 Found 1698 hits with Last Name = 'gong' and Initial = 'y'
Found 1698 hits with Last Name = 'gong' and Initial = 'y' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50605126

(CHEMBL5187340)Show SMILES COc1cc(ccc1NC(=O)[C@@H]1N[C@@H](CC(C)(C)C)[C@@](CNCC2CCNCC2)([C@H]1c1cccc(Cl)c1F)c1ccc(Cl)cc1F)C(O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00095
BindingDB Entry DOI: 10.7270/Q2V69PP3 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50114539
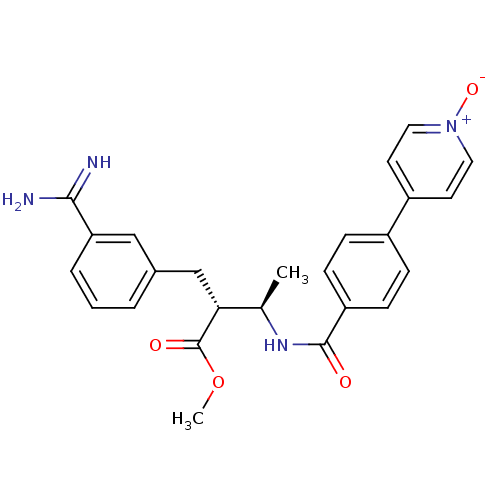
((2R,3R)-2-(3-Carbamimidoyl-benzyl)-3-[4-(1-oxy-pyr...)Show SMILES COC(=O)[C@H](Cc1cccc(c1)C(N)=N)[C@@H](C)NC(=O)c1ccc(cc1)-c1cc[n+]([O-])cc1 Show InChI InChI=1S/C25H26N4O4/c1-16(22(25(31)33-2)15-17-4-3-5-21(14-17)23(26)27)28-24(30)20-8-6-18(7-9-20)19-10-12-29(32)13-11-19/h3-14,16,22H,15H2,1-2H3,(H3,26,27)(H,28,30)/t16-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inibition of Coagulation factor Xa |
Bioorg Med Chem Lett 12: 1671-4 (2002)
BindingDB Entry DOI: 10.7270/Q2BV7FXG |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50605132

(CHEMBL5171665)Show SMILES COc1cc(ccc1NC(=O)[C@@H]1N[C@@H](CC(C)(C)C)[C@@](CNS(=O)(=O)c2ccccc2C(O)=O)([C@H]1c1cccc(Cl)c1F)c1ccc(Cl)cc1F)C(O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00095
BindingDB Entry DOI: 10.7270/Q2V69PP3 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50114544
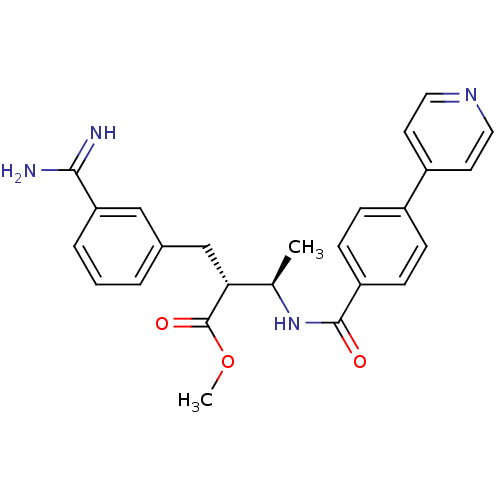
((2R,3R)-2-(3-Carbamimidoyl-benzyl)-3-(4-pyridin-4-...)Show SMILES COC(=O)[C@H](Cc1cccc(c1)C(N)=N)[C@@H](C)NC(=O)c1ccc(cc1)-c1ccncc1 Show InChI InChI=1S/C25H26N4O3/c1-16(22(25(31)32-2)15-17-4-3-5-21(14-17)23(26)27)29-24(30)20-8-6-18(7-9-20)19-10-12-28-13-11-19/h3-14,16,22H,15H2,1-2H3,(H3,26,27)(H,29,30)/t16-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inibition of Coagulation factor Xa |
Bioorg Med Chem Lett 12: 1671-4 (2002)
BindingDB Entry DOI: 10.7270/Q2BV7FXG |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50085393
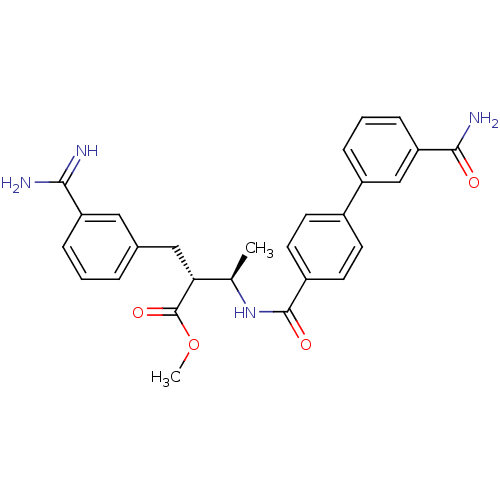
((2R,3R)-2-(3-Carbamimidoyl-benzyl)-3-[(3'-carbamoy...)Show SMILES COC(=O)[C@H](Cc1cccc(c1)C(N)=N)[C@@H](C)NC(=O)c1ccc(cc1)-c1cccc(c1)C(N)=O Show InChI InChI=1S/C27H28N4O4/c1-16(23(27(34)35-2)14-17-5-3-7-21(13-17)24(28)29)31-26(33)19-11-9-18(10-12-19)20-6-4-8-22(15-20)25(30)32/h3-13,15-16,23H,14H2,1-2H3,(H3,28,29)(H2,30,32)(H,31,33)/t16-,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer
Curated by ChEMBL
| Assay Description
In vitro inhibition of coagulation factor Xa. |
Bioorg Med Chem Lett 10: 217-21 (2000)
BindingDB Entry DOI: 10.7270/Q2M32TZG |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50114543

