 Found 87 hits with Last Name = 'igarashi' and Initial = 'y'
Found 87 hits with Last Name = 'igarashi' and Initial = 'y' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50241913
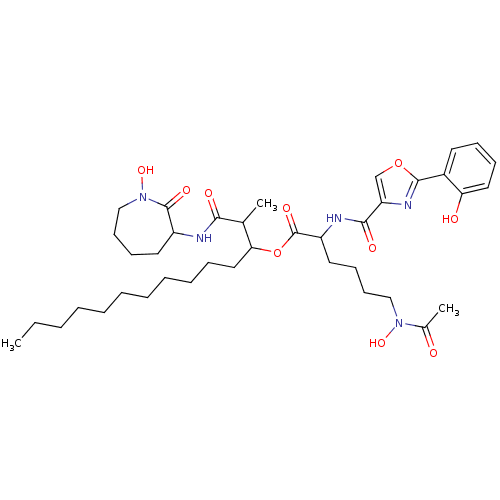
(CHEMBL499519 | GNF-PF-5618 | nocardimicin B)Show SMILES CCCCCCCCCCCC(OC(=O)C(CCCCN(O)C(C)=O)NC(=O)c1coc(n1)-c1ccccc1O)C(C)C(=O)NC1CCCCN(O)C1=O Show InChI InChI=1S/C39H59N5O10/c1-4-5-6-7-8-9-10-11-12-23-34(27(2)35(47)40-30-20-15-18-25-44(52)38(30)49)54-39(50)31(21-16-17-24-43(51)28(3)45)41-36(48)32-26-53-37(42-32)29-19-13-14-22-33(29)46/h13-14,19,22,26-27,30-31,34,46,51-52H,4-12,15-18,20-21,23-25H2,1-3H3,(H,40,47)(H,41,48) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human muscarinic M3 receptor expressed in insect Sf9 cells |
J Nat Prod 68: 1061-5 (2005)
Article DOI: 10.1021/np050091j
BindingDB Entry DOI: 10.7270/Q2R21155 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50241913

(CHEMBL499519 | GNF-PF-5618 | nocardimicin B)Show SMILES CCCCCCCCCCCC(OC(=O)C(CCCCN(O)C(C)=O)NC(=O)c1coc(n1)-c1ccccc1O)C(C)C(=O)NC1CCCCN(O)C1=O Show InChI InChI=1S/C39H59N5O10/c1-4-5-6-7-8-9-10-11-12-23-34(27(2)35(47)40-30-20-15-18-25-44(52)38(30)49)54-39(50)31(21-16-17-24-43(51)28(3)45)41-36(48)32-26-53-37(42-32)29-19-13-14-22-33(29)46/h13-14,19,22,26-27,30-31,34,46,51-52H,4-12,15-18,20-21,23-25H2,1-3H3,(H,40,47)(H,41,48) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to human muscarinic M4 receptor expressed in insect Sf9 cells |
J Nat Prod 68: 1061-5 (2005)
Article DOI: 10.1021/np050091j
BindingDB Entry DOI: 10.7270/Q2R21155 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50241915
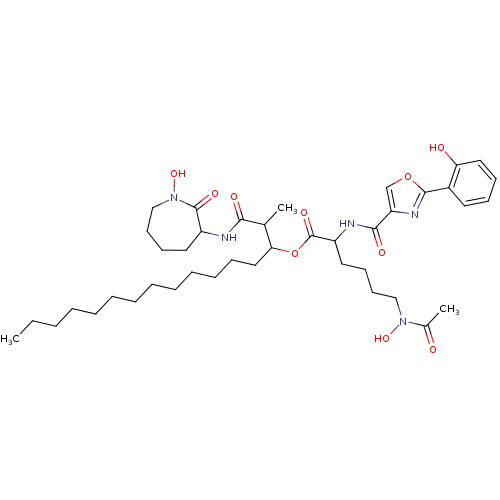
(CHEMBL450986 | nocardimicin D)Show SMILES CCCCCCCCCCCCCC(OC(=O)C(CCCCN(O)C(C)=O)NC(=O)c1coc(n1)-c1ccccc1O)C(C)C(=O)NC1CCCCN(O)C1=O Show InChI InChI=1S/C41H63N5O10/c1-4-5-6-7-8-9-10-11-12-13-14-25-36(29(2)37(49)42-32-22-17-20-27-46(54)40(32)51)56-41(52)33(23-18-19-26-45(53)30(3)47)43-38(50)34-28-55-39(44-34)31-21-15-16-24-35(31)48/h15-16,21,24,28-29,32-33,36,48,53-54H,4-14,17-20,22-23,25-27H2,1-3H3,(H,42,49)(H,43,50) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human muscarinic M3 receptor expressed in insect Sf9 cells |
J Nat Prod 68: 1061-5 (2005)
Article DOI: 10.1021/np050091j
BindingDB Entry DOI: 10.7270/Q2R21155 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50241913

(CHEMBL499519 | GNF-PF-5618 | nocardimicin B)Show SMILES CCCCCCCCCCCC(OC(=O)C(CCCCN(O)C(C)=O)NC(=O)c1coc(n1)-c1ccccc1O)C(C)C(=O)NC1CCCCN(O)C1=O Show InChI InChI=1S/C39H59N5O10/c1-4-5-6-7-8-9-10-11-12-23-34(27(2)35(47)40-30-20-15-18-25-44(52)38(30)49)54-39(50)31(21-16-17-24-43(51)28(3)45)41-36(48)32-26-53-37(42-32)29-19-13-14-22-33(29)46/h13-14,19,22,26-27,30-31,34,46,51-52H,4-12,15-18,20-21,23-25H2,1-3H3,(H,40,47)(H,41,48) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to human muscarinic M1 receptor expressed in insect Sf9 cells |
J Nat Prod 68: 1061-5 (2005)
Article DOI: 10.1021/np050091j
BindingDB Entry DOI: 10.7270/Q2R21155 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50241913

