

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostaglandin E2 receptor EP4 subtype (Mus musculus (Mouse)) | BDBM50101856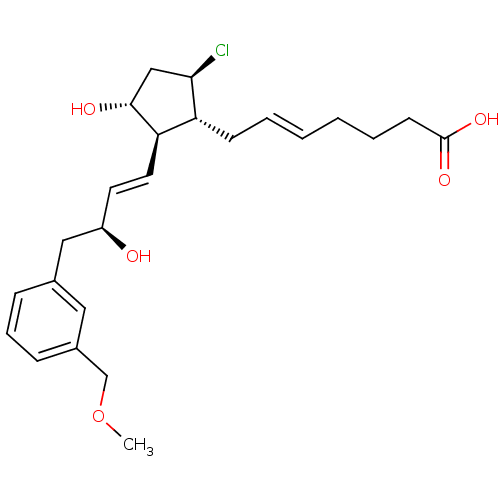 ((E)-7-{(1R,2R,3R,5R)-5-Chloro-3-hydroxy-2-[(E)-(S)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Affinity for mouse Prostanoid EP4 receptor expressed in CHO cells | Bioorg Med Chem Lett 11: 2033-5 (2001) BindingDB Entry DOI: 10.7270/Q24B30K2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Mus musculus (Mouse)) | BDBM50101861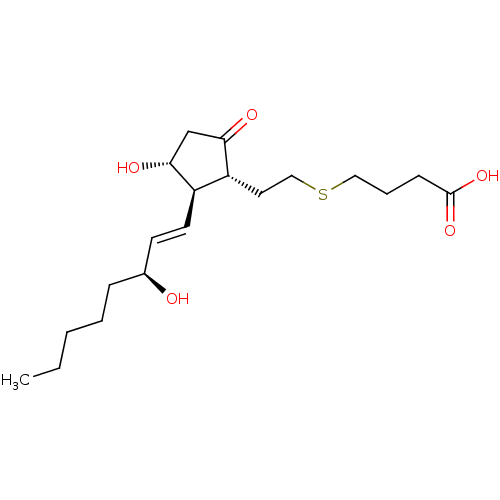 (4-{(S)-2-[(R)-3-Hydroxy-2-((1R,2R)-3-hydroxy-oct-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Affinity for mouse Prostanoid EP3 receptor expressed in CHO cells | Bioorg Med Chem Lett 11: 2033-5 (2001) BindingDB Entry DOI: 10.7270/Q24B30K2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Mus musculus (Mouse)) | BDBM50101858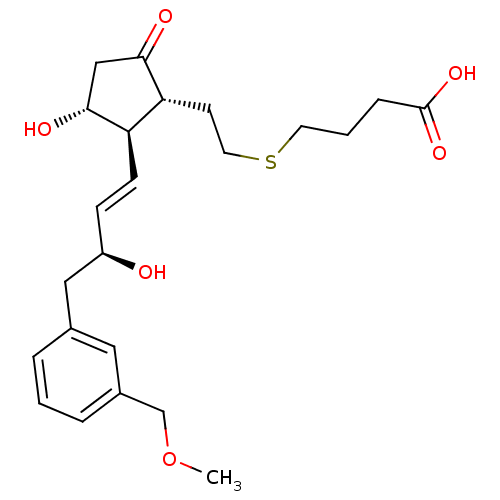 (4-(2-((1R,2R,3R)-3-hydroxy-2-((S,E)-3-hydroxy-4-(3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Effective concentration for increased intracellular c-AMP production by mouse Prostanoid EP4 receptor | Bioorg Med Chem Lett 11: 2033-5 (2001) BindingDB Entry DOI: 10.7270/Q24B30K2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Mus musculus (Mouse)) | BDBM50101836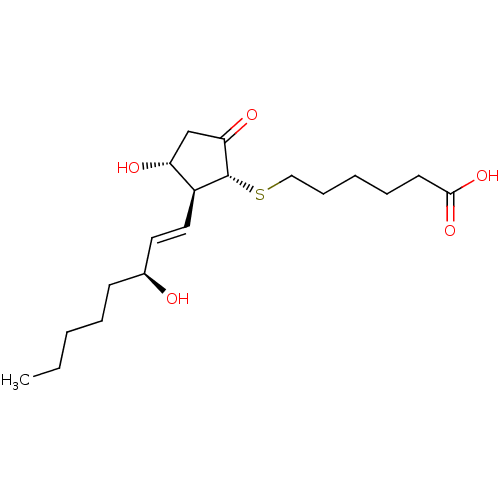 (6-[(S)-(R)-3-Hydroxy-2-((1S,2R)-3-hydroxy-oct-1-en...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Affinity for mouse Prostanoid EP4 receptor expressed in CHO cells | Bioorg Med Chem Lett 11: 2033-5 (2001) BindingDB Entry DOI: 10.7270/Q24B30K2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Mus musculus (Mouse)) | BDBM50101860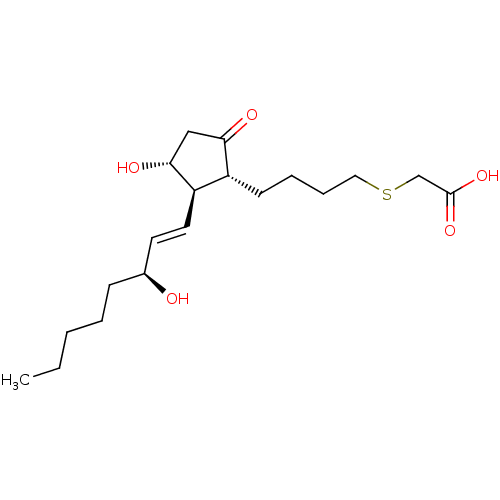 (CHEMBL64188 | {(S)-4-[(R)-3-Hydroxy-2-((1R,2R)-3-h...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Affinity for mouse Prostanoid EP3 receptor expressed in CHO cells | Bioorg Med Chem Lett 11: 2033-5 (2001) BindingDB Entry DOI: 10.7270/Q24B30K2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Mus musculus (Mouse)) | BDBM50101861 (4-{(S)-2-[(R)-3-Hydroxy-2-((1R,2R)-3-hydroxy-oct-1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Affinity for mouse Prostanoid EP3 receptor expressed in CHO cells | Bioorg Med Chem Lett 11: 2033-5 (2001) BindingDB Entry DOI: 10.7270/Q24B30K2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Mus musculus (Mouse)) | BDBM50101862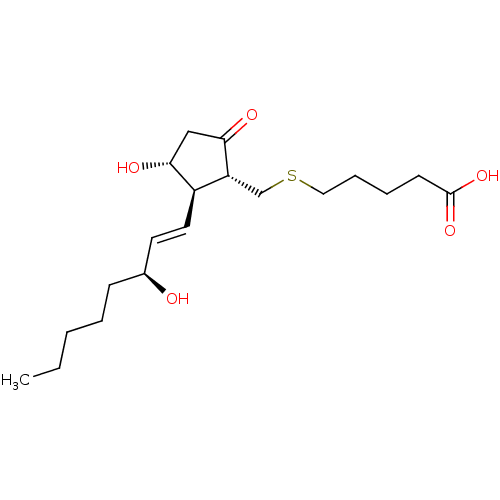 (5-[(S)-(R)-3-Hydroxy-2-((1R,2R)-3-hydroxy-oct-1-en...