

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50176988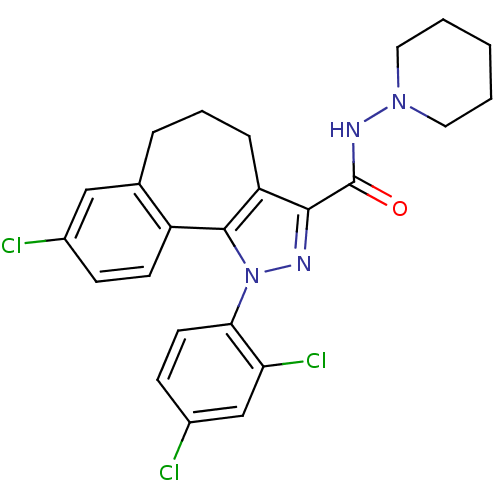 (8-Chloro-1-(2,4-dichloro-phenyl)-1,3a,4,5,6,10b-he...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.000350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Binding affinity to CB1 receptor (unknown origin) | J Med Chem 51: 3526-39 (2008) Article DOI: 10.1021/jm8000778 BindingDB Entry DOI: 10.7270/Q29K4B0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM106803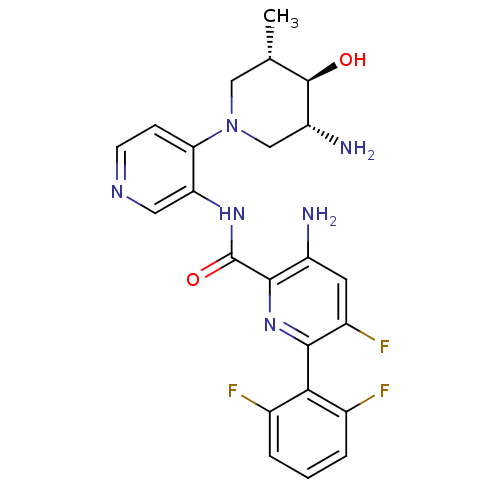 (US8592455, 3) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of PIM1 (unknown origin) | ACS Med Chem Lett 4: 1193-7 (2013) Article DOI: 10.1021/ml400307j BindingDB Entry DOI: 10.7270/Q23X8840 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM106896 (US8592455, 96) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of PIM3 (unknown origin) | ACS Med Chem Lett 4: 1193-7 (2013) Article DOI: 10.1021/ml400307j BindingDB Entry DOI: 10.7270/Q23X8840 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM106896 (US8592455, 96) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of PIM1 (unknown origin) | ACS Med Chem Lett 4: 1193-7 (2013) Article DOI: 10.1021/ml400307j BindingDB Entry DOI: 10.7270/Q23X8840 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-3 (Homo sapiens (Human)) | BDBM50160957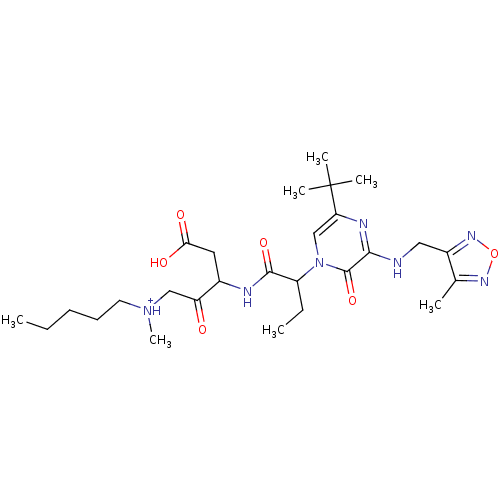 (CHEMBL179503 | [3-(2-{5-tert-Butyl-3-[(4-methyl-fu...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory concentration against recombinant human caspase-3 in neuronal precursor (NT2) cells | Bioorg Med Chem Lett 15: 1173-80 (2005) Article DOI: 10.1016/j.bmcl.2004.12.006 BindingDB Entry DOI: 10.7270/Q2D50MGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-2 (Homo sapiens (Human)) | BDBM106803 (US8592455, 3) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of PIM2 (unknown origin) | ACS Med Chem Lett 4: 1193-7 (2013) Article DOI: 10.1021/ml400307j BindingDB Entry DOI: 10.7270/Q23X8840 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-2 (Homo sapiens (Human)) | BDBM106896 (US8592455, 96) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of PIM2 (unknown origin) | ACS Med Chem Lett 4: 1193-7 (2013) Article DOI: 10.1021/ml400307j BindingDB Entry DOI: 10.7270/Q23X8840 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50445133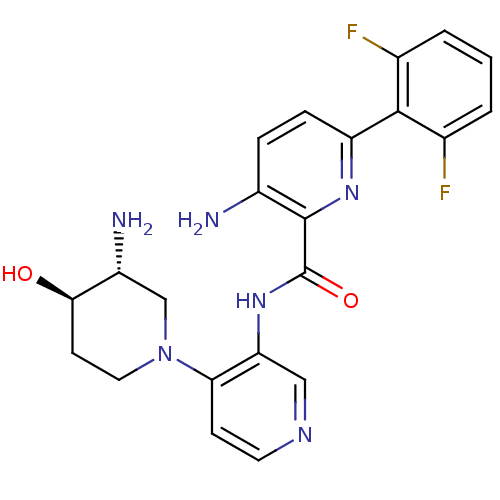 (CHEMBL3103869) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of PIM1 (unknown origin) | ACS Med Chem Lett 4: 1193-7 (2013) Article DOI: 10.1021/ml400307j BindingDB Entry DOI: 10.7270/Q23X8840 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50445124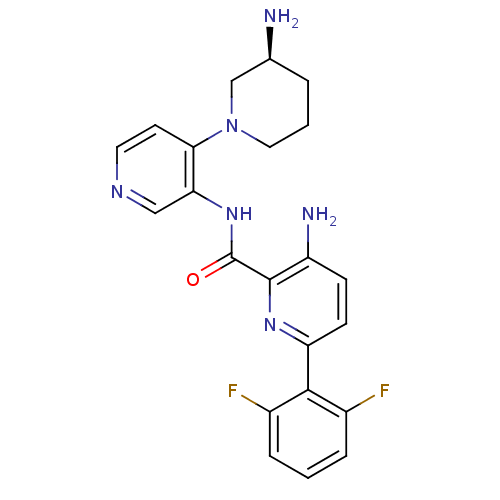 (CHEMBL3103868) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of PIM1 (unknown origin) using Biotin-AGAGRSRHSSYPAGT-OH as substrate after 2 hrs by AlphaScreen assay | ACS Med Chem Lett 4: 1193-7 (2013) Article DOI: 10.1021/ml400307j BindingDB Entry DOI: 