

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prothrombin (Homo sapiens (Human)) | BDBM50069294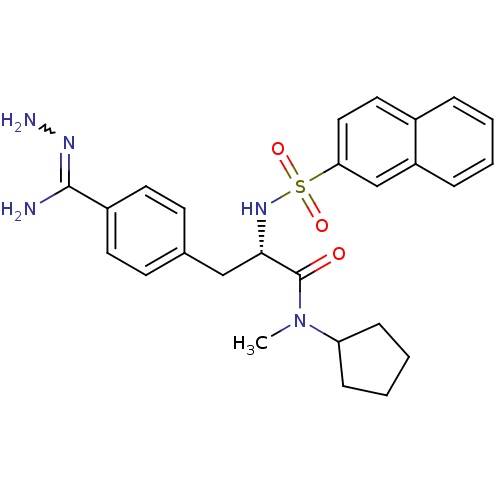 ((S)-3-[4-(carbohydrazonamidol)-phenyl]-N-cyclopent...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Compound was tested for inhibitory activity against human thrombin | Bioorg Med Chem Lett 8: 631-4 (1999) BindingDB Entry DOI: 10.7270/Q2CR5SHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50069294 ((S)-3-[4-(carbohydrazonamidol)-phenyl]-N-cyclopent...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human thrombin | Bioorg Med Chem Lett 8: 2563-8 (1999) BindingDB Entry DOI: 10.7270/Q20Z72FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50069294 ((S)-3-[4-(carbohydrazonamidol)-phenyl]-N-cyclopent...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Compound was tested for inhibitory activity against bovine thrombin | Bioorg Med Chem Lett 8: 631-4 (1999) BindingDB Entry DOI: 10.7270/Q2CR5SHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50071729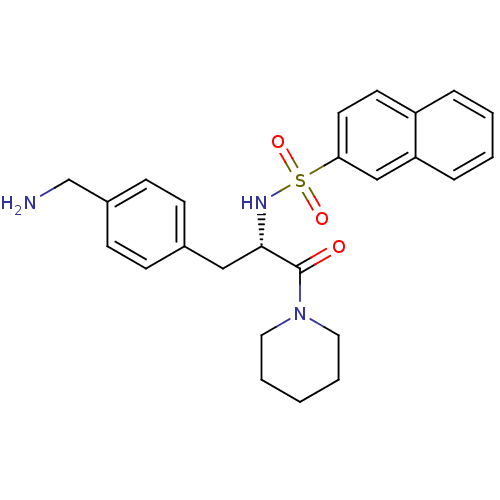 (CHEMBL313826 | Naphthalene-2-sulfonic acid [(S)-1-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human thrombin | Bioorg Med Chem Lett 8: 2563-8 (1999) BindingDB Entry DOI: 10.7270/Q20Z72FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50071723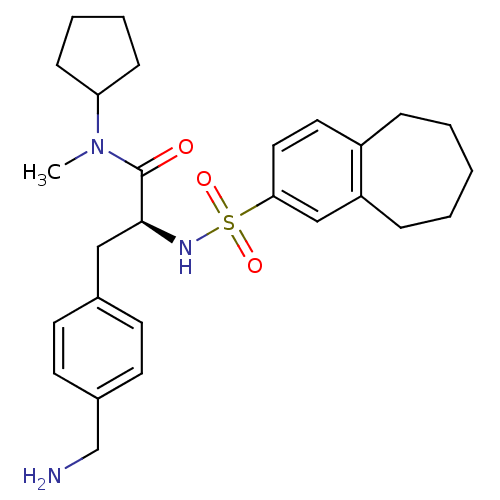 ((S)-3-(4-Aminomethyl-phenyl)-N-cyclopentyl-N-methy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human thrombin | Bioorg Med Chem Lett 8: 2563-8 (1999) BindingDB Entry DOI: 10.7270/Q20Z72FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50069292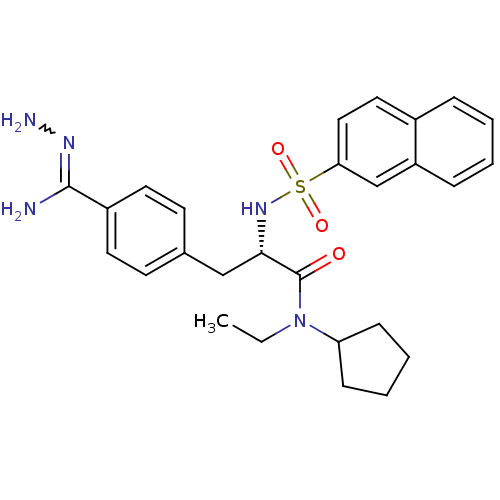 (CHEMBL156082 | N-ethyl-N-cyclopentyl-3-(4-hydrazon...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Compound was tested for inhibitory activity against bovine thrombin | Bioorg Med Chem Lett 8: 631-4 (1999) BindingDB Entry DOI: 10.7270/Q2CR5SHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50071725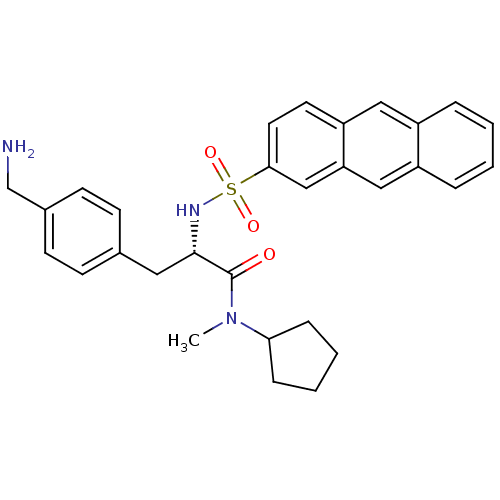 ((S)-3-(4-Aminomethyl-phenyl)-2-(anthracene-2-sulfo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human thrombin | Bioorg Med Chem Lett 8: 2563-8 (1999) BindingDB Entry DOI: 10.7270/Q20Z72FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50071722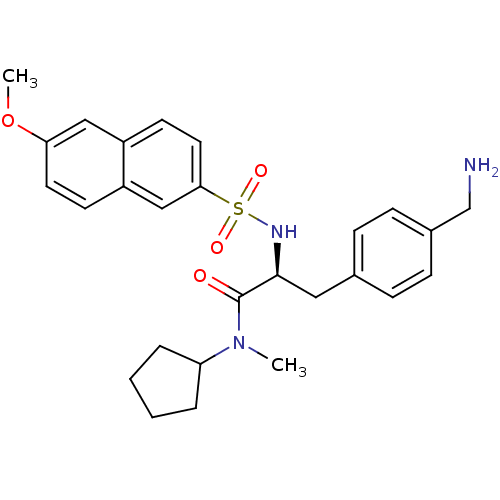 ((S)-3-(4-Aminomethyl-phenyl)-N-cyclopentyl-2-(6-me...