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Affinity for mouse Prostanoid EP3 receptor expressed in CHO cells | Bioorg Med Chem Lett 11: 2033-5 (2001) BindingDB Entry DOI: 10.7270/Q24B30K2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Mus musculus (Mouse)) | BDBM50101860 (CHEMBL64188 | {(S)-4-[(R)-3-Hydroxy-2-((1R,2R)-3-h...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Affinity for mouse Prostanoid EP4 receptor expressed in CHO cells | Bioorg Med Chem Lett 11: 2033-5 (2001) BindingDB Entry DOI: 10.7270/Q24B30K2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Mus musculus (Mouse)) | BDBM50101855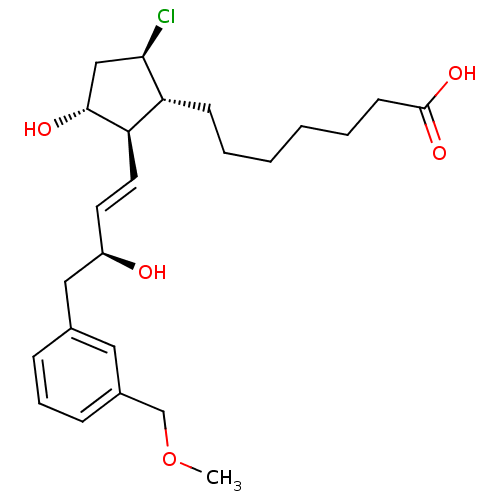 (7-{(1R,2R,3R,5R)-5-Chloro-3-hydroxy-2-[(E)-(S)-3-h...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Affinity for mouse Prostanoid EP4 receptor expressed in CHO cells | Bioorg Med Chem Lett 11: 2033-5 (2001) BindingDB Entry DOI: 10.7270/Q24B30K2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Mus musculus (Mouse)) | BDBM50101853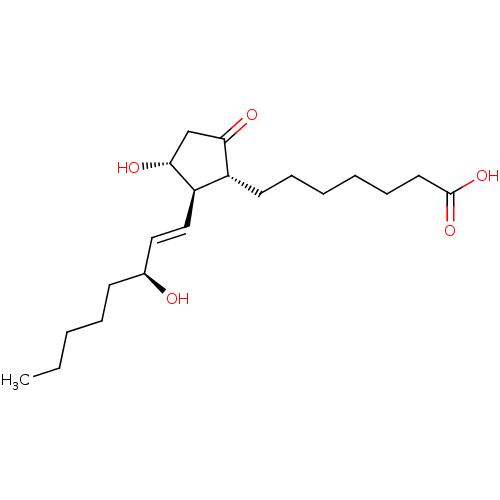 ((11alpha,13E,15S)-11,15-dihydroxy-9-oxoprost-13-en...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Affinity for mouse Prostanoid EP4 receptor expressed in CHO cells | Bioorg Med Chem Lett 11: 2033-5 (2001) BindingDB Entry DOI: 10.7270/Q24B30K2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Mus musculus (Mouse)) | BDBM50101853 ((11alpha,13E,15S)-11,15-dihydroxy-9-oxoprost-13-en...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Affinity for mouse Prostanoid EP4 receptor expressed in CHO cells | Bioorg Med Chem Lett 11: 2033-5 (2001) BindingDB Entry DOI: 10.7270/Q24B30K2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Mus musculus (Mouse)) | BDBM50101857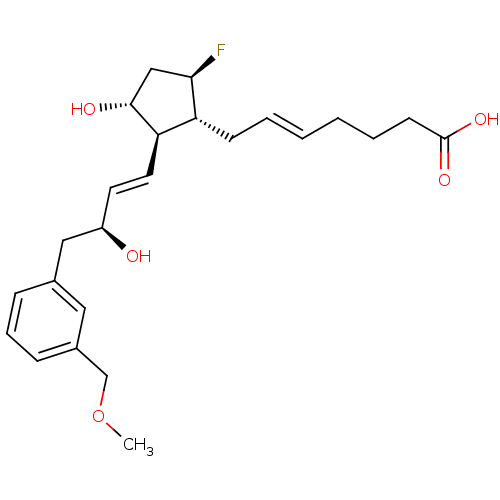 ((E)-7-{(1R,2R,3R,5R)-5-Fluoro-3-hydroxy-2-[(E)-(S)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Affinity for mouse Prostanoid EP4 receptor expressed in CHO cells | Bioorg Med Chem Lett 11: 2033-5 (2001) BindingDB Entry DOI: 10.7270/Q24B30K2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Mus musculus (Mouse)) | BDBM50101863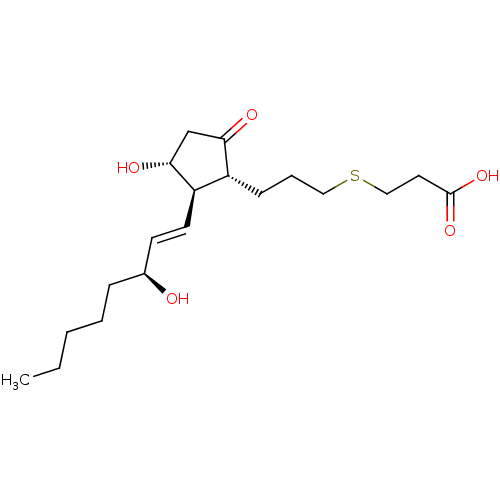 (3-{(S)-3-[(R)-3-Hydroxy-2-((1R,2R)-3-hydroxy-oct-1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Affinity for mouse Prostanoid EP3 receptor expressed in CHO cells | Bioorg Med Chem Lett 11: 2033-5 (2001) BindingDB Entry DOI: 10.7270/Q24B30K2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Mus musculus (Mouse)) | BDBM50101836 (6-[(S)-(R)-3-Hydroxy-2-((1S,2R)-3-hydroxy-oct-1-en...