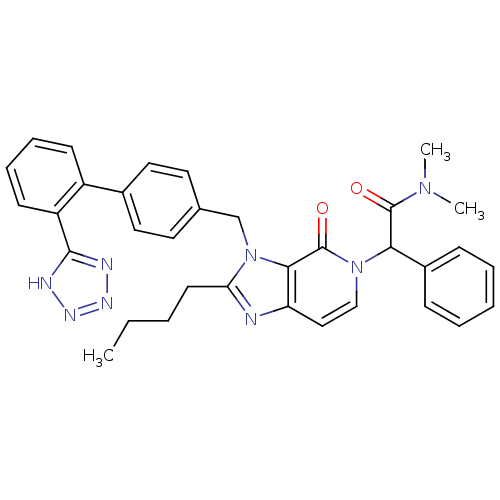
(2-{2-Butyl-4-oxo-3-[2'-(1H-tetrazol-5-yl)-biphenyl...)Show SMILES CCCCc1nc2ccn(C(C(=O)N(C)C)c3ccccc3)c(=O)c2n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C34H34N8O2/c1-4-5-15-29-35-28-20-21-41(30(33(43)40(2)3)25-11-7-6-8-12-25)34(44)31(28)42(29)22-23-16-18-24(19-17-23)26-13-9-10-14-27(26)32-36-38-39-37-32/h6-14,16-21,30H,4-5,15,22H2,1-3H3,(H,36,37,38,39) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity determined against angiotensin II AT1 receptor in rat adrenal cortex preparation |
Bioorg Med Chem Lett 5: 2665-2670 (1995)
Article DOI: 10.1016/0960-894X(95)00477-B
BindingDB Entry DOI: 10.7270/Q2ZW1KVW |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50450370
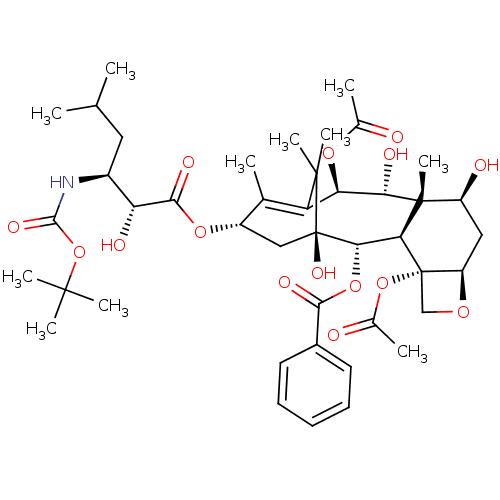
(CHEMBL313691)Show SMILES [H][C@@]12C[C@H](O)[C@@]3(C)[C@@H](O)[C@H](OC(C)=O)C4=C(C)[C@H](C[C@@](O)([C@@H](OC(=O)c5ccccc5)[C@]3([H])[C@@]1(CO2)OC(C)=O)C4(C)C)OC(=O)[C@H](O)[C@H](CC(C)C)NC(=O)OC(C)(C)C |c:14| Show InChI InChI=1S/C43H61NO15/c1-21(2)17-26(44-38(52)59-39(6,7)8)31(48)37(51)56-27-19-43(53)35(57-36(50)25-15-13-12-14-16-25)33-41(11,28(47)18-29-42(33,20-54-29)58-24(5)46)34(49)32(55-23(4)45)30(22(27)3)40(43,9)10/h12-16,21,26-29,31-35,47-49,53H,17-20H2,1-11H3,(H,44,52)/t26-,27-,28-,29+,31+,32+,33-,34-,35-,41+,42-,43+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity determined against angiotensin II AT1 receptor in rat adrenal cortex preparation |
Bioorg Med Chem Lett 5: 2665-2670 (1995)
Article DOI: 10.1016/0960-894X(95)00477-B
BindingDB Entry DOI: 10.7270/Q2ZW1KVW |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(RAT) | BDBM50070873
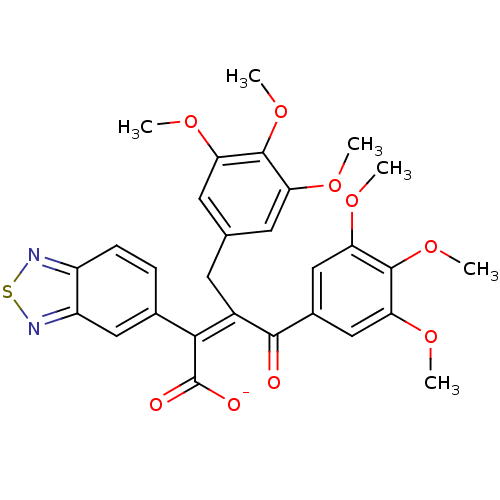