((2R,3R)-2-(3-Carbamimidoyl-benzyl)-3-[4-(1-oxy-pyr...)Show SMILES COC(=O)[C@H](Cc1cccc(c1)C(N)=N)[C@@H](C)NC(=O)c1ccc(cc1)-c1cccc[n+]1[O-] Show InChI InChI=1S/C25H26N4O4/c1-16(21(25(31)33-2)15-17-6-5-7-20(14-17)23(26)27)28-24(30)19-11-9-18(10-12-19)22-8-3-4-13-29(22)32/h3-14,16,21H,15H2,1-2H3,(H3,26,27)(H,28,30)/t16-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inibition of Coagulation factor Xa |
Bioorg Med Chem Lett 12: 1671-4 (2002)
BindingDB Entry DOI: 10.7270/Q2BV7FXG |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50085402
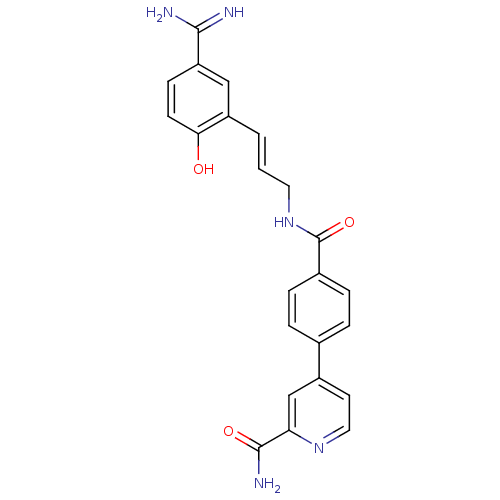
(4-{4-[3-(5-Carbamimidoyl-2-hydroxy-phenyl)-allylca...)Show SMILES NC(=N)c1ccc(O)c(\C=C\CNC(=O)c2ccc(cc2)-c2ccnc(c2)C(N)=O)c1 Show InChI InChI=1S/C23H21N5O3/c24-21(25)18-7-8-20(29)17(12-18)2-1-10-28-23(31)15-5-3-14(4-6-15)16-9-11-27-19(13-16)22(26)30/h1-9,11-13,29H,10H2,(H3,24,25)(H2,26,30)(H,28,31)/b2-1+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer
Curated by ChEMBL
| Assay Description
In vitro inhibition of coagulation factor Xa. |
Bioorg Med Chem Lett 10: 217-21 (2000)
BindingDB Entry DOI: 10.7270/Q2M32TZG |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50605125
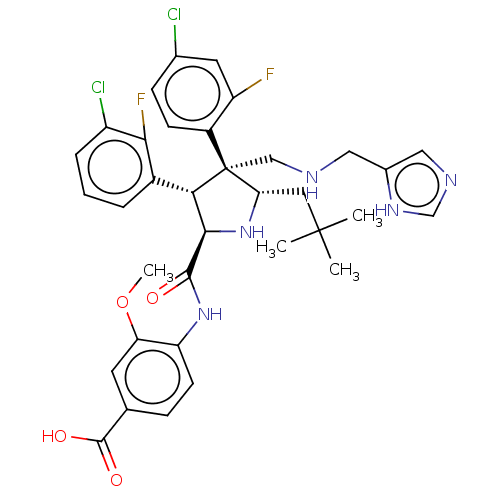
(CHEMBL5197279)Show SMILES COc1cc(ccc1NC(=O)[C@@H]1N[C@@H](CC(C)(C)C)[C@@](CNCc2cnc[nH]2)([C@H]1c1cccc(Cl)c1F)c1ccc(Cl)cc1F)C(O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00095
BindingDB Entry DOI: 10.7270/Q2V69PP3 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50114540

(3-{4-[(1R,2R)-3-(3-Carbamimidoyl-phenyl)-2-methoxy...)Show SMILES COC(=O)[C@H](Cc1cccc(c1)C(N)=N)[C@@H](C)NC(=O)c1ccc(cc1)-c1ccc[n+](C)c1 Show InChI InChI=1S/C26H28N4O3/c1-17(23(26(32)33-3)15-18-6-4-7-21(14-18)24(27)28)29-25(31)20-11-9-19(10-12-20)22-8-5-13-30(2)16-22/h4-14,16-17,23H,15H2,1-3H3,(H3-,27,28,29,31)/p+1/t17-,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inibition of Coagulation factor Xa |
Bioorg Med Chem Lett 12: 1671-4 (2002)
BindingDB Entry DOI: 10.7270/Q2BV7FXG |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50123781
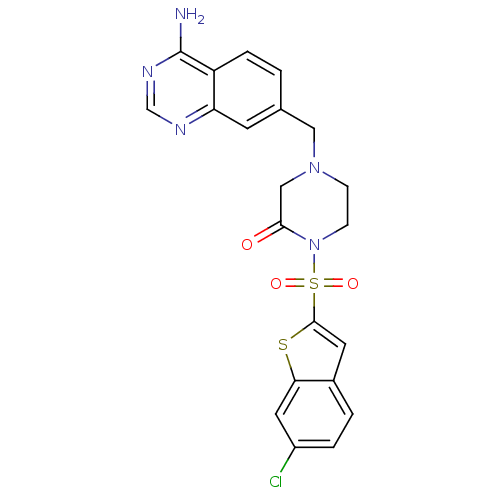
(4-(4-Amino-quinazolin-7-ylmethyl)-1-(6-chloro-benz...)Show SMILES Nc1ncnc2cc(CN3CCN(C(=O)C3)S(=O)(=O)c3cc4ccc(Cl)cc4s3)ccc12 Show InChI InChI=1S/C21H18ClN5O3S2/c22-15-3-2-14-8-20(31-18(14)9-15)32(29,30)27-6-5-26(11-19(27)28)10-13-1-4-16-17(7-13)24-12-25-21(16)23/h1-4,7-9,12H,5-6,10-11H2,(H2,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity (in vitro) of the compound towards human Coagulation factor X was determined at 10 mg/kg peroral dose |
J Med Chem 46: 681-4 (2003)
Article DOI: 10.1021/jm020384z
BindingDB Entry DOI: 10.7270/Q2KW5FDH |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50114537
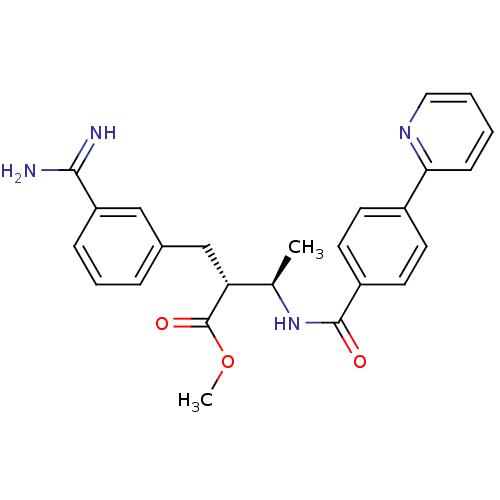