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Affinity for mouse Prostanoid EP3 receptor expressed in CHO cells | Bioorg Med Chem Lett 11: 2033-5 (2001) BindingDB Entry DOI: 10.7270/Q24B30K2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Mus musculus (Mouse)) | BDBM50101864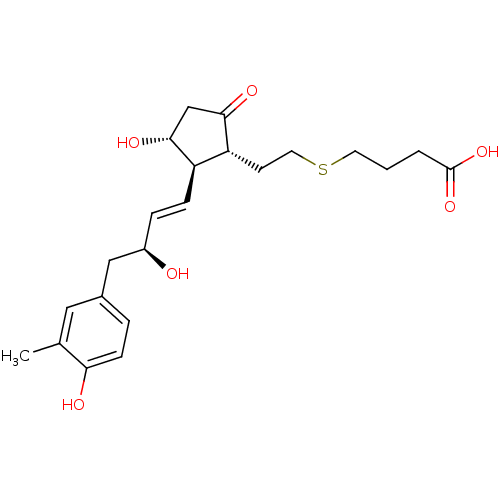 (4-(2-{(1R,2R,3R)-3-Hydroxy-2-[(E)-(S)-3-hydroxy-4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Affinity for mouse Prostanoid EP4 receptor expressed in CHO cells | Bioorg Med Chem Lett 11: 2033-5 (2001) BindingDB Entry DOI: 10.7270/Q24B30K2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Mus musculus (Mouse)) | BDBM50101853 ((11alpha,13E,15S)-11,15-dihydroxy-9-oxoprost-13-en...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Affinity for mouse Prostanoid EP3 receptor expressed in CHO cells | Bioorg Med Chem Lett 11: 2033-5 (2001) BindingDB Entry DOI: 10.7270/Q24B30K2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Mus musculus (Mouse)) | BDBM50101853 ((11alpha,13E,15S)-11,15-dihydroxy-9-oxoprost-13-en...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Affinity for mouse Prostanoid EP4 receptor expressed in CHO cells | Bioorg Med Chem Lett 11: 2033-5 (2001) BindingDB Entry DOI: 10.7270/Q24B30K2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Mus musculus (Mouse)) | BDBM50101865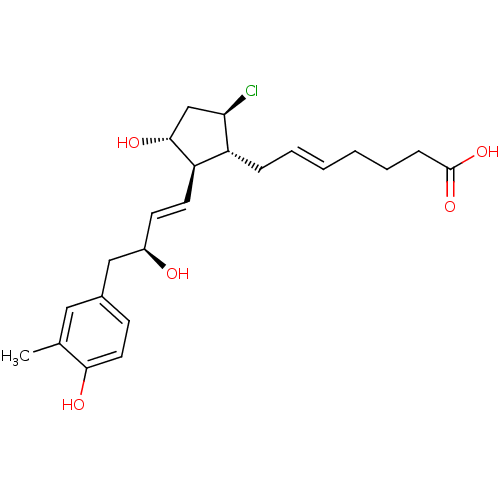 ((E)-7-{(1R,2R,3R,5R)-5-Chloro-3-hydroxy-2-[(E)-(S)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Affinity for mouse Prostanoid EP4 receptor expressed in CHO cells | Bioorg Med Chem Lett 11: 2033-5 (2001) BindingDB Entry DOI: 10.7270/Q24B30K2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Mus musculus (Mouse)) | BDBM50101862 (5-[(S)-(R)-3-Hydroxy-2-((1R,2R)-3-hydroxy-oct-1-en...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Affinity for mouse Prostanoid EP2 receptor expressed in CHO cells | Bioorg Med Chem Lett 11: 2033-5 (2001) BindingDB Entry DOI: 10.7270/Q24B30K2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM149937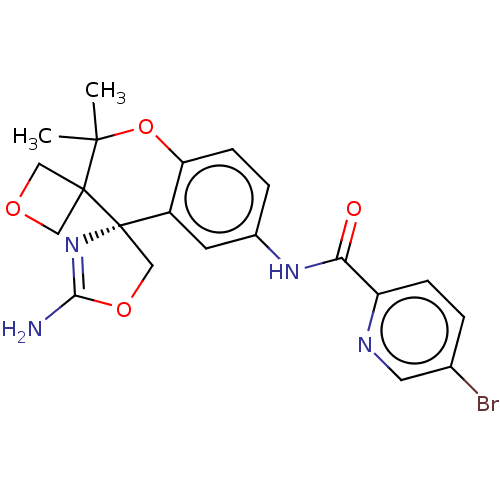 (US8975415, 219 | US9242973, 219) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 10.2 | n/a | 44.4 | n/a | n/a | n/a | n/a | n/a | n/a |
CoMentis, Inc. US Patent | Assay Description Potency of test compounds were determined by measurement of their inhibition of BACE1 activity toward a fluorescent substrate. Experiments were perfo... | US Patent US8975415 (2015) BindingDB Entry DOI: 10.7270/Q2SB44GN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM149936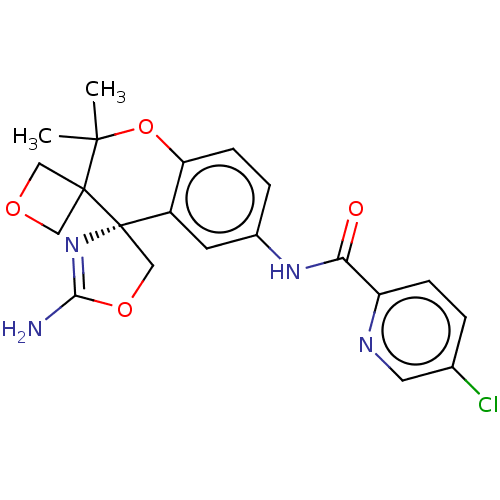 (US8975415, 218 | US9242973, 218) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 10.3 | n/a | 44.7 | n/a | n/a | n/a | n/a | n/a | n/a |