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human thrombin | Bioorg Med Chem Lett 8: 2563-8 (1999) BindingDB Entry DOI: 10.7270/Q20Z72FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50070783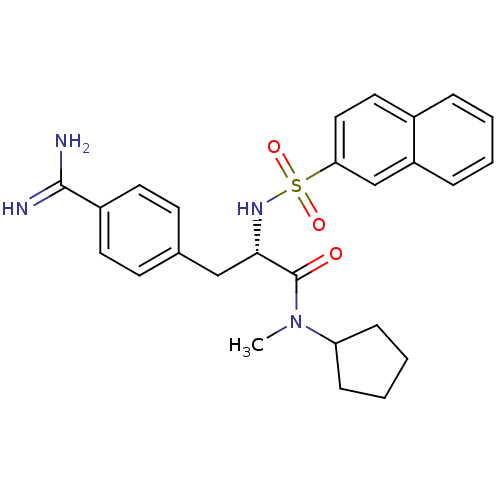 ((S)-3-(4-Carbamimidoyl-phenyl)-N-cyclopentyl-N-met...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human thrombin | Bioorg Med Chem Lett 8: 2563-8 (1999) BindingDB Entry DOI: 10.7270/Q20Z72FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50069055 ((S)-3-(4-Aminomethyl-phenyl)-N-cyclopentyl-N-methy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human thrombin | Bioorg Med Chem Lett 8: 2563-8 (1999) BindingDB Entry DOI: 10.7270/Q20Z72FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50071726 ((S)-3-(4-Aminomethyl-phenyl)-N-cyclopentyl-N-methy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human thrombin | Bioorg Med Chem Lett 8: 2563-8 (1999) BindingDB Entry DOI: 10.7270/Q20Z72FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50071724 ((S)-3-(4-Aminomethyl-phenyl)-N-cyclopentyl-2-(5-di...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human thrombin | Bioorg Med Chem Lett 8: 2563-8 (1999) BindingDB Entry DOI: 10.7270/Q20Z72FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50069053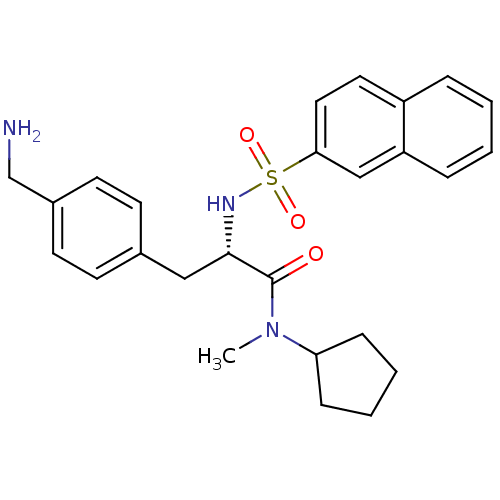 ((S)-3-(4-Aminomethyl-phenyl)-N-cyclopentyl-N-methy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human thrombin | Bioorg Med Chem Lett 8: 2563-8 (1999) BindingDB Entry DOI: 10.7270/Q20Z72FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50071721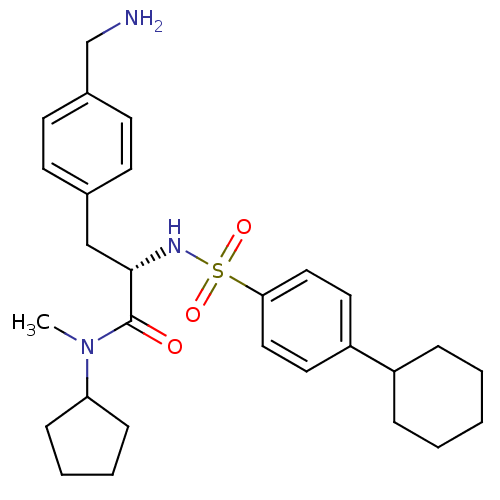 ((S)-3-(4-Aminomethyl-phenyl)-2-(4-cyclohexyl-benze...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human thrombin | Bioorg Med Chem Lett 8: 2563-8 (1999) BindingDB Entry DOI: 10.7270/Q20Z72FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50069293 (CHEMBL440188 | N-methyl-N-n-butyl-3-(4-hydrazonofo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Compound was tested for inhibitory activity against bovine thrombin | Bioorg Med Chem Lett 8: 631-4 (1999) BindingDB Entry DOI: 10.7270/Q2CR5SHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50071728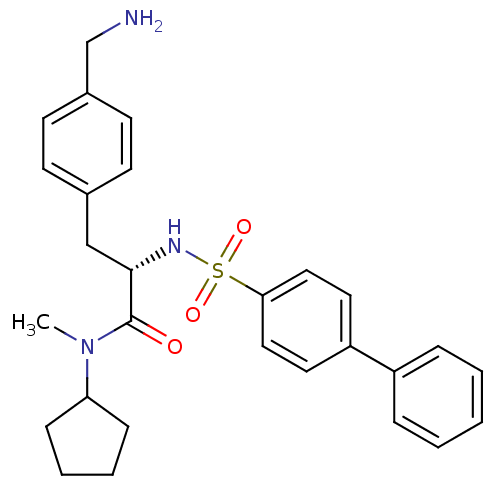 ((S)-3-(4-Aminomethyl-phenyl)-2-(biphenyl-4-sulfony...