(CHEMBL499519 | GNF-PF-5618 | nocardimicin B)Show SMILES CCCCCCCCCCCC(OC(=O)C(CCCCN(O)C(C)=O)NC(=O)c1coc(n1)-c1ccccc1O)C(C)C(=O)NC1CCCCN(O)C1=O Show InChI InChI=1S/C39H59N5O10/c1-4-5-6-7-8-9-10-11-12-23-34(27(2)35(47)40-30-20-15-18-25-44(52)38(30)49)54-39(50)31(21-16-17-24-43(51)28(3)45)41-36(48)32-26-53-37(42-32)29-19-13-14-22-33(29)46/h13-14,19,22,26-27,30-31,34,46,51-52H,4-12,15-18,20-21,23-25H2,1-3H3,(H,40,47)(H,41,48) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to human muscarinic M2 receptor expressed in insect Sf9 cells |
J Nat Prod 68: 1061-5 (2005)
Article DOI: 10.1021/np050091j
BindingDB Entry DOI: 10.7270/Q2R21155 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50241916
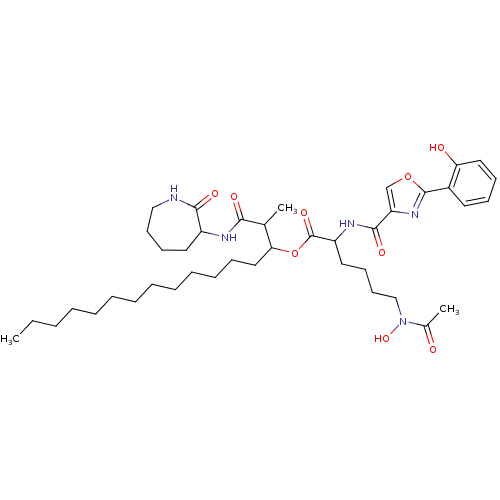
(CHEMBL510340 | Noccardimicin E)Show SMILES CCCCCCCCCCCCCC(OC(=O)C(CCCCN(O)C(C)=O)NC(=O)c1coc(n1)-c1ccccc1O)C(C)C(=O)NC1CCCCNC1=O Show InChI InChI=1S/C41H63N5O9/c1-4-5-6-7-8-9-10-11-12-13-14-25-36(29(2)37(49)43-32-22-17-19-26-42-38(32)50)55-41(52)33(23-18-20-27-46(53)30(3)47)44-39(51)34-28-54-40(45-34)31-21-15-16-24-35(31)48/h15-16,21,24,28-29,32-33,36,48,53H,4-14,17-20,22-23,25-27H2,1-3H3,(H,42,50)(H,43,49)(H,44,51) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human muscarinic M3 receptor expressed in insect Sf9 cells |
J Nat Prod 68: 1061-5 (2005)
Article DOI: 10.1021/np050091j
BindingDB Entry DOI: 10.7270/Q2R21155 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50241914

(CHEMBL507760 | nocardimicin C)Show SMILES CCCCCCCCCCCC(OC(=O)C(CCCCN(O)C(C)=O)NC(=O)c1coc(n1)-c1ccccc1O)C(C)C(=O)NC1CCCCNC1=O Show InChI InChI=1S/C39H59N5O9/c1-4-5-6-7-8-9-10-11-12-23-34(27(2)35(47)41-30-20-15-17-24-40-36(30)48)53-39(50)31(21-16-18-25-44(51)28(3)45)42-37(49)32-26-52-38(43-32)29-19-13-14-22-33(29)46/h13-14,19,22,26-27,30-31,34,46,51H,4-12,15-18,20-21,23-25H2,1-3H3,(H,40,48)(H,41,47)(H,42,49) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human muscarinic M3 receptor expressed in insect Sf9 cells |
J Nat Prod 68: 1061-5 (2005)
Article DOI: 10.1021/np050091j
BindingDB Entry DOI: 10.7270/Q2R21155 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M5
(Homo sapiens (Human)) | BDBM50241913

(CHEMBL499519 | GNF-PF-5618 | nocardimicin B)Show SMILES CCCCCCCCCCCC(OC(=O)C(CCCCN(O)C(C)=O)NC(=O)c1coc(n1)-c1ccccc1O)C(C)C(=O)NC1CCCCN(O)C1=O Show InChI InChI=1S/C39H59N5O10/c1-4-5-6-7-8-9-10-11-12-23-34(27(2)35(47)40-30-20-15-18-25-44(52)38(30)49)54-39(50)31(21-16-17-24-43(51)28(3)45)41-36(48)32-26-53-37(42-32)29-19-13-14-22-33(29)46/h13-14,19,22,26-27,30-31,34,46,51-52H,4-12,15-18,20-21,23-25H2,1-3H3,(H,40,47)(H,41,48) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to human muscarinic M5 receptor expressed in insect Sf9 cells |
J Nat Prod 68: 1061-5 (2005)
Article DOI: 10.1021/np050091j
BindingDB Entry DOI: 10.7270/Q2R21155 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50241912
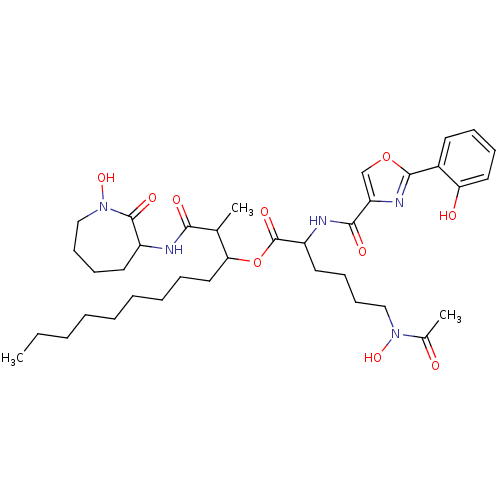
(CHEMBL505197 | Nocardimicin A)Show SMILES CCCCCCCCCC(OC(=O)C(CCCCN(O)C(C)=O)NC(=O)c1coc(n1)-c1ccccc1O)C(C)C(=O)NC1CCCCN(O)C1=O Show InChI InChI=1S/C37H55N5O10/c1-4-5-6-7-8-9-10-21-32(25(2)33(45)38-28-18-13-16-23-42(50)36(28)47)52-37(48)29(19-14-15-22-41(49)26(3)43)39-34(46)30-24-51-35(40-30)27-17-11-12-20-31(27)44/h11-12,17,20,24-25,28-29,32,44,49-50H,4-10,13-16,18-19,21-23H2,1-3H3,(H,38,45)(H,39,46) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human muscarinic M3 receptor expressed in insect Sf9 cells |
J Nat Prod 68: 1061-5 (2005)
Article DOI: 10.1021/np050091j
BindingDB Entry DOI: 10.7270/Q2R21155 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50241917