CoMentis, Inc. US Patent | Assay Description Potency of test compounds were determined by measurement of their inhibition of BACE1 activity toward a fluorescent substrate. Experiments were perfo... | US Patent US8975415 (2015) BindingDB Entry DOI: 10.7270/Q2SB44GN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Mus musculus (Mouse)) | BDBM50101863 (3-{(S)-3-[(R)-3-Hydroxy-2-((1R,2R)-3-hydroxy-oct-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Effective concentration for increased intracellular c-AMP production by mouse Prostanoid EP4 receptor | Bioorg Med Chem Lett 11: 2033-5 (2001) BindingDB Entry DOI: 10.7270/Q24B30K2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP2 subtype (Mus musculus (Mouse)) | BDBM50101856 ((E)-7-{(1R,2R,3R,5R)-5-Chloro-3-hydroxy-2-[(E)-(S)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Affinity for mouse Prostanoid EP2 receptor expressed in CHO cells | Bioorg Med Chem Lett 11: 2033-5 (2001) BindingDB Entry DOI: 10.7270/Q24B30K2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Mus musculus (Mouse)) | BDBM50101853 ((11alpha,13E,15S)-11,15-dihydroxy-9-oxoprost-13-en...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Affinity for mouse Prostanoid EP1 receptor expressed in CHO cells | Bioorg Med Chem Lett 11: 2033-5 (2001) BindingDB Entry DOI: 10.7270/Q24B30K2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Mus musculus (Mouse)) | BDBM50101853 ((11alpha,13E,15S)-11,15-dihydroxy-9-oxoprost-13-en...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Affinity for mouse Prostanoid EP4 receptor expressed in CHO cells | Bioorg Med Chem Lett 11: 2033-5 (2001) BindingDB Entry DOI: 10.7270/Q24B30K2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Mus musculus (Mouse)) | BDBM50101863 (3-{(S)-3-[(R)-3-Hydroxy-2-((1R,2R)-3-hydroxy-oct-1...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Affinity for mouse Prostanoid EP1 receptor expressed in CHO cells | Bioorg Med Chem Lett 11: 2033-5 (2001) BindingDB Entry DOI: 10.7270/Q24B30K2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Mus musculus (Mouse)) | BDBM50101859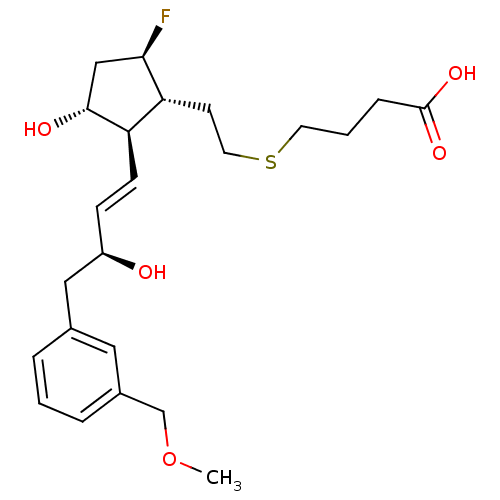 (4-(2-{(1R,2R,3R,5R)-5-Fluoro-3-hydroxy-2-[(E)-(S)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Affinity for mouse Prostanoid EP4 receptor expressed in CHO cells | Bioorg Med Chem Lett 11: 2033-5 (2001) BindingDB Entry DOI: 10.7270/Q24B30K2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM149939 (US8975415, 221 | US9242973, 221) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 37.8 | n/a | 81.8 | n/a | n/a | n/a | n/a | n/a | n/a |