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human thrombin | Bioorg Med Chem Lett 8: 2563-8 (1999) BindingDB Entry DOI: 10.7270/Q20Z72FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50069296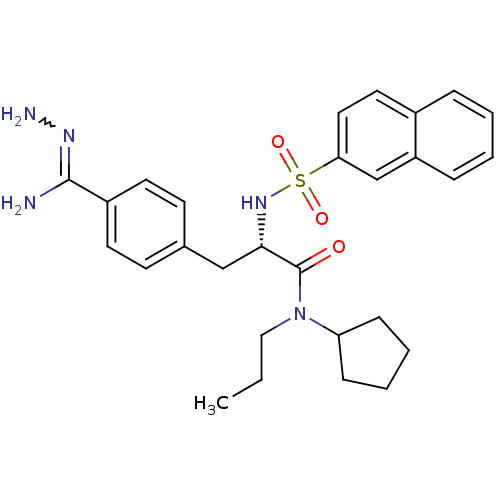 (CHEMBL347371 | N-(n-propyl)-N-cyclopentyl-3-(4-hyd...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 93 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Compound was tested for inhibitory activity against bovine thrombin | Bioorg Med Chem Lett 8: 631-4 (1999) BindingDB Entry DOI: 10.7270/Q2CR5SHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50071727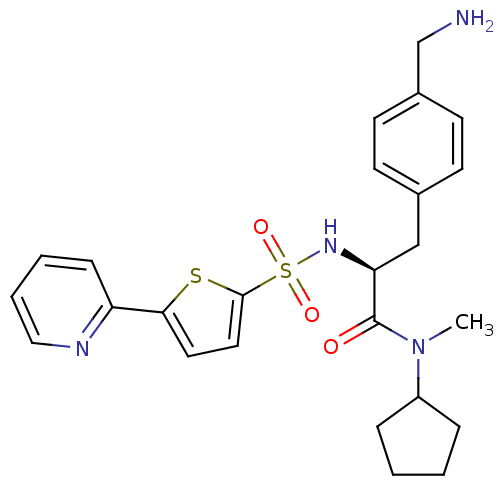 ((S)-3-(4-Aminomethyl-phenyl)-N-cyclopentyl-N-methy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 107 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human thrombin | Bioorg Med Chem Lett 8: 2563-8 (1999) BindingDB Entry DOI: 10.7270/Q20Z72FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50069298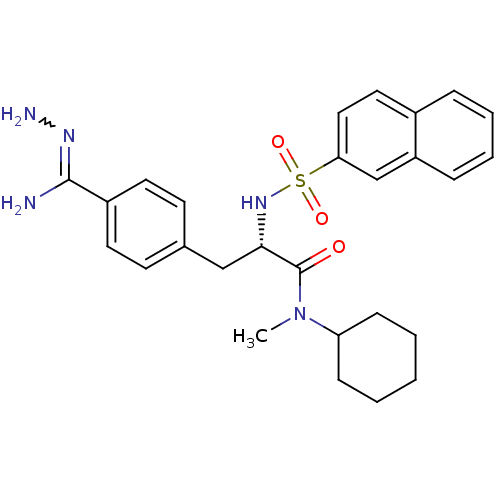 (CHEMBL434678 | N-methyl-N-cyclohexyl-3-(4-hydrazon...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 152 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Compound was tested for inhibitory activity against bovine thrombin | Bioorg Med Chem Lett 8: 631-4 (1999) BindingDB Entry DOI: 10.7270/Q2CR5SHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50069299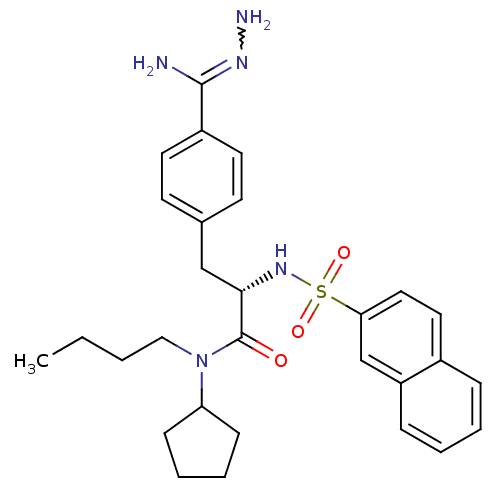 (CHEMBL155317 | N-(n-butyl)-N-cyclopentyl-3-(4-hydr...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 231 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Compound was tested for inhibitory activity against bovine thrombin | Bioorg Med Chem Lett 8: 631-4 (1999) BindingDB Entry DOI: 10.7270/Q2CR5SHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50069295 (CHEMBL348175 | N-methyl-N-cyclopropyl-3-(4-hydrazo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 247 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Compound was tested for inhibitory activity against bovine thrombin | Bioorg Med Chem Lett 8: 631-4 (1999) BindingDB Entry DOI: 10.7270/Q2CR5SHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50069300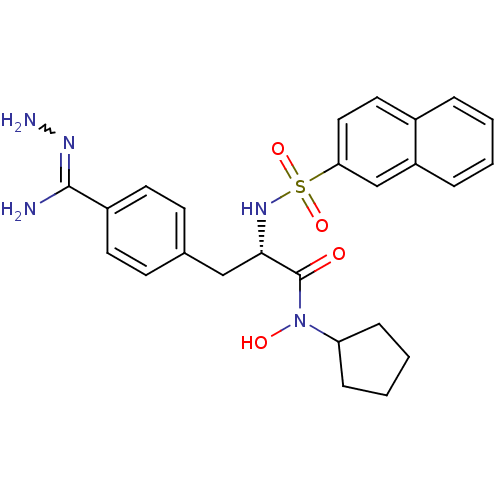 (CHEMBL350901 | N-hydroxy-N-cyclopentyl-3-(4-hydraz...