(CHEMBL301577 | Sodium; (Z)-2-benzo[1,2,5]thiadiazo...)Show SMILES COc1cc(C\C(C(=O)c2cc(OC)c(OC)c(OC)c2)=C(\C([O-])=O)c2ccc3nsnc3c2)cc(OC)c1OC Show InChI InChI=1S/C29H28N2O9S/c1-35-21-10-15(11-22(36-2)27(21)39-5)9-18(25(29(33)34)16-7-8-19-20(12-16)31-41-30-19)26(32)17-13-23(37-3)28(40-6)24(14-17)38-4/h7-8,10-14H,9H2,1-6H3,(H,33,34)/p-1/b25-18- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA
Curated by ChEMBL
| Assay Description
In vitro ability to inhibit specific [125I]ET1 binding to rat aorta membranes Endothelin A receptor |
Bioorg Med Chem Lett 8: 1771-6 (1999)
BindingDB Entry DOI: 10.7270/Q2TM7BM4 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50285813
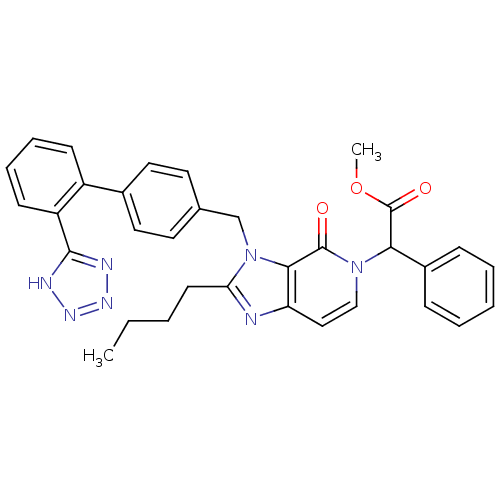
(CHEMBL316063 | {2-Butyl-4-oxo-3-[2'-(1H-tetrazol-5...)Show SMILES CCCCc1nc2ccn(C(C(=O)OC)c3ccccc3)c(=O)c2n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C33H31N7O3/c1-3-4-14-28-34-27-19-20-39(29(33(42)43-2)24-10-6-5-7-11-24)32(41)30(27)40(28)21-22-15-17-23(18-16-22)25-12-8-9-13-26(25)31-35-37-38-36-31/h5-13,15-20,29H,3-4,14,21H2,1-2H3,(H,35,36,37,38) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity determined against angiotensin II AT1 receptor in rat adrenal cortex preparation |
Bioorg Med Chem Lett 5: 2665-2670 (1995)
Article DOI: 10.1016/0960-894X(95)00477-B
BindingDB Entry DOI: 10.7270/Q2ZW1KVW |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(RABBIT) | BDBM50282611
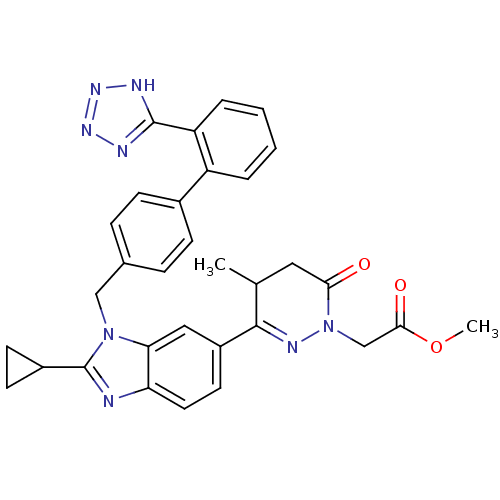
((3-{2-Cyclopropyl-3-[2'-(1H-tetrazol-5-yl)-bipheny...)Show SMILES COC(=O)CN1N=C(C(C)CC1=O)c1ccc2nc(C3CC3)n(Cc3ccc(cc3)-c3ccccc3-c3nnn[nH]3)c2c1 |c:6| Show InChI InChI=1S/C32H30N8O3/c1-19-15-28(41)40(18-29(42)43-2)36-30(19)23-13-14-26-27(16-23)39(32(33-26)22-11-12-22)17-20-7-9-21(10-8-20)24-5-3-4-6-25(24)31-34-37-38-35-31/h3-10,13-14,16,19,22H,11-12,15,17-18H2,1-2H3,(H,34,35,37,38) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against Angiotensin II receptor, type 1 induced contractions in isolated rabbit aortic rings |