((2R,3R)-2-(3-Carbamimidoyl-benzyl)-3-(4-pyridin-2-...)Show SMILES COC(=O)[C@H](Cc1cccc(c1)C(N)=N)[C@@H](C)NC(=O)c1ccc(cc1)-c1ccccn1 Show InChI InChI=1S/C25H26N4O3/c1-16(21(25(31)32-2)15-17-6-5-7-20(14-17)23(26)27)29-24(30)19-11-9-18(10-12-19)22-8-3-4-13-28-22/h3-14,16,21H,15H2,1-2H3,(H3,26,27)(H,29,30)/t16-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inibition of Coagulation factor Xa |
Bioorg Med Chem Lett 12: 1671-4 (2002)
BindingDB Entry DOI: 10.7270/Q2BV7FXG |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50605123
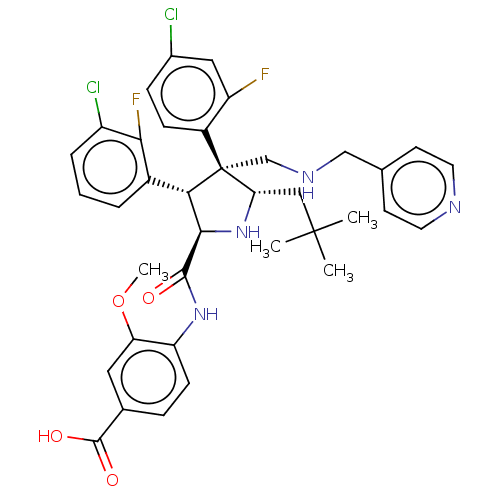
(CHEMBL5181559)Show SMILES COc1cc(ccc1NC(=O)[C@@H]1N[C@@H](CC(C)(C)C)[C@@](CNCc2ccncc2)([C@H]1c1cccc(Cl)c1F)c1ccc(Cl)cc1F)C(O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00095
BindingDB Entry DOI: 10.7270/Q2V69PP3 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12596
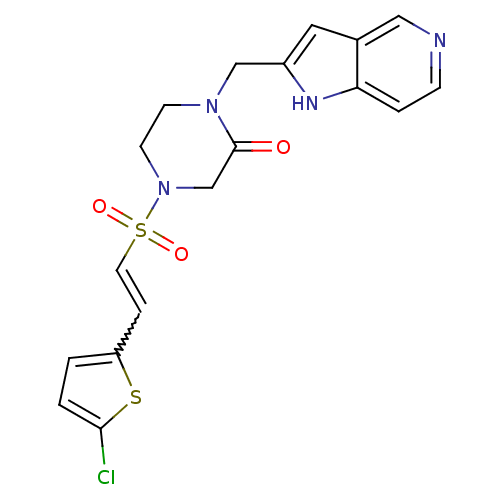
(4-{[(E)-2-(5-CHLOROTHIEN-2-YL)VINYL]SULFONYL}-1-(1...)Show SMILES Clc1ccc(C=CS(=O)(=O)N2CCN(Cc3cc4cnccc4[nH]3)C(=O)C2)s1 |w:5.4| Show InChI InChI=1S/C18H17ClN4O3S2/c19-17-2-1-15(27-17)4-8-28(25,26)23-7-6-22(18(24)12-23)11-14-9-13-10-20-5-3-16(13)21-14/h1-5,8-10,21H,6-7,11-12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity (in vitro) of the compound towards human Coagulation factor X was determined at 5 mg/kg peroral dose |
J Med Chem 46: 681-4 (2003)
Article DOI: 10.1021/jm020384z
BindingDB Entry DOI: 10.7270/Q2KW5FDH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50114548
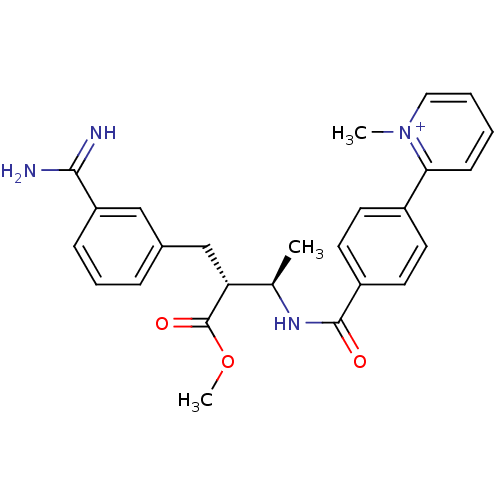
(2-{4-[(1R,2R)-3-(3-Carbamimidoyl-phenyl)-2-methoxy...)Show SMILES COC(=O)[C@H](Cc1cccc(c1)C(N)=N)[C@@H](C)NC(=O)c1ccc(cc1)-c1cccc[n+]1C Show InChI InChI=1S/C26H28N4O3/c1-17(22(26(32)33-3)16-18-7-6-8-21(15-18)24(27)28)29-25(31)20-12-10-19(11-13-20)23-9-4-5-14-30(23)2/h4-15,17,22H,16H2,1-3H3,(H3-,27,28,29,31)/p+1/t17-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inibition of Coagulation factor Xa |
Bioorg Med Chem Lett 12: 1671-4 (2002)
BindingDB Entry DOI: 10.7270/Q2BV7FXG |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50605114
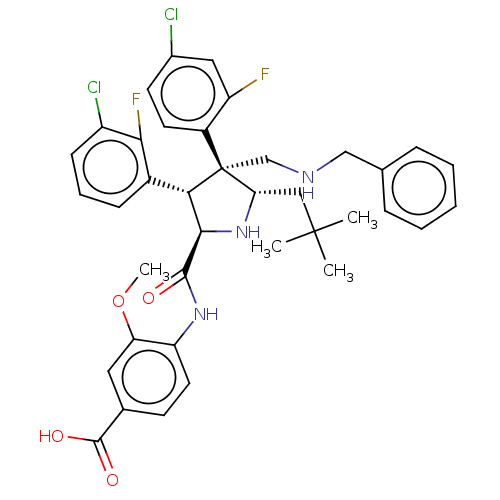
(CHEMBL5173513)Show SMILES COc1cc(ccc1NC(=O)[C@@H]1N[C@@H](CC(C)(C)C)[C@@](CNCc2ccccc2)([C@H]1c1cccc(Cl)c1F)c1ccc(Cl)cc1F)C(O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00095
BindingDB Entry DOI: 10.7270/Q2V69PP3 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50605147
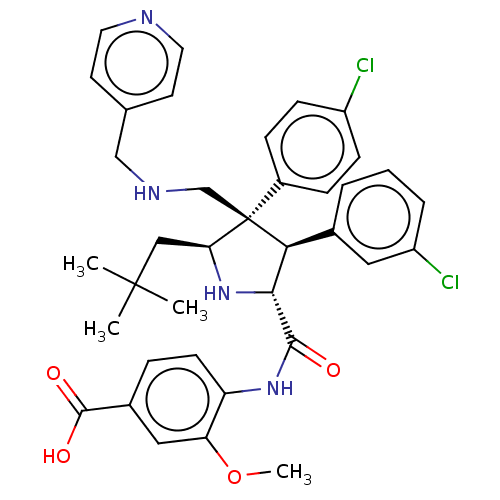
(CHEMBL5169689)Show SMILES COc1cc(ccc1NC(=O)[C@@H]1N[C@@H](CC(C)(C)C)[C@@](CNCc2ccncc2)([C@H]1c1cccc(Cl)c1)c1ccc(Cl)cc1)C(O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00095
BindingDB Entry DOI: 10.7270/Q2V69PP3 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50605116