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 367 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Compound was tested for inhibitory activity against bovine thrombin | Bioorg Med Chem Lett 8: 631-4 (1999) BindingDB Entry DOI: 10.7270/Q2CR5SHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50069294 ((S)-3-[4-(carbohydrazonamidol)-phenyl]-N-cyclopent...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description The compound was tested for inhibitory activity against Coagulation factor X | Bioorg Med Chem Lett 8: 631-4 (1999) BindingDB Entry DOI: 10.7270/Q2CR5SHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50069297 (1-[3-[4-amino(amineimino)methylphenyl]-2-(2-naphth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Compound was tested for inhibitory activity against bovine thrombin | Bioorg Med Chem Lett 8: 631-4 (1999) BindingDB Entry DOI: 10.7270/Q2CR5SHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-methyl-5'-thioadenosine phosphorylase (Mus musculus) | BDBM50368297 (CHEMBL62847) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description The inhibitory constant(Ki) for MTA phosphorylase activity was determined by using a mouse liver enzyme preparation | J Med Chem 34: 2600-6 (1991) BindingDB Entry DOI: 10.7270/Q2765FX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-methyl-5'-thioadenosine phosphorylase (Mus musculus) | BDBM50368299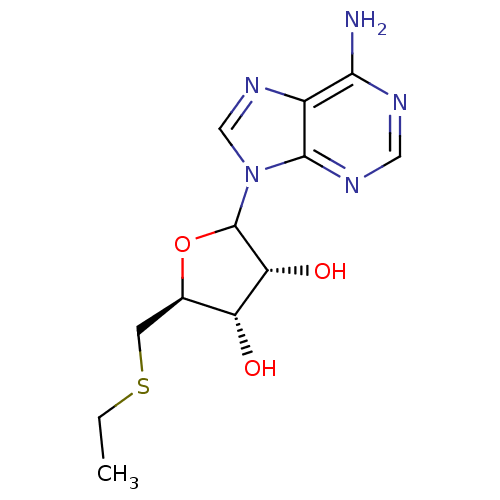 (CHEMBL608305) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description The inhibitory constant(Ki) for MTA phosphorylase activity was determined by using a mouse liver enzyme preparation | J Med Chem 34: 2600-6 (1991) BindingDB Entry DOI: 10.7270/Q2765FX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-methyl-5'-thioadenosine phosphorylase (Mus musculus) | BDBM50368302 (CHEMBL609198) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description The inhibitory constant(Ki) for MTA phosphorylase activity was determined by using a mouse liver enzyme preparation | J Med Chem 34: 2600-6 (1991) BindingDB Entry DOI: 10.7270/Q2765FX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypsin (Homo sapiens (Human)) | BDBM50069294 ((S)-3-[4-(carbohydrazonamidol)-phenyl]-N-cyclopent...