CoMentis, Inc. US Patent | Assay Description Potency of test compounds were determined by measurement of their inhibition of BACE1 activity toward a fluorescent substrate. Experiments were perfo... | US Patent US8975415 (2015) BindingDB Entry DOI: 10.7270/Q2SB44GN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM149749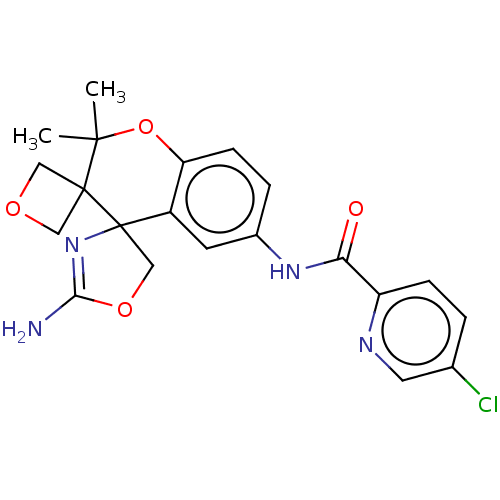 (US8975415, 27 | US9242973, 27) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 38.2 | n/a | 82.2 | n/a | n/a | n/a | n/a | n/a | n/a |
CoMentis, Inc. US Patent | Assay Description Potency of test compounds were determined by measurement of their inhibition of BACE1 activity toward a fluorescent substrate. Experiments were perfo... | US Patent US8975415 (2015) BindingDB Entry DOI: 10.7270/Q2SB44GN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP2 subtype (Mus musculus (Mouse)) | BDBM50101853 ((11alpha,13E,15S)-11,15-dihydroxy-9-oxoprost-13-en...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Effective concentration for increased intracellular c-AMP production by mouse Prostanoid EP4 receptor | Bioorg Med Chem Lett 11: 2033-5 (2001) BindingDB Entry DOI: 10.7270/Q24B30K2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP2 subtype (Mus musculus (Mouse)) | BDBM50101853 ((11alpha,13E,15S)-11,15-dihydroxy-9-oxoprost-13-en...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Affinity for mouse Prostanoid EP2 receptor expressed in CHO cells | Bioorg Med Chem Lett 11: 2033-5 (2001) BindingDB Entry DOI: 10.7270/Q24B30K2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM149938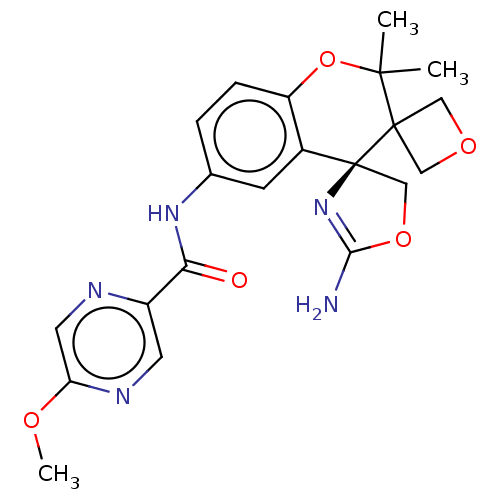 (US8975415, 220 | US9242973, 220) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 42.9 | n/a | 76.5 | n/a | n/a | n/a | n/a | n/a | n/a |
CoMentis, Inc. US Patent | Assay Description Potency of test compounds were determined by measurement of their inhibition of BACE1 activity toward a fluorescent substrate. Experiments were perfo... | US Patent US8975415 (2015) BindingDB Entry DOI: 10.7270/Q2SB44GN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP2 subtype (Mus musculus (Mouse)) | BDBM50101860 (CHEMBL64188 | {(S)-4-[(R)-3-Hydroxy-2-((1R,2R)-3-h...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Affinity for mouse Prostanoid EP2 receptor expressed in CHO cells | Bioorg Med Chem Lett 11: 2033-5 (2001) BindingDB Entry DOI: 10.7270/Q24B30K2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM149752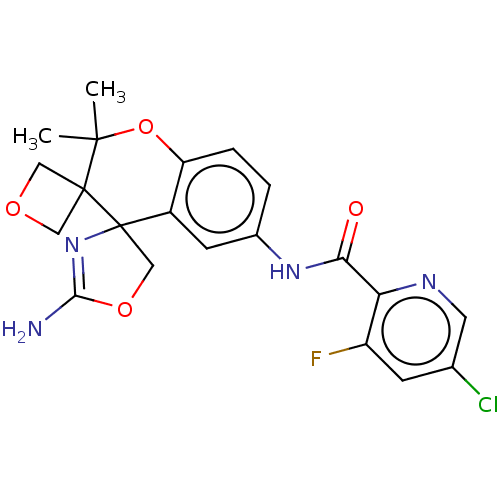 (US8975415, 30 | US9242973, 30) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 51.5 | n/a | 97.5 | n/a | n/a | n/a | n/a | n/a | n/a |