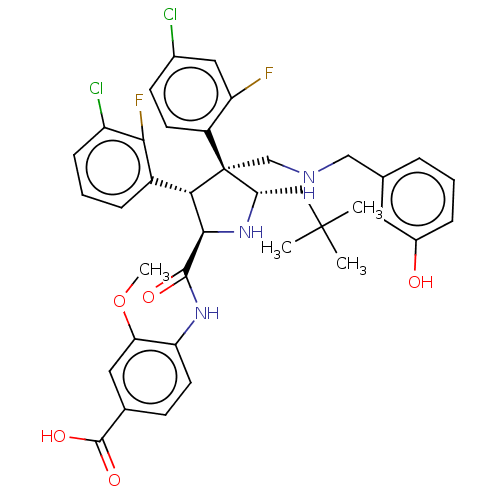
(CHEMBL5181296)Show SMILES COc1cc(ccc1NC(=O)[C@@H]1N[C@@H](CC(C)(C)C)[C@@](CNCc2cccc(O)c2)([C@H]1c1cccc(Cl)c1F)c1ccc(Cl)cc1F)C(O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00095
BindingDB Entry DOI: 10.7270/Q2V69PP3 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50605124
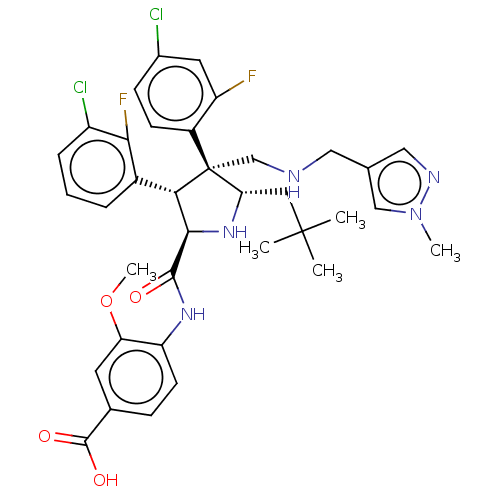
(CHEMBL5196069)Show SMILES COc1cc(ccc1NC(=O)[C@@H]1N[C@@H](CC(C)(C)C)[C@@](CNCc2cnn(C)c2)([C@H]1c1cccc(Cl)c1F)c1ccc(Cl)cc1F)C(O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00095
BindingDB Entry DOI: 10.7270/Q2V69PP3 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50605117
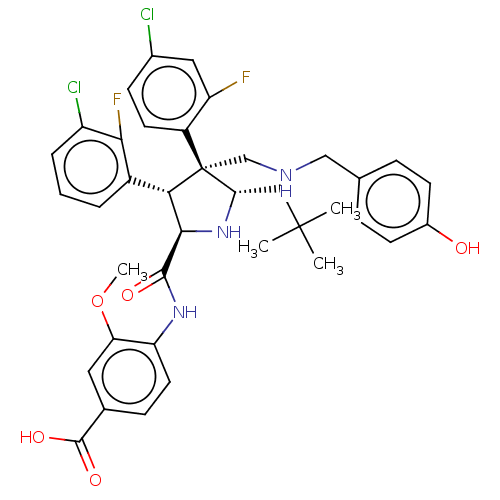
(CHEMBL5205792)Show SMILES COc1cc(ccc1NC(=O)[C@@H]1N[C@@H](CC(C)(C)C)[C@@](CNCc2ccc(O)cc2)([C@H]1c1cccc(Cl)c1F)c1ccc(Cl)cc1F)C(O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00095
BindingDB Entry DOI: 10.7270/Q2V69PP3 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50605102
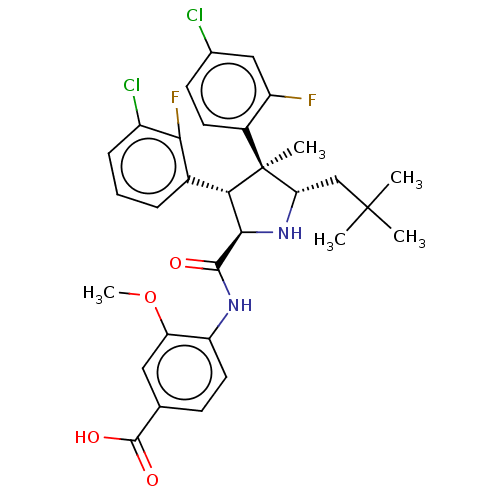
(CHEMBL5196857)Show SMILES COc1cc(ccc1NC(=O)[C@@H]1N[C@@H](CC(C)(C)C)[C@@](C)([C@H]1c1cccc(Cl)c1F)c1ccc(Cl)cc1F)C(O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00095
BindingDB Entry DOI: 10.7270/Q2V69PP3 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50605134
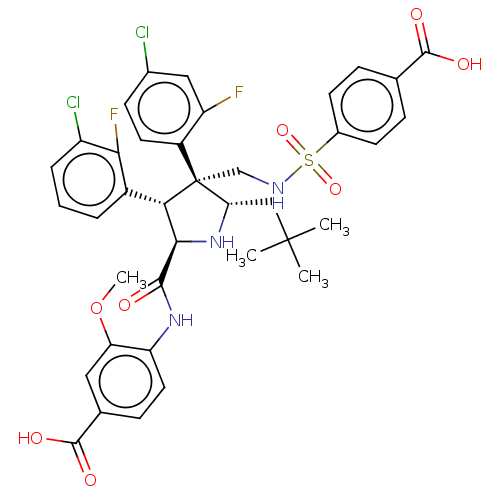
(CHEMBL5207803)Show SMILES COc1cc(ccc1NC(=O)[C@@H]1N[C@@H](CC(C)(C)C)[C@@](CNS(=O)(=O)c2ccc(cc2)C(O)=O)([C@H]1c1cccc(Cl)c1F)c1ccc(Cl)cc1F)C(O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00095
BindingDB Entry DOI: 10.7270/Q2V69PP3 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50605146
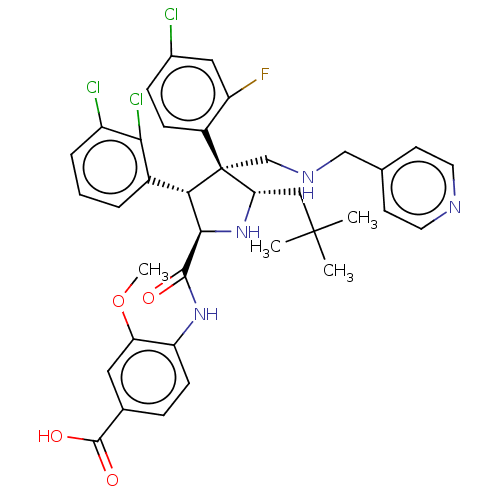
(CHEMBL5179430)Show SMILES COc1cc(ccc1NC(=O)[C@@H]1N[C@@H](CC(C)(C)C)[C@@](CNCc2ccncc2)([C@H]1c1cccc(Cl)c1Cl)c1ccc(Cl)cc1F)C(O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00095
BindingDB Entry DOI: 10.7270/Q2V69PP3 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50605111
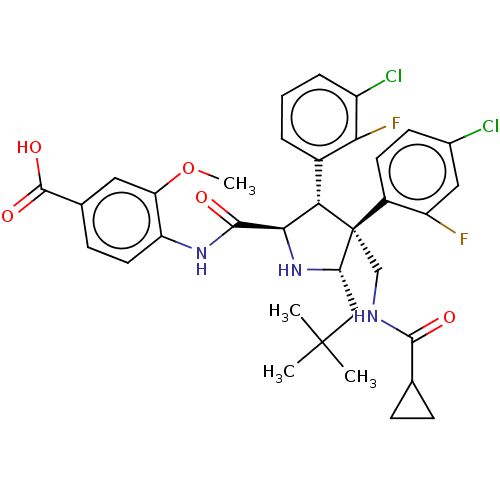
(CHEMBL5173985)Show SMILES COc1cc(ccc1NC(=O)[C@@H]1N[C@@H](CC(C)(C)C)[C@@](CNC(=O)C2CC2)([C@H]1c1cccc(Cl)c1F)c1ccc(Cl)cc1F)C(O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00095
BindingDB Entry DOI: 10.7270/Q2V69PP3 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50114547

((2R,3R)-2-(3-Carbamimidoyl-benzyl)-3-[4-(1-oxy-pyr...)Show SMILES COC(=O)[C@H](Cc1cccc(c1)C(N)=N)[C@@H](C)NC(=O)c1ccc(cc1)-c1ccc[n+]([O-])c1 Show InChI InChI=1S/C25H26N4O4/c1-16(22(25(31)33-2)14-17-5-3-6-20(13-17)23(26)27)28-24(30)19-10-8-18(9-11-19)21-7-4-12-29(32)15-21/h3-13,15-16,22H,14H2,1-2H3,(H3,26,27)(H,28,30)/t16-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inibition of Coagulation factor Xa |