(CHEMBL508807 | Nocardimicin F)Show SMILES CCCCCCCCCCCCCCCC(OC(=O)C(CCCCN(O)C(C)=O)NC(=O)c1coc(n1)-c1ccccc1O)C(C)C(=O)NC1CCCCN(O)C1=O Show InChI InChI=1S/C43H67N5O10/c1-4-5-6-7-8-9-10-11-12-13-14-15-16-27-38(31(2)39(51)44-34-24-19-22-29-48(56)42(34)53)58-43(54)35(25-20-21-28-47(55)32(3)49)45-40(52)36-30-57-41(46-36)33-23-17-18-26-37(33)50/h17-18,23,26,30-31,34-35,38,50,55-56H,4-16,19-22,24-25,27-29H2,1-3H3,(H,44,51)(H,45,52) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human muscarinic M3 receptor expressed in insect Sf9 cells |
J Nat Prod 68: 1061-5 (2005)
Article DOI: 10.1021/np050091j
BindingDB Entry DOI: 10.7270/Q2R21155 |
More data for this
Ligand-Target Pair | |
Tissue alpha-L-fucosidase
(Homo sapiens (Human)) | BDBM50104412
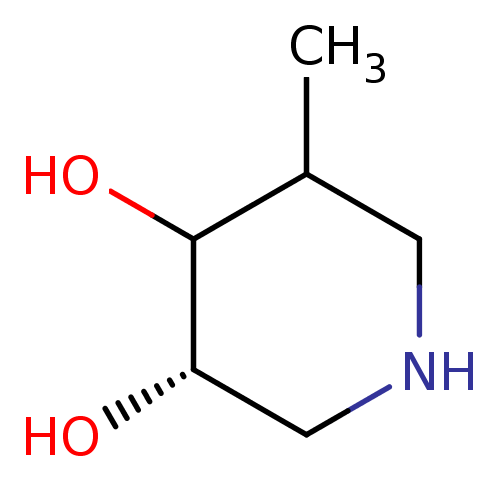
((S)-5-Methyl-piperidine-3,4-diol | CHEMBL86305)Show InChI InChI=1S/C6H13NO2/c1-4-2-7-3-5(8)6(4)9/h4-9H,2-3H2,1H3/t4?,5-,6?/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 8.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 6.8 | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of alpha-fucosidase from bovine kidney(sigma F 5884) at pH 6.8 |
Bioorg Med Chem Lett 6: 553-558 (1996)
Article DOI: 10.1016/0960-894X(96)00068-6
BindingDB Entry DOI: 10.7270/Q2ZS2WHG |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50259847
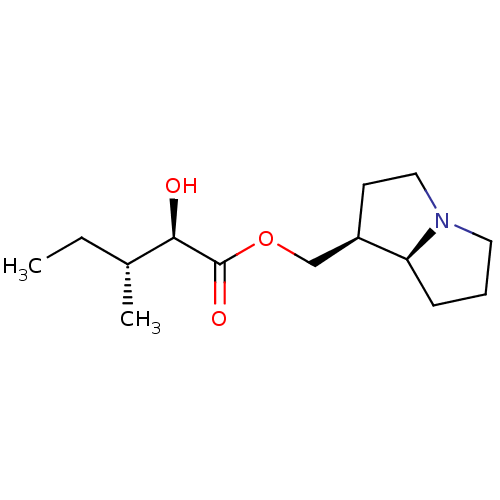
(CHEMBL480464 | cremastrine)Show SMILES CC[C@@H](C)[C@@H](O)C(=O)OC[C@H]1CCN2CCC[C@@H]12 |r| Show InChI InChI=1S/C14H25NO3/c1-3-10(2)13(16)14(17)18-9-11-6-8-15-7-4-5-12(11)15/h10-13,16H,3-9H2,1-2H3/t10-,11-,12+,13-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 126 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human cloned muscarinic M3 receptor expressed in insect Sf9 cells |
J Nat Prod 68: 572-3 (2005)
Article DOI: 10.1021/np049650x
BindingDB Entry DOI: 10.7270/Q2HH6JVJ |
More data for this
Ligand-Target Pair | |
Accessory gene regulator protein A
(Staphylococcus aureus) | BDBM50499936

(CHEMBL3742171)Show SMILES CC(C)[C@@H]1NC(=O)C[C@@H](OC(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@@H](C)NC1=O)C(C)C)C(C)C)\C=C\c1ccccc1 |r| Show InChI InChI=1S/C29H42N4O6/c1-16(2)23-27(36)30-19(7)26(35)32-24(17(3)4)28(37)33-25(18(5)6)29(38)39-21(15-22(34)31-23)14-13-20-11-9-8-10-12-20/h8-14,16-19,21,23-25H,15H2,1-7H3,(H,30,36)(H,31,34)(H,32,35)(H,33,37)/b14-13+/t19-,21+,23+,24+,25+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Toyama Prefectural University
Curated by ChEMBL
| Assay Description
Inhibition of agr-mediated quorum sensing in Staphylococcus aureus after 5 hrs by luminescence analysis |
J Nat Prod 78: 2827-31 (2015)
Article DOI: 10.1021/acs.jnatprod.5b00540
BindingDB Entry DOI: 10.7270/Q20868BF |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50259847

(CHEMBL480464 | cremastrine)Show SMILES CC[C@@H](C)[C@@H](O)C(=O)OC[C@H]1CCN2CCC[C@@H]12 |r| Show InChI InChI=1S/C14H25NO3/c1-3-10(2)13(16)14(17)18-9-11-6-8-15-7-4-5-12(11)15/h10-13,16H,3-9H2,1-2H3/t10-,11-,12+,13-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 498 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human cloned muscarinic M4 receptor expressed in insect Sf9 cells |
J Nat Prod 68: 572-3 (2005)
Article DOI: 10.1021/np049650x
BindingDB Entry DOI: 10.7270/Q2HH6JVJ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50259847

(CHEMBL480464 | cremastrine)Show SMILES CC[C@@H](C)[C@@H](O)C(=O)OC[C@H]1CCN2CCC[C@@H]12 |r| Show InChI InChI=1S/C14H25NO3/c1-3-10(2)13(16)14(17)18-9-11-6-8-15-7-4-5-12(11)15/h10-13,16H,3-9H2,1-2H3/t10-,11-,12+,13-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 505 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human cloned muscarinic M1 receptor expressed in insect Sf9 cells |
J Nat Prod 68: 572-3 (2005)
Article DOI: 10.1021/np049650x
BindingDB Entry DOI: 10.7270/Q2HH6JVJ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50241913