Bioorg Med Chem Lett 4: 1297-1302 (1994)
Article DOI: 10.1016/S0960-894X(01)80348-6
BindingDB Entry DOI: 10.7270/Q2G44QRD |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(RABBIT) | BDBM50403244
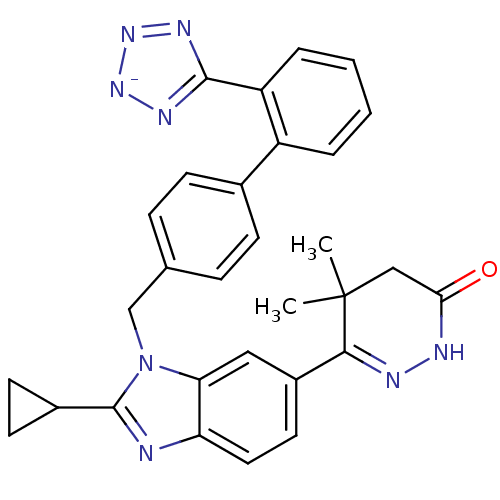
(CHEMBL2079785)Show SMILES CC1(C)CC(=O)NN=C1c1ccc2nc(C3CC3)n(Cc3ccc(cc3)-c3ccccc3-c3nn[n-]n3)c2c1 |c:7| Show InChI InChI=1S/C30H28N8O/c1-30(2)16-26(39)32-33-27(30)21-13-14-24-25(15-21)38(29(31-24)20-11-12-20)17-18-7-9-19(10-8-18)22-5-3-4-6-23(22)28-34-36-37-35-28/h3-10,13-15,20H,11-12,16-17H2,1-2H3,(H2,32,34,35,36,37,39)/p-1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against Angiotensin II receptor, type 1 induced contractions in isolated rabbit aortic rings |
Bioorg Med Chem Lett 4: 1297-1302 (1994)
Article DOI: 10.1016/S0960-894X(01)80348-6
BindingDB Entry DOI: 10.7270/Q2G44QRD |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50285821
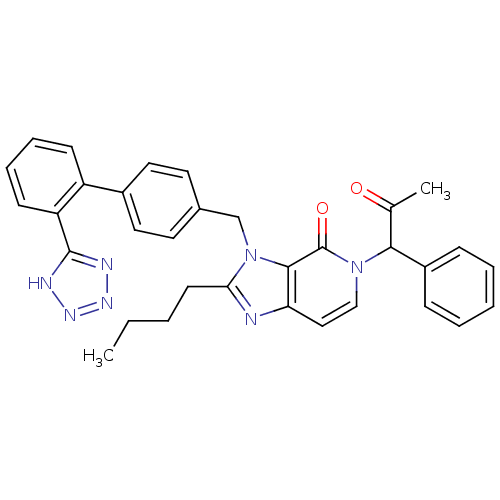
(2-Butyl-5-(2-oxo-1-phenyl-propyl)-3-[2'-(1H-tetraz...)Show SMILES CCCCc1nc2ccn(C(C(C)=O)c3ccccc3)c(=O)c2n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C33H31N7O2/c1-3-4-14-29-34-28-19-20-39(30(22(2)41)25-10-6-5-7-11-25)33(42)31(28)40(29)21-23-15-17-24(18-16-23)26-12-8-9-13-27(26)32-35-37-38-36-32/h5-13,15-20,30H,3-4,14,21H2,1-2H3,(H,35,36,37,38) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity determined against angiotensin II AT1 receptor in rat adrenal cortex preparation |
Bioorg Med Chem Lett 5: 2665-2670 (1995)
Article DOI: 10.1016/0960-894X(95)00477-B
BindingDB Entry DOI: 10.7270/Q2ZW1KVW |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50285816