Bioorg Med Chem Lett 12: 1671-4 (2002)
BindingDB Entry DOI: 10.7270/Q2BV7FXG |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50605120
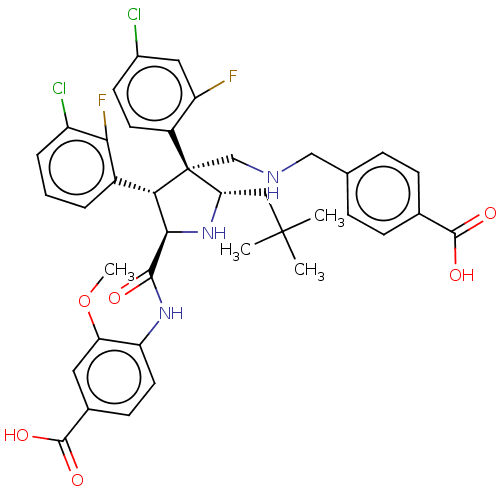
(CHEMBL5185334)Show SMILES COc1cc(ccc1NC(=O)[C@@H]1N[C@@H](CC(C)(C)C)[C@@](CNCc2ccc(cc2)C(O)=O)([C@H]1c1cccc(Cl)c1F)c1ccc(Cl)cc1F)C(O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00095
BindingDB Entry DOI: 10.7270/Q2V69PP3 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50085405
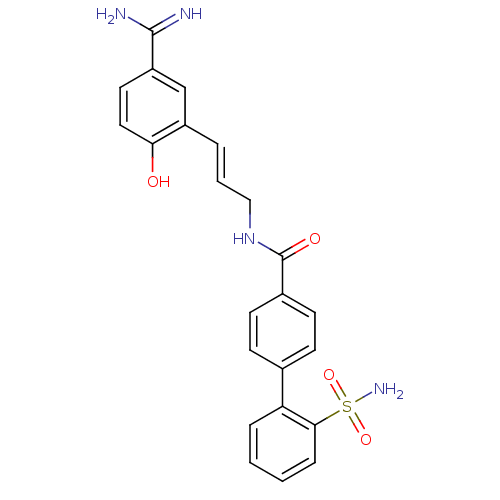
(2'-Sulfamoyl-biphenyl-4-carboxylic acid [3-(5-carb...)Show SMILES NC(=N)c1ccc(O)c(\C=C\CNC(=O)c2ccc(cc2)-c2ccccc2S(N)(=O)=O)c1 Show InChI InChI=1S/C23H22N4O4S/c24-22(25)18-11-12-20(28)17(14-18)4-3-13-27-23(29)16-9-7-15(8-10-16)19-5-1-2-6-21(19)32(26,30)31/h1-12,14,28H,13H2,(H3,24,25)(H,27,29)(H2,26,30,31)/b4-3+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer
Curated by ChEMBL
| Assay Description
In vitro inhibition of coagulation factor Xa. |
Bioorg Med Chem Lett 10: 217-21 (2000)
BindingDB Entry DOI: 10.7270/Q2M32TZG |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50605127
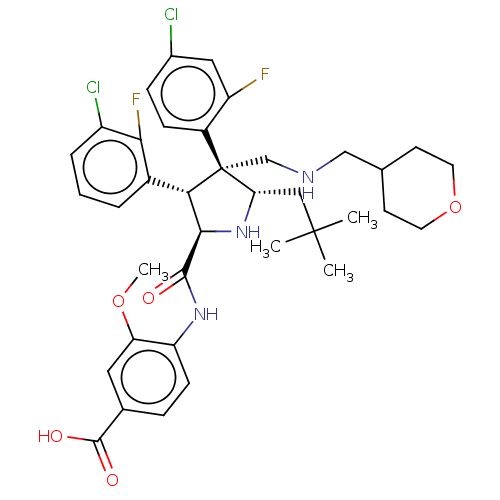
(CHEMBL5185988)Show SMILES COc1cc(ccc1NC(=O)[C@@H]1N[C@@H](CC(C)(C)C)[C@@](CNCC2CCOCC2)([C@H]1c1cccc(Cl)c1F)c1ccc(Cl)cc1F)C(O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00095
BindingDB Entry DOI: 10.7270/Q2V69PP3 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50605121
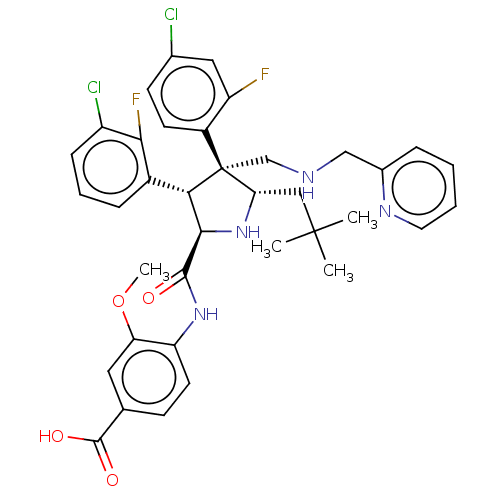
(CHEMBL5176086)Show SMILES COc1cc(ccc1NC(=O)[C@@H]1N[C@@H](CC(C)(C)C)[C@@](CNCc2ccccn2)([C@H]1c1cccc(Cl)c1F)c1ccc(Cl)cc1F)C(O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00095
BindingDB Entry DOI: 10.7270/Q2V69PP3 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50605106

(CHEMBL5199459)Show SMILES CCNC[C@@]1([C@H](CC(C)(C)C)N[C@H]([C@@H]1c1cccc(Cl)c1F)C(=O)Nc1ccc(cc1OC)C(O)=O)c1ccc(Cl)cc1F |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00095
BindingDB Entry DOI: 10.7270/Q2V69PP3 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50605130
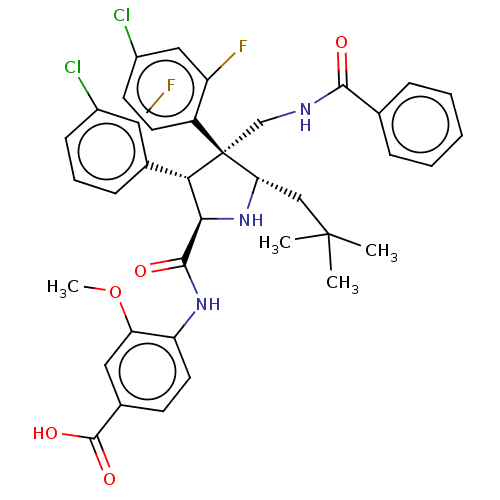
(CHEMBL5195734)Show SMILES COc1cc(ccc1NC(=O)[C@@H]1N[C@@H](CC(C)(C)C)[C@@](CNC(=O)c2ccccc2)([C@H]1c1cccc(Cl)c1F)c1ccc(Cl)cc1F)C(O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00095
BindingDB Entry DOI: 10.7270/Q2V69PP3 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50605113
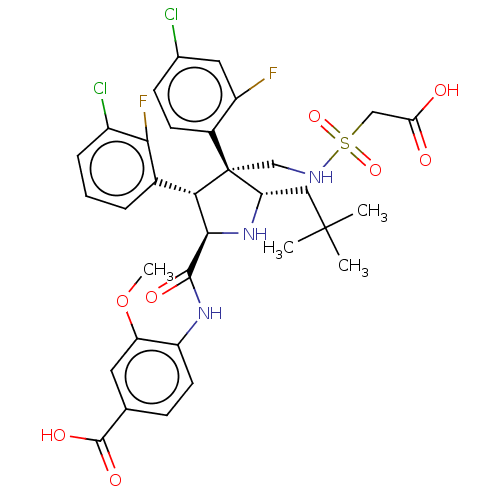
(CHEMBL5183808)Show SMILES COc1cc(ccc1NC(=O)[C@@H]1N[C@@H](CC(C)(C)C)[C@@](CNS(=O)(=O)CC(O)=O)([C@H]1c1cccc(Cl)c1F)c1ccc(Cl)cc1F)C(O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00095
BindingDB Entry DOI: 10.7270/Q2V69PP3 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50605119