(CHEMBL499519 | GNF-PF-5618 | nocardimicin B)Show SMILES CCCCCCCCCCCC(OC(=O)C(CCCCN(O)C(C)=O)NC(=O)c1coc(n1)-c1ccccc1O)C(C)C(=O)NC1CCCCN(O)C1=O Show InChI InChI=1S/C39H59N5O10/c1-4-5-6-7-8-9-10-11-12-23-34(27(2)35(47)40-30-20-15-18-25-44(52)38(30)49)54-39(50)31(21-16-17-24-43(51)28(3)45)41-36(48)32-26-53-37(42-32)29-19-13-14-22-33(29)46/h13-14,19,22,26-27,30-31,34,46,51-52H,4-12,15-18,20-21,23-25H2,1-3H3,(H,40,47)(H,41,48) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human muscarinic M3 receptor expressed in insect Sf9 cells |
J Nat Prod 68: 1061-5 (2005)
Article DOI: 10.1021/np050091j
BindingDB Entry DOI: 10.7270/Q2R21155 |
More data for this
Ligand-Target Pair | |
Accessory gene regulator protein A
(Staphylococcus aureus) | BDBM50499937
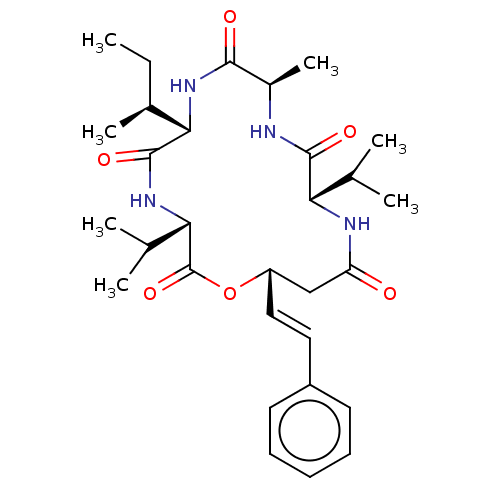
(CHEBI:67522 | Turnagainolide A)Show SMILES [H][C@]1(NC(=O)[C@@H](C)NC(=O)[C@@H](NC(=O)C[C@@H](OC(=O)[C@@H](NC1=O)C(C)C)\C=C\c1ccccc1)C(C)C)[C@@H](C)CC |r| Show InChI InChI=1S/C30H44N4O6/c1-8-19(6)26-29(38)33-25(18(4)5)30(39)40-22(15-14-21-12-10-9-11-13-21)16-23(35)32-24(17(2)3)28(37)31-20(7)27(36)34-26/h9-15,17-20,22,24-26H,8,16H2,1-7H3,(H,31,37)(H,32,35)(H,33,38)(H,34,36)/b15-14+/t19-,20+,22-,24-,25-,26-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Toyama Prefectural University
Curated by ChEMBL
| Assay Description
Inhibition of agr-mediated quorum sensing in Staphylococcus aureus after 5 hrs by luminescence analysis |
J Nat Prod 78: 2827-31 (2015)
Article DOI: 10.1021/acs.jnatprod.5b00540
BindingDB Entry DOI: 10.7270/Q20868BF |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50481798
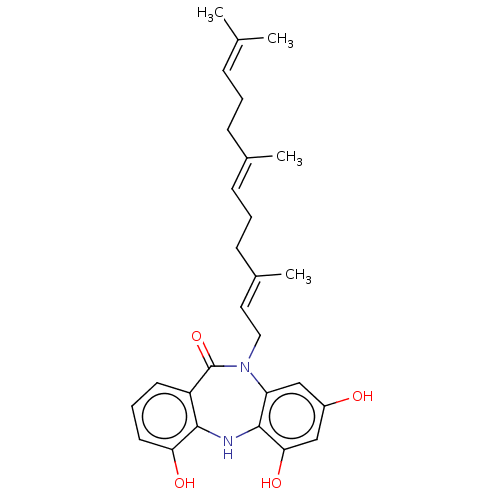
(Amo-01 | BU-4664L | Diazepinomicin | ECO-4601 | TL...)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-[#7]-1-c2cc(-[#8])cc(-[#8])c2-[#7]-c2c(-[#8])cccc2-[#6]-1=O Show InChI InChI=1S/C28H34N2O4/c1-18(2)8-5-9-19(3)10-6-11-20(4)14-15-30-23-16-21(31)17-25(33)27(23)29-26-22(28(30)34)12-7-13-24(26)32/h7-8,10,12-14,16-17,29,31-33H,5-6,9,11,15H2,1-4H3/b19-10+,20-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 994 | n/a | n/a | n/a | n/a | n/a | n/a |
Toyama Prefectural University
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 20: 963-5 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.055
BindingDB Entry DOI: 10.7270/Q2GB26W9 |
More data for this
Ligand-Target Pair | |
Phosphatidylcholine:ceramide cholinephosphotransferase 2
(Homo sapiens (Human)) | BDBM50182491

(CHEBI:69015 | Malabaricone C)Show InChI InChI=1S/C21H26O5/c22-16-13-12-15(14-20(16)26)8-5-3-1-2-4-6-9-17(23)21-18(24)10-7-11-19(21)25/h7,10-14,22,24-26H,1-6,8-9H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Noncompetitive inhibition of SMS2 (unknown origin) stably expressed in mouse ZS cells using 5 to 50 uM C6-NBD-ceramide as substrate preincubated for ... |
ACS Med Chem Lett 10: 1154-1158 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00171
BindingDB Entry DOI: 10.7270/Q2WS8XPS |
More data for this
Ligand-Target Pair | |
Phosphatidylcholine:ceramide cholinephosphotransferase 2
(Homo sapiens (Human)) | BDBM50182487

(Malabaricone A)Show InChI InChI=1S/C21H26O3/c22-18(21-19(23)15-10-16-20(21)24)14-9-4-2-1-3-6-11-17-12-7-5-8-13-17/h5,7-8,10,12-13,15-16,23-24H,1-4,6,9,11,14H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Noncompetitive inhibition of SMS2 (unknown origin) stably expressed in mouse ZS cells using 5 to 50 uM C6-NBD-ceramide as substrate preincubated for ... |
ACS Med Chem Lett 10: 1154-1158 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00171
BindingDB Entry DOI: 10.7270/Q2WS8XPS |
More data for this
Ligand-Target Pair | |
Phosphatidylcholine:ceramide cholinephosphotransferase 2
(Homo sapiens (Human)) | BDBM50182486
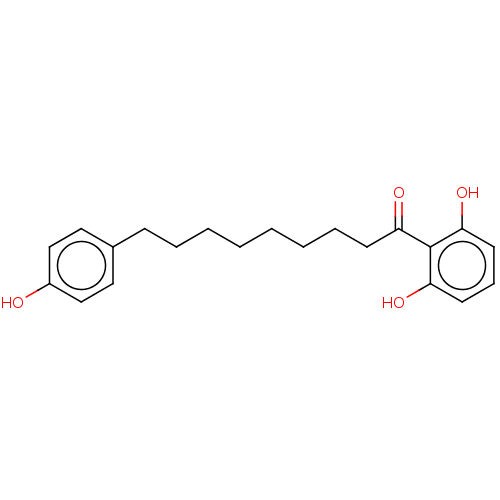
(MALABARICONE B)Show InChI InChI=1S/C21H26O4/c22-17-14-12-16(13-15-17)8-5-3-1-2-4-6-9-18(23)21-19(24)10-7-11-20(21)25/h7,10-15,22,24-25H,1-6,8-9H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Noncompetitive inhibition of SMS2 (unknown origin) stably expressed in mouse ZS cells using 5 to 50 uM C6-NBD-ceramide as substrate preincubated for ... |
ACS Med Chem Lett 10: 1154-1158 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00171
BindingDB Entry DOI: 10.7270/Q2WS8XPS |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50241913

(CHEMBL499519 | GNF-PF-5618 | nocardimicin B)Show SMILES CCCCCCCCCCCC(OC(=O)C(CCCCN(O)C(C)=O)NC(=O)c1coc(n1)-c1ccccc1O)C(C)C(=O)NC1CCCCN(O)C1=O Show InChI InChI=1S/C39H59N5O10/c1-4-5-6-7-8-9-10-11-12-23-34(27(2)35(47)40-30-20-15-18-25-44(52)38(30)49)54-39(50)31(21-16-17-24-43(51)28(3)45)41-36(48)32-26-53-37(42-32)29-19-13-14-22-33(29)46/h13-14,19,22,26-27,30-31,34,46,51-52H,4-12,15-18,20-21,23-25H2,1-3H3,(H,40,47)(H,41,48) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to human muscarinic M4 receptor expressed in insect Sf9 cells |
J Nat Prod 68: 1061-5 (2005)
Article DOI: 10.1021/np050091j
BindingDB Entry DOI: 10.7270/Q2R21155 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50241915