CoMentis, Inc. US Patent | Assay Description Potency of test compounds were determined by measurement of their inhibition of BACE1 activity toward a fluorescent substrate. Experiments were perfo... | US Patent US8975415 (2015) BindingDB Entry DOI: 10.7270/Q2SB44GN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Mus musculus (Mouse)) | BDBM50101861 (4-{(S)-2-[(R)-3-Hydroxy-2-((1R,2R)-3-hydroxy-oct-1...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Affinity for mouse Prostanoid EP1 receptor expressed in CHO cells | Bioorg Med Chem Lett 11: 2033-5 (2001) BindingDB Entry DOI: 10.7270/Q24B30K2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM149942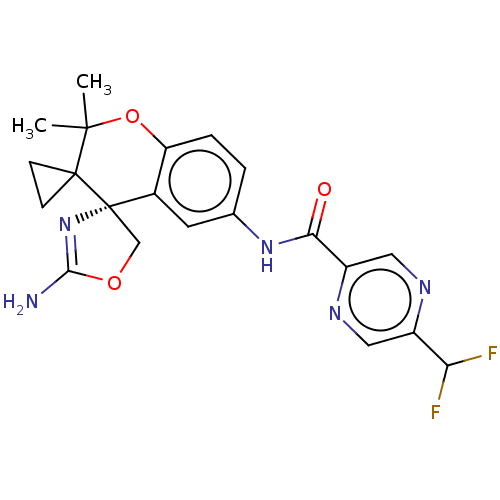 (US8975415, 224 | US9242973, 224) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 52.7 | n/a | 91.4 | n/a | n/a | n/a | n/a | n/a | n/a |
CoMentis, Inc. US Patent | Assay Description Potency of test compounds were determined by measurement of their inhibition of BACE1 activity toward a fluorescent substrate. Experiments were perfo... | US Patent US8975415 (2015) BindingDB Entry DOI: 10.7270/Q2SB44GN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Mus musculus (Mouse)) | BDBM50101858 (4-(2-((1R,2R,3R)-3-hydroxy-2-((S,E)-3-hydroxy-4-(3...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Affinity for mouse Prostanoid EP3 receptor expressed in CHO cells | Bioorg Med Chem Lett 11: 2033-5 (2001) BindingDB Entry DOI: 10.7270/Q24B30K2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP2 subtype (Mus musculus (Mouse)) | BDBM50101857 ((E)-7-{(1R,2R,3R,5R)-5-Fluoro-3-hydroxy-2-[(E)-(S)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Affinity for mouse Prostanoid EP2 receptor expressed in CHO cells | Bioorg Med Chem Lett 11: 2033-5 (2001) BindingDB Entry DOI: 10.7270/Q24B30K2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM149941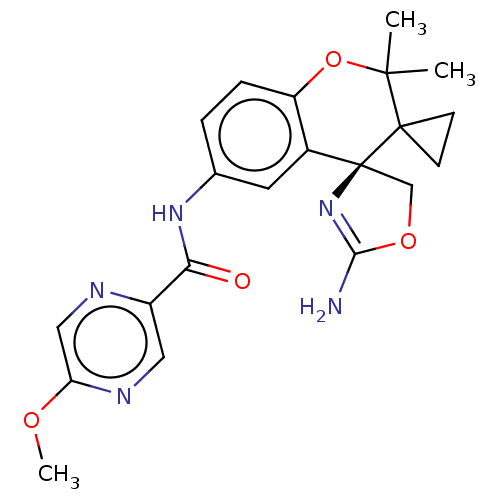 (US8975415, 223 | US9242973, 223) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 61.2 | n/a | 99.1 | n/a | n/a | n/a | n/a | n/a | n/a |
CoMentis, Inc. US Patent | Assay Description Potency of test compounds were determined by measurement of their inhibition of BACE1 activity toward a fluorescent substrate. Experiments were perfo... | US Patent US8975415 (2015) BindingDB Entry DOI: 10.7270/Q2SB44GN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM149914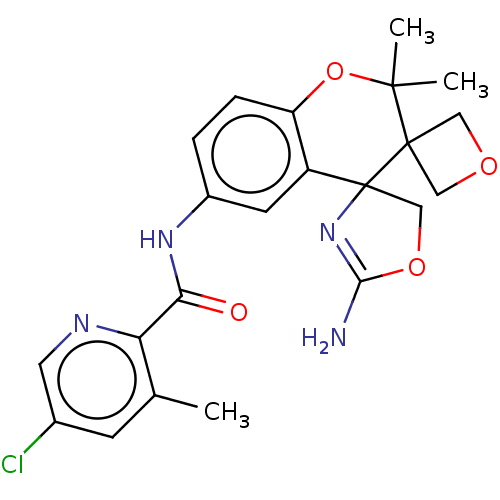 (US8975415, RP195 | US9242973, 195) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 64.5 | n/a | 109 | n/a | n/a | n/a | n/a | n/a | n/a |