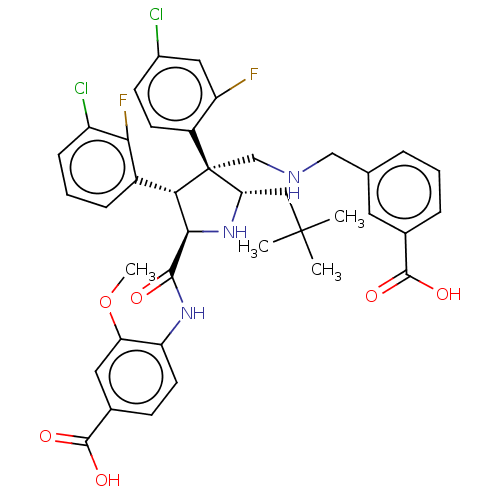
(CHEMBL5190459)Show SMILES COc1cc(ccc1NC(=O)[C@@H]1N[C@@H](CC(C)(C)C)[C@@](CNCc2cccc(c2)C(O)=O)([C@H]1c1cccc(Cl)c1F)c1ccc(Cl)cc1F)C(O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00095
BindingDB Entry DOI: 10.7270/Q2V69PP3 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50605118
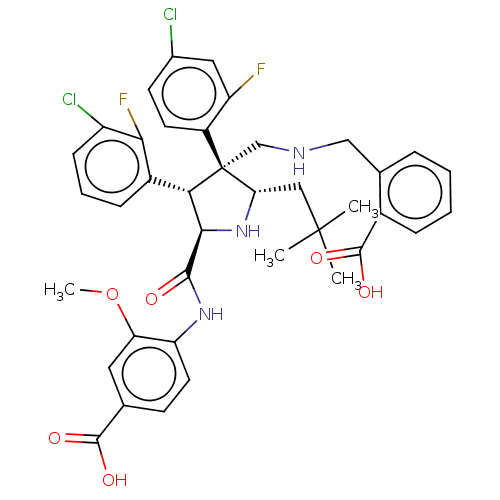
(CHEMBL5200925)Show SMILES COc1cc(ccc1NC(=O)[C@@H]1N[C@@H](CC(C)(C)C)[C@@](CNCc2ccccc2C(O)=O)([C@H]1c1cccc(Cl)c1F)c1ccc(Cl)cc1F)C(O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00095
BindingDB Entry DOI: 10.7270/Q2V69PP3 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50605110
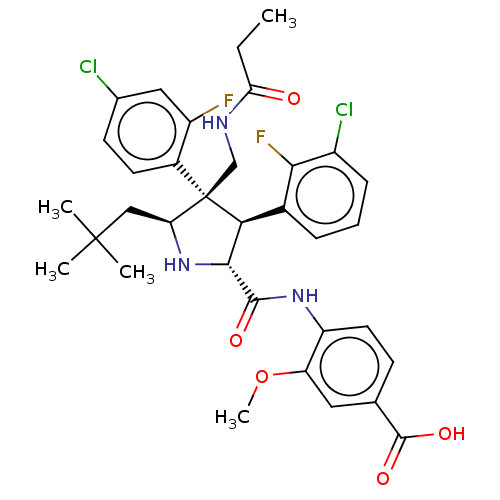
(CHEMBL5186698)Show SMILES CCC(=O)NC[C@@]1([C@H](CC(C)(C)C)N[C@H]([C@@H]1c1cccc(Cl)c1F)C(=O)Nc1ccc(cc1OC)C(O)=O)c1ccc(Cl)cc1F |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00095
BindingDB Entry DOI: 10.7270/Q2V69PP3 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50605122
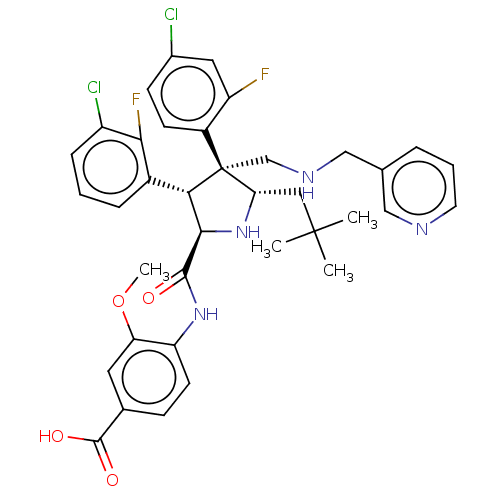
(CHEMBL5188708)Show SMILES COc1cc(ccc1NC(=O)[C@@H]1N[C@@H](CC(C)(C)C)[C@@](CNCc2cccnc2)([C@H]1c1cccc(Cl)c1F)c1ccc(Cl)cc1F)C(O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00095
BindingDB Entry DOI: 10.7270/Q2V69PP3 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50605109
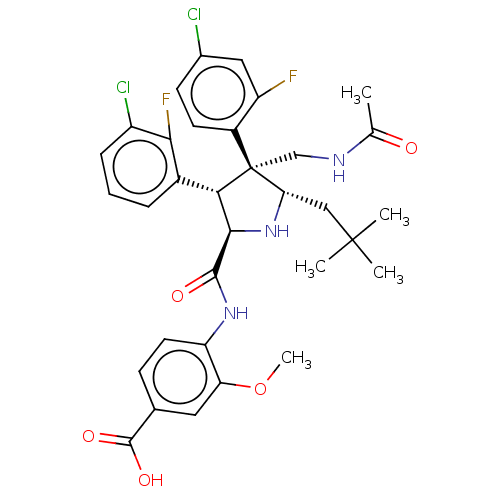
(CHEMBL5194615)Show SMILES COc1cc(ccc1NC(=O)[C@@H]1N[C@@H](CC(C)(C)C)[C@@](CNC(C)=O)([C@H]1c1cccc(Cl)c1F)c1ccc(Cl)cc1F)C(O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00095
BindingDB Entry DOI: 10.7270/Q2V69PP3 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50114542
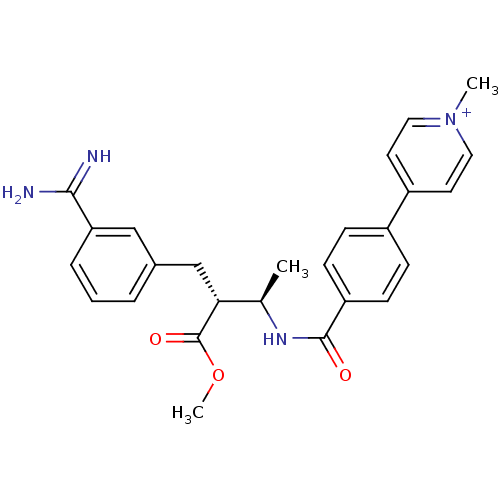
(4-{4-[(1R,2R)-3-(3-Carbamimidoyl-phenyl)-2-methoxy...)Show SMILES COC(=O)[C@H](Cc1cccc(c1)C(N)=N)[C@@H](C)NC(=O)c1ccc(cc1)-c1cc[n+](C)cc1 Show InChI InChI=1S/C26H28N4O3/c1-17(23(26(32)33-3)16-18-5-4-6-22(15-18)24(27)28)29-25(31)21-9-7-19(8-10-21)20-11-13-30(2)14-12-20/h4-15,17,23H,16H2,1-3H3,(H3-,27,28,29,31)/p+1/t17-,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inibition of Coagulation factor Xa |
Bioorg Med Chem Lett 12: 1671-4 (2002)
BindingDB Entry DOI: 10.7270/Q2BV7FXG |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50605115
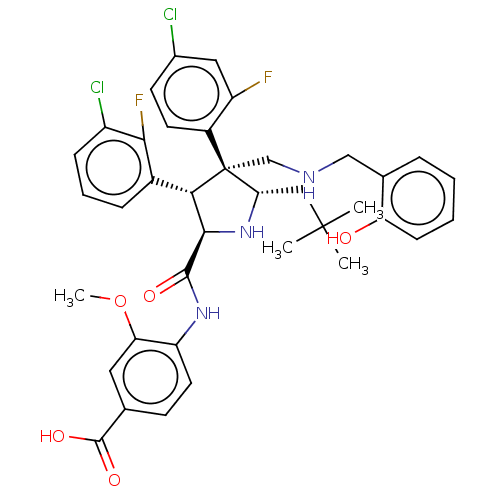
(CHEMBL5191798)Show SMILES COc1cc(ccc1NC(=O)[C@@H]1N[C@@H](CC(C)(C)C)[C@@](CNCc2ccccc2O)([C@H]1c1cccc(Cl)c1F)c1ccc(Cl)cc1F)C(O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00095
BindingDB Entry DOI: 10.7270/Q2V69PP3 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12595