(CHEMBL450986 | nocardimicin D)Show SMILES CCCCCCCCCCCCCC(OC(=O)C(CCCCN(O)C(C)=O)NC(=O)c1coc(n1)-c1ccccc1O)C(C)C(=O)NC1CCCCN(O)C1=O Show InChI InChI=1S/C41H63N5O10/c1-4-5-6-7-8-9-10-11-12-13-14-25-36(29(2)37(49)42-32-22-17-20-27-46(54)40(32)51)56-41(52)33(23-18-19-26-45(53)30(3)47)43-38(50)34-28-55-39(44-34)31-21-15-16-24-35(31)48/h15-16,21,24,28-29,32-33,36,48,53-54H,4-14,17-20,22-23,25-27H2,1-3H3,(H,42,49)(H,43,50) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human muscarinic M3 receptor expressed in insect Sf9 cells |
J Nat Prod 68: 1061-5 (2005)
Article DOI: 10.1021/np050091j
BindingDB Entry DOI: 10.7270/Q2R21155 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M5
(Homo sapiens (Human)) | BDBM50259847

(CHEMBL480464 | cremastrine)Show SMILES CC[C@@H](C)[C@@H](O)C(=O)OC[C@H]1CCN2CCC[C@@H]12 |r| Show InChI InChI=1S/C14H25NO3/c1-3-10(2)13(16)14(17)18-9-11-6-8-15-7-4-5-12(11)15/h10-13,16H,3-9H2,1-2H3/t10-,11-,12+,13-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human cloned muscarinic M5 receptor expressed in insect Sf9 cells |
J Nat Prod 68: 572-3 (2005)
Article DOI: 10.1021/np049650x
BindingDB Entry DOI: 10.7270/Q2HH6JVJ |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50481798

(Amo-01 | BU-4664L | Diazepinomicin | ECO-4601 | TL...)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-[#7]-1-c2cc(-[#8])cc(-[#8])c2-[#7]-c2c(-[#8])cccc2-[#6]-1=O Show InChI InChI=1S/C28H34N2O4/c1-18(2)8-5-9-19(3)10-6-11-20(4)14-15-30-23-16-21(31)17-25(33)27(23)29-26-22(28(30)34)12-7-13-24(26)32/h7-8,10,12-14,16-17,29,31-33H,5-6,9,11,15H2,1-4H3/b19-10+,20-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Toyama Prefectural University
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem Lett 20: 963-5 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.055
BindingDB Entry DOI: 10.7270/Q2GB26W9 |
More data for this
Ligand-Target Pair | |
Phosphatidylcholine:ceramide cholinephosphotransferase 2
(Homo sapiens (Human)) | BDBM50182491

(CHEBI:69015 | Malabaricone C)Show InChI InChI=1S/C21H26O5/c22-16-13-12-15(14-20(16)26)8-5-3-1-2-4-6-9-17(23)21-18(24)10-7-11-19(21)25/h7,10-14,22,24-26H,1-6,8-9H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of SMS2 (unknown origin) stably expressed in mouse ZS cells using C6-NBD-ceramide as substrate preincubated for 30 mins followed by substr... |
ACS Med Chem Lett 10: 1154-1158 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00171
BindingDB Entry DOI: 10.7270/Q2WS8XPS |
More data for this
Ligand-Target Pair | |
Phosphatidylcholine:ceramide cholinephosphotransferase 2
(Homo sapiens (Human)) | BDBM50242188

(6-(8'Z-pentadecenyl)-salicylicacid | 6-[8'(Z)-pent...)Show InChI InChI=1S/C22H34O3/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-16-19-17-15-18-20(23)21(19)22(24)25/h7-8,15,17-18,23H,2-6,9-14,16H2,1H3,(H,24,25)/b8-7- | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of SMS2 (unknown origin) |
ACS Med Chem Lett 10: 1154-1158 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00171
BindingDB Entry DOI: 10.7270/Q2WS8XPS |
More data for this
Ligand-Target Pair | |
Phosphatidylcholine:ceramide cholinephosphotransferase 1
(Homo sapiens (Human)) | BDBM50242188

(6-(8'Z-pentadecenyl)-salicylicacid | 6-[8'(Z)-pent...)Show InChI InChI=1S/C22H34O3/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-16-19-17-15-18-20(23)21(19)22(24)25/h7-8,15,17-18,23H,2-6,9-14,16H2,1H3,(H,24,25)/b8-7- | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of SMS1 (unknown origin) |
ACS Med Chem Lett 10: 1154-1158 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00171
BindingDB Entry DOI: 10.7270/Q2WS8XPS |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M5
(Homo sapiens (Human)) | BDBM50241913

(CHEMBL499519 | GNF-PF-5618 | nocardimicin B)Show SMILES CCCCCCCCCCCC(OC(=O)C(CCCCN(O)C(C)=O)NC(=O)c1coc(n1)-c1ccccc1O)C(C)C(=O)NC1CCCCN(O)C1=O Show InChI InChI=1S/C39H59N5O10/c1-4-5-6-7-8-9-10-11-12-23-34(27(2)35(47)40-30-20-15-18-25-44(52)38(30)49)54-39(50)31(21-16-17-24-43(51)28(3)45)41-36(48)32-26-53-37(42-32)29-19-13-14-22-33(29)46/h13-14,19,22,26-27,30-31,34,46,51-52H,4-12,15-18,20-21,23-25H2,1-3H3,(H,40,47)(H,41,48) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to human muscarinic M5 receptor expressed in insect Sf9 cells |
J Nat Prod 68: 1061-5 (2005)
Article DOI: 10.1021/np050091j
BindingDB Entry DOI: 10.7270/Q2R21155 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50241913

(CHEMBL499519 | GNF-PF-5618 | nocardimicin B)Show SMILES CCCCCCCCCCCC(OC(=O)C(CCCCN(O)C(C)=O)NC(=O)c1coc(n1)-c1ccccc1O)C(C)C(=O)NC1CCCCN(O)C1=O Show InChI InChI=1S/C39H59N5O10/c1-4-5-6-7-8-9-10-11-12-23-34(27(2)35(47)40-30-20-15-18-25-44(52)38(30)49)54-39(50)31(21-16-17-24-43(51)28(3)45)41-36(48)32-26-53-37(42-32)29-19-13-14-22-33(29)46/h13-14,19,22,26-27,30-31,34,46,51-52H,4-12,15-18,20-21,23-25H2,1-3H3,(H,40,47)(H,41,48) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to human muscarinic M2 receptor expressed in insect Sf9 cells |
J Nat Prod 68: 1061-5 (2005)
Article DOI: 10.1021/np050091j
BindingDB Entry DOI: 10.7270/Q2R21155 |
More data for this
Ligand-Target Pair | |
Phosphatidylcholine:ceramide cholinephosphotransferase 1
(Homo sapiens (Human)) | BDBM50182491