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Compound was tested for inhibitory activity against bovine trypsin | Bioorg Med Chem Lett 8: 631-4 (1999) BindingDB Entry DOI: 10.7270/Q2CR5SHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50071725 ((S)-3-(4-Aminomethyl-phenyl)-2-(anthracene-2-sulfo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Binding affinity of the compound towards bovine trypsin was evaluated in vitro | Bioorg Med Chem Lett 8: 2563-8 (1999) BindingDB Entry DOI: 10.7270/Q20Z72FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-methyl-5'-thioadenosine phosphorylase (Mus musculus) | BDBM50368298 (CHEMBL611249) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description The inhibitory constant(Ki) for MTA phosphorylase activity was determined by using a mouse liver enzyme preparation | J Med Chem 34: 2600-6 (1991) BindingDB Entry DOI: 10.7270/Q2765FX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50071721 ((S)-3-(4-Aminomethyl-phenyl)-2-(4-cyclohexyl-benze...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Binding affinity of the compound towards bovine trypsin was evaluated in vitro | Bioorg Med Chem Lett 8: 2563-8 (1999) BindingDB Entry DOI: 10.7270/Q20Z72FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50071723 ((S)-3-(4-Aminomethyl-phenyl)-N-cyclopentyl-N-methy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Binding affinity of the compound towards bovine trypsin was evaluated in vitro | Bioorg Med Chem Lett 8: 2563-8 (1999) BindingDB Entry DOI: 10.7270/Q20Z72FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-methyl-5'-thioadenosine phosphorylase (Mus musculus) | BDBM50368301 (CHEMBL606448) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description The inhibitory constant(Ki) for MTA phosphorylase activity was determined by using a mouse liver enzyme preparation | J Med Chem 34: 2600-6 (1991) BindingDB Entry DOI: 10.7270/Q2765FX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50069301 (CHEMBL155311 | N-hydroxyethyl-N-cyclopentyl-3-(4-h...