CoMentis, Inc. US Patent | Assay Description Potency of test compounds were determined by measurement of their inhibition of BACE1 activity toward a fluorescent substrate. Experiments were perfo... | US Patent US8975415 (2015) BindingDB Entry DOI: 10.7270/Q2SB44GN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM149920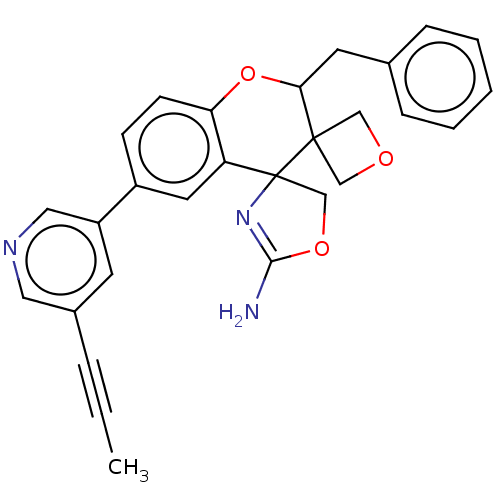 (US8975415, RP201 | US8975415, RP203 | US9242973, R...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 67.6 | n/a | 109 | n/a | n/a | n/a | n/a | n/a | n/a |
CoMentis, Inc. US Patent | Assay Description Potency of test compounds were determined by measurement of their inhibition of BACE1 activity toward a fluorescent substrate. Experiments were perfo... | US Patent US8975415 (2015) BindingDB Entry DOI: 10.7270/Q2SB44GN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM149920 (US8975415, RP201 | US8975415, RP203 | US9242973, R...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 71.1 | n/a | 113 | n/a | n/a | n/a | n/a | n/a | n/a |
CoMentis, Inc. US Patent | Assay Description Potency of test compounds were determined by measurement of their inhibition of BACE1 activity toward a fluorescent substrate. Experiments were perfo... | US Patent US8975415 (2015) BindingDB Entry DOI: 10.7270/Q2SB44GN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM149916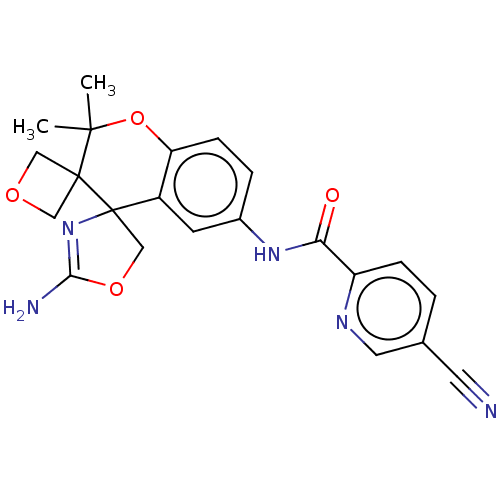 (US8975415, RP197 | US9242973, 197) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 72 | n/a | 118 | n/a | n/a | n/a | n/a | n/a | n/a |
CoMentis, Inc. US Patent | Assay Description Potency of test compounds were determined by measurement of their inhibition of BACE1 activity toward a fluorescent substrate. Experiments were perfo... | US Patent US8975415 (2015) BindingDB Entry DOI: 10.7270/Q2SB44GN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP2 subtype (Mus musculus (Mouse)) | BDBM50101861 (4-{(S)-2-[(R)-3-Hydroxy-2-((1R,2R)-3-hydroxy-oct-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Affinity for mouse Prostanoid EP2 receptor expressed in CHO cells | Bioorg Med Chem Lett 11: 2033-5 (2001) BindingDB Entry DOI: 10.7270/Q24B30K2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM149943 (US8975415, 227 | US9242973, 227) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 79.2 | n/a | 107 | n/a | n/a | n/a | n/a | n/a | n/a |