(4-[(6-CHLORO-1-BENZOTHIEN-2-YL)SULFONYL]-1-{[1-(2-...)Show SMILES OCCn1c(CN2CCN(CC2=O)S(=O)(=O)c2cc3ccc(Cl)cc3s2)cc2cnccc12 Show InChI InChI=1S/C22H21ClN4O4S2/c23-17-2-1-15-10-22(32-20(15)11-17)33(30,31)26-6-5-25(21(29)14-26)13-18-9-16-12-24-4-3-19(16)27(18)7-8-28/h1-4,9-12,28H,5-8,13-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity (in vitro) of the compound towards human Coagulation factor X was determined at 10 mg/kg peroral dose |
J Med Chem 46: 681-4 (2003)
Article DOI: 10.1021/jm020384z
BindingDB Entry DOI: 10.7270/Q2KW5FDH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50123766

(4-(6-Chloro-benzo[b]thiophene-2-sulfonyl)-1-(1-met...)Show SMILES Cn1c(CN2CCN(CC2=O)S(=O)(=O)c2cc3ccc(Cl)cc3s2)cc2cnccc12 Show InChI InChI=1S/C21H19ClN4O3S2/c1-24-17(8-15-11-23-5-4-18(15)24)12-25-6-7-26(13-20(25)27)31(28,29)21-9-14-2-3-16(22)10-19(14)30-21/h2-5,8-11H,6-7,12-13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro binding affinity for human Coagulation factor X |
J Med Chem 46: 681-4 (2003)
Article DOI: 10.1021/jm020384z
BindingDB Entry DOI: 10.7270/Q2KW5FDH |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50114538
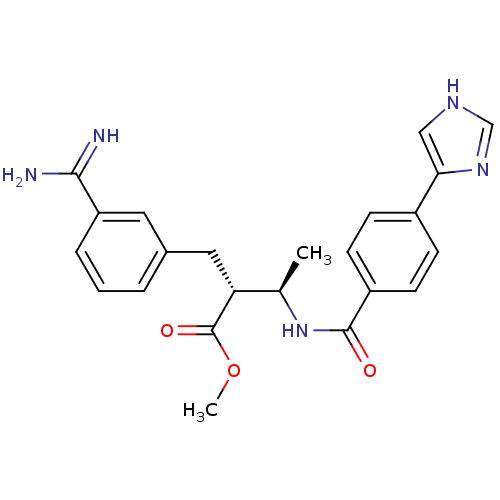
((2R,3R)-2-(3-Carbamimidoyl-benzyl)-3-[4-(1H-imidaz...)Show SMILES COC(=O)[C@H](Cc1cccc(c1)C(N)=N)[C@@H](C)NC(=O)c1ccc(cc1)-c1c[nH]cn1 Show InChI InChI=1S/C23H25N5O3/c1-14(19(23(30)31-2)11-15-4-3-5-18(10-15)21(24)25)28-22(29)17-8-6-16(7-9-17)20-12-26-13-27-20/h3-10,12-14,19H,11H2,1-2H3,(H3,24,25)(H,26,27)(H,28,29)/t14-,19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inibition of Coagulation factor Xa |
Bioorg Med Chem Lett 12: 1671-4 (2002)
BindingDB Entry DOI: 10.7270/Q2BV7FXG |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50123788
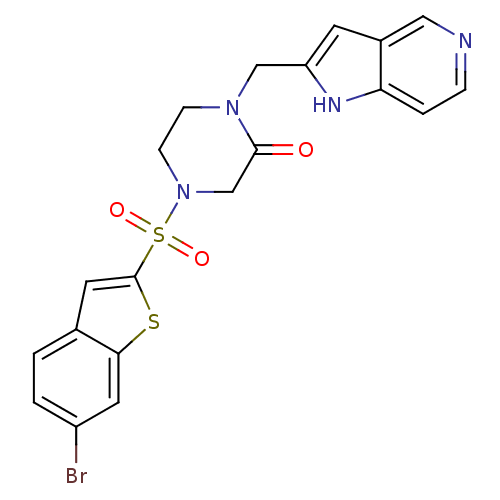
(4-(6-Bromo-benzo[b]thiophene-2-sulfonyl)-1-(1H-pyr...)Show SMILES Brc1ccc2cc(sc2c1)S(=O)(=O)N1CCN(Cc2cc3cnccc3[nH]2)C(=O)C1 Show InChI InChI=1S/C20H17BrN4O3S2/c21-15-2-1-13-8-20(29-18(13)9-15)30(27,28)25-6-5-24(19(26)12-25)11-16-7-14-10-22-4-3-17(14)23-16/h1-4,7-10,23H,5-6,11-12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro binding affinity for human Coagulation factor X |
J Med Chem 46: 681-4 (2003)
Article DOI: 10.1021/jm020384z
BindingDB Entry DOI: 10.7270/Q2KW5FDH |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50605129
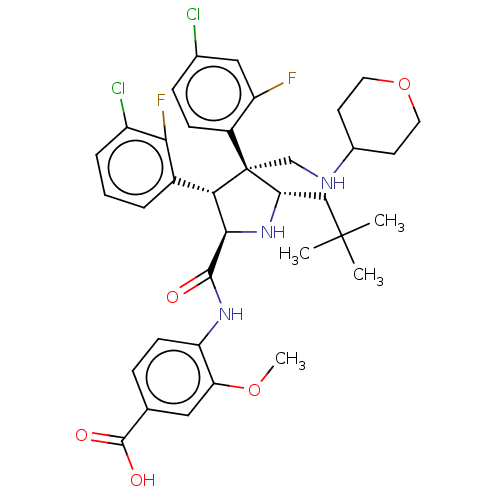
(CHEMBL5196353)Show SMILES COc1cc(ccc1NC(=O)[C@@H]1N[C@@H](CC(C)(C)C)[C@@](CNC2CCOCC2)([C@H]1c1cccc(Cl)c1F)c1ccc(Cl)cc1F)C(O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00095
BindingDB Entry DOI: 10.7270/Q2V69PP3 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50605133
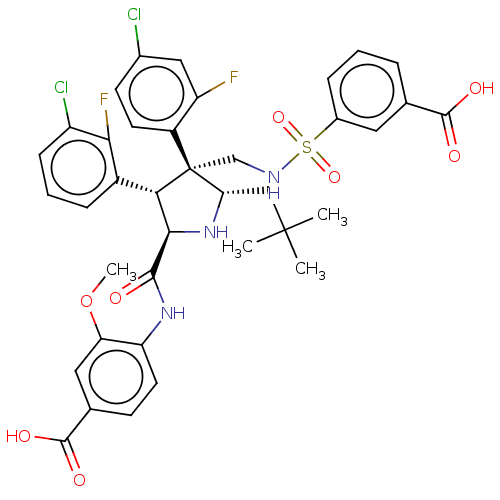