(CHEBI:69015 | Malabaricone C)Show InChI InChI=1S/C21H26O5/c22-16-13-12-15(14-20(16)26)8-5-3-1-2-4-6-9-17(23)21-18(24)10-7-11-19(21)25/h7,10-14,22,24-26H,1-6,8-9H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Noncompetitive inhibition of SMS1 (unknown origin) stably expressed in mouse ZS cells using 5 to 50 uM C6-NBD-ceramide as substrate preincubated for ... |
ACS Med Chem Lett 10: 1154-1158 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00171
BindingDB Entry DOI: 10.7270/Q2WS8XPS |
More data for this
Ligand-Target Pair | |
Phosphatidylcholine:ceramide cholinephosphotransferase 1
(Homo sapiens (Human)) | BDBM50182487

(Malabaricone A)Show InChI InChI=1S/C21H26O3/c22-18(21-19(23)15-10-16-20(21)24)14-9-4-2-1-3-6-11-17-12-7-5-8-13-17/h5,7-8,10,12-13,15-16,23-24H,1-4,6,9,11,14H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Noncompetitive inhibition of SMS1 (unknown origin) stably expressed in mouse ZS cells using 5 to 50 uM C6-NBD-ceramide as substrate preincubated for ... |
ACS Med Chem Lett 10: 1154-1158 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00171
BindingDB Entry DOI: 10.7270/Q2WS8XPS |
More data for this
Ligand-Target Pair | |
Phosphatidylcholine:ceramide cholinephosphotransferase 1
(Homo sapiens (Human)) | BDBM50182486

(MALABARICONE B)Show InChI InChI=1S/C21H26O4/c22-17-14-12-16(13-15-17)8-5-3-1-2-4-6-9-18(23)21-19(24)10-7-11-20(21)25/h7,10-15,22,24-25H,1-6,8-9H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Noncompetitive inhibition of SMS1 (unknown origin) stably expressed in mouse ZS cells using 5 to 50 uM C6-NBD-ceramide as substrate preincubated for ... |
ACS Med Chem Lett 10: 1154-1158 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00171
BindingDB Entry DOI: 10.7270/Q2WS8XPS |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50241913

(CHEMBL499519 | GNF-PF-5618 | nocardimicin B)Show SMILES CCCCCCCCCCCC(OC(=O)C(CCCCN(O)C(C)=O)NC(=O)c1coc(n1)-c1ccccc1O)C(C)C(=O)NC1CCCCN(O)C1=O Show InChI InChI=1S/C39H59N5O10/c1-4-5-6-7-8-9-10-11-12-23-34(27(2)35(47)40-30-20-15-18-25-44(52)38(30)49)54-39(50)31(21-16-17-24-43(51)28(3)45)41-36(48)32-26-53-37(42-32)29-19-13-14-22-33(29)46/h13-14,19,22,26-27,30-31,34,46,51-52H,4-12,15-18,20-21,23-25H2,1-3H3,(H,40,47)(H,41,48) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to human muscarinic M1 receptor expressed in insect Sf9 cells |
J Nat Prod 68: 1061-5 (2005)
Article DOI: 10.1021/np050091j
BindingDB Entry DOI: 10.7270/Q2R21155 |
More data for this
Ligand-Target Pair | |
Accessory gene regulator protein A
(Staphylococcus aureus) | BDBM50499939
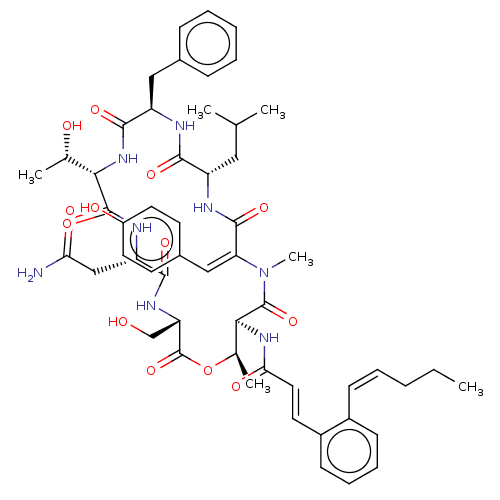
(CHEMBL3740058)Show SMILES [H][C@]1(NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](CC(C)C)NC(=O)\C(=C/c2ccc(O)cc2)N(C)C(=O)[C@@H](NC(=O)\C=C\c2ccccc2\C=C/CCC)[C@@H](C)OC(=O)[C@H](CO)NC(=O)[C@H](CC(N)=O)NC1=O)[C@H](C)O |r| Show InChI InChI=1S/C54H68N8O13/c1-7-8-10-17-36-18-13-14-19-37(36)22-25-45(67)60-47-33(5)75-54(74)42(30-63)59-49(69)41(29-44(55)66)58-52(72)46(32(4)64)61-50(70)40(27-34-15-11-9-12-16-34)56-48(68)39(26-31(2)3)57-51(71)43(62(6)53(47)73)28-35-20-23-38(65)24-21-35/h9-25,28,31-33,39-42,46-47,63-65H,7-8,26-27,29-30H2,1-6H3,(H2,55,66)(H,56,68)(H,57,71)(H,58,72)(H,59,69)(H,60,67)(H,61,70)/b17-10-,25-22+,43-28+/t32-,33+,39-,40+,41-,42-,46-,47-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Toyama Prefectural University
Curated by ChEMBL
| Assay Description
Inhibition of agr-mediated quorum sensing in Staphylococcus aureus after 5 hrs by luminescence analysis |
J Nat Prod 78: 2827-31 (2015)
Article DOI: 10.1021/acs.jnatprod.5b00540
BindingDB Entry DOI: 10.7270/Q20868BF |
More data for this
Ligand-Target Pair | |
Phosphatidylcholine:ceramide cholinephosphotransferase 2
(Homo sapiens (Human)) | BDBM50182486

(MALABARICONE B)Show InChI InChI=1S/C21H26O4/c22-17-14-12-16(13-15-17)8-5-3-1-2-4-6-9-18(23)21-19(24)10-7-11-20(21)25/h7,10-15,22,24-25H,1-6,8-9H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of SMS2 (unknown origin) stably expressed in mouse ZS cells using C6-NBD-ceramide as substrate preincubated for 30 mins followed by substr... |
ACS Med Chem Lett 10: 1154-1158 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00171
BindingDB Entry DOI: 10.7270/Q2WS8XPS |
More data for this
Ligand-Target Pair | |
Phosphatidylcholine:ceramide cholinephosphotransferase 1
(Homo sapiens (Human)) | BDBM50182491