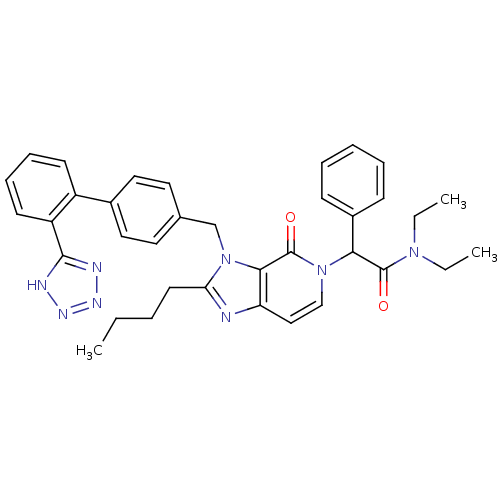
(2-{2-Butyl-4-oxo-3-[2'-(1H-tetrazol-5-yl)-biphenyl...)Show SMILES CCCCc1nc2ccn(C(C(=O)N(CC)CC)c3ccccc3)c(=O)c2n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C36H38N8O2/c1-4-7-17-31-37-30-22-23-43(32(27-13-9-8-10-14-27)35(45)42(5-2)6-3)36(46)33(30)44(31)24-25-18-20-26(21-19-25)28-15-11-12-16-29(28)34-38-40-41-39-34/h8-16,18-23,32H,4-7,17,24H2,1-3H3,(H,38,39,40,41) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity determined against angiotensin II AT1 receptor in rat adrenal cortex preparation |
Bioorg Med Chem Lett 5: 2665-2670 (1995)
Article DOI: 10.1016/0960-894X(95)00477-B
BindingDB Entry DOI: 10.7270/Q2ZW1KVW |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50285177

(6-Propyl-1-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmet...)Show SMILES CCCc1cc(=O)[nH]c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C21H20N6O2/c1-2-5-16-12-19(28)22-21(29)27(16)13-14-8-10-15(11-9-14)17-6-3-4-7-18(17)20-23-25-26-24-20/h3-4,6-12H,2,5,13H2,1H3,(H,22,28,29)(H,23,24,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for binding affinity against angiotensin II receptor in rat adrenal cortex |
Bioorg Med Chem Lett 5: 2071-2076 (1995)
Article DOI: 10.1016/0960-894X(95)00370-9
BindingDB Entry DOI: 10.7270/Q2SF2W4J |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50285835
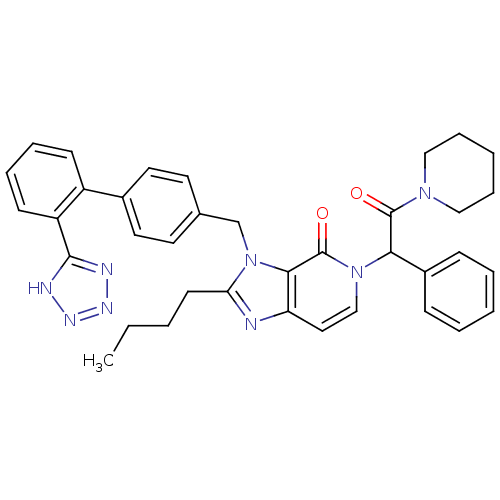
(2-Butyl-5-(2-oxo-1-phenyl-2-piperidin-1-yl-ethyl)-...)Show SMILES CCCCc1nc2ccn(C(C(=O)N3CCCCC3)c3ccccc3)c(=O)c2n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C37H38N8O2/c1-2-3-16-32-38-31-21-24-44(33(28-12-6-4-7-13-28)36(46)43-22-10-5-11-23-43)37(47)34(31)45(32)25-26-17-19-27(20-18-26)29-14-8-9-15-30(29)35-39-41-42-40-35/h4,6-9,12-15,17-21,24,33H,2-3,5,10-11,16,22-23,25H2,1H3,(H,39,40,41,42) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity determined against angiotensin II AT1 receptor in rat adrenal cortex preparation |