10.7270/Q23X8840 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM106803 (US8592455, 3) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of PIM3 (unknown origin) | ACS Med Chem Lett 4: 1193-7 (2013) Article DOI: 10.1021/ml400307j BindingDB Entry DOI: 10.7270/Q23X8840 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM110961 (US8614206, 518) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of recombinant human PIM1 using FAM-pimtide as substrate after 90 mins by Z-LYTE assay | J Med Chem 62: 2140-2153 (2019) Article DOI: 10.1021/acs.jmedchem.8b01857 BindingDB Entry DOI: 10.7270/Q2Q52SVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50474596 (CHEMBL79332) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Ability of compound to compete with 50 pM of 5-[125I]iodo-A-85,380 for receptor binding sites in rat brain membranes at room temperature | J Med Chem 47: 2453-65 (2004) Article DOI: 10.1021/jm030432v BindingDB Entry DOI: 10.7270/Q21C20MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50474584 (CHEMBL79387) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Ability of compound to compete with 50 pM of 5-[125I]iodo-A-85,380 for receptor binding sites in rat brain membranes at room temperature | J Med Chem 47: 2453-65 (2004) Article DOI: 10.1021/jm030432v BindingDB Entry DOI: 10.7270/Q21C20MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50430798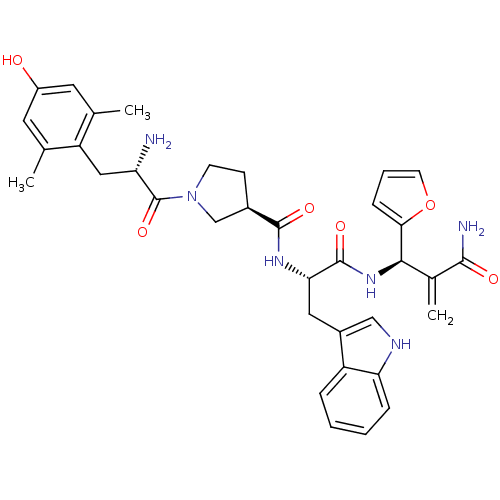 (CHEMBL2335120) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00372 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from FLAG-tagged mu-type opioid receptor (unknown origin) expressed in HEK293 cells after 60 mins by liquid scintillation c... | J Med Chem 56: 3102-14 (2013) Article DOI: 10.1021/jm400195y BindingDB Entry DOI: 10.7270/Q2KS6SW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50474594 (CHEMBL86050) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Ability of compound to compete with 50 pM of 5-[125I]iodo-A-85,380 for receptor binding sites in rat brain membranes at room temperature | J Med Chem 47: 2453-65 (2004) Article DOI: 10.1021/jm030432v BindingDB Entry DOI: 10.7270/Q21C20MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-2 (Homo sapiens (Human)) | BDBM50445124 (CHEMBL3103868) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of PIM2 (unknown origin) using Biotin-AGAGRSRHSSYPAGT-OH as substrate after 2 hrs by AlphaScreen assay | ACS Med Chem Lett 4: 1193-7 (2013) Article DOI: 10.1021/ml400307j BindingDB Entry DOI: 10.7270/Q23X8840 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50474583 (CHEMBL86051) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Ability of compound to compete with 50 pM of 5-[125I]iodo-A-85,380 for receptor binding sites in rat brain membranes at room temperature | J Med Chem 47: 2453-65 (2004) Article DOI: 10.1021/jm030432v BindingDB Entry DOI: 10.7270/Q21C20MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50474587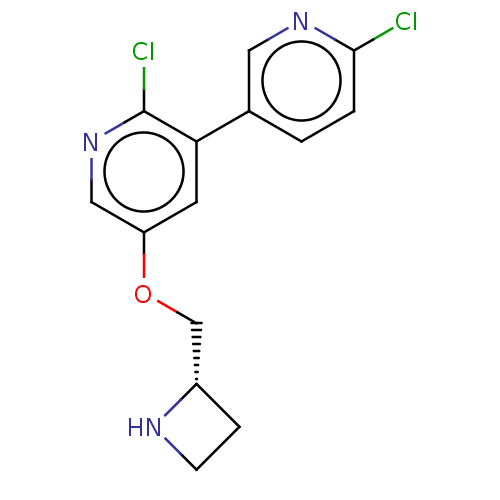 (CHEMBL314718) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Ability of compound to compete with 50 pM of 5-[125I]iodo-A-85,380 for receptor binding sites in rat brain membranes at room temperature | J Med Chem 47: 2453-65 (2004) Article DOI: 10.1021/jm030432v BindingDB Entry DOI: 10.7270/Q21C20MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50474595 (CHEMBL79594) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Ability of compound to compete with 50 pM of 5-[125I]iodo-A-85,380 for receptor binding sites in rat brain membranes at room temperature | J Med Chem 47: 2453-65 (2004) Article DOI: 10.1021/jm030432v BindingDB Entry DOI: 10.7270/Q21C20MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50474582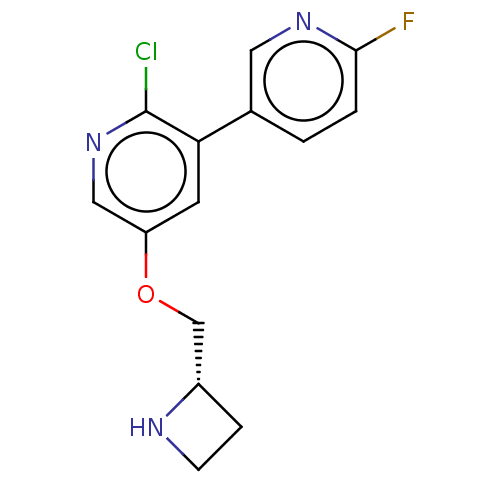 (CHEMBL83444) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Ability of compound to compete with 50 pM of 5-[125I]iodo-A-85,380 for receptor binding sites in rat brain membranes at room temperature | J Med Chem 47: 2453-65 (2004) Article DOI: 10.1021/jm030432v BindingDB Entry DOI: 10.7270/Q21C20MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM50505054 (CHEMBL4455188) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of recombinant human PIM3 using FAM-pimtide as substrate after 90 mins by Z-LYTE assay | J Med Chem 62: 2140-2153 (2019) Article DOI: 10.1021/acs.jmedchem.8b01857 BindingDB Entry DOI: 10.7270/Q2Q52SVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50505052 (CHEMBL3623150) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of recombinant human PIM1 using FAM-pimtide as substrate after 90 mins by Z-LYTE assay | J Med Chem 62: 2140-2153 (2019) Article DOI: 10.1021/acs.jmedchem.8b01857 BindingDB Entry DOI: 10.7270/Q2Q52SVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM227170 (US9328106, 118) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of recombinant human PIM3 using FAM-pimtide as substrate after 90 mins by Z-LYTE assay | J Med Chem 62: 2140-2153 (2019) Article DOI: 10.1021/acs.jmedchem.8b01857 BindingDB Entry DOI: 10.7270/Q2Q52SVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-3 (Homo sapiens (Human)) | BDBM50160974 (CHEMBL366927 | [3-(2-{5-tert-Butyl-3-[(4-methyl-fu...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory concentration against recombinant human caspase-3 in neuronal precursor (NT2) cells | Bioorg Med Chem Lett 15: 1173-80 (2005) Article DOI: 10.1016/j.bmcl.2004.12.006 BindingDB Entry DOI: 10.7270/Q2D50MGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50474593 (CHEMBL313877) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Ability of compound to compete with 50 pM of 5-[125I]iodo-A-85,380 for receptor binding sites in rat brain membranes at room temperature | J Med Chem 47: 2453-65 (2004) Article DOI: 10.1021/jm030432v BindingDB Entry DOI: 10.7270/Q21C20MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50474591 (CHEMBL79515) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Ability of compound to compete with 50 pM of 5-[125I]iodo-A-85,380 for receptor binding sites in rat brain membranes at room temperature | J Med Chem 47: 2453-65 (2004) Article DOI: 10.1021/jm030432v BindingDB Entry DOI: 10.7270/Q21C20MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM50445124 (CHEMBL3103868) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of PIM3 (unknown origin) using Biotin-AGAGRSRHSSYPAGT-OH as substrate after 2 hrs by AlphaScreen assay | ACS Med Chem Lett 4: 1193-7 (2013) Article DOI: 10.1021/ml400307j BindingDB Entry DOI: 10.7270/Q23X8840 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM227170 (US9328106, 118) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of recombinant human PIM1 using FAM-pimtide as substrate after 90 mins by Z-LYTE assay | J Med Chem 62: 2140-2153 (2019) Article DOI: 10.1021/acs.jmedchem.8b01857 BindingDB Entry DOI: 10.7270/Q2Q52SVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-2 (Homo sapiens (Human)) | BDBM50445133 (CHEMBL3103869) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of PIM2 (unknown origin) | ACS Med Chem Lett 4: 1193-7 (2013) Article DOI: 10.1021/ml400307j BindingDB Entry DOI: 10.7270/Q23X8840 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM106870 (US8592455, 70) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of PIM1 kinase (unknown origin) using Biotin-AGAGRSRHSSYPAGT-OH as substrate after 2 hrs by alphascreen assay | J Med Chem 58: 8373-86 (2015) Article DOI: 10.1021/acs.jmedchem.5b01275 BindingDB Entry DOI: 10.7270/Q2H41VGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50474598 (CHEMBL79702) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Ability of compound to compete with 50 pM of 5-[125I]iodo-A-85,380 for receptor binding sites in rat brain membranes at room temperature | J Med Chem 47: 2453-65 (2004) Article DOI: 10.1021/jm030432v BindingDB Entry DOI: 10.7270/Q21C20MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM50445133 (CHEMBL3103869) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of PIM3 (unknown origin) | ACS Med Chem Lett 4: 1193-7 (2013) Article DOI: 10.1021/ml400307j BindingDB Entry DOI: 10.7270/Q23X8840 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM50505051 (CHEMBL4437940) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of recombinant human PIM3 using FAM-pimtide as substrate after 90 mins by Z-LYTE assay | J Med Chem 62: 2140-2153 (2019) Article DOI: 10.1021/acs.jmedchem.8b01857 BindingDB Entry DOI: 10.7270/Q2Q52SVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50505059 (CHEMBL4459538) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of recombinant human PIM1 using FAM-pimtide as substrate after 90 mins by Z-LYTE assay | J Med Chem 62: 2140-2153 (2019) Article DOI: 10.1021/acs.jmedchem.8b01857 BindingDB Entry DOI: 10.7270/Q2Q52SVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50474592 (CHEMBL83738) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Ability of compound to compete with 50 pM of 5-[125I]iodo-A-85,380 for receptor binding sites in rat brain membranes at room temperature | J Med Chem 47: 2453-65 (2004) Article DOI: 10.1021/jm030432v BindingDB Entry DOI: 10.7270/Q21C20MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50474585 (CHEMBL312132) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Ability of compound to compete with 50 pM of 5-[125I]iodo-A-85,380 for receptor binding sites in rat brain membranes at room temperature | J Med Chem 47: 2453-65 (2004) Article DOI: 10.1021/jm030432v BindingDB Entry DOI: 10.7270/Q21C20MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50474590 (CHEMBL309292) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Ability of compound to compete with 50 pM of 5-[125I]iodo-A-85,380 for receptor binding sites in rat brain membranes at room temperature | J Med Chem 47: 2453-65 (2004) Article DOI: 10.1021/jm030432v BindingDB Entry DOI: 10.7270/Q21C20MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM106870 (US8592455, 70) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of PIM3 kinase (unknown origin) using Biotin-AGAGRSRHSSYPAGT-OH as substrate after 2 hrs by alphascreen assay | J Med Chem 58: 8373-86 (2015) Article DOI: 10.1021/acs.jmedchem.5b01275 BindingDB Entry DOI: 10.7270/Q2H41VGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50474586 (CHEMBL80040) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Ability of compound to compete with 50 pM of 5-[125I]iodo-A-85,380 for receptor binding sites in rat brain membranes at room temperature | J Med Chem 47: 2453-65 (2004) Article DOI: 10.1021/jm030432v BindingDB Entry DOI: 10.7270/Q21C20MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM50505052 (CHEMBL3623150) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of recombinant human PIM3 using FAM-pimtide as substrate after 90 mins by Z-LYTE assay | J Med Chem 62: 2140-2153 (2019) Article DOI: 10.1021/acs.jmedchem.8b01857 BindingDB Entry DOI: 10.7270/Q2Q52SVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50049757 (()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Ability of compound to compete with 50 pM of 5-[125I]iodo-A-85,380 for receptor binding sites in rat brain membranes at room temperature | J Med Chem 47: 2453-65 (2004) Article DOI: 10.1021/jm030432v BindingDB Entry DOI: 10.7270/Q21C20MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM110700 (US8614206, 120 | US8614206, 125 | US8614206, 400) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of recombinant human PIM3 using FAM-pimtide as substrate after 90 mins by Z-LYTE assay | J Med Chem 62: 2140-2153 (2019) Article DOI: 10.1021/acs.jmedchem.8b01857 BindingDB Entry DOI: 10.7270/Q2Q52SVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50474588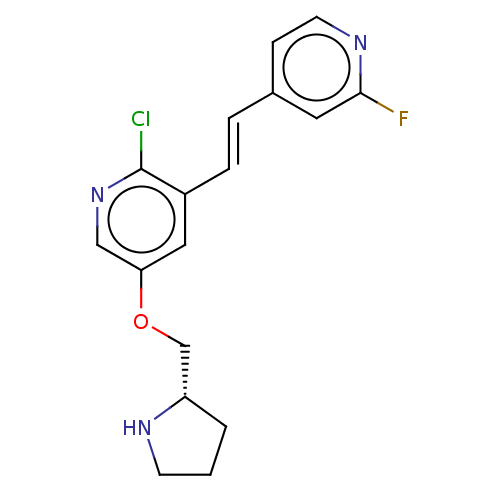 (CHEMBL310197) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Ability of compound to compete with 50 pM of 5-[125I]iodo-A-85,380 for receptor binding sites in rat brain membranes at room temperature | J Med Chem 47: 2453-65 (2004) Article DOI: 10.1021/jm030432v BindingDB Entry DOI: 10.7270/Q21C20MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50430801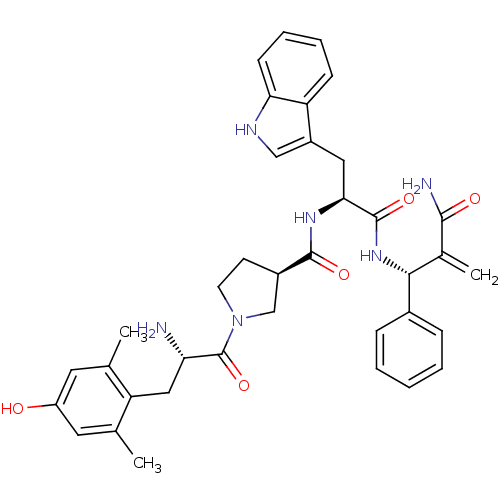 (CHEMBL2334776) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00986 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from FLAG-tagged mu-type opioid receptor (unknown origin) expressed in HEK293 cells after 60 mins by liquid scintillation c... | J Med Chem 56: 3102-14 (2013) Article DOI: 10.1021/jm400195y BindingDB Entry DOI: 10.7270/Q2KS6SW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM50505050 (CHEMBL4439756) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of recombinant human PIM3 using FAM-pimtide as substrate after 90 mins by Z-LYTE assay | J Med Chem 62: 2140-2153 (2019) Article DOI: 10.1021/acs.jmedchem.8b01857 BindingDB Entry DOI: 10.7270/Q2Q52SVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM50505061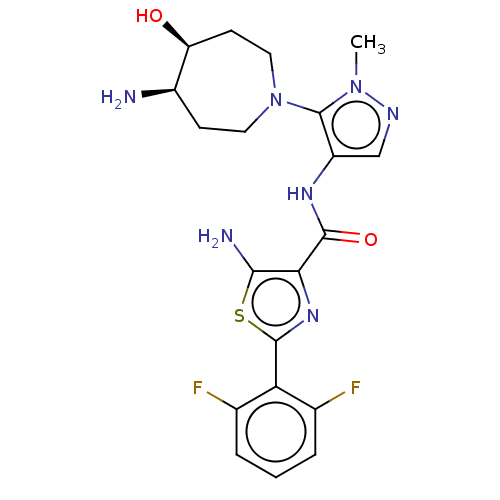 (CHEMBL4453890) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of recombinant human PIM3 using FAM-pimtide as substrate after 90 mins by Z-LYTE assay | J Med Chem 62: 2140-2153 (2019) Article DOI: 10.1021/acs.jmedchem.8b01857 BindingDB Entry DOI: 10.7270/Q2Q52SVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50208446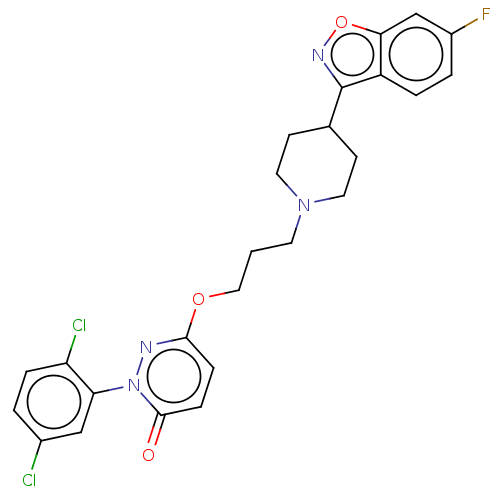 (CHEMBL3884161) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from 5-HT1A receptor in Sprague-Dawley rat cerebral cortex incubated for 30 mins by liquid scintillation counting analy... | Eur J Med Chem 124: 713-728 (2016) Article DOI: 10.1016/j.ejmech.2016.09.008 BindingDB Entry DOI: 10.7270/Q2SQ92C5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM50505053 (CHEMBL4469964) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of recombinant human PIM3 using FAM-pimtide as substrate after 90 mins by Z-LYTE assay | J Med Chem 62: 2140-2153 (2019) Article DOI: 10.1021/acs.jmedchem.8b01857 BindingDB Entry DOI: 10.7270/Q2Q52SVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM110961 (US8614206, 518) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of recombinant human PIM3 using FAM-pimtide as substrate after 90 mins by Z-LYTE assay | J Med Chem 62: 2140-2153 (2019) Article DOI: 10.1021/acs.jmedchem.8b01857 BindingDB Entry DOI: 10.7270/Q2Q52SVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50505057 (CHEMBL3676285) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of recombinant human PIM1 using FAM-pimtide as substrate after 90 mins by Z-LYTE assay | J Med Chem 62: 2140-2153 (2019) Article DOI: 10.1021/acs.jmedchem.8b01857 BindingDB Entry DOI: 10.7270/Q2Q52SVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 63831 total ) | Next | Last >> |