(CHEBI:69015 | Malabaricone C)Show InChI InChI=1S/C21H26O5/c22-16-13-12-15(14-20(16)26)8-5-3-1-2-4-6-9-17(23)21-18(24)10-7-11-19(21)25/h7,10-14,22,24-26H,1-6,8-9H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of SMS1 (unknown origin) stably expressed in mouse ZS cells using C6-NBD-ceramide as substrate preincubated for 30 mins followed by substr... |
ACS Med Chem Lett 10: 1154-1158 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00171
BindingDB Entry DOI: 10.7270/Q2WS8XPS |
More data for this
Ligand-Target Pair | |
Phosphatidylcholine:ceramide cholinephosphotransferase 1
(Homo sapiens (Human)) | BDBM50182486

(MALABARICONE B)Show InChI InChI=1S/C21H26O4/c22-17-14-12-16(13-15-17)8-5-3-1-2-4-6-9-18(23)21-19(24)10-7-11-20(21)25/h7,10-15,22,24-25H,1-6,8-9H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of SMS1 (unknown origin) stably expressed in mouse ZS cells using C6-NBD-ceramide as substrate preincubated for 30 mins followed by substr... |
ACS Med Chem Lett 10: 1154-1158 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00171
BindingDB Entry DOI: 10.7270/Q2WS8XPS |
More data for this
Ligand-Target Pair | |
Phosphatidylcholine:ceramide cholinephosphotransferase 1
(Homo sapiens (Human)) | BDBM50182487

(Malabaricone A)Show InChI InChI=1S/C21H26O3/c22-18(21-19(23)15-10-16-20(21)24)14-9-4-2-1-3-6-11-17-12-7-5-8-13-17/h5,7-8,10,12-13,15-16,23-24H,1-4,6,9,11,14H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of SMS1 (unknown origin) stably expressed in mouse ZS cells using C6-NBD-ceramide as substrate preincubated for 30 mins followed by substr... |
ACS Med Chem Lett 10: 1154-1158 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00171
BindingDB Entry DOI: 10.7270/Q2WS8XPS |
More data for this
Ligand-Target Pair | |
Phosphatidylcholine:ceramide cholinephosphotransferase 2
(Homo sapiens (Human)) | BDBM50182487

(Malabaricone A)Show InChI InChI=1S/C21H26O3/c22-18(21-19(23)15-10-16-20(21)24)14-9-4-2-1-3-6-11-17-12-7-5-8-13-17/h5,7-8,10,12-13,15-16,23-24H,1-4,6,9,11,14H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of SMS2 (unknown origin) stably expressed in mouse ZS cells using C6-NBD-ceramide as substrate preincubated for 30 mins followed by substr... |
ACS Med Chem Lett 10: 1154-1158 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00171
BindingDB Entry DOI: 10.7270/Q2WS8XPS |
More data for this
Ligand-Target Pair | |
Accessory gene regulator protein A
(Staphylococcus aureus) | BDBM50499938
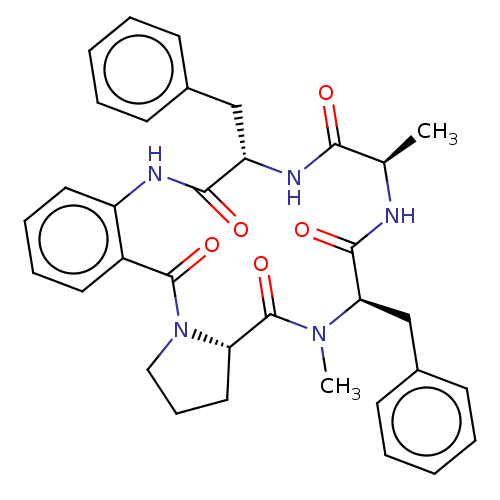
(CHEMBL3741390)Show SMILES [H][C@@]12CCCN1C(=O)c1ccccc1NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](C)NC(=O)[C@@H](Cc1ccccc1)N(C)C2=O |r| Show InChI InChI=1S/C34H37N5O5/c1-22-30(40)37-27(20-23-12-5-3-6-13-23)31(41)36-26-17-10-9-16-25(26)33(43)39-19-11-18-28(39)34(44)38(2)29(32(42)35-22)21-24-14-7-4-8-15-24/h3-10,12-17,22,27-29H,11,18-21H2,1-2H3,(H,35,42)(H,36,41)(H,37,40)/t22-,27+,28+,29-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Toyama Prefectural University
Curated by ChEMBL
| Assay Description
Inhibition of agr-mediated quorum sensing in Staphylococcus aureus after 5 hrs by luminescence analysis |
J Nat Prod 78: 2827-31 (2015)
Article DOI: 10.1021/acs.jnatprod.5b00540
BindingDB Entry DOI: 10.7270/Q20868BF |
More data for this
Ligand-Target Pair | |
Phosphatidylcholine:ceramide cholinephosphotransferase 2
(Homo sapiens (Human)) | BDBM50182488

(CHEMBL3819036)Show InChI InChI=1S/C21H26O5/c22-16-11-9-15(10-12-16)7-5-3-1-2-4-6-8-18(24)21-19(25)13-17(23)14-20(21)26/h9-14,22-23,25-26H,1-8H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of SMS2 (unknown origin) stably expressed in mouse ZS cells using C6-NBD-ceramide as substrate preincubated for 30 mins followed by substr... |
ACS Med Chem Lett 10: 1154-1158 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00171
BindingDB Entry DOI: 10.7270/Q2WS8XPS |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50241916

(CHEMBL510340 | Noccardimicin E)Show SMILES CCCCCCCCCCCCCC(OC(=O)C(CCCCN(O)C(C)=O)NC(=O)c1coc(n1)-c1ccccc1O)C(C)C(=O)NC1CCCCNC1=O Show InChI InChI=1S/C41H63N5O9/c1-4-5-6-7-8-9-10-11-12-13-14-25-36(29(2)37(49)43-32-22-17-19-26-42-38(32)50)55-41(52)33(23-18-20-27-46(53)30(3)47)44-39(51)34-28-54-40(45-34)31-21-15-16-24-35(31)48/h15-16,21,24,28-29,32-33,36,48,53H,4-14,17-20,22-23,25-27H2,1-3H3,(H,42,50)(H,43,49)(H,44,51) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human muscarinic M3 receptor expressed in insect Sf9 cells |
J Nat Prod 68: 1061-5 (2005)
Article DOI: 10.1021/np050091j
BindingDB Entry DOI: 10.7270/Q2R21155 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50241914

(CHEMBL507760 | nocardimicin C)Show SMILES CCCCCCCCCCCC(OC(=O)C(CCCCN(O)C(C)=O)NC(=O)c1coc(n1)-c1ccccc1O)C(C)C(=O)NC1CCCCNC1=O Show InChI InChI=1S/C39H59N5O9/c1-4-5-6-7-8-9-10-11-12-23-34(27(2)35(47)41-30-20-15-17-24-40-36(30)48)53-39(50)31(21-16-18-25-44(51)28(3)45)42-37(49)32-26-52-38(43-32)29-19-13-14-22-33(29)46/h13-14,19,22,26-27,30-31,34,46,51H,4-12,15-18,20-21,23-25H2,1-3H3,(H,40,48)(H,41,47)(H,42,49) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human muscarinic M3 receptor expressed in insect Sf9 cells |
J Nat Prod 68: 1061-5 (2005)
Article DOI: 10.1021/np050091j
BindingDB Entry DOI: 10.7270/Q2R21155 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50259847