Bioorg Med Chem Lett 5: 2665-2670 (1995)
Article DOI: 10.1016/0960-894X(95)00477-B
BindingDB Entry DOI: 10.7270/Q2ZW1KVW |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Bos taurus) | BDBM50285173
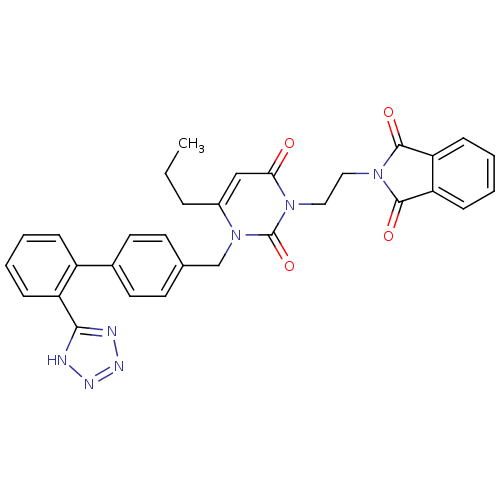
(2-(2-{2,6-Dioxo-4-propyl-3-[2'-(1H-tetrazol-5-yl)-...)Show SMILES CCCc1cc(=O)n(CCN2C(=O)c3ccccc3C2=O)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C31H27N7O4/c1-2-7-22-18-27(39)36(16-17-37-29(40)25-10-5-6-11-26(25)30(37)41)31(42)38(22)19-20-12-14-21(15-13-20)23-8-3-4-9-24(23)28-32-34-35-33-28/h3-6,8-15,18H,2,7,16-17,19H2,1H3,(H,32,33,34,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for binding affinity against angiotensin II receptor in rat adrenal cortex |
Bioorg Med Chem Lett 5: 2071-2076 (1995)
Article DOI: 10.1016/0960-894X(95)00370-9
BindingDB Entry DOI: 10.7270/Q2SF2W4J |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50285167
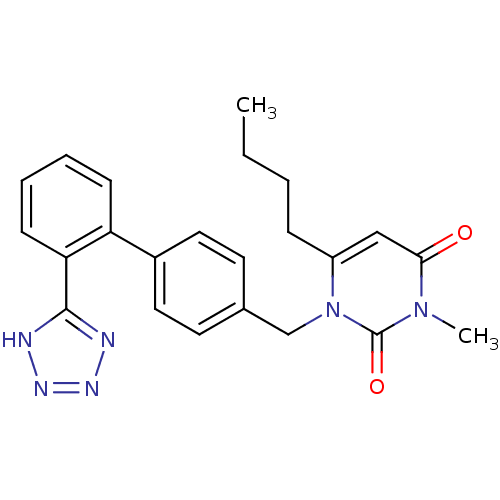
(6-Butyl-3-methyl-1-[2'-(1H-tetrazol-5-yl)-biphenyl...)Show SMILES CCCCc1cc(=O)n(C)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C23H24N6O2/c1-3-4-7-18-14-21(30)28(2)23(31)29(18)15-16-10-12-17(13-11-16)19-8-5-6-9-20(19)22-24-26-27-25-22/h5-6,8-14H,3-4,7,15H2,1-2H3,(H,24,25,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for binding affinity against angiotensin II receptor in rat adrenal cortex |
Bioorg Med Chem Lett 5: 2071-2076 (1995)
Article DOI: 10.1016/0960-894X(95)00370-9
BindingDB Entry DOI: 10.7270/Q2SF2W4J |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data