(CHEMBL5186741)Show SMILES COc1cc(ccc1NC(=O)[C@@H]1N[C@@H](CC(C)(C)C)[C@@](CNS(=O)(=O)c2cccc(c2)C(O)=O)([C@H]1c1cccc(Cl)c1F)c1ccc(Cl)cc1F)C(O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00095
BindingDB Entry DOI: 10.7270/Q2V69PP3 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50605139
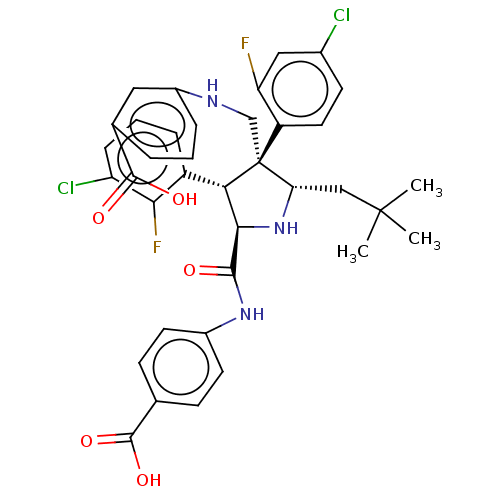
(CHEMBL5184577)Show SMILES CC(C)(C)C[C@@H]1N[C@H]([C@H](c2cccc(Cl)c2F)[C@@]1(CNc1cccc(c1)C(O)=O)c1ccc(Cl)cc1F)C(=O)Nc1ccc(cc1)C(O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00095
BindingDB Entry DOI: 10.7270/Q2V69PP3 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50605108
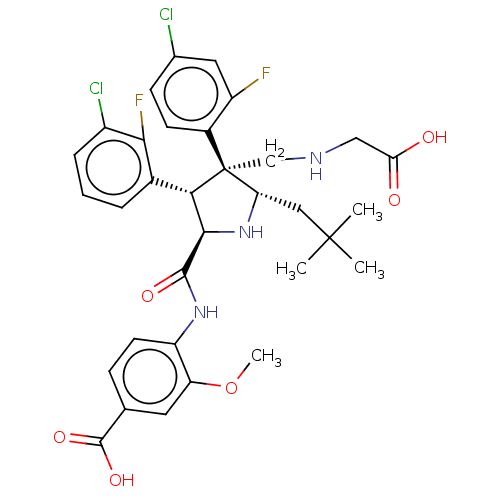
(CHEMBL5204732)Show SMILES COc1cc(ccc1NC(=O)[C@@H]1N[C@@H](CC(C)(C)C)[C@@](CNCC(O)=O)([C@H]1c1cccc(Cl)c1F)c1ccc(Cl)cc1F)C(O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00095
BindingDB Entry DOI: 10.7270/Q2V69PP3 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50605137
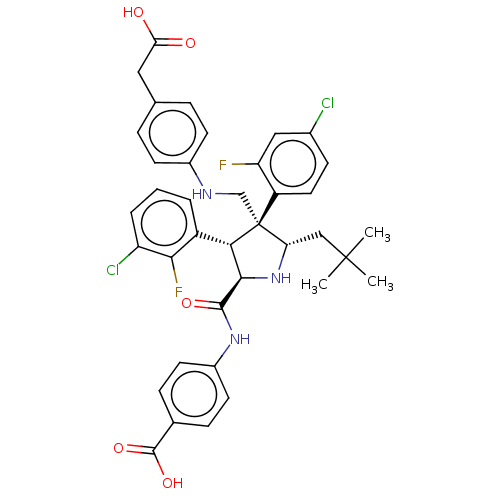
(CHEMBL5183859)Show SMILES CC(C)(C)C[C@@H]1N[C@H]([C@H](c2cccc(Cl)c2F)[C@@]1(CNc1ccc(CC(O)=O)cc1)c1ccc(Cl)cc1F)C(=O)Nc1ccc(cc1)C(O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00095
BindingDB Entry DOI: 10.7270/Q2V69PP3 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50123782
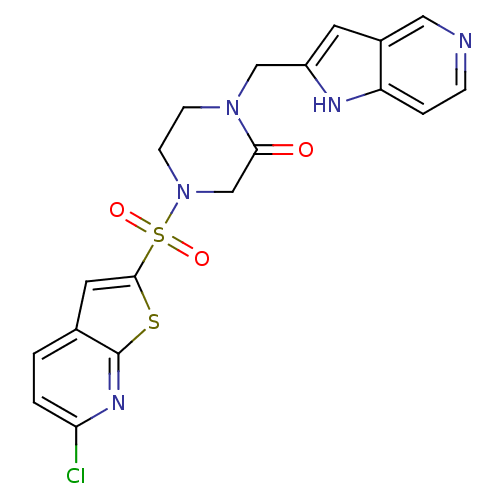
(4-(6-Chloro-thieno[2,3-b]pyridine-2-sulfonyl)-1-(1...)Show SMILES Clc1ccc2cc(sc2n1)S(=O)(=O)N1CCN(Cc2cc3cnccc3[nH]2)C(=O)C1 Show InChI InChI=1S/C19H16ClN5O3S2/c20-16-2-1-12-8-18(29-19(12)23-16)30(27,28)25-6-5-24(17(26)11-25)10-14-7-13-9-21-4-3-15(13)22-14/h1-4,7-9,22H,5-6,10-11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro binding affinity for human Coagulation factor X |
J Med Chem 46: 681-4 (2003)
Article DOI: 10.1021/jm020384z
BindingDB Entry DOI: 10.7270/Q2KW5FDH |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50123767
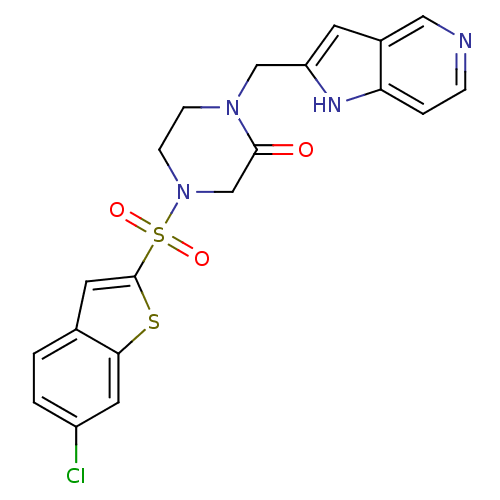
(4-(6-Chloro-benzo[b]thiophene-2-sulfonyl)-1-(1H-py...)Show SMILES Clc1ccc2cc(sc2c1)S(=O)(=O)N1CCN(Cc2cc3cnccc3[nH]2)C(=O)C1 Show InChI InChI=1S/C20H17ClN4O3S2/c21-15-2-1-13-8-20(29-18(13)9-15)30(27,28)25-6-5-24(19(26)12-25)11-16-7-14-10-22-4-3-17(14)23-16/h1-4,7-10,23H,5-6,11-12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro binding affinity for human Coagulation factor X |
J Med Chem 46: 681-4 (2003)
Article DOI: 10.1021/jm020384z
BindingDB Entry DOI: 10.7270/Q2KW5FDH |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50605112
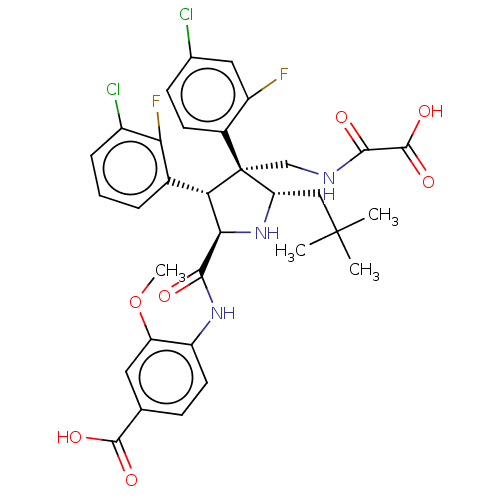
(CHEMBL5181817)Show SMILES COc1cc(ccc1NC(=O)[C@@H]1N[C@@H](CC(C)(C)C)[C@@](CNC(=O)C(O)=O)([C@H]1c1cccc(Cl)c1F)c1ccc(Cl)cc1F)C(O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00095
BindingDB Entry DOI: 10.7270/Q2V69PP3 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data