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Compound was tested for inhibitory activity against bovine thrombin | Bioorg Med Chem Lett 8: 631-4 (1999) BindingDB Entry DOI: 10.7270/Q2CR5SHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50069055 ((S)-3-(4-Aminomethyl-phenyl)-N-cyclopentyl-N-methy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Binding affinity of the compound towards bovine trypsin was evaluated in vitro | Bioorg Med Chem Lett 8: 2563-8 (1999) BindingDB Entry DOI: 10.7270/Q20Z72FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50071726 ((S)-3-(4-Aminomethyl-phenyl)-N-cyclopentyl-N-methy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Binding affinity of the compound towards bovine trypsin was evaluated in vitro | Bioorg Med Chem Lett 8: 2563-8 (1999) BindingDB Entry DOI: 10.7270/Q20Z72FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-methyl-5'-thioadenosine phosphorylase (Mus musculus) | BDBM50368296 (CHEMBL608607) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description The inhibitory constant(Ki) for MTA phosphorylase activity was determined by using a mouse liver enzyme preparation | J Med Chem 34: 2600-6 (1991) BindingDB Entry DOI: 10.7270/Q2765FX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50069053 ((S)-3-(4-Aminomethyl-phenyl)-N-cyclopentyl-N-methy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.36E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Binding affinity of the compound towards bovine trypsin was evaluated in vitro | Bioorg Med Chem Lett 8: 2563-8 (1999) BindingDB Entry DOI: 10.7270/Q20Z72FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50071722 ((S)-3-(4-Aminomethyl-phenyl)-N-cyclopentyl-2-(6-me...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.42E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Binding affinity of the compound towards bovine trypsin was evaluated in vitro | Bioorg Med Chem Lett 8: 2563-8 (1999) BindingDB Entry DOI: 10.7270/Q20Z72FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50071724 ((S)-3-(4-Aminomethyl-phenyl)-N-cyclopentyl-2-(5-di...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.71E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Binding affinity of the compound towards bovine trypsin was evaluated in vitro | Bioorg Med Chem Lett 8: 2563-8 (1999) BindingDB Entry DOI: 10.7270/Q20Z72FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-methyl-5'-thioadenosine phosphorylase (Mus