(CHEMBL480464 | cremastrine)Show SMILES CC[C@@H](C)[C@@H](O)C(=O)OC[C@H]1CCN2CCC[C@@H]12 |r| Show InChI InChI=1S/C14H25NO3/c1-3-10(2)13(16)14(17)18-9-11-6-8-15-7-4-5-12(11)15/h10-13,16H,3-9H2,1-2H3/t10-,11-,12+,13-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human cloned muscarinic M2 receptor expressed in insect Sf9 cells |
J Nat Prod 68: 572-3 (2005)
Article DOI: 10.1021/np049650x
BindingDB Entry DOI: 10.7270/Q2HH6JVJ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50241912

(CHEMBL505197 | Nocardimicin A)Show SMILES CCCCCCCCCC(OC(=O)C(CCCCN(O)C(C)=O)NC(=O)c1coc(n1)-c1ccccc1O)C(C)C(=O)NC1CCCCN(O)C1=O Show InChI InChI=1S/C37H55N5O10/c1-4-5-6-7-8-9-10-21-32(25(2)33(45)38-28-18-13-16-23-42(50)36(28)47)52-37(48)29(19-14-15-22-41(49)26(3)43)39-34(46)30-24-51-35(40-30)27-17-11-12-20-31(27)44/h11-12,17,20,24-25,28-29,32,44,49-50H,4-10,13-16,18-19,21-23H2,1-3H3,(H,38,45)(H,39,46) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human muscarinic M3 receptor expressed in insect Sf9 cells |
J Nat Prod 68: 1061-5 (2005)
Article DOI: 10.1021/np050091j
BindingDB Entry DOI: 10.7270/Q2R21155 |
More data for this
Ligand-Target Pair | |
Phosphatidylcholine:ceramide cholinephosphotransferase 1
(Homo sapiens (Human)) | BDBM50182488

(CHEMBL3819036)Show InChI InChI=1S/C21H26O5/c22-16-11-9-15(10-12-16)7-5-3-1-2-4-6-8-18(24)21-19(25)13-17(23)14-20(21)26/h9-14,22-23,25-26H,1-8H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of SMS1 (unknown origin) stably expressed in mouse ZS cells using C6-NBD-ceramide as substrate preincubated for 30 mins followed by substr... |
ACS Med Chem Lett 10: 1154-1158 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00171
BindingDB Entry DOI: 10.7270/Q2WS8XPS |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50241917

(CHEMBL508807 | Nocardimicin F)Show SMILES CCCCCCCCCCCCCCCC(OC(=O)C(CCCCN(O)C(C)=O)NC(=O)c1coc(n1)-c1ccccc1O)C(C)C(=O)NC1CCCCN(O)C1=O Show InChI InChI=1S/C43H67N5O10/c1-4-5-6-7-8-9-10-11-12-13-14-15-16-27-38(31(2)39(51)44-34-24-19-22-29-48(56)42(34)53)58-43(54)35(25-20-21-28-47(55)32(3)49)45-40(52)36-30-57-41(46-36)33-23-17-18-26-37(33)50/h17-18,23,26,30-31,34-35,38,50,55-56H,4-16,19-22,24-25,27-29H2,1-3H3,(H,44,51)(H,45,52) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human muscarinic M3 receptor expressed in insect Sf9 cells |
J Nat Prod 68: 1061-5 (2005)
Article DOI: 10.1021/np050091j
BindingDB Entry DOI: 10.7270/Q2R21155 |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM50182798
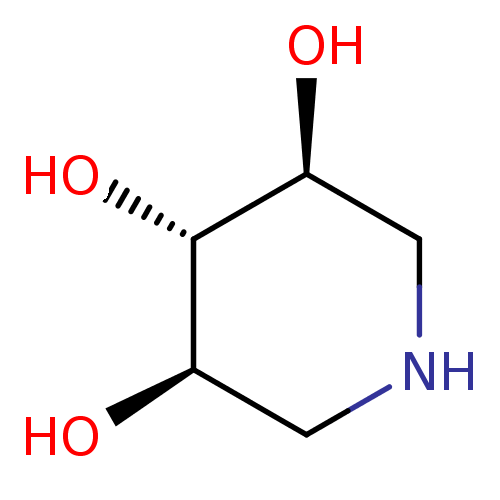
((3R,4r,5S)-piperidine-3,4,5-triol | 1,5-Dideoxy-1,...)Show InChI InChI=1S/C5H11NO3/c7-3-1-6-2-4(8)5(3)9/h3-9H,1-2H2/t3-,4+,5+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| n/a | n/a | 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of beta-glucosidase from almonds(sigma G 4511). |
Bioorg Med Chem Lett 6: 553-558 (1996)
Article DOI: 10.1016/0960-894X(96)00068-6
BindingDB Entry DOI: 10.7270/Q2ZS2WHG |
More data for this
Ligand-Target Pair | |
Cholesterol side-chain cleavage enzyme, mitochondrial
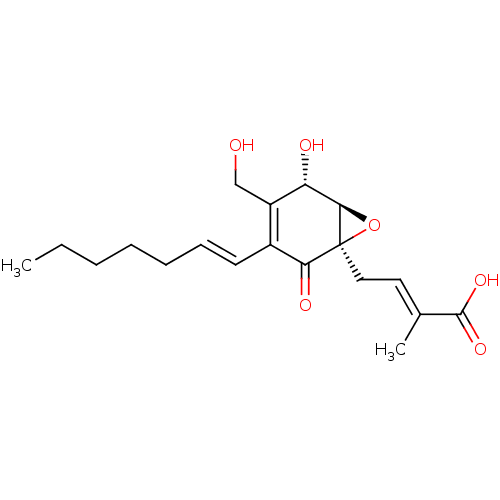
(Rattus norvegicus) | BDBM50483195
(Ambuic Acid)Show SMILES [H][C@]12O[C@@]1(C\C=C(/C)C(O)=O)C(=O)C(\C=C\CCCCC)=C(CO)[C@@H]2O |r,t:21| Show InChI InChI=1S/C19H26O6/c1-3-4-5-6-7-8-13-14(11-20)15(21)17-19(25-17,16(13)22)10-9-12(2)18(23)24/h7-9,15,17,20-21H,3-6,10-11H2,1-2H3,(H,23,24)/b8-7+,12-9+/t15-,17+,19-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyushu University
Curated by ChEMBL
| Assay Description
Inhibition of gelatinase production in Enterococcus faecalis OG1RF after 5 hrs |
Antimicrob Agents Chemother 53: 580-6 (2009)
Article DOI: 10.1128/AAC.00995-08
BindingDB Entry DOI: 10.7270/Q2XS5Z7J |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data