CoMentis, Inc. US Patent | Assay Description Potency of test compounds were determined by measurement of their inhibition of BACE1 activity toward a fluorescent substrate. Experiments were perfo... | US Patent US8975415 (2015) BindingDB Entry DOI: 10.7270/Q2SB44GN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM149924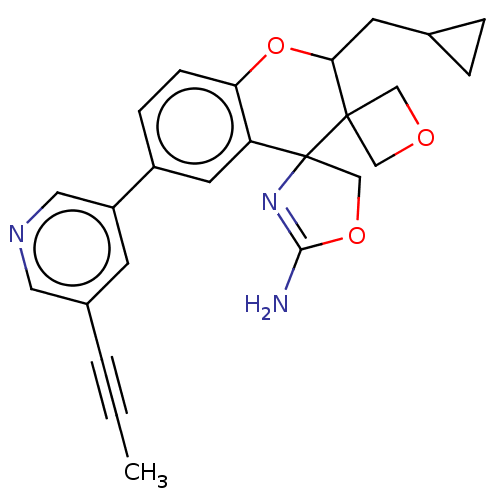 (US8975415, RP205 | US9242973, RP205) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 82.5 | n/a | 126 | n/a | n/a | n/a | n/a | n/a | n/a |
CoMentis, Inc. US Patent | Assay Description Potency of test compounds were determined by measurement of their inhibition of BACE1 activity toward a fluorescent substrate. Experiments were perfo... | US Patent US8975415 (2015) BindingDB Entry DOI: 10.7270/Q2SB44GN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM149909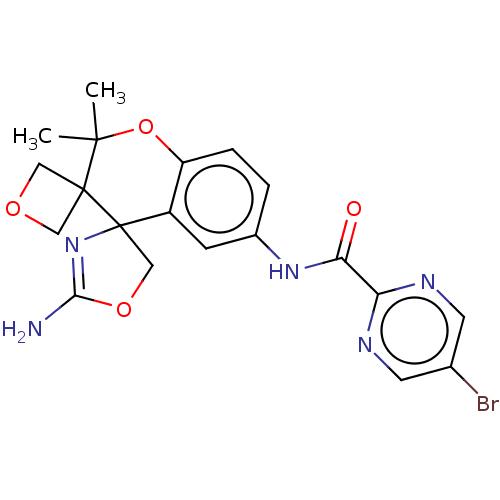 (US8975415, RP190 | US9242973, 190) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 83.5 | n/a | 125 | n/a | n/a | n/a | n/a | n/a | n/a |
CoMentis, Inc. US Patent | Assay Description Potency of test compounds were determined by measurement of their inhibition of BACE1 activity toward a fluorescent substrate. Experiments were perfo... | US Patent US8975415 (2015) BindingDB Entry DOI: 10.7270/Q2SB44GN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM149811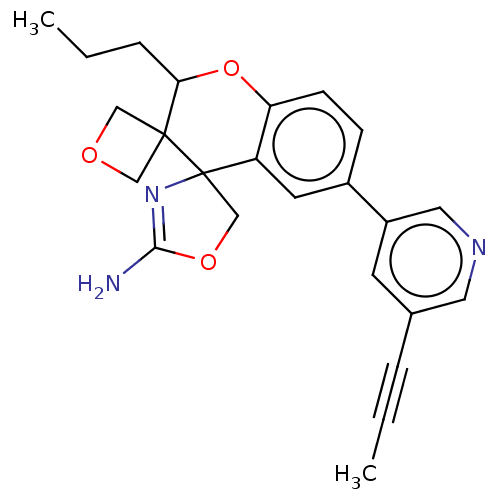 (US8975415, RP92 | US9242973, RP92) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 87.9 | n/a | 124 | n/a | n/a | n/a | n/a | n/a | n/a |
CoMentis, Inc. US Patent | Assay Description Potency of test compounds were determined by measurement of their inhibition of BACE1 activity toward a fluorescent substrate. Experiments were perfo... | US Patent US8975415 (2015) BindingDB Entry DOI: 10.7270/Q2SB44GN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM149829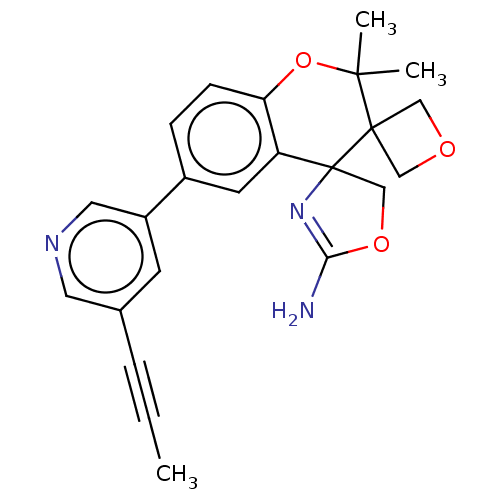 (US8975415, RP110 | US9242973, RP110) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 90.4 | n/a | 137 | n/a | n/a | n/a | n/a | n/a | n/a |
CoMentis, Inc. US Patent | Assay Description Potency of test compounds were determined by measurement of their inhibition of BACE1 activity toward a fluorescent substrate. Experiments were perfo... | US Patent US8975415 (2015) BindingDB Entry DOI: 10.7270/Q2SB44GN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP2 subtype (Mus musculus (Mouse)) | BDBM50101862 (5-[(S)-(R)-3-Hydroxy-2-((1R,2R)-3-hydroxy-oct-1-en...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 91 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Affinity for mouse Prostanoid EP2 receptor expressed in CHO cells | Bioorg Med Chem Lett 11: 2033-5 (2001) BindingDB Entry DOI: 10.7270/Q24B30K2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1238 total ) | Next | Last >> |