musculus) | BDBM50368300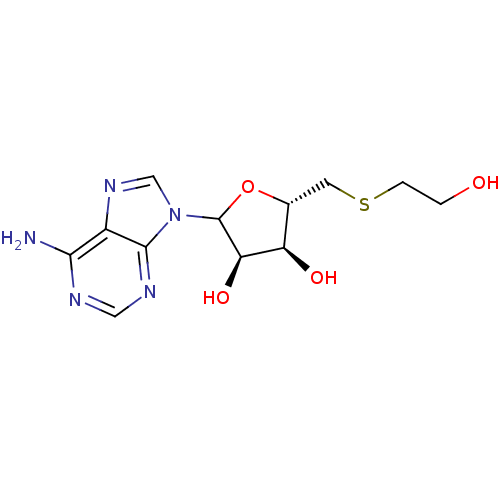 (CHEMBL608307) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description The inhibitory constant(Ki) for MTA phosphorylase activity was determined by using a mouse liver enzyme preparation | J Med Chem 34: 2600-6 (1991) BindingDB Entry DOI: 10.7270/Q2765FX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM50069294 ((S)-3-[4-(carbohydrazonamidol)-phenyl]-N-cyclopent...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.72E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description The compound was tested for inhibitory activity against tissue plasminogen activator | Bioorg Med Chem Lett 8: 631-4 (1999) BindingDB Entry DOI: 10.7270/Q2CR5SHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50069294 ((S)-3-[4-(carbohydrazonamidol)-phenyl]-N-cyclopent...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 4.79E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description The compound was tested for inhibitory activity against human plasmin | Bioorg Med Chem Lett 8: 631-4 (1999) BindingDB Entry DOI: 10.7270/Q2CR5SHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calpain-1 catalytic subunit (Homo sapiens (Human)) | BDBM50073850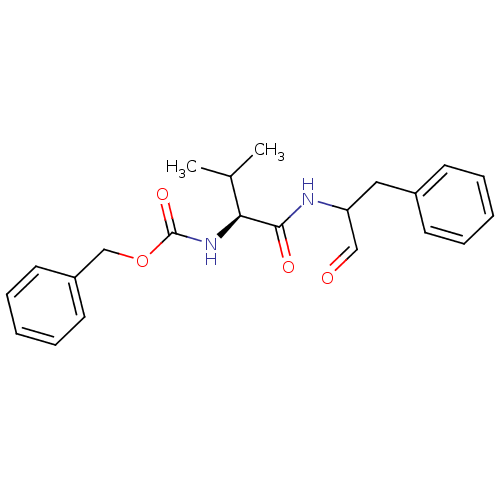 ((S)-2-((S)-2-Benzyloxycarbonylamino-3-methyl-butyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science & Technology Curated by ChEMBL | Assay Description Inhibitory concentration against human plasma mu-calpain | Bioorg Med Chem Lett 15: 2857-60 (2005) Article DOI: 10.1016/j.bmcl.2005.03.095 BindingDB Entry DOI: 10.7270/Q2Z31Z5J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM50554673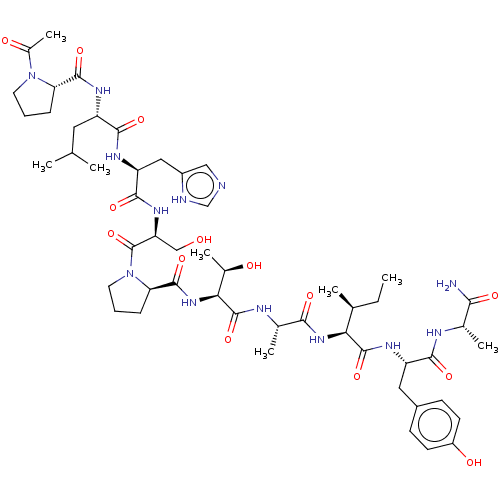 (CHEMBL4760204) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of 5-carboxyfluorescein-labeled PBD-binding peptide from PLk1 PBD (unknown origin) expressed in bacterial expression system incubated fo... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01451 BindingDB Entry DOI: 10.7270/Q2W38105 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calpain-1 catalytic subunit (Homo sapiens (Human)) | BDBM50167706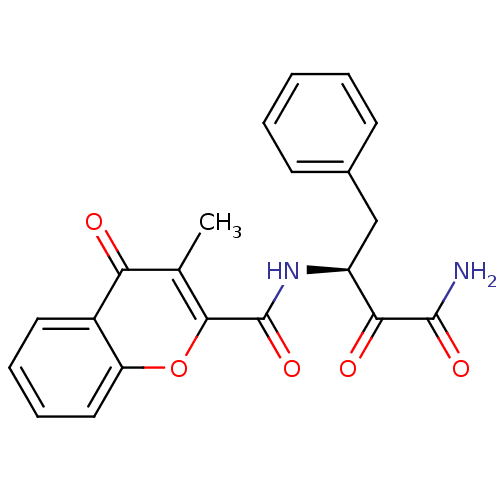 ((S)-N-(4-amino-3,4-dioxo-1-phenylbutan-2-yl)-3-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science & Technology Curated by ChEMBL | Assay Description Inhibitory concentration against human plasma mu-calpain | Bioorg Med Chem Lett 15: 2857-60 (2005) Article DOI: 10.1016/j.bmcl.2005.03.095 BindingDB Entry DOI: 10.7270/Q2Z31Z5J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50040212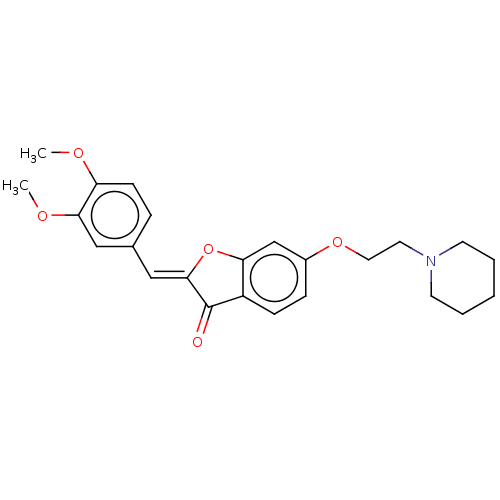 (CHEMBL3359473) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University Curated by ChEMBL | Assay Description Inhibition of ICR mouse brain AChE using acetylthiocholine iodide as substrate preincubated for 10 mins before substrate addition by modified Ellman'... | Bioorg Med Chem 23: 231-40 (2014) Article DOI: 10.1016/j.bmc.2014.11.004 BindingDB Entry DOI: 10.7270/Q2V126FS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calpain-1 catalytic subunit (Homo sapiens (Human)) | BDBM50167708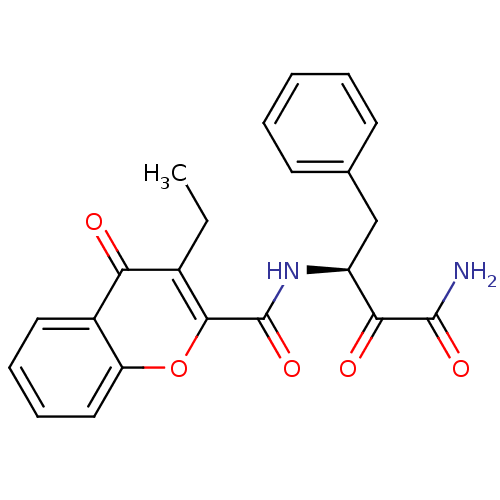 (3-Ethyl-4-oxo-4H-chromene-2-carboxylic acid ((S)-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science & Technology Curated by ChEMBL | Assay Description Inhibitory concentration against human plasma mu-calpain | Bioorg Med Chem Lett 15: 2857-60 (2005) Article DOI: 10.1016/j.bmcl.2005.03.095 BindingDB Entry DOI: 10.7270/Q2Z31Z5J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calpain-1 catalytic subunit (Homo sapiens (Human)) | BDBM50167707 (3-Methyl-4-oxo-4H-chromene-2-carboxylic acid ((S)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science & Technology Curated by ChEMBL | Assay Description Inhibitory concentration against human plasma mu-calpain | Bioorg Med Chem Lett 15: 2857-60 (2005) Article DOI: 10.1016/j.bmcl.2005.03.095 BindingDB Entry DOI: 10.7270/Q2Z31Z5J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calpain-1 catalytic subunit (Homo sapiens (Human)) | BDBM50167705 (3-Methyl-4-oxo-4H-chromene-2-carboxylic acid ((S)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 720 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science & Technology Curated by ChEMBL | Assay Description Inhibitory concentration against human plasma mu-calpain | Bioorg Med Chem Lett 15: 2857-60 (2005) Article DOI: 10.1016/j.bmcl.2005.03.095 BindingDB Entry DOI: 10.7270/Q2Z31Z5J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 150 total